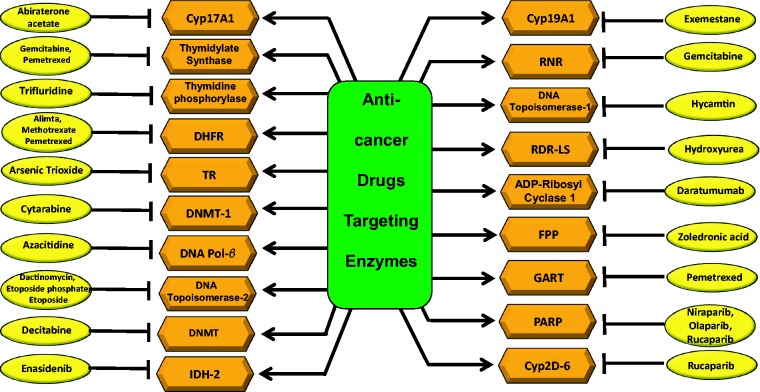
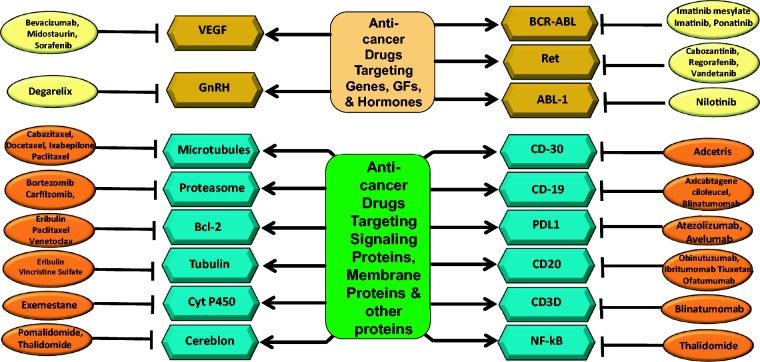
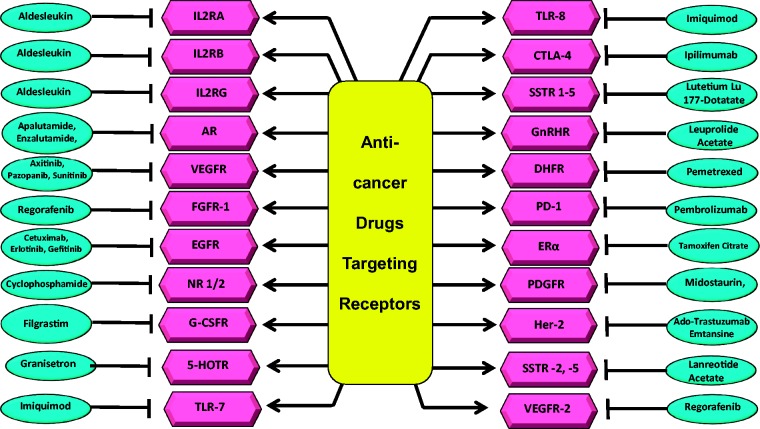
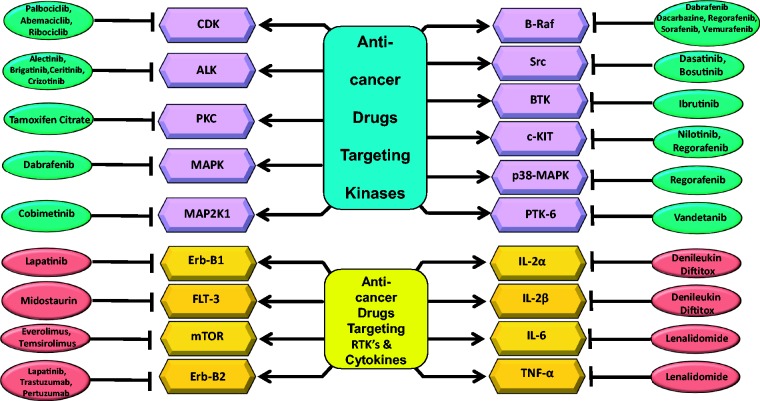
Short abstract
Although better science and technology has been linked with better health care, however, reality is much different. Although America and most of Europe are equipped with most advanced science and technology, paradoxically cancer incidence is highest in the world. This indicates that science and technology alone is not sufficient in treating diseases like cancer. It is also now well recognized that more than 95% of the drugs/compounds that kill either cancer cells in culture or regress the tumors in animals, fail in phase I clinical trials in humans, indicating that most pre-clinical models of cancer are inadequate. In addition, most of the anticancer drugs that are approved by the regulatory agencies such as FDA either has no effect on the overall survival of the cancer patient or may provide an increase in few months in overall survival. This is despite the fact that most targeted therapies that are currently available are highly expensive; thus suggesting the lack of affordability. This review is meant to focus on some of these problems in detail and then provide potential solutions since most cancers are caused by multiple genes, and thus multi-targeted therapies are needed such as natural products which are inexpensive, safe and have been used for thousands of years for both prevention and treatment of cancer.
Impact statement
The success rate for cancer drugs which enter into phase 1 clinical trials is utterly less. Why the vast majority of drugs fail is not understood but suggests that pre-clinical studies are not adequate for human diseases. In 1975, as per the Tufts Center for the Study of Drug Development, pharmaceutical industries expended 100 million dollars for research and development of the average FDA approved drug. By 2005, this figure had more than quadrupled, to $1.3 billion. In order to recover their high and risky investment cost, pharmaceutical companies charge more for their products. However, there exists no correlation between drug development cost and actual sale of the drug. This high drug development cost could be due to the reason that all patients might not respond to the drug. Hence, a given drug has to be tested in large number of patients to show drug benefits and obtain significant results.
Keywords: Cancer, drugs, patient survival, pre-clinical, clinical, cost
Introduction
Cancer is a group of more than 200 neoplastic diseases, caused by diverse deregulated cell signaling cascades.1 It represents the leading causes of morbidity as well as mortality across the globe and over the coming two decades, its incidence is predicted to increase by approximately 70%.2,3 Among all the cancers, lung cancer is reported to be most commonly diagnosed one followed by female breast cancer, prostate cancer, and colorectal cancer. Notably, lung cancer also represents the most common cause of death due to cancer followed by colorectal cancer, stomach cancer, and liver cancer.4 Further, lung cancer is the most common cancer and the leading cause of cancer death among males, whereas breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death among females.4,5 Consumption of tobacco and alcohol, obesity, insufficient physical activity, exposure to ultraviolet radiation, and various dietary factors which include insufficient fruit, non-starchy vegetables, and fiber; red/processed meat are predicted to be strongly associated with the risk of diverse cancer types.6–8 Cancer occurs as a result of the dysregulation of as many as 500 different genes which may happen over a very long duration of time (20–30 years) till the symptoms become apparent.1 Large-scale sequencing of human cancer genome has revealed 1007 somatic mutations in 274 megabases of DNA which corresponds to the coding exons of 518 protein kinase genes in 210 different human cancers comprising of 169 primary tumors, two early cultures, and 39 immortal cancer cell lines. The majority of the somatic mutations are predicted to be ‘‘passengers’’ with no role in the development of cancer, whereas “driver” mutations were well evinced to play role in oncogenesis.9 These mutations can be of different classes such as missense mutations, silent mutations, nonsense mutations, frame-shift mutations, insertions/deletions, and non-coding mutations. Among these, single nucleotide mutations are most common in kidney clear-cell carcinoma, hepatocellular carcinoma, acute myeloid leukemia, colorectal carcinoma, glioblastoma multiform, and endometrial carcinoma excluding the serous-like subtype. On the other hand, majority of lung and head and neck squamous cell carcinomas and all serous ovarian and breast carcinomas display copy-number variations.10 Notably, not only the variants in protein-coding regions of the genome but also the somatic and germline variants occurring in non-coding parts play important role in promoting tumorogenesis by affecting gene expression through diverse mechanisms.11 An integrative analysis of a total of 930 tumor whole genomes and matched transcriptomes identified a network of 193 non-coding loci in which mutations were found to disrupt the expression of target gene.12 Clustered regularly interspaced short palindromic repeats (CRISPR) barcoding also offers a well-suited method to functionally annotate specific mutations and study the sub-clonal mutations’ dynamics in heterogeneous tumor populations.13 A recent meta-analysis performed at the Broad Institute which includes 2957 whole exomes and 126 whole genomes from 27 cancer types clearly depicted the mutational heterogeneity in diverse cancer types.14 Further, Roerink et al. carried out a study to examine the nature as well as extent of intratumor diversification by characterizing organoids obtained from various single cells from three colorectal cancers and adjacent normal intestinal crypts. They found that colorectal cancer cells exhibited mutational diversification with more somatic mutations compared to normal colorectal cells.15
Cancer progresses in a stepwise fashion where the initiated cells, nodules, polyps or the development of papillomas progress further leading to more malignant condition. As mentioned, altered gene expression and their aberrant function are the key features of cancer. The alteration of proto-oncogenes into oncogenes may give rise to malignancy. Further, mutations can also convert proto-oncogenes into carcinogenic oncogenes.16,17 For instance, mutations in ras gene is observed in diverse tumors with varied incidence rate. Mutations in 12, 13, or 61 codon of one of the three ras genes namely, K-ras H-ras, and N-ras convert them into active oncogenes. Importantly, the highest incidences have been reported in adenocarcinomas of the pancreas (90%), whereas 50% in the colon and thyroid tumors and 30% in case of lung tumors and myeloid leukemia.18 Further, activation of nuclear factor kappa B (NF-κB) has also been linked with multiple processes in cancer cells such as proliferation, invasion, metastasis, angiogenesis, chemoresistance, and radioresistance.17,19,20 Another important signal which is reported to get overactivated in diverse range of tumors is phosphatidylinositol 3,5 trisphosphate production by phosphatidylinositol 3-kinase (PI3K), which in turn elicits a cascade of cellular responses including growth, proliferation, survival, and motility of cells ultimately leading to tumor progression.21 Further, dysregulation of the Keap1-Nrf2 pathway which is actively involved in the regulation of cytoprotective responses to oxidative and electrophilic stress was also reported in cancer cells.22 Again, Wnt signaling is another key cascade involved in the regulation of development and stemness, was found to play predominant role in different cancer types.23 In addition, different oxygen-derived species like hydrogen peroxide, superoxide radical, hydroxyl radical, and singlet oxygen are implicated to have active role in carcinogenesis.16 Furthermore, germline polymorphisms or mutations in DNA damage response (DDR) genes may also lead to the development of cancer.24
Problems with pre-clinical models for human cancer
The paradigm of translational oncology has shifted remarkably over the past decade, characterized by the launching of highly sophisticated molecular tools into the clinic.25 However, there exists a missing connection between pre-clinical data and clinical findings. Although, a significantly huge amount of money is spent in the pre-clinical settings for target validation and drug optimization, most of the therapies fail in the clinical trials till date. This can be due to the reason that the models used in the pre-clinical setting are not the adequate ones to effectively mimic human responses.26
Cell lines
Almost three-fourth of the total publications in cancer research are based on the application of 112 different cell lines.9 There are multiple reasons behind the usage of cancer cell lines as pre-clinical model of cancer such as they are cost-effective, immortalized, mostly homogeneous, easily perpetuated, and genetically alterable. Further, they possessed several intrinsic characteristics of cancer and have many genetic profiles and genomic modifications similar to that of primary human cancers.27,28 Further, cell lines confidently recapitulate the oncogenic alterations such as integrating somatic mutations, DNA methylation, copy number alterations, and gene expression identified in tumors. Moreover, it exerts usefulness in correlating the drug sensitivity/resistance with the alterations indicating the significance of tissue lineage in regulating drug response.29 Owing to these, they possess manifold intrinsic advantages for cancer research and development of novel cancer therapies. Notably, although highly convenient, these models are associated with several limitations as well.9,27 For example, existence of genomic instability which may result in differences between the original tumor and the respective cell line, culture conditions that can alter the morphology, gene expression pattern, genomic profile, cellular pathways and culture environment from that of the original tumor, loss of natural tumor heterogeneity, etc.27 Further, the generic transformations that occur upon culturing of the cancer cells are not restored when regrown in vivo. Besides, cancer cells in the in vitro condition grow in absence of stroma which include lymphatic vessels and blood, associated fibroblasts and immune cells, and lack a complex extracellular matrix. Therefore, in vitro data often exhibits fundamental mismatch with those obtained from clinical findings and hence this can be regarded as one prime reason behind the failure of novel drug development.28 In a study conducted by Pathak et al. suggested that in order to evaluate the efficacy of a novel compound against cancer, the use of only murine cells is not sufficient but a variety of cancer cells, including those of human origin should be used. They observed that anvirzel, an extract of oleander (Nerium oleander), and oleandrin, the compound derived from it, effectively killed human cancer cells, but were unable to do the same in murine cancer cells. From these results, it is evident that anvirzel and oleandrin function in a species-specific manner and hence evaluating their effect only in murine cells will not help in getting proper idea of their effectiveness against human cancers.30
Animal models
Not only with in vitro, there are several problems associated with the in vivo models of cancer as well. For in vivo studies, different small-animal models of human cancer have been generated such as inbred strains that develop cancer spontaneously, rodents wherein cancer is induced through postnatal exposure to chemical mutagens or intrauterine, and mice in which tumors are induced with the help of bacterial or viral infection.31 Mice and humans are believed to possess various notable similarities naturally as they are known to have diverged from each other.32 Hence various mouse models are extensively used in cancer research as they provide prime resource for cancer chemoprevention studies as well for developing novel cancer therapeutics through recapitulation of human disease in mice and also enabling the researchers to study both normal and aberrant gene interactions in different tumor types.33 The most commonly used animal model is athymic nude mice model because they have inhibited immune system due to which they are unable to reject human tumors. Besides, transgenic, gene knock out, xenograft, orthotopic, zebrafish, and several other animal models are used for carrying out different experiments.9 These models are extensively used to study the processes of carcinogenesis, role of specific gene target in cancer together with cancer drug development and thus they offer a strong basis for evaluating the various aspects of translational and clinical oncology.9,28
The foremost shortcomings of the use of these animal models are their inability to recapitulate the link between the tumor and its microenvironment completely and the requisite of an immunocompromised host.31 Basically, these animal models do not have the ability to reflect all the features of human cancer impeccably. This can be attributed to the presence of non-malignant cells and extracellular matrix within the microenvironment for the maintenance of tumor. Notably, the interactions between malignant and non-malignant cells decide the tumor fate and which in turn determines novel therapeutic regimen. However, replacement of these individual components with animal counterparts in the tumor site may change the tumor microenvironment remarkably. Further, inhibited immune system of animals in order to avoid rejection of human implants results in compromised normal function of the immune cells in the tumor microenvironment.28 The risks of over dependence on mice has been highlighted in a recent ‘experiment of nature’ wherein the mice deficient in RIPK1, a protein kinase that regulates cell death, immediately died right after birth due to the essential role of RIPK1. However, this deleterious effect of RIPK1 was not observed in case of patients with inactivated RIPK1 gene as the loss of RIP1 solely induced immunodeficiency in the human.34
In addition, cell line xenograft models possess multiple limitations despite their diverse range of applications such as genomic divergence upon extensive passages like differed gene expression, chromosome rearrangements, karyotype alterations, and disrupted growth rates.28 Recently, patient-derived xenograft (PDX) mice models have gained notable attention in cancer research. However, the engraftment rate of different cancer tissues into mouse is highly variable and therefore some human tumor subtypes are not represented in these PDX mouse collections.35 Notably, latest advances in the in vitro 3D culture techniques such as organoids have offered new opportunities to develop more physiological and novel human cancer models for translation of basic cancer research into novel treatment approaches for patients with cancer.36 Further, human organ chips which recreate organ-level physical microenvironments, tissue–tissue interfaces, and vascular perfusion also helps in studying tissue development and pathophysiology. It serves as an important tool for studying how molecular, cellular, physical, and chemical cues work alone as well as together, to impact human tissue development and disease.37
Failure of most anticancer drugs in phase I trials in human
Despite the advances in understanding of cancer biology and deriving different novel therapeutic targets, the translation of these understanding into therapies is poor due to higher failure rate (90%). The high failure rate could be due to non-consideration of factors such as clinical translation, drug delivery, drug pharmacokinetics, pre-clinical models, and tumor physiology, which are critical factors.38
Phase I trials in human are carried out to evaluate the safety and determine the maximum tolerated dose (MTD) of the drug of interest. While carrying out the same, the investigators of the trial must attribute the adverse effects as related or unrelated to drug studied. In general, the side effects related to drug studied are only taken into consideration which defines the MTD.39 These adverse events are known as dose-limiting toxicities (DLTs) which change according to the cancer types and the studied drug and should be pre-defined clearly before starting the study. Notably, the toxicities generated should not be life-threatening or irreversible to be regarded as DLTs; they simply have to be severe enough to terminate the treatment with that dose of the drug.40
Challenges associated with phase I trials
Phase I oncology trials are associated with several inherent challenges. Patients who did not respond to the all existing standard therapies are generally enrolled in this phase of trial. Therefore, there exist very limited treatment options for them with utterly short-life expectancy.41 Over the last two decades, a good deal of studies have entered from pre-clinical to the phase I trial in human. However, the response rate obtained was around 4–10% only with a median overall survival (OS) of six months.42 For instance, Smith et al. conducted two phase I clinical trials on the combination of idelalisib, rituximab, and lenalidomide in follicular lymphoma and mantle cell lymphoma. The doublets lenalidomide–rituximab and idelalisib–rituximab were combined safely and found effective in several clinical lymphoma settings. But several adverse effects such as fevers, hypotension, and rash, hepatotoxicity and pulmonary infiltrates were observed. Therefore, both the trials were dismissed.43 Another phase I trial was conducted to access the safety and tolerability of idelalisib, lenalidomide, and rituximab in case of relapsed and refractory lymphoma. The trial enrolled a total of 11 patients of whom three had mantle cell lymphoma and eight had follicular lymphoma. In this trial, four patients were found to experience dose-limiting toxicities after 9–20 days of initiation of treatment, coinciding with rituximab infusions. Therefore, both studies were amended to eliminate rituximab, however two patients developed grade 3 rashes and for one patient grade 3 aspartate aminotransferase elevation was reported. As this trial was unsuccessful in meeting the primary endpoint of safety and tolerability, it was terminated. Collectively, this phase I trial led to the interpretation that the combination of idelalisib, lenalidomide, and rituximab is highly toxic and these trials serve as warning notes as new combinations are designed.44 Further, the results of a phase I clinical trial of BEZ235 in advanced renal cell carcinoma (RCC) patients was reported by Carlo et al. in the year 2016. BEZ235 is a dual pan-class phosphoinositide 3-kinases (PI3K) and mammalian target of rapamycin (mTOR) inhibitor which is presently undergoing phase I/II clinical trials in solid tumors and hematologic malignancies. In this study, Carlo et al. reported increased administration of BEZ235 resulted in increased incidence of grade 3–4 side effects in 50% of the patients without objective responses in the evaluable patients. As pan-PI3K inhibitor attempts to stop the effect of total PI3K in tumor, it fails to exploit dependency to a particular isoform which in turn could avoid redundant effects through inhibition of other isoforms which have no role to play.45,46 Therefore, a new clinical trial is undergoing to assess the effectiveness of isoform specific PI3Ka inhibitor namely BYL719, together with everolimus in advanced RCC and pancreatic neuroendocrine tumor (PNET) patients.45 Therefore, besides, effectiveness, emerging toxicities, and tolerability issues should be cautiously evaluated while assessing the biological agents either alone or in combination and should strictly be performed in clinical setting.
Attrition and phase I trials
Importantly, attrition is the foremost concern in anticancer drug development as up to 95% of drugs checked in phase I trials are not achieving the marketing authorization causing the process of drug development an extremely inefficient and costly affair.47 In the United States, drug costs are also the focus of political discourse.48 It is imperative that this problem is addressed throughout the entire drug development process in order to improve efficiency which will consequently benefit the patients with more profitable drugs. Mainly three approaches must be followed to decrease the cancer drug attrition rates; first better pre-clinical models must be included for the study, secondly clinical trials must incorporate the predictive and pharmacodynamic biomarkers and there should be more collaboration between the industry, academia, and regulators to guarantee that the interests of all the stakeholders are met.47
Efficacy of anticancer drugs approved and patient survival
Cancer is an utterly complex cluster of diseases with complicated processes of clinical development for onco-therapies. Before a new drug or biologic finally gets approved by the Food and Drug Administration (FDA), it passes through ample in vitro and in vivo research, and clinical trials spanning several years. Due to the complexity of cancer, clinical research for these anticancer drugs takes around 1.5 years on average longer than drugs for other diseases.49 Long time taken for developing novel cancer therapeutics can partly be attributed to the slow progress in clinical development.50 In the last two decades, the number of therapeutic regimens developed against cancer has doubled annually which can be attributed to the marked improvements achieved in cancer research through human genome sequencing, identification of vital signaling pathways, growth factors, and their receptors resulting in approximately 1884 phase I, 3436 phase II, and 1025 phase III cancer clinical trials49 (Table 1). But surprisingly, the success rate for cancer drugs from phase III clinical trials to final approval remains as low as 3.4% only.52 A recent study reported that drugs and agents against cancer which are sponsored by small and medium-size companies possess remarkably less chance of getting approved. Not only that, the extent of benefit obtained in a clinical trial is found to be often less compared to the one predicted at the time of trial design as evinced by an analysis of 235 recently published data of phase III randomized clinical trials (RCTs), where 62% of the trials failed to obtain statistically significant results.53 As a matter of fact, the median progress in survival of patients with solid tumors treated with 71 drugs was found to be 2.1 months only.54
Table 1.
Efficacy of drugs against cancer approved by FDA in the last 10 years.
| Drug | Cancer | Trial | Efficacy |
|---|---|---|---|
| Drugs approved in 2018 | |||
| Abemaciclib (VERZENIO) vs. PBO | Breast cancer | MONARCH 3 | 28.2 vs. 14.8 moa |
| Abiraterone acetate (Zytiga) + prednisone | CSPC | LATITUDE | Median OS was not estimable |
| Afatinib (Gilotrif) | NSCLC | LUX-Lung 2, | 66%b |
| LUX-Lung 3, | |||
| LUX-Lung 6 | |||
| Apalutamide (Erleada) vs. PBO | Prostate cancer | SPARTAN | 40.5 vs. 16.2 mod |
| Brentuximab vedotin (Adcetris) + Chemotherapy vs. chemotherapy | cHL | ECHELON-1 | Median PFS was not reached |
| Bevacizumab (Avastin) + CP vs. CP | Ovarian cancer | GOG-0218 | 12.8 vs. 12.0 moa; 43.8 vs. 40.6 moc |
| Cemiplimab-rwlc (LIBTAYO) | CSCC | R2810-ONC-1423, R2810-ONC-1540 |
47%b (metastatic); 49%b (locally advanced |
| Dabrafenib (TAFINLAR) + trametinib (MEKINIST) vs. PBO | Melanoma | COMBI-AD | Improved RFS in treatment |
| Dabrafenib + trametinib | Thyroid cancer | BRF117019 | 61%b |
| Dacomitinib vs. gefitinib | NSCLC | ARCHER | 14.7 vs. 9.2 moa |
| Durvalumab (Imfinzi) vs. PBO | NSCLC | PACIFIC | 16.8 vs. 5.6 moa |
| Duvelisib (COPIKTRA) vs. ofatumumab | CLL, SLL | NCT02004522 | 16.4 vs. 9.1 moa; 78% vs. 39%b |
| Encorafenib (BRAFTOVI) + binimetinib (MEKTOVI) vs. vemurafenib | Melanoma | COLUMBUS | 14.9 vs. 7.3 moa; 63% vs. 40%b |
| Enzalutamide (XTANDI) vs. PBO | CRPC | PROSPER | 36.6 vs. 14.7 mod |
| Iobenguane I 131 (AZEDRA) | PPGL | Study IB12B | – |
| Ipilimumab (YERVOY) + Nivolumab | CRC | CheckMate-142 | 46%b |
| Ivosidenib (Tibsovo) | AML | AG120-C-001 | 32.8%g |
| Lenvatinib (Lenvima) vs. sorafenib | HCC | REFLECT | 7.3 vs. 3.6 moa; 13.6 vs. 12.3 moc |
| Linatumomab (Blincyto) | ALL | BLAST | 35.2 mof (CR1); 12.3 mof (CR2) |
| Lutetium Lu 177 dotatate (LUTATHERA) | GEP-NETs | NETTER-1 | 16%b |
| Nilotinib (TASIGNA) | Ph+ CML-CP | CAMN107A2120 | MMR 64.0% |
| Nivolumab (Opdivo) + ipilimumab (Yervoy) vs. sunitinib | RCC | CheckMate-214 | 41.6% vs. 26.5%b |
| Nivolumab (Opdivo) | SCLC | CheckMate-032 | 12%b |
| Olaparib (Lynparza) vs. chemotherapy | Breast cancer | OlympiAD | 7.0 vs. 4.2 moa |
| Osimertinib (Tagrisso) vs. SOC | NSCLC | FLAURA | 18.9 vs. 10.2 moa; 77% vs. 69%b |
| Pembrolizumab (Keytruda) | PMBCL | KEYNOTE‑170 | 45%b |
| Pembrolizumab (Keytruda) | Cervical cancer | KEYNOTE-158 | 14.3%b |
| Pembrolizumab (KEYTRUDA) + PemC vs. PemC | NSCLC | KEYNOTE-189 | 8.8 vs. 4.9 moa; 48% vs.19%b |
| Ribociclib (Kisqali) vs. PBO | Breast cancer | MONALEESA-7 | 27.5 vs. 13.8 moa |
| Rucaparib (Rubraca) vs. PBO | Peritoneal cancer | ARIEL3 | 10.8 vs. 5.4 moa |
| Tisagenlecleucel (KYMRIAH) | B-cell lymphoma | JULIET | 50%b; 32%g |
| Venetoclax (VENCLEXTA) + RTX vs. bendamustine + RTX | CLL/SLL | MURANO | 92% vs. 72%b |
| Drugs approved in 2017 | |||
| Abemaciclib (VERZENIO) | Breast cancer | MONARCH 1 | 19.7%b |
| Acalabrutinib (Calquence) | MCL | LY-004 | 81%b; 40%g |
| Alectinib (ALECENSA) vs. crizotinib | NSCLC | ALEX | 25.7 vs. 10.4 moa; 79% vs. 72%b |
| Avelumab (BAVENCIO) | Urothelial cancer | – | 16.1%b |
| Avelumab (BAVENCIO) | MCC | JAVELIN Merkel 200 | 33%b |
| Axicabtagene ciloleucel (YESCARTA) | B-cell lymphoma | – | 72%b; 51%g |
| Blinatumomab (BLINCYTO) vs. SOC chemotherapy | ALL | TOWER | 7.7 vs. 4.0 moc |
| Bosutinib (BOSULIF) vs. imatinib | PH + CML | BFORE | MMR 47.2% vs. 36.9% |
| Brentuximab vedotin (ADCETRIS) | pcALCL vs. MTX/bexarotene | ALCANZA | 17 vs. 4 moa; 16% vs. 2%g |
| Brigatinib (ALUNBRIG) 90 mg/day vs. 180 mg/day | NSCLC | ALTA | 48% vs. 53%b |
| Cabozantinib (Cabometyx) vs. sunitinib | RCC | CABOSUN | 8.6 vs. 5.3 moa |
| Ceritinib (ZYKADIA) vs. Pt-pemetrexed | NSCLC | ASCEND-4 | 16.6 vs. 8.1 moa; 73%b |
| Copanlisib (ALIQOPA) | FL | Phase II | 58.7%b |
| Dabrafenib (TAFINLAR) + trametinib (MEKINIST) | NSCLC | BRF113928 | 63%b |
| Dasatinib (SPRYCEL) | PH + CML | Phase I & II | – |
| Enasidenib (IDHIFA) | AML | AG221-C-001 | 23%g |
| Gemtuzumab ozogamicin (Mylotarg) vs. BSC | AML | AML-19 | 4.9 vs. 3.6 moc |
| Lenalidomide (Revlimid) vs. PBO | MM | CALGB 100104, | 111 vs. 106 moc (CALGB); |
| IFM 2005-02 | 84 vs. 88 moc (IFM) | ||
| L-encap-DAU + ARA-C (VYXEOS) vs. DAU + ARA-C | AML | CLTR0310-301 | 9.6 vs. 5.9 moc |
| Midostaurin (RYDAPT) vs. PBO | AML | – | Significant increase in OS |
| Neratinib (NERLYNX) vs. PBO | Breast cancer | ExteNET | IDFS- 94.2% vs. 91.9% |
| Niraparib (ZEJULA) vs. PBO | Peritoneal cancer | NOVA | 21 vs. 5.5 moa (BRCA mutation); 9.3 vs. 3.9 moa (no BRCA mutation) |
| Nivolumab (OPDIVO) | HCC | CheckMate-040 | 14.3%b |
| Nivolumab (OPDIVO) | CRC | CheckMate-142 | 32%b |
| Nivolumab (OPDIVO) | Urothelial cancer | – | 19.6%b |
| Nivolumab (OPDIVO) vs. ipilimumab | Melanoma | CheckMate-238 | Recurrences/deaths- 34% vs. 45.5% |
| Obinutuzumab (GAZYVA) vs. RTX | FL | GALLIUM | 91% vs. 88%b |
| Olaparib (Lynparza) vs. PBO | Ovarian cancer | SOLO-21 | 9.1 vs. 5.5 moa |
| Osimertinib (TAGRISSO) vs. chemotherapy | NSCLC | AURA3 | 10.1 vs. 4.4 moa; 65% vs. 29%b |
| Ozogamicin (BESPONSA) | ALL | INO-VATE ALL | 35.8%g |
| Palbociclib (IBRANCE) + letrozole vs. PBO | Breast cancer | PALOMA-2 | 24.8 vs. 14.5 moa |
| Pembrolizumab (KEYTRUDA) | Gastric cancer | KEYNOTE 059 | 13.3%b |
| Pembrolizumab (KEYTRUDA) | cHL | – | 69%b |
| Pembrolizumab (KEYTRUDA) vs. Pt-chemotherapy | Urothelial cancer | KEYNOTE-045 | 21% vs. 11%b; 10.3 vs. 7.4 moc |
| Pembrolizumab (KEYTRUDA) + PemC vs. PemC | NSCLC | KEYNOTE-021 | 13.0 vs. 9 moa; 55% vs. 29%b |
| Pertuzumab (PERJETA) + trastuzumab vs. PBO | Breast cancer | APHINITY | IDFS-8.2% vs. 10.6% |
| Ribociclib (KISQALI) + LET vs. PBO+ LET | Breast cancer | MONALEESA-2 | 52.7% vs. 37.1%b |
| Drugs approved in 2016 | |||
| Abozantinib (CABOMETYX) vs. EVR | RCC | – | 7.4 vs. 3.8 moa; 21.4 vs. 16.5 moc |
| Atezolizumab (Tecentriq) | Urothelial cancer | – | 14.8%b |
| Atezolizumab (TECENTRIQ) vs. Dox | NSCLC | OAK, POPLAR | 13.8 vs. 9.6 moc (OAK); 12.6 vs. 9.7 moc (POPLAR) |
| Daratumumab (DARZALEX) + lenalido- mide +DXM vs. lenalidomide + DXM | MM | POLLUX | Median PFS was not reached |
| Eenetoclax (VENCLEXTA) | CLL | – | 80%b |
| Eribulin (HALAVEN®) + dacarbazine | Liposarcoma | – | 13.5 vs. 11.3 moc |
| Lenvatinib +EVR vs. EVR | RCC | – | 14.6 vs. 5.5 moa |
| Nivolumab (Opdivo) | cHL | – | 65%b; 8.7 moe |
| Nivolumab (OPDIVO) vs. ICC | SCCHN | CheckMate-141 | 7.5 vs. 5.1 moc |
| Obinutuzumab (Gazyva)+ bendamustine vs. bendamustine | FL | – | Median PFS was not reached |
| Palbociclib (IBRANCE) + fulvestrant vs. PBO + fulvestrant | Breast cancer | – | 9.5 vs. 4.6 moa |
| Pembrolizumab (KEYTRUDA | HNSCC | – | 16%b; 2.4–27.7 moe |
| Pembrolizumab (KEYTRUDA) vs. Pt - chemotherapy | NSCLC | – | 10.3 vs. 6.0 moa |
| Rizotinib (Xalkori) | NSCLC | – | 66%b; 18 moe |
| Rucaparib (RUBRACA) | Ovarian cancer | – | 54%b; 9.2 moe |
| Drugs approved in 2015 | |||
| Alectinib (ALECENSA) | NSCLC | – | 61%b; 9.1 moe |
| Brentuximab vedotin (ADCETRIS) vs. PBO | cHL | PBO controlled | 42.9 vs. 24.1 moa |
| Carfilzomib (Kyprolis) + lenalidomide + DXM vs. lenalidomide + DXM | MM | PX-171-009 ASPIRE | 26.3 vs. 17.6 moa |
| Cobimetinib (COTELLIC) + vemurafenib vs. vemurafenib | Melanoma | Controlled | 2.3 vs. 7.2 moa; 70% vs. 50%b |
| Dabrafenib + trametinib vs. dabrafenib + PBO | Melanoma | – | 9.3 vs. 8.8 moa; 66% vs. 51%b; 25.1 vs. 18.7 moc |
| Daratumumab (DARZALEX) | MM | Open label | 29%b |
| Gefitinib (IRESSA) vs. CBP/PTX | NSCLC | Open-label | 10.9 vs. 7.4 moa; 67% vs. 41%b; 9.6 vs. 5.5moe |
| Ipilimumab (Yervoy) vs. PBO | Melanoma | Controlled | 26 vs. 17 mof |
| Irinotecan liposome (ONIVYDE) + 5FU/LV vs. 5FU/LV | Pancreatic cancer | Open-label | 3.1 vs. 1.5 moa; 6.1 vs. 4.2 moc |
| Ixazomib (NINLARO) + lenalidomide + DXM vs. PBO + lenalidomide + DXM | MM | – | 20.6 vs. 14.7 moa |
| Lenvatinib (Lenvima) vs. PBO | Thyroid cancer | E7080-G00-303 | 18.3 vs. 3.6 moa; 65% vs. 2%b |
| Lotuzumab (EMPLICITI) + enalidomide + DXM vs. lenalidomide +DXM | MM | – | 19.4 vs. 14 moa; 78.5% vs. 65.5%b |
| Necitumumab (PORTRAZZA) + GC vs. GC | NSCLC | – | 5.7 vs. 5.5 moa; 31% vs. 29%b |
| Nivolumab (Opdivo) vs. Dox | NSCLC | Open-label | 19% vs. 12%b; 12.2 vs. 9.4 moc |
| Nivolumab (Opdivo) vs. EVR | RCC | – | 21.5% vs. 3.9%b; 25.0 vs. 19.6 moc |
| Nivolumab (Opdivo) + ipilimumab vs. ipilimumab | Melanoma | Active-controlled | 8.9 vs. 4.7 moa; 60% vs. 11%b |
| Osimertinib (TAGRISSO) | NSCLC | Open label | 57%b (study 1); 61%b (study 2) |
| Palbociclib (IBRANCE) + LET vs. LET | Breast cancer | Open-label | 20.2 vs. 10.2 moa; 55.4% vs. 39.4%b |
| Panobinostat (FARYDAK) + bortezomib + DXM vs. PBO + bortezomib + DXM | MM | PBO-controlled | 10.6 vs. 5.8 moa; 8.5% vs. 41.4%b |
| Pembrolizumab (KEYTRUDA) | NSCLC | Open-label | 41.0%b |
| Pembrolizumab (Keytruda) Q2W vs. Q3W vs. ipilimumab | Melanoma | – | 34% vs. 33% vs. 12%b |
| Ramucirumab (CYRAMZA) + FOLFIRI vs. PBO + FOLFIR | CRC | – | 5.7 vs. 4.5 moa; 13.3 vs. 11.7 moc |
| Sonidegib (Odomzo) | BCC | – | 58%b |
| Trabectedin (Yondelis) vs. DTIC | Liposarcoma | Open‑label | 4.2 vs. 1.5 moa; 7% vs. 6%b; 13.7 vs. 13.1 moc |
| Trifluridine/tipiracil (LONSURF) vs. PBO | CRC | PBO-controlled | 7.1 vs. 5.3 moc |
| Drugs approved in 2014 | |||
| Amucirumab (Cyramza) + BSC vs. PBO + BSC | GEJ | I4T-IE-JVBD | 5.2 vs. 3.8 moc |
| Belinostat (BELEODAQ) | PTCL | – | 25.8%b; 8.4 moe |
| Bevacizumab (Avastin) + PTX vs. PTX | Ovarian/fallopian tube/peritoneal cancer | AURELIA | 6.8 vs. 3.4 moa; 16.6 vs. 13.3 moc |
| Bevacizumab + chemotherapy vs. chemotherapy | Cervical cancer | – | 16.8 vs. 12.9 moc |
| Blinatumomab (BLINCYTO) | R/R ALL | MT103-211 | 6.7 moe |
| ceritinib (ZYKADIA) | NSCLC | Open-label | 44%b; 7.1 moe |
| Ibrutinib (IMBRUVICA) | MCL | – | 58.3%b |
| Idelalisib (Zydelig) + RTX vs. PBO + RTX | CLL | PBO-controlled | Median PFS was not reached |
| Lanreotide (somatuline depot) vs. PBO | GEP-NETs | Trial 2-55-52030-726 | Median PFS was not reached |
| Laparib (Lynparza) | Ovarian cancer | – | 34%b; 7.9 moe |
| Nivolumab (OPDIVO) vs. ICC | Melanoma | Open-label | 32%b |
| Ofatumumab (Arzerra) + CBC vs. CBC | CLL | Open-label | 22.4 vs. 13.1 moa |
| Pembrolizumab (KEYTRUDA) | Melanoma | Trial P001 | 24%b |
| Ramucirumab (Cyramza) + PTX vs. PBO + PTX | GEJ | I4T-IE-JVBE | 9.6 vs. 7.4 moc |
| Ramucirumab (Cyramza) + Dox vs. PBO+ Dox | NSCLC | I4T-MC-JVBA | 10.5 vs. 9.1 moc |
| Trametinib (Mekinist) + dabrafenib vs. dabrafenib | Melanoma | Open-label | 76% vs. 54%b; 10.5 vs. 5.6 moe |
| Drugs approved in 2013 | |||
| Ado-tras emtansine (KADCYLA) vs. LAP + CAP | Breast cancer | Open-label | 9.6 vs. 6.4 moa; 30.9 vs. 25.1 moc |
| Afatinib (Gilotrif) vs. chemotherapy | NSCLC | Open-label | 11.1 vs. 6.9 moa; 50.4% vs. 19.1%b |
| Bevacizumab (Avastin) + chemotherapy vs. chemotherapy | CRC | Open-label | 5.7 vs. 4.0 moa; 11.2 vs. 9.8 moc |
| Crizotinib (Xalkori) vs. chemotherapy | NSCLC | Open-label | 7.7 vs. 3.0 moa; 7.4 vs. 5.6 moe |
| Dabrafenib (TAFINLAR) vs. dacarbazine | Melanoma | Open-label | 5.1 vs. 2.7 moa; 52% vs. 17%b |
| Ibrutinib (IMBRUVICA) | MCL | – | 66%b; 17.5 moe |
| Obinutuzumab (GAZYVA) + CBC vs. CBC | CLL | Open-label | 23.0 vs. 11.1 moa |
| Lenalidomide (REVLIMID) | MCL | – | 26%b; 16.6 moe |
| Pertuzumab (PERJETA) + Tras + Dox vs. Tras + Dox | Breast cancer | – | pCR rate 39.3% vs. 21.5% |
| Pomalidomide (POMALYST) + DXM vs. pomalidomide | MM | CC-4047-MM-002 | 29% vs. 7%b |
| PTX (albumin-bound) + GEM vs. GEM | Pancreatic cancer | Open-label | 5.5 vs. 3.7 moa; 23% vs. 7%b |
| Ra 223 dichloride (Xofigo) vs. PBO | Prostate cancer | Open-label | 14.0 vs. 11.2 moc |
| Sorafenib (NEXAVAR) vs. PBO | Thyroid cancer | PBO-controlled | 10.8 vs. 5.8 moa; 2% vs. 1%b |
| Trametinib (MEKINIST) vs. chemotherapy | Melanoma | Open-label | 4.8 vs. 1.5 moa; 22% vs. 8%b |
| Drugs approved in 2012 | |||
| Axitinib (Inlyta) vs. sorafenib | RCC | Open-label | 6.7 vs. 4.7 moa |
| Cabozantinib (COMETRIQ) vs. PBO | Thyroid cancer | PBO controlled | 11.2 vs. 4.0 moa |
| Carfilzomib injection (Kyprolis) | MM | – | 22.9%b |
| Cetuximab (Erbitux) + FOLFIRI vs. FOLFIRI | CRC | CRYSTAL | 8.9 vs. 8.1 moa; 19.6 vs. 18.5 moc |
| Enzalutamide (XTANDI) vs. PBO | Prostate cancer | PBO controlled | 18.4 vs. 13.6 moc |
| Everolimus (Afinitor) + exemestane vs. Exemestane + PBO | Breast cancer | – | 7.8 vs. 3.2 moa; 12.6% vs. 1.7%b |
| Pazopanib (VOTRIENT) vs. PBO | STS | PBO controlled | 4.6 vs. 1.6 moa; 12.6 vs. 10.7 moc |
| Pertuzumab injection (PERJETA) + Tras + Dox vs. PBO + Tras + Dox | Breast cancer | PBO controlled | 18.5 vs. 12.4 moa |
| PTX (albumin-bound; ABRAXANE) vs. PTX | NSCLC | Protocol CA031 | 33% vs. 25%b; 6.9 vs. 6.0 moe |
| Regorafenib (Stivarga) vs. PBO | CRC | Study 14387 | 2.0 vs. 1.7 moa |
| RTX infusion | NHL | RATE trail | – |
| VinCRIStine sulfate LIPOSOME injection (Marqibo) | ALL | HBS407 | 4.6%g |
| Vismodegib (ERIVEDGE) | BCC | – | 30.3%b; 7.6 moe |
| Ziv-aflibercept injection (Zaltrap) + FOLFIRI vs. FOLFIRI + PBO | CRC | Phase III | 6.9 vs. 4.7 moa; 13.5 vs. 12.06 moc |
| Drugs approved in 2011 | |||
| Abiraterone acetate (Zytiga) +prednisone | Prostate cancer | RPC | 14.8 vs. 10.9 moc |
| AEC (Erwinaze) | ALL | EMTP | – |
| Brentuximab Vedotin | ALCL | – | 86%b |
| Cetuximab (Erbitux) + 5-FU | SCCHN | Clinical | 19.1 vs. 18.2 moc |
| Crizotinib (XALKORI) | NSCLC | – | 50%b |
| Denosumab (Prolia) vs. PBO | Prostate/Breast cancer | DBPC | Significant effect 24 vs. 12 mo |
| Everolimus (Afinitor) vs. PBO | Pancreatic cancer | RC | 11.0 vs. 4.6 moa |
| Ipilimumab injection (YERVOY) tumor vaccine | Melanoma | DBPC | 10 vs. 6 moc |
| Rituximab (Rituxan) | B-cell NHL | Phase III | 46%a |
| Sunitinib (Suten) vs. PBO | Pancreatic cancer | RC | 10.2 vs. 5.4 moa |
| Vemurafenib (ZELBORAF) vs. dacarbazine prednisone vs. PBO | Melanoma | Random | OS 6.2 vs. 4.5 mo |
| Vandetanib vs. PBO | Thyroid cancer | DBPC | No significant OS |
| Drugs approved in 2010 | |||
| Cabazitaxel (Jevtana) vs. mitoxantrone | Prostate cancer | Random | Median survivals 15.1 vs. 12.7 mo |
| Dasatinib (Sprycel) vs. imatinib | CML | RC | MMR 52.1% vs. 33.8% |
| Erlotinib (Tarceva) | NSCLC | DBPC | hazard ratios 0.69a, 0.77c |
| Eribulin mesylate (Halaven) | Breast cancer | EMBRACE | 10.6 moc |
| Nilotinib (Tasigna) vs. imatinib | CML | RC | MMR 44% vs. 22% |
| Rituximab (Rituxan) vs. FC | CLL | ML17102 | 39.8 vs. 31.5 moa |
| Rituximab (Rituxan) vs. FC | CLL | BO17072 | 39.8 vs. 31.5 moa |
| Tykerb (lapatinib) vs. PBO + letrozole | Breast cancer | RPC | 35.4 vs. 13.0 weeksa |
5FU: fluorouracil; AEC: asparaginase Erwinia chrysanthemi; ALL: acute lymphoblastic leukemia; ALCL: anaplastic large cell lymphoma; AML: acute myeloid leukemia; ARA-C: cytarabine; BCC: basal cell carcinoma; BSC: best supportive care; CAP: capecitabine; CBP: carboplatin; CBC: chlorambucil; CCyR: complete cytogenic response; cHL: classical Hodgkin lymphoma; CLL: chronic lymphocytic leukemia; cp: carboplatin + paclitaxel; CR: complete remission; CRPC: castration-resistant prostate cancer; CSCC: cutaneous squamous cell carcinoma; CSPC: castration-sensitive prostate cancer; DAU: daunorubicin; DBPC:double-blind, placebo-controlled; DoR: duration of response; DXM: dexamethasone; Dox:docetaxel; DTIC: dacarbazine; EVR: everolimus; FC: fludarabine and cyclophosphamide; FL: follicular lymphoma; FOLFIRI: folinic acid + 5-fluorouracil + irinotecan; GC: gemcitabine and cisplatin; GEP-NETs: gastroenteropancreatic neuroendocrine tumors; GEJ: gastric or gastroesophagal junction; ICC: investigator’s choice of chemotherapy; IDFS: invasive disease-free survival; LAP: lapatinib; L-encap: liposome encapsulated; LET: letrozole; LV: leucovorin; MCC: Merkel cell carcinoma; MCL: mantle cell lymphoma; mCRC: metastatic colorectal cancer; MFS: metastasis-free survival; MMR: MajoR molecular response; mo: months; MRD: minimal residual disease; MTX: methotrexate; MM: multiple myeloma; NHL: Non-Hodgkin’s lymphoma; NSCLC: non-small cell lung cancer; ORR: overall response rate; ORR4: objective response rate lasting 4 months; OS: overall survival; PBO: placebo; pcALCL: primary cutaneous anaplastic large cell lymphoma; PemC: premetrexed and carboplatin; PFS: progression-free survival; PH + CML: Philadelphia chromosome positive chronic myelogenous leukemia; Ph + CML-CP: Philadelphia chromosome positive chronic myeloid leukemia in chronic phase; PHEO: pheochromocytoma; PMBCL: primary mediastinal large B-cell lymphoma; pCR: pathological complete response; PFS: progression-free survival; Pt: platinum; PPGL: pheochromocytoma/paraganglioma; PTCL: peripheral T-cell lymphoma; PTX: paclitaxel; Q2W: 10 mg/kg intravenously every 2 weeks; Q3W: 10 mg/kg every 3 weeks; Ra: radium; RC: randomized controlled; RCC: renal cell carcinoma; RPC: randomized, placebo-controlled; R/R ALL: relapsed or refractory B-cell precursor acute lymphoblastic leukemia; RFS: relapse-free survival; RTX: rituximab; SCCHN: squamous cell carcinoma of the head and neck; SCLC: small cell lung cancer; SLL: small lymphocytic lymphoma; SOC: standard-of-care; STS: soft tissue sarcoma; Tras: trastuzumab.
aMedian PFS.
bORR.
cMedian OS.
dMFS.
eMedian DoR.
fRFS.
gCR rate.
This information has been gathered from: https://www.fda.gov.51
There are a few examples of transformative cancer medicines with huge benefits to the patients.55 For instance, in 2001, imatinib was approved for second-line therapy against chronic myeloid leukemia (CML) on the basis of hematologic and cytogenic response rates in trial participants. Six years after initial approval, the Insulin Resistance Intervention After Stroke study reported imatinib-treated CML patients had 0% disease progression rate with an estimated OS rate of 88%.49 However, in most of the cases, the available cancer drugs exert survival benefits only to a marginal extent. In an evaluation of 71 drugs approved for treating solid tumors between 2002 and 2012, the median improvement in OS in pivotal trials was found to be only 2.1 months. Further, out of 47 consecutive approvals for cancer drugs, only 9% showed a relative improvement in survival of 25% or an absolute increase of 2.5 months.56 Another report also showed that out of the 62 new active anticancer molecules approved by the FDA and EMA during 2003–2013, 53 were assessed by the Australian, English, or French, or health technology assessment agencies through May 2015. Among these 53 drugs, 23 increased OS by three months or more, six of them improved the OS by less than three months, and eight caused increased OS by an unknown magnitude whereas the remaining16 failed to increase OS over best alternative treatments. Although 22 of 53 new medicines were found to cause an increase in quality of life (QoL), 24 reduced patient safety.55 A systemic review and analysis was conducted by Jawed et al. to ascertain what percentage of the life expectancy gain in locally advanced and metastatic colorectal cancer (mCRC) in the past 20 years is as a result of novel therapies versus improvements in supportive care or secular trends and to thereby inform approaches for treatment development. The OS of patients with mCRC showed gradual improvement over the last two decades, with gains from chemotherapy, lead-time bias, and improved locoregional strategies and supportive care. First-line therapies showed modest but consistent gains; however, gains due to second-line therapies have been unsatisfactory.57
Noteworthy to mention, the biological rebellion has offered us diverse targets against cancer; consequently, a plethora of relatively specific drugs are in the process of development whereas some of them are already in use after attaining approval. Although some patients are benefiting from those drugs, but the results of large randomized trials are often very discouraging.58 For instance, almost two decades back, Mendelsohn and Baselga developed the first agents which target epidermal growth factor receptor (EGFR) pathway and showed quite encouraging outcomes in the in vitro setting. However, it exerted utterly low response rates in phase II trials in lung cancer whereas no significant response was observed in case of renal, colon, ovarian, and breast cancer. Similarly, gefitinib which showed 9–19% response rate initially was approved for non-small cell lung cancer (NSCLC). However, in subsequent randomized trial, the survival advantage was not achieved and hence the FDA limited the scope of its approval.59 Further, combination of BRAF and MEK inhibitors, which was expected to be highly effective and mitigate drug response in the patients with melanoma showed complete response in only 13% of the patients with a median progression-free survival (PFS) of 11.4 months in all patients exemplifying the need of better therapies.60 A phase III trial, ICON7 was conducted in 1528 women with ovarian cancer to access the efficacy of bevacizumab with standard chemotherapy. It was found that bevacizumab in combination with platinum-based chemotherapy failed to increase the OS but an OS benefit was observed in poor-prognosis patients, thus indicating the importance of optimal usage of bevacizumab for the management of ovarian cancer.61 In addition, arsenic has been used for treating chronic myelogenous leukemia (CML) in the 19th century in western medicine. Notably, arsenic trioxide (As2O3) has been found to induce complete remissions in acute promyelocytic leukemia (APL) patients. At the same time, As2O3 has been a well-known carcinogen and its administration was found to exert adverse effects on patients leading to its discontinued use against CML. Although, it is usually well-tolerated by APL patients, side effects are encountered in a majority of patients receiving As2O3. Thus it is evident that As2O3 is toxic to cells other than APL cells clearly suggesting it to have other effects or targets.62
Accelerated approval of drugs
Accelerated approval is an expedited regulatory pathway that permits a drug to get approval from the FDA on the basis of an endpoint which is regarded “reasonably likely to predict a clinical benefit”. Drugs obtained accelerated approval must be further assessed in post-marketing studies to confirm the desired clinical benefit and may be promoted to “regular” approval if clinical benefit is confirmed or may be withdrawn from the market otherwise.63 Immune checkpoint inhibitors like nivolumab or pembrolizumab target the programmed death receptor 1/programmed death ligand 1 (PD-1/PDL-1) and PDL-2 interaction.64 The evaluation of the efficacy of PD-1 blockade in cancer patients having advanced mismatch repair-deficient (dMMR) cancers in 12 different tumor types showed sensitivity to immune checkpoint blockade, irrespective of the tissue of origin.65 The FDA granted accelerated approval to pembrolizumab (Keytruda, Merck & Co) for the treatment of patients having unresectable or metastatic, microsatellite instable-high (MSI-H) or dMMR solid tumors that have progressed prior therapy and have no suitable treatment options. Till date, this is the first drug approved for use on the basis of a molecular biomarker, not traditional histopathologic diagnosis.66 The approval was based on data obtained from 149 MSI-H or dMMR cancer patients registered across five uncontrolled, multi-cohort, multicenter, single-arm clinical trials. A maximum of 24 months of treatment was administered and overall response rate (ORR) obtained was 39.6% whereas responses lasted six months or more for 78% of those who responded to pembrolizumab. The common adverse effects to pembrolizumab include constipation, cough, decreased appetite, diarrhea, dyspnea, fatigue, musculoskeletal pain, nausea, pyrexia, and rash. Pembrolizumab exerted immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis.51,66,67 Another drug which achieved FDA’s accelerated approval is ponatinib, a BCR-ABL tyrosine kinase inhibitor (TKI), the sixth drug approved for the management of CML or Philadelphia chromosome–positive acute lymphoblastic leukemia and indicated for patients showing refractory or intolerance to prior TKI therapy (Iclusig, Ariad Pharmaceuticals). On 31 October 2013, the FDA suspended marketing and sales of ponatinib. Just seven weeks later, the FDA partially reversed that decision, permitting use of the drug under a narrower indication. The primary advantage or benefit of this drug is its distinctive ability to target a gatekeeper mutation in BCR-ABL, the T315I transition, which confers resistance against all other approved TKI drugs; however, during withdrawal of ponatinib, the FDA indicated that the adversity of the drug, i.e. a substantial rise in arterial and venous thrombosis, outweighed the benefits.68
Use of surrogate endpoints
The efficacy of drugs approved via traditional and accelerated approval processes has been accepted by FDA through the use of surrogate endpoints. However, the method of surrogate endpoints brings the uncertainty regarding the risks and benefits of a drug as clinical value is not checked directly. Thereby it can introduce useless or even harmful therapies if the prediction of the benefit to the cancer patients could not be made or even if the drug has the inferior effect than expected benefit with larger than expected adverse effects.69 Kim and Prasad investigated the surrogate–survival correlation in 55 FDA surrogate-based approved cancer drugs. It was found that 14 out of 25 accelerated approvals and 11 out of 30 traditional approvals lacked formal analyses of the strength of the surrogate–survival correlation, thus implying the need to reconsider this method of drug approval.70 Most trial-level meta-analyses showed poor correlation between surrogate end points and the OS.71 It has been reported that 18 out of the 36 cancer drugs approved by the FDA based on surrogate endpoints between 2008 and 2012 did not show OS benefit.72 An analysis of 65 eligible trials by Prasad et al. showed that more than half (52%) of reported correlations were of low strength, 25% showed medium strength, and only 23% were highly associated with survival. Hence, supportive evidence of the use of surrogate end points as means of approval of new cancer drugs and determining treatment options for cancer patients is limited.71 Therefore, one of the prime reasons for inflated cost of cancer therapy is that new drugs which have no significant clinical benefit get approved by the FDA and are available in the market at prices similar to the most expensive ones.72 Further, Kemp and Prasad demonstrated that the trend of using surrogates in oncology is common and increasing. Although, the association of surrogates used and clinically meaningful outcomes is either unknown or weak. Therefore, there should be restricted use of surrogate outcomes in situations where it has shown robust ability to predict meaningful benefits, in cases which are difficult to handle with limited treatment options.73 Another study suggested that if the drug approval is based on the surrogate end point like the response rate or PFS, the subsequent studies must be performed and the drug’s effect on OS must be clarified.74 The findings of Wilkerson and Fojo suggested that the PFS cannot be taken as a surrogate for OS. It is however, the measure of benefit during therapy and cannot predict the tumor growth after termination of the treatment. Therefore, PFS should not replace OS in regulatory approval consideration.75
Randomized clinical trials
Randomized clinical trials (RCTs) are the main assessment carried out for novel therapeutics where study drugs have been traditionally tested in the sickest patients and later developed in a more wider population.76 It is observed that in most of the clinical settings, there is statistically significant under-representation of the elderly patients; aged ≥70 years.77 Therefore, although the benefit of most cancer drugs are seen in carefully selected, young, healthy populations, evidence exist that the real-world use of novel anticancer drugs may fail to retain the same benefits observed in clinical trials. For example, sorafenib tosylate, an oral TKI, is the only FDA-approved therapy for advanced or metastatic hepatocellular cancer. The pivotal, placebo-controlled trial leading to FDA approval enrolled patients with a median age of 65 years. In this population, sorafenib extended median survival by 2.8 months resulting in its approval. However, in reality, they fail to validate the results of the pivotal sorafenib trial. Second, they show that in actuality, the outcomes are far poorer than that of the trial.56 Therefore, strategies may be required to evaluate cancer therapies for the elderly patients in clinical trials and develop cancer care among the elderly people.56 However, to the few sections of biomedical authors, medical practices have been analogized with the parachute as they believe that all the medical practices carry large magnitude of benefit and performing RCTs are unnecessary. However, the ground reality is at far and the most parachute analogies in medicine can be treated as inappropriate, incorrect, or misused.78
Genome driven treatment approaches
Notably in the present days, patients eligible for genome-driven treatment has increased, which could help a minority of patients with advanced cancer. The estimated number of patients qualified for genome-targeted therapy was 28,729 out of 564,830 patients with metastatic cancer in 2006. By 2018, this number had improved to 50,811 (8.33%) from a total of 609,640. In case of genome-informed therapy, the eligible number of patients was 59,301 (10.50%) out of 564,830 in 2006.79 On 6 March 2018, the US FDA granted the first marketing authorization to a personal genomics and biotechnology company namely 23andMe, in order to access three BRCA1 or BRCA2 mutations to identify women with higher risk of breast cancer. This authorization was based on the accuracy and reproducibility of the test for three BRCA mutations that are most prevalent in people of Ashkenazi Jewish descent, but which also occur in the general population. Consumers simply need to mail a swab of saliva to 23andMe and get the results in a time span of 6–8 weeks at $199.3.80 However, sometimes in these online gene tests, direct-to-consumer results and third-party analyses may be wrong. For instance, Dr Joshua Clayton of 29-year-old age sent a sample of his saliva to 23andMe in order to learn about his ancestry. His report was a quite ordinary one with no new disclosures. But when he sent the profile created by 23andMe to a separate company called Promethease for an extensive in-depth analysis for genetic mutation, he was detected with a mutation linked to Lynch syndrome, a serious genetic disorder which leads to potentially deadly cancers at an early age. However, after subsequent genetic test at another company with expertise in medical diagnostics, he finally got to know that he did not have the mutation as the result was wrong. Therefore, the third-party analysis of raw DNA is not as rigorous as that done in a certified laboratory and hence their results cannot be considered as conclusive at all.81 Further, in the present days, people suffering from advanced cancer have been offered the hope of precision cancer medicine (PCM).82 PCM is a concept in which targeted therapies are adapted to match the complexity of the cancer genome.83 It employs genetic testing of the patients to find the most suitable drugs targeting specific mutation in the tumor, hoping for facilitating better outcome of the patients. However, precision treatments neither improve the patients’ survival nor induce better outcome in controlled studies. Thus, precision strategy requires further verification before using it for primary care.84 In addition, this method of treatment faces the criticism of overhyping as the pace of development of the genome-guided drug is very slow and patients who are likely to be benefitted from these drugs are very less in number.82
Blood-based approaches
One of the recently developed method based on the blood test namely tumor mutational burden (TMB) might prove to be beneficial in cancer treatment. It would be of more help where collecting tissues from the cancer patients is difficult and moreover, it is less invasive. The need of the hour is to develop those tests which can help in the prediction of immunotherapy especially the checkpoint inhibitors which can enable the immune cells to attack the tumors. The recently approved drug targeting has transformed cancer care and TMB test can certainly improve the treatment. Due to the high importance, FDA has designated the blood TMB test a “breakthrough device”.85 In 2016, the US FDA approved the use of Epi proColon, the first blood-based screening test for colon cancer that relies on the detection of the methylated septin 9 gene (SEPT9). This screening method addresses the limitation of conventional methods of screening such as stool-based tests and may potentially increase the number of individuals who undergo colon cancer screening. However, a clinical trial showed that SEPT9 test considerably enhanced sensitivity but significantly reduced specificity compared to fecal immunohistochemistry testing. Hence, whether this blood-based assay will reduce colorectal cancer mortality remains questionable.86
Analysis of cytostatic and cytotoxic agent
Ever since the traditional cancer chemotherapy and novel target-based agents were developed, the significance of cytostasis in therapies against cancer has always been controversial. Cytostatic drugs stop cancer cell proliferation without killing them. Notably, agents which are presently considered as cytotoxic have been observed to cause cytostasis or clinically stable disease for many years and were considered as ineffective. Therefore, the method of analysis of value of a putative cytostatic agent in a randomized phase III study should be different from that of a cytotoxic agent.87
Drug development duration and dosing options
Aforementioned, it takes almost 6–12 years to develop an anticancer drug starting from discovery to final approval. To illustrate the significance of rapid drug approval, Stewart et al. calculated life‐years possibly saved if selected agents were approved faster using 27 trials showing survival benefits. They found that if the time required to take a drug from discovery to approval is lessened to five years, the median life‐years saved per example would have been 523,890 globally. This clearly implies that a considerable amount of life‐years could be saved plausibly through increased efficiency of novel drug development for advanced neoplasms.88 Further, in the development of different oral agents against cancer, dosing options are random and limited by pills’ size. Prasad et al. reported that this limited dosing options frequently resulted in large dose adjustments in response to toxicity which might lead to reduced real-world clinical effectiveness of oral anticancer agents resulting in differed outcomes than those achieved in registration trials.89 The immigration policy that halts the entry of best from coming to train as well as work in the United States and discourages the American trainees and faculty from traveling to other countries is a regressive step which will ultimately harm the patients and America’s place as a global leader in healthcare and innovation.90
Non-inferiority trials
Intensified interest in comparative effectiveness research has made one-on-one assessments between drugs against cancer quite common. One strategy is non-inferiority trials, which often rely on points other than efficacy, like safety, QoL, convenience, and cost to update treatment decisions. As no specific guidelines exist in this regard, therefore these comparisons can emphasize randomly on specified endpoints or draw conclusions regardless of limited participation and treatment time surveyed and thus risks abusing of patient-reported outcomes.91 Further, the phrase "unmet medical need" requires a clearer definition and standardization as it is generally used to describe cancers that are rare, with little or no curative potential and poor survival outcome. However, it is also used to refer commonly diagnosed cancers, indolent, having several treatment options with better survival rate. Lu et al. identified 237 cancer indications which are regarded by the authors as "unmet medical need”. Out of these 237 indications, the term was mostly used for breast cancer indications (30/237 citations) followed by lung (24/237), hepatocellular (18/237), and prostate cancer (13/237).92
Clarified ethical conduct and geriatric oncology in low- and middle-income countries
In addition, conducting RCTs for cancer medicine in low- and middle-income countries have raised questions such as, what could be the suitable control arms and what the obligation of trials sponsors towards the host communities. It is noteworthy that a placebo-controlled trial can be ethical if the tested treatment has a feasibility of being employed in the host community. Many of these trials would not have been possible in developed countries like US as majority of clinicians would have objected in subjecting their patients where they have 50% chance of being randomly assigned to interventions considered inferior by previous studies. Thus, it is imperative to clarify the ethical conduct of clinical trials in the developing countries.93 Additionally, the recent days have witnessed a faster pace of population aging in middle-income countries as compared to the high-income countries. Mexico which belongs to middle-income countries having the second largest economy in Latin America is also undergoing rapid population aging and the number of new cancer cases in the overall population is expected to increase up to 75% by 2030 and nearly 60% in the elderly population (aged ≥65). The elderly population of Mexico suffers extreme poverty with low education attainment and devoid of any health insurance schemes. As a result of these problems, the elderly people in Mexico are more prone to the effects of the rising cancer burden and encounters difficulty in measuring high-quality cancer care. Therefore, it is recommended that geriatric oncology should be Mexico’s urgent public policy.94
Emphasis on the consideration of proven therapies
Further, reports suggest that cancer drugs or combinations that have not completed the phase I, II, or III stages of drug development have been used to treat cancer patients who have exhausted recommended treatment options. Mailankody and Prasad believed that unsafe drugs or combinations should not be applied regardless of theoretical efficacy or cost.95 The systematic evaluation of cancer drugs approved by the EMA between 2009 and 2013 showed that the most drugs came to the market without any evidence of benefit on the QoL or survival of cancer patients. In addition, if survival gains over existing treatment options or placebo were observed, they were found to be minimal.96 The study by Lammers et al. indicated that the substantial percentage (36.0%) of approved cancer drug have not presented efficacy data within 30 days of approval. Moreover, it was observed that the efficacy data are difficult to find from other sources. Adding to this problem, the current policies adopted by newer, costly drugs, and broadening market share may dampen the sponsors from applying for the formal approval, depending on the robust studies, for the off-label uses of drugs.97 In order to reduce the delay and improve the access of cancer drugs including those which were earlier considered but not approved by National Institute for Health and Care Excellence, the NHS Cancer Drugs Fund was established in 2010. However, the evaluation of its impact on the society has suggested that it has not served meaningful value to the cancer patients.98 The process of overturning an accepted practice such as diagnostic test, medication, or procedure is known as the medical reversal. It can be due to the inferior effect to a pre-existing, less intensive, or less invasive one. It can be the result of the inferior effect than no intervention. The need of the hour is to prove the efficacy of the interventions rather than just assuming that all the new therapy certainly leads to a better outcome. The emphasis should be on the consideration of only proven therapies along with the preparedness for the setbacks.99
Hazard ratio
In comparison to Kaplan–Meier plot, which emphasizes the number of patients persisting to do well at the completion of the time of interest, the hazard rate and hazard ratio focus on the opposite that is the patients who have not done well and would face a hazardous event. The hazard ratios data give the clearer picture of the treatment success and should be included in all the reports of clinical trials.100
Thus it is evident that clinical trials are one of the main aspects for determining the safety and efficacy of drugs. However, it is observed that they are quite expensive, time-consuming, and require ample resources. Nevertheless, it is worth to invest in high-quality clinical trial data to get the proper and strong evidence to ultimately benefit the cancer patients.101
Cost of anticancer drugs approved by the FDA
The economic burden imposed by cancer is escalating due to the rise in the cost of cancer drugs at an unprecedented rate102 (Table 2). In US, cetuximab treatment for 18 weeks against NSCLC costs around $80,000 in average, which translates into $800,000 to extend the life of one patient by a year. Similarly, bevacizumab costs $90,000 to treat an average patient whereas erlotinib and sorafenib cost approximately $16,000–$34,000 for one patient.132 Notably, in the past decade, funding by the government of US and others for cancer research has stagnated, whereas the demand for investment has grown remarkably due to the ever increasing incidence of cancer across the globe.102 A study by Moore et al. suggested that the high cost of the trials was associated with proving the efficacy of new agents as non-inferior to the already available drugs. Moreover, trials are expensive due to the involvement of the larger patient populations so as to attain statistical power to provide evidence for smaller therapeutic regimen.227 The average launch price of anticancer drugs, adjusted for inflation and health benefits, was found to increase by 10% annually from 1995 to 2013.228 As per the report by the IMS Institute for Healthcare Informatics, cancer drugs’ global market has reached $100 billion in annual sales.229 It was reported that from 138 pivotal clinical trials conducted during the year 2015–2016, 59 novel therapeutic agents were approved by the FDA, at the expense worth approximately $19.0 million.227 Remarkably, the anticancer drugs are much more expensive than the drugs from other divisions of health care which raises concern to the patients, physicians, policy researchers, and also the society.230,231 In addition, novel radiotherapy technology which includes proton therapy also comes with a massively high price tag.232 Recently, an analysis of US Securities and Exchange Commission filings for drug companies with no drugs on the US market which obtained FDA approval for cancer drug from 1 January 2006 to 31 December 2015 was carried out including 10 companies and drugs. They found that the median time taken by these companies to develop a drug was 7.3 years. The study also reported that five drugs received accelerated approval from the FDA, five received regular approval and the median cost of drug development was $648.0 million.233 Further, a report by Mailankody and Prasad has indicated that the price of cancer drugs is not based on novelty as the median wholesale price of 30 next-in-class drugs approved over a period of five years and 21 novel drugs were found to be almost same.230 Recently, there has been a drastic hike in the price of older drugs. For instance, Turing Pharmaceuticals increased the price of one tablet by 5000% (from $13.50 to $750). Again, the price of EpiPen was increased to more than $600 which earlier costed $100. The rise in the price of older drugs is highly objectionable considering the fact that the expenses for research and development was occurred quite before and almost definitely been recouped.234 Additionally, it is also noteworthy that there exists a very little difference in prices of drugs approved on the basis of response rate with those approved depending on the time-to-event end points.230 These clearly imply that the current pricing models of cancer drugs are highly non-rational. Further, the fact that the prices of drugs against cancer vary around the world and that no uniformity exists in the variation.235 The survey on the official prices of 31 cancer drugs in 18 different countries as published by Vogler and colleagues has shown sizable differences in price for the same drug in these countries. Thus, the price of cancer is not only limited to human suffering, but it increases the burden on the national GDPs as well due to premature mortality and morbidity.236 This strongly demands the need for greater transparency.235 Further, aiming to lower the costs and improve the value, the Centers for Medicare & Medicaid Services has proposed several measures such as improving incentive for better clinical care, discounting or eliminating patient cost sharing, feedback on physician’s prescription, and pricing of drugs based on its effectiveness.237 Although the cost-effectiveness analyses showed the tremendous cost of some cancer drugs as reasonable, majority of novel hematologic malignancy drugs failed to meet the value for the price. Further, empirical evidence revealed that most cancer drugs do not reach the conventional cost effectiveness thresholds, thus implying the crucial requirement to reconsider the current pricing of cancer drugs.238 The examination of the work for the contribution of National Institutes of Health (NIH) funding to published research linked with 210 new molecular entities (NMEs) approved by the FDA during 2010–2016 suggested that the NIH contributes to new drug approvals through research is greater than earlier expected. Moreover, it was found that the NIH research budget was more concentrated on the basic research for translating the new products. Any decrease in the funding can result in the slow pace of the research which could cause the delay in the outcome in terms of the emergence of new drugs in the near future.239 The examination of the presence of financial ties to drug makers among academics with research productivity showed a positive correlation. However, further analysis must be extended to validate these findings and if proven some policies must be framed to provide alternative incentives to physicians who could not make industry payments.240 The analysis of the differences in the guidelines for the approval of anticancer drugs by the National Comprehensive Cancer Network (NCNN) and FDA indicated that the NCCN recommendations are weak. It was observed that the NCCN defends the coverage of expensive, toxic cancer drugs based on weak indication.241 Further, the study on evaluating the benefits of the US FDA’s pediatric exclusivity program extension (2007–2012) by Sinha et al. has suggested that it provided significant information about the safety and efficacy of drugs used for pediatric population. Although, it was observed that the cost to consumers was high and the clinical trial was costlier.242
Table 2.
Cost of FDA approved drugs for cancer.
| Drug name | Target | Cancer | Treatment cost | References |
|---|---|---|---|---|
| Abemaciclib | CDK-4,-6 | Breast cancer | $10,948/month | 103,104 |
| Abiraterone acetate | CYP17A1 | Prostate cancer | $5000/month | 105 |
| Adcetris | CD30 | Lymphoma | $100,000 | 106 |
| Ado-Trastuzumab Emtansine |
HER-2 | Breast cancer | $5325.25 (160 mg vial) | 107 |
| Afatinib | ErbB | NSCLC | EUR12,364/10 years | 108 |
| Aldesleukin | IL2RA, IL2RB, IL2RG | RCC | $6000–8000/course | 103,109 |
| Alectinib | ALK | NSCLC | £87,000(average)/32 months | 110 |
| Apalutamide | AR | Prostate cancer | $10,000 | 111 |
| Arsenic trioxide | TR | APL | $15,582/28 days | 103,112 |
| Asparaginase | Asparagine | ALL | $42.00 (10,000 IU vial) | 103,113 |
| Atezolizumab | PDL-1 | Bladder cancer | $12,500/month | 114 |
| Avelumab | PDL-1 | mMCC | $9275.00/28 days | 115 |
| Axicabtagene ciloleucel | CD-19 | BCL | $373,000 | 116 |
| Axitinib | VEGFR | RCC | £27,000/6.4 months | 117 |
| Azacitidine | DNMT1 | Leukemia | $6000/28 days | 103,118 |
| Bendamustine | Mitotic checkpoints | CLL, B-cell NHL | $8640/1 TC | 119 |
| Bevacizumab | VEGF | Colon cancer | £1848.80/month | 120 |
| Bexarotene | RXR | CTCL | $214.67 (1 capsule) | 103,121 |
| Blinatumomab | CD19,CD3D | ALL | $55,594.20/28 days | 103,122 |
| Bortezomib | Proteasome | MM | $121,007/year | 123 |
| Bosutinib | Abl, Src kinases | CML | £44,799/year | 124 |
| Busulfan | DNA | Leukemia | €1.18/mg (oral) | 103,125 |
| Brigatinib | ALK | NSCLC | $17,100/month | 126 |
| Cabazitaxel | Microtubule | Prostate cancer | £3696/1 TC | 127 |
| Cabozantinib | MET, VEGFR2, RET | RCC | £4800/1 TC | 128,129 |
| Carfilzomib | Proteasome | MM | $10,000/28 cycles | 130 |
| Ceritinib | ALK | NSCLC | $111,468/6 months | 131 |
| Cetuximab | EGFR | NSCLC | $80,000/18 weeks | 132 |
| Chlorambucil | – | CLL | $22,417 | 133 |
| Cobimetinib | MAP2K1 | Melanoma | €79,433 | 103, 134 |
| Crizotinib | ALK | NSCLC | €6457/month | 135 |
| Cyclophosphamide | NR1I2 | Breast cancer | $1751/visit | 103,136 |
| Cytarabine | DNA polymerase beta | Leukemia | $12.08/2 g | 103,137 |
| Dabrafenib | B-raf, ERK, MAPK | Melanoma | £1400/year | 103,138, 139 |
| Dacarbazine | B-raf | Melanoma | $3600/month | 140 |
| Dactinomycin | DNA topoisomerase 2 | GTN | $308.01/5 days | 103,141 |
| Daratumumab | ADP-ribosyl cyclase 1 | Multiple myeloma | R$596,335/year | 103,142 |
| Dasatinib | Src | CML | £30,477.00/year | 143 |
| Decitabine | DNA methyltransferase | AML | $170,506/year | 103,144 |
| Degarelix | GnRH | Prostate cancer | $4411/year | 103,145 |
| Denileukin diftitox | IL-2α, IL-2 β | CTCL | $1648/300 mcg vial | 103,146 |
| Dinutuximab | Ganglioside GD2 | Neuroblastoma | £127,800/course | 103,147 |
| Docetaxel | Microtubule | Breast, prostate cancer | US$16,235/9.5 TC | 103,148 |
| Doxorubicin HCL | – | Ovarian cancer | 9614.72 euros | 149 |
| Enasidenib | IDH2 | AML | $24,872/month | 150 |
| Enzalutamide | Androgen receptor | Prostate cancer | $60,000/8 months | 103,151 |
| Eribulin | Bcl-2, Tubulin beta-1 chain | Breast cancer | Rs. 4.8 lakh | 103,152 |
| Eribulin mesylate | – | Breast cancer | €18,694/month | 153 |
| Erlotinib | EGFR | NSCLC | £6800/125 days | 154 |
| Etoposide phosphate | DNA topoisomerase | SCLC | $26,026.70/6 cycles | 103,155 |
| Etoposide | DNA topoisomerase 2-α; -β | SCLC | $26,764.48/6 cycles | 103,155 |
| Everolimus | mTOR | RCC | $186/day | 103,156 |
| Exemestane | CYP19A1 | Breast cancer | $180/month | 103,157 |
| Filgrastim | G-CSFR | Breast cancer | €7915/year | 103,158 |
| Fluorouracil injectionwith leucovorin | TS | – | $933/8 months | 103,159 |
| Fluorouracil-Topical | TS | Skin cancer | $2444/year | 103,160 |
| FOLFIRI | – | Colorectal cancer | $36,922/10-day cycle | 103,161 |
| FOLFIRINOX | – | Pancreatic cancer | $13,404 | 103,162 |
| Fulvestrant | Estrogen receptor alpha | Breast cancer | £1084/month | 103,163 |
| Gefitinib | EGFR | Lung cancer | $1029.94/month | 103,164 |
| Gemcitabine | TS, RNR | Pancreatic cancer | $1363/month | 165 |
| Glucarpidase | Methotrexate | Cancers | $27,000/1000 unit vial | 103,166 |
| Granisetron | 5-HOTR | – | MYR 73.5 | 103,167 |
| Hycamtin | DNA topoisomerase 1 | Ovarian cancer | $7832.07 | 103,168 |
| Hydroxyurea | RDR-LS | CML | 15,566 pound sterlings | 103,169 |
| Ibritumomab Tiuxetan | CD20 | NHL | £8535/15 years | 103,170 |
| Ibrutinib | Bruton’s tyrosine kinase | CLL | $18,506/month | 171 |
| Idelalisib | P110δ | CLL, FL, SLL | $14,449/20 months | 103,170 |
| Imatinib mesylate | BCR-ABL, RET | CML | 110,103 pound sterlings | 103,172 |
| Imatinib | BCR-ABL, RET, ABL1 | CML | $60,390/year | 103,173 |
| Imiquimod | TLR-7,8 | Skin cancer | €526 (mean cost)/year | 103,174 |
| Inotuzumab Ozogamicin | CD22 | ALL | $57,623.40/21 days | 103, 175 |
| Ipilimumab | CTLA4 | Melanoma | $30,000/injection | 103,176 |
| Ixabepilone | Microtubule | Breast cancer | $4609.81(75 mg dose) | 177 |
| Lanreotide acetate | SSTR-2,-5 | NET | $84,856/year | 103,178 |
| Lapatinib | ErbB1, ErbB2 | Breast cancer | £20,969/year | 179 |
| Lenalidomide | TNF-α, IL-6 | MM | $63,385/year | 180,181 |
| Lenvatinib | – | Thyroid cancer | $15,000/month | 182 |
| Leuprolide acetate | GnRHR | Prostate cancer | $1532/kit | 103,183 |
| Lomustine | Stathmin-4, DNA | Brain tumor | $648.88/100 mg | 103,184 |
| Lutetium Lu 177- Dotatate | SSTR-1,-2,-3,-4,-5 | GEP-NETs | $47,500/dose | 103,185 |
| Melphalan | DNA | Myeloma | $27,000 | 103,186 |
| Midostaurin | PDGF-Rβ & Rα, VEGF, FLT3 | AML | $14,990/28 days | 103,187 |
| Mitomycin C | – | Bladder cancer | £220.74/instillation | 103,188 |
| Necitumumab | EGFR | NSCLC | $1745/month | 103,189 |
| Nelarabine | POLA1 | ALL | $4000.00/day | 103,190 |
| Nilotinib | ABL1, c–Kit | CML | $10,360/month | 103,191 |
| Niraparib | PARP | Ovarian cancer | $20,032/month | 192 |
| Obinutuzumab | CD20 | CLL | $41,300/month | 103,193 |
| Ofatumumab | CD20 | CLL | £63,542/year | 103,194 |
| Olaparib | PARP | Ovarian cancer | $13,440/month | 195 |
| Osimertinib | EGFR | Lung cancer | $17,028.90/month | 164 |
| Paclitaxel | Microtubule, BCl-2 | Breast cancer | $865/cycle | 103,196 |
| Palbociclib | CDK 4 and 6 | Breast cancer | $9850/4 weeks | 197 |
| Panitumumab | EGFR | CRC | $100,000/year | 103,198 |
| Pazopanib | VEGFR, PDGFR | RCC | £2745.96 (average)/6 weeks | 199 |
| Pembrolizumab | PD-1 | Cervical cancer | $51.79/mg | 103,200 |
| Pemetrexed | TS, purH, DHFR, GART | NSCLC | $24,000 | 103,201 |
| Pertuzumab | ERBB2 | Breast cancer | $187,000/course | 103,202 |
| Pomalidomide | Cereblon | MM | $13.700/4 weeks TC | 103,203 |
| Ponatinib | BCR-ABL | CML | $199,000/year | 103,204 |
| Radium 223 Dichloride | DNA | Prostate cancer | $69,000/course (6 injections) | 103,205 |
| Regorafenib | VEGFR1-3, c-KIT, TIE-2, RET, | Colorectal cancer | 10,080 yuan/week | 206,207 |
| PDGFR-β, FGFR-1, RAF-1,B-raf, p38 MAPK | ||||
| Ribociclib | CDK-4,-6 | Breast cancer | $10,950(for 600 mg)/28 days | 103,208 |
| Rituximab | C1QB, C1QC | CLL | $13,702/dose | 103,209 |
| Rucaparib | PARP, CYP2D6 | Ovarian cancer | $13,740/30 days | 103,210 |
| Sipuleucel-T | PAP | Prostate cancer | $22,683/month | 103,211 |
| Sonidegib | Smoothened homolog | BCC | $146,876/year | 103,212 |
| Sorafenib | B-raf; VEGF | RCC | $6064/month | 103,213 |
| Sunitinib | VEGFR, PDGFR | RCC | £3139/6 weeks TC | 214 |
| Tamoxifen citrate | ER-α,-β, PKC | Breast cancer | $167/month | 103,213 |
| Temozolomide | – | Brain cancer | $2195/month | 103,213 |
| Temsirolimus | mTOR | RCC | $5000/month | 215 |
| Thalidomide | Cereblon, TNF, NF-kB | MM | $3555.74 | 103,216 |
| Thioguanine | – | Leukemia | $122/month | 103,213 |
| Tisagenlecleucel | CD19 | Leukemia | $475,000/infusion | 103,217 |
| Trabectedin | – | Sarcomas | $10,408.52/28 days | 103,218 |
| Trastuzumab | ERBB2, EGFR | Breast cancer | $70,000/year | 103,219 |
| Trifluridine | Thymidine phosphorylase | Colorectal cancer | $10,947.70/TC | 220 |
| Vandetanib | VEGF-A, EGFR, PTK6, RET, Ang1R | Thyroid cancer | $5460.00/28 days | 103,221 |
| Vemurafenib | B-raf | Melanoma | $13,000/month | 103,140 |
| Venetoclax | Bcl-2 | CML | $1760.88/28 days | 103,222 |
| Vincristine sulfate | Tubulin | ALL | $46,800.00/28 days | 223 |
| Vismodegib | Smoothened homolog | BCC | $75,000/10 months | 103,224 |
| Ziv-Aflibercept | VEGF | CRC | $1600/4 mL | 103,225 |
| Zoledronic acid | FPP, GGPS1, hydroxylapatite | MM | $140/dose | 103,226 |
5-HOTR: 5-hydroxytryptamine receptor 3A; ALL: acute lymphoblastic lymphoma; AML: acute myelogenous leukemia; Ang1R: angiopoietin-1 receptor; APL: acute promyelocytic leukemia; BCC: basal cell carcinoma; BCL: B-cell lymphoma; C1QC: complement C1q subcomponent subunit C; C1QB: complement C1q subcomponent subunit B; CDK: cyclin-dependent kinase; CLL: chronic lymphocytic leukemia; CTCL: cutaneous T-cell lymphoma; CTLA4: cytotoxic T-lymphocyte protein 4; CYP19A1: cytochrome P450 19A1; CYP2D6: cytochrome P450 2D6; DHFR: dihydrofolate reductase; DNMT1: DNA (cytosine-5)-methyltransferase 1; EGFR: epidermal growth factor receptor; FL: follicular lymphoma; FPP: farnesyl pyrophosphate; GART: trifunctional purine biosynthetic protein adenosine-3; G-CSFR: granulocyte colony-stimulating factor receptor; GEP-NETs: gastroenteropancreatic neuroendocrine tumors; GGPS1: geranylgeranyl pyrophosphate synthase; GnRH: gonadotropin-releasing hormone receptor; GnRHR: gonadotropin-releasing hormone receptor; GTN: gestational trophoblastic neoplasia; IDH2: isocitrate dehydrogenase-2; IL2RA: interleukin-2 receptor subunit alpha; IL2RB: interleukin-2 receptor subunit beta; IL2RG: cytokine receptor common subunit gamma; MAP2K1: mitogen-activated protein kinase kinase 1; mMCC: metastatic Merkel cell carcinoma; MM: multiple myeloma; NET: neuroendocrine tumor; NHL: Non-Hodgkin’s lymphoma; NR1I2: nuclear receptor subfamily 1 group I member 2; P110δ: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta; PARP: poly(ADP-ribose) polymerase; PD-1: programmed cell death protein 1; PDGF-R: platelet-derived growth factor receptors; PD-L1: programmed cell death 1 ligand 1; PKC: protein kinase C; POLA1: DNA polymerase alpha catalytic subunit; PTK6: protein-tyrosine kinase 6; PurH: bifunctional purine biosynthesis protein PURH; RCC: renal cell carcinoma; RDR-LS: ribonucleoside-diphosphate reductase large subunit; RNR: ribonucleotide reductase; RXR: retinoic acid receptor; SLL: small lymphocytic lymphoma; SSTR: somatostatin receptor; TC: treatment cycle; TLR: toll-like receptor; TNFRSF8: tumor necrosis factor receptor superfamily member 8; TR: thioredoxin reductase; TS: thymidylate synthase; VEGF: vascular endothelial growth factor.
Potential solutions to the problem for prevention and treatment of cancer
Development of new drugs till the final approval by FDA is extremely expensive which costs around two billion dollars. Although the chief reason behind this excessive high cost remains unknown, high failure rates of trials related to novel drug discovery at the pre-clinical and clinical settings contribute enormously to it. Although many mono-targeted therapies have been developed for diverse cancers, such strategies have had little effect in the prevention or treatment of different malignancies.243 Targeted therapy which involves different strategies such as monoclonal antibodies, prodrug, small molecule inhibitors, and nano-particulate antibody conjugates has gained enormous attention in the recent days due to their specificity towards cancer cells without causing toxicity to off-target cells. However, newest findings suggest that tumor heterogeneity with reference to molecular targets leads to failure of these targeted therapies in many cases.244 Some potential solutions for the prevention and treatment of cancer are illustrated below.
Natural therapies
Since ages, compounds derived from Mother Nature, especially plants have been the primary source of medicine and health not only due to their safety, affordability, effectiveness but also due to their ability to modulate multiple cell signaling pathways. Further, increasing lines of evidence clearly imply that more than 70% of the current drugs are of natural origin.245–247 Notably, a vast majority of world’s population relies on plants for their primary healthcare.245 Reports suggest that people of Southeast Asian countries possess lower risk of developing colon, gastrointestinal, prostate, breast, and some other cancers due to their dietary habits as dietary constituents are considered to offer protection against diverse cancers.248 Dietary phytochemicals contain various active components with potent chemopreventive properties such as curcumin, genistein, resveratrol, diallyl sulfide, S-allyl cysteine, allicin, lycopene, capsaicin, diosgenin, [6]-gingerol, ellagic acid, ursolic acid, silymarin, anethol, catechins, eugenol, isoeugenol, isothiocyanates, indole-3-carbinol, isoflavones, phytosterols, folic acid, β-carotene, and flavonoids. These components are extremely safe, possess multi-targeting ability, cost-effective, bioavailable, and also serve as better ligands for biologically active proteins.249,250 A recent paper reported that insectivorous plants which are rich in secondary metabolites provide benefits against cancer. For instance, metabolites like napthoquinones, phenolic acids, and flavonoids are present in the plants such as Drosera indica, Dionaea muscipula, Darlingtonia, and Sarracenia, which contribute enormously to their potent anticancer property.251
For the period of 2005 to 2007, a total of 13 different drugs based on natural products were approved and five of them represented the first members of new classes of drugs: exenatide, ziconotide, ixabepilone, retapamulin, and trabectedin. Further, the structures of these natural products possess high chemical diversity, biochemical specificity, and various other molecular features, making them highly promising as lead structures for drug design.252,253 Despite these advantages and the past achievements, research into natural products in drug discovery screening in the pharmaceutical companies have decreased remarkably in the last decade. This might be due to the apparent difficulties associated with natural products which include technical obstructions in screening them in high-throughput assays against different molecular targets.254 For instance, one such highly promising medicinal plant is Azadirachta indica, commonly known as neem, belonging to Mahogany family. Various parts of this plant have been used for the treatment of diverse human ailments since olden times and also showed anticancer effect in the pre-clinical findings. Despite the identification of more than 300 components from neem, the effect of only very few were assessed in details.245 Deciphering the effect and mechanism of action of all the compounds present in such precious medicinal plants hold immense prospect in the development of novel therapeutic strategies against diverse cancer types. Notably, recent advances in genomics and structural biology provide a clearer picture of the diversity of proteins targeted by molecules of natural origin thereby developing an interest in natural products for drug discovery.249,252,253,255 Overthrowing the technical disadvantages associated with natural product research definitely provides better opportunities to unravel the biological features of previously inaccessible natural product sources. Further, chemical diversity of natural products is perfectly suitable to provide the core scaffolds for novel drugs and therefore further developments in using newer natural products and chemical libraries based on them possess enormous prospect in drug discovery crusades.253 Additionally, a method of drug discovery involving the generation of natural products’ based molecular diversity in combination with synthetic procedures undoubtedly displays the most well-suited solution to discover and develop effective drugs.256
Multi-targeted agents
Till date, different drugs have been developed against various targets such as tyrosine kinases, diverse membrane proteins, and enzymes for the treatment of varied cancer types and they also obtained FDA approval (Figures 1–4). Interestingly, one leading paradigm in drug discovery is to develop regimens with high selectivity to act on individual drug targets.257,258 With this mono-targeted approach, many novel entities have been designed and further got approval as drugs.257 However, these drugs had hardly exerted efficacy against cancer as it is a complex disease characterized by diverse molecular and genetic variations. Due to this enormous biological diversity, targeting a single target is not sufficient to combat cancer. Instead, multi-targeting approach holds prospect in this regard.259,260 Multi-targeted therapeutics can be accomplished either through combination of single targeted drugs or via administration of a multi-targeted agent. The combinatorial treatment approach using agents with distinctive molecular mechanisms is considered to be highly promising for better efficacy as use of multiple agents is frequently limited by drug–drug interactions and dose-limiting toxicities. In addition, the use of a single agent is generally much more cost-effective than two separate agents.257,261 Some other important yet added advantages of using a single multi-targeted agent include the avoidance of different bio-availabilities as well as pharmacokinetics and metabolism of each component within the combination regimen. Further, the simplified dosing regimen would greatly aid in enhancing the therapeutic efficacy and exert minimal side effects.257 These findings clearly indicate that the use of more unspecific agents with ability to modulate different targets simultaneously offer high prospect.259 Increasing lines of evidence suggest that “natural products” such as isoflavones, indole-3-carbinol, and curcumin inhibited the growth and induced apoptosis of cancer cells effectively by targeting multiple signaling pathways in vitro without causing much toxicity to the normal cells. Therefore, these non-toxic “natural products” could be of immense use in combination with conventional chemotherapeutic agents as well for treating diverse human malignancies effectively without exerting much toxicity.261–263 For example, curcumin, a component of a spice native to India, was found to exhibit potent antioxidant, anti-inflammatory, antiviral, antibacterial, antifungal, and anticancer activities. It modulates diverse transcription factors, inflammatory cytokines, enzymes, kinases, and various other proteins and can regulate the growth of tumor cells effectively through modulation of multiple cell signaling cascades. Further, it was also reported that curcumin can interact with almost all the targets regulated by FDA-approved anticancer drugs.264–266 Again, silymarin, another multi-targeting agent also showed anti-inflammatory as well as anti-metastatic activity and modulated the expressions of cell cycle regulators and proteins involved in apoptosis.267 Recent studies identified niclosamide as an effective anticancer agent with ability to inhibit Wnt/β-catenin, mTORC1, STAT3, NF-κB, and Notch signaling pathways. Further, it was also reported to target mitochondria in cancer cells to induce cell cycle arrest, growth inhibition, and apoptosis.268 In addition, tocotrienols, analogs of vitamin E also have gained considerable attention due to their effectiveness and their ability to inflect various targets which are strongly involved in cancer cell proliferation, survival, invasion, angiogenesis, and metastasis. Further, tocotrienols can chemosensitize cancer cells to celecoxib, doxorubicin, erlotinib, gefitinib, gemcitabine, paclitaxel, statin, etc. effectively.269,270
Figure 1.
Different drugs approved by the FDA which target enzymes. (A color version of this figure is available in the online journal.)
Figure 4.
Different drugs approved by the FDA which target other signaling molecules. (A color version of this figure is available in the online journal.)
Figure 2.
Different drugs approved by the FDA which target receptors. (A color version of this figure is available in the online journal.)
Figure 3.
Different drugs approved by the FDA which target kinases, RTKs and cytokines. (A color version of this figure is available in the online journal.)
Thus it is well affirmed that targeting different biochemical and molecular signaling pathways provides the most well suited and effective strategy to deal with carcinogenesis and overcome resistance to mono-targeted agents. Therefore, exploration of more of these multi-targeted agents, especially those of natural origin and their thorough investigation in pre-clinical and clinical setting would definitely pave way towards successful prevention and treatment of diverse neoplasms.
Cost
Cost of novel anticancer drugs are extremely high which affects the patients and payers globally.271 For the last 25 years, well-meaning bureaucratic functionaries have introduced numerous new guidelines without consulting any clinical investigators or field testing which resulted in delayed development of new treatments, subdued innovation together with driven up drug costs remarkably.272 The use of costly therapies with minimal benefits for their approved and unproven indications contributes hugely to the growing cost of cancer care, also known as “financial toxicity” which results in poor health-related and non-health-related issues to the patients.273,274 Moreover, the survival benefits of certain newer anticancer drugs may be just a few months more than that of the already existing treatment but at a reasonably higher cost.271 Considering the complication of this matter, evidently, no single solution will suffice. Adopting approaches like macroeconomic basis of cancer costs’ re-engineering, education of policy makers, and a transparent regulatory system might offer some potent solutions to this problem.275 A fair drug price is of vital importance which not only reflects its true benefit but also the societal and personal costs. Further, deciding the price of cancer drugs solely by pharmaceutical companies could make our health care system as well as Medicare completely penniless and is absolutely unfair not only to the cancer patients, but also to the whole society.276 Another plausible approach is the introduction of generic and biosimilar drugs. Generic drugs are same as brand-name drugs which are used in similar treatment programs for the approved indication and also at comparable dosing levels. On the other hand, biosimilar drugs possess high similarity with the FDA-approved biological agent, with no clinically meaningful differences with regard to safety, efficacy, or purity. Filgrastim-sndz which was developed in 2015 is the first FDA-approved biosimilar drug as an alternative to filgrastim to treat neutropenia in cancer patients. Compared to filgrastim, the cost of biosimilar filgrastim-sndz is around 15% lower in the USA and 30% lower in Europe.231 Thus they offer a relatively inexpensive treatment choice and may provide relief to the increasing costs of cancer drugs. However, design of strategies to obtain enhanced uptake of biosimilar drugs is highly critical. Another plausible solution is the development and use of biomarkers of drug response to enable reduced use of costly drugs which are improbable to benefit the patients on the basis of disease characteristics. For example, screening for PD-1 and/or PDL-1 provides targeted treatment and reduces the use of expensive PD-1/PDL-1 inhibitors in patients who would not benefit. However, impact of biomarker screening may depend on the approval status of a drug in relation to the discovery of the biomarker.277 Aforementioned, FDA approved pembrolizumab based on molecular biomarker for patients bearing MSI-H or dMMR solid tumors which have progressed on prior therapy. Approval of such drugs has interweaved the translation of basic science to clinical settings, restricting treatment on the basis of genetic subgroups and the flexibility of the FDA to approve drugs on the basis of early as well as favorable data.66 In addition, the application of dose individualization concept depending on body surface area may cause reduced expenditure of anticancer drugs. A study was conducted to determine if the rational application of dose individualization leads to reduction in anticancer drugs’ costs where 18 different anticancer drugs were given 939 times. They found that if dosage was sternly based on body surface area, drug costs would have been 509,664 Euro. Rounding off to total ampoules with a dose margin of maximum 10% would have caused 8.6% lessening of cost.278
Safety
The cancer drugs approved by FDA are mostly associated with adverse side effects. Therefore strategies to overcome these toxicities are of vital importance. For instance, doxorubicin (adriamycin) exerts notable anticancer effect, particularly in solid tumors. Further, it was reported to exhibit a higher therapeutic index compared to some other anticancer drugs like daunorubicin, with minor change in its structure. However, cardiotoxicity generated due to its use presents a major limitation. Nevertheless, it can be prevented by liposomal encapsulation as liposomal-encapsulated doxorubicin is reported to exert less cardio toxicity and it was also approved by FDA for treating ovarian cancer and multiple myeloma. Various reports suggest that treatment related toxicities can be prevented through different pharmacologic substances such as α-tocopherol, ascorbate, vitamin E, and N-acetylcystein. Vitamin E and ascorbate are antioxidants which were found to inhibit the formation of free radicals. Qishenyiqi pills were also reported to cause improved cardiac activity through inhibiting the apoptosis of myocardial cells. Again, N-acetyl cysteine is shown to increase non-protein sulfhydryl contents of heart, thereby preventing drug induced cardiomyopathy.279 Therefore encapsulation with these substances may provide protection against cardiotoxicity induced by anticancer drugs. In addition, cytoprotective agents reduce the toxicity related to anticancer treatment and also help to increase the dose as well as dose intensity of radio and chemotherapy. One such organic thiophosphate is amifostine, which provides selective protection to the normal tissues as well different organs without exerting much toxicity. Clinical studies depicted that amifostine provided protection against nephrotoxicity, neurotoxicity, myelotoxicity, mucositis, and esophagitis in patients treated with radio and chemotherapy. Amifostine is well tolerated in 740 or 910 mg/m2 doses and interestingly, studies in both pre-clinical and clinical settings did not report to hinder the antitumor efficacy due to its use.280
Prevention and treatment
It is well evinced that only 5–10% of all cancer cases is attributed to genetic defects, whereas environment toxins, unhealthy lifestyle, and diet account for the remaining 90–95% cases. Of all cancer-related deaths, almost 25–30% are due to use of tobacco, 30–35% are associated with unhealthy diet, approximately 15–20% are due to infections, and the remaining are caused by other factors such as exposure to radiation, stress, physical inactivity, and environmental pollutants.281 As mentioned, maintaining a healthy diet plays an inevitable role in cancer prevention. Low use of fibers, consumption of red meat and an imbalance of omega-3, -6 fats may enhance the risk of cancer. On the other hand, high intake of fruits and vegetables may considerably lower the risk of cancer. Moreover, use of digestive enzymes and probiotics as oral supplements is another anticancer dietary measure.282 The chemo-protective effect of fruits and vegetables also holds true for different cancer types including cancers of the colon, esophagus, endometrium, oral cavity, lung, pharynx, pancreas, and stomach.283,284 Therefore, by embracing a healthy diet which include smoking cessation, increased intake of fruits and vegetables, moderate use of alcohol, caloric restriction, minimal meat consumption, use of whole grains, and maintaining a healthy lifestyle regime including regular exercise, less direct exposure to sunlight, use of vaccinations, and regular check-ups, this deadly disease can be prevented.285
In addition, personalized cancer medicine offers a huge benefit in the treatment of cancer. It can be done through a national facilitated access program and registry for off-label use of targeted anticancer drugs. With the help of such program, patients can be benefited by receiving the targeted agent matched to their tumor profile.286 Again, centrosome clustering mechanisms are also attractive theranostic targets against cancer.285 When cancer is diagnosed, besides medical consultation, cancer patients seek advice and counsel from close family and friends. Hence, medical advice in the care of a friend, loved one, or close associate by an oncologist can be of immense help.287 Further, the Ayurveda, Siddha, and Unani (ASU) education system require a reform at every level to produce expert, resourceful and informed graduates who can certainly contribute to the betterment of the society.288
Conclusion
Cancer is an extremely complex disease caused by the deregulation of multiple genes, proteins, and pathways. Advancements in molecular biology and high throughput screening technologies led to the development of different target specific drugs for the treatment of cancer. But unfortunately, none of them is effective and devoid of toxicities and hence fails to combat cancer despite the fact they are extremely expensive. As a matter of fact, in almost all the cases, taking a single drug against cancer from discovery to testing to market costs around $1 billion. This review summarizes the advantages as well as limitations associated with various aspects of cancer diagnosis as well as treatment. While cell lines and animal models possess ample advantages for cancer research and development of novel cancer therapies, reports indicate that these pre-clinical models are highly incomplete and data obtained from them shows a fundamental mismatch with those obtained from clinical findings. This presents a major obstacle in the development of effective anticancer drugs. It is well evinced that inflection of numerous transduction cascades presents a convincing tactic to the actuality of carcinogenesis and the growing issue of emerging chemoresistance. Therefore, a multidisciplinary drug discovery approach which includes the generation of novel molecular multiplicity from natural sources, together with synthetic procedures might offer key solution to the discovery and development of safe, effective and affordable drugs against diverse neoplasms.
Author Contributions
BBA and ABK contributed to the study design and writing. DB, BLS, and NKR performed bibliographic search, writing, and artwork. KKT and KB contributed to figures, tables, and reference editing. SCG and MS contributed to proofread and writing.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Department of Biotechnology awarded to Ajaikumar B Kunnumakkara by Department of Biotechnology (DBT), Government of India. The author Bethsebie Lalduhsaki Sailo acknowledges the DST INSPIRE and Kishore Banik acknowledges the UGC New Delhi for providing them the fellowship.
References
- 1.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol 2012; 3:328–51 [PMC free article] [PubMed] [Google Scholar]
- 2.Bordoloi D, Sailo BL, Manteghi N, Padmavathi G, Kunnumakkara AB. Introduction and basic concepts of cancer. Cancer cell chemoresistance and chemosensitization. Hackensack, NJ: World Scientific, 2018, pp.1–14. [Google Scholar]
- 3.Bordoloi D, Banik K, Shabnam B, Padmavathi G, Monisha J, Arfuso F, Dharmarajan A, Mao X, Lim LHK, Wang L, Fan L, Hui KM, Kumar AP, Sethi G, Kunnumakkara AB. TIPE family of proteins and its implications in different chronic diseases. Int J Mol Sci 2018; 19:E2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68:394–424 [DOI] [PubMed] [Google Scholar]
- 5.Thakur KK, Bordoloi D, Kunnumakkara AB. Alarming burden of triple-negative breast cancer in India. Clin Breast Cancer 2018; 18:e393–9 [DOI] [PubMed] [Google Scholar]
- 6.Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: a global review. Cancer Epidemiol 2016; 44:203–21 [DOI] [PubMed] [Google Scholar]
- 7.Harsha C, Banik K, Bordoloi D, Kunnumakkara AB. Antiulcer properties of fruits and vegetables: a mechanism based perspective. Food Chem Toxicol 2017; 108:104–19 [DOI] [PubMed] [Google Scholar]
- 8.Khwairakpam AD, Bordoloi D, Thakur KK, Monisha J, Arfuso F, Sethi G, Mishra S, Kumar AP, Kunnumakkara AB. Possible use of Punica granatum (pomegranate) in cancer therapy. Pharmacol Res 2018; 133:53–64 [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Danda D, Gupta S, Gehlot P. Models for prevention and treatment of cancer: problems vs promises. Biochem Pharmacol 2009; 78:1083–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi S, Lin S, Li Y, Zhao W, Mills GB, Sahni N. Functional variomics and network perturbation: connecting genotype to phenotype in cancer. Nat Rev Genet 2017; 18:395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurana E, Fu Y, Chakravarty D, Demichelis F, Rubin MA, Gerstein M. Role of non-coding sequence variants in cancer. Nat Rev Genet 2016; 17:93–108 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Bojorquez-Gomez A, Velez DO, Xu G, Sanchez KS, Shen JP, Chen K, Licon K, Melton C, Olson KM, Yu MK, Huang JK, Carter H, Farley EK, Snyder M, Fraley SI, Kreisberg JF, Ideker T. A global transcriptional network connecting noncoding mutations to changes in tumor gene expression. Nat Genet 2018; 50:613–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drost J, Clevers H. Who is in the driver's seat: tracing cancer genes using CRISPR-barcoding. Mol Cell 2016; 63:352–4 [DOI] [PubMed] [Google Scholar]
- 14.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet 2013; 14:703–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roerink SF, Sasaki N, Lee-Six H, Young MD, Alexandrov LB, Behjati S, Mitchell TJ, Grossmann S, Lightfoot H, Egan DA, Pronk A, Smakman N, van Gorp J, Anderson E, Gamble SJ, Alder C, van de Wetering M, Campbell PJ, Stratton MR, Clevers H. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 2018; 556:457–62 [DOI] [PubMed] [Google Scholar]
- 16.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 2006; 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Sung B. NF-κB in cancer: a matter of life and death. Cancer Discov 2011; 1:469–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989; 49:4682–9 [PubMed] [Google Scholar]
- 19.Monisha J, Roy NK, Bordoloi D, Kumar A, Golla R, Kotoky J, Padmavathi G, Kunnumakkara AB. Nuclear factor kappa B: a potential target to persecute head and neck cancer. Curr Drug Targets 2017; 18:232–53 [DOI] [PubMed] [Google Scholar]
- 20.Padmavathi G, Roy NK, Bordoloi D, Arfuso F, Mishra S, Sethi G, Bishayee A, Kunnumakkara AB. Butein in health and disease: a comprehensive review. Phytomedicine 2017; 25:118–27 [DOI] [PubMed] [Google Scholar]
- 21.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2:489–501 [DOI] [PubMed] [Google Scholar]
- 22.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 2013; 1:45–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017; 36:1461–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 2012; 12:801–17 [DOI] [PubMed] [Google Scholar]
- 25.Doroshow JH, Kummar S. Translational research in oncology-10 years of progress and future prospects. Nat Rev Clin Oncol 2014; 11:649–62 [DOI] [PubMed] [Google Scholar]
- 26.Wilding JL, Bodmer WF. Cancer cell lines for drug discovery and development. Cancer Res 2014; 74:2377–84 [DOI] [PubMed] [Google Scholar]
- 27.Daniela F, Filomena A, Raquel C. The importance of cancer cell lines as in vitro models in cancer methylome analysis and anticancer drugs testing. Oncogenomics Cancer Proteomics 2013;139–66 [Google Scholar]
- 28.Gardaneh M. Human cancer modeling: recapitulating tumor heterogeneity towards personalized medicine. Multidiscip Cancer Invest 2017; 1:1–12 [Google Scholar]
- 29.Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, Aben N, Gonçalves E, Barthorpe S, Lightfoot H, Cokelaer T, Greninger P, van Dyk E, Chang H, de Silva H, Heyn H, Deng X, Egan RK, Liu Q, Mironenko T, Mitropoulos X, Richardson L, Wang J, Zhang T, Moran S, Sayols S, Soleimani M, Tamborero D, Lopez-Bigas N, Ross-Macdonald P, Esteller M, Gray NS, Haber DA, Stratton MR, Benes CH, Wessels LFA, Saez-Rodriguez J, McDermott U, Garnett MJ. A landscape of pharmacogenomic interactions in cancer. Cell 2016; 166:740–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak S, Multani AS, Narayan S, Kumar V, Newman RA. Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anticancer Drugs 2000; 11:455–63 [DOI] [PubMed] [Google Scholar]
- 31.Gutmann DH, Hunter-Schaedle K, Shannon KM. Harnessing preclinical mouse models to inform human clinical cancer trials. J Clin Invest 2006; 116:847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 2015; 163:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Moore L, Ji P. Mouse models for cancer research. Chin J Cancer 2011; 30:149–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurtley S. Humans as model of human disease. Res Sci J 2018; 361:763–4 [Google Scholar]
- 35.Jackson SJ, Thomas GJ. Human tissue models in cancer research: looking beyond the mouse. Dis Model Mech 2017; 10:939–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer 2018; 18:407–18 [DOI] [PubMed] [Google Scholar]
- 37.Ingber DE. Developmentally inspired human 'organs on chips'. Development 2018; 145:dev156125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams DJ. The Valley of Death in anticancer drug development: a reassessment. Trends Pharmacol Sci 2012; 33:173–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eaton A, Iasonos A, Gounder MM, Pamer EG, Drilon A, Vulih D, Smith GL, Ivy SP, Spriggs DR, Hyman DM. Toxicity attribution in phase I trials: evaluating the effect of dose on the frequency of related and unrelated toxicities. Clin Cancer Res 2016; 22:553–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun TM. The current design of oncology phase I clinical trials: progressing from algorithms to statistical models. Chin Clin Oncol 2014; 3:2. [DOI] [PubMed] [Google Scholar]
- 41.Mark V, Hendrik-Tobias A. Challenges of patient selection for phase I oncology trials. Drug Discov Dev IntechOpen 2015;65–82 [Google Scholar]
- 42.Molife LR, Alam S, Olmos D, Puglisi M, Shah K, Fehrmann R, Trani L, Tjokrowidjaja A, de Bono JS, Banerji U, Kaye SB. Defining the risk of toxicity in phase I oncology trials of novel molecularly targeted agents: a single centre experience. Ann Oncol 2012; 23:1968–73 [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Pitcher BN, Jung SH, Bartlett NL, Wagner-Johnston N, Park SI, Richards KL, Cashen AF, Jaslowski A, Smith SE, Cheson BD, Hsi E, Leonard JP. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017; 4:e176–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonali MS, Brandelyn NP, Sin-Ho J, Nancy LB, Nina WJ, Steven IP, Kristy LR, Amanda FC, Anthony J, Scott ES, Bruce DC, Eric H, John PL. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017; 4:Pe176–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pongas G, Fojo T. BEZ235: when promising science meets clinical reality. Oncologist 2016; 21:1033–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy NK, Bordoloi D, Monisha J, Padmavathi G, Kotoky J, Golla R, Kunnumakkara AB. Specific targeting of Akt kinase isoforms: taking the precise path for prevention and treatment of cancer. Curr Drug Targets 2017; 18:421–35 [DOI] [PubMed] [Google Scholar]
- 47.Moreno L, Pearson AD. How can attrition rates be reduced in cancer drug discovery? Expert Opin Drug Discov 2013; 8:363–8 [DOI] [PubMed] [Google Scholar]
- 48.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates?. Nat Rev Drug Discov 2004; 3:711–5 [DOI] [PubMed] [Google Scholar]
- 49.Goss TF, Picard EH, Tarab A. Recognizing value in oncology innovation. Boston, MA: Boston Healthcare, 2012 [Google Scholar]
- 50.Stein WD, Figg WD, Dahut W, Stein AD, Hoshen MB, Price D, Bates SE, Fojo T. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: a novel strategy for evaluation of clinical trial data. Oncologist 2008; 13:1046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration, https://www.fda.gov (accessed 31 August 2018)
- 52.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics 2019; 20:273–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amiri-Kordestani L, Fojo T. Why do phase III clinical trials in oncology fail so often. J Natl Cancer Inst 2012; 104:568–9 [DOI] [PubMed] [Google Scholar]
- 54.Prasad V. Do cancer drugs improve survival or quality of life? BMJ 2017; 359:j4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salas-Vega S, Iliopoulos O, Mossialos E. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol 2017; 3:382–90 [DOI] [PubMed] [Google Scholar]
- 56.Mailankody S, Prasad V. Overall survival in cancer drug trials as a new surrogate end point for overall survival in the real world. JAMA Oncol 2017; 3:889–90 [DOI] [PubMed] [Google Scholar]
- 57.Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal cancer survival gains and novel treatment regimens: a systematic review and analysis. JAMA Oncol 2015; 1:787–95 [DOI] [PubMed] [Google Scholar]
- 58.Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much?. Clin Cancer Res 2010; 16:5972–80 [DOI] [PubMed] [Google Scholar]
- 59.Bates SE, Fojo T. Epidermal growth factor receptor inhibitors: a moving target?. Clin Cancer Res 2005; 11:7203–5 [DOI] [PubMed] [Google Scholar]
- 60.Massey PR, Prasad V, Figg WD, Fojo T. Multiplying therapies and reducing toxicity in metastatic melanoma. Cancer Biol Ther 2015; 16:1014–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Park-Simon TW, Rustin G, Joly F, Mirza MR, Plante M, Quinn M, Poveda A, Jayson GC, Stark D, Swart AM, Farrelly L, Kaplan R, Parmar MK, Perren TJ. ICON7 trial investigators. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015; 16:928–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fojo T, Bates S. Arsenic trioxide (As(2)O(3)): still a mystery. Cell Cycle 2002; 1:183–6 [DOI] [PubMed] [Google Scholar]
- 63.Wilson WH, Schenkein DP, Jernigan CL, Woodcock J, Schilsky RL. Reevaluating the accelerated approval process for oncology drugs. Clin Cancer Res 2013; 19:2804–9 [DOI] [PubMed] [Google Scholar]
- 64.Fuereder T. Immunotherapy for head and neck squamous cell carcinoma. Memo 2016; 9:66–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA., Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357:409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type-FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol 2018; 4:157–8 [DOI] [PubMed] [Google Scholar]
- 67.Prasad V, Kaestner V, Mailankody S. Clarification of the FDA accelerated agnostic approval of pembrolizumab and the opportunities arising from the required confirmatory studies-reply. JAMA Oncol 2018; 4:1300–1 [DOI] [PubMed] [Google Scholar]
- 68.Prasad V, Mailankody S. The accelerated approval of oncologic drugs: lessons from ponatinib. JAMA 2014; 311:353. [DOI] [PubMed] [Google Scholar]
- 69.New Drug Approval, United States Government Accountability Office. FDA needs to enhance its oversight of drugs approved on the basis of surrogate endpoints, https://www.gao.gov/new.items/d09866.pdf (2009, accessed 3 September 2018)
- 70.Kim C, Prasad V. Strength of validation for surrogate end points used in the US Food and Drug Administration's approval of oncology drugs. Mayo Clin Proc 2016; 91:713–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med 2015; 175:1389–98 [DOI] [PubMed] [Google Scholar]
- 72.Rupp T, Zuckerman D. Quality of life, overall survival, and costs of cancer drugs approved based on surrogate endpoints. JAMA Intern Med 2017; 177:276–7 [DOI] [PubMed] [Google Scholar]
- 73.Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions. And are they currently overused? BMC Med 2017; 15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med 2015; 175:1992–4 [DOI] [PubMed] [Google Scholar]
- 75.Wilkerson J, Fojo T. Progression-free survival is simply a measure of a drug's effect while administered and is not a surrogate for overall survival. Cancer J 2009; 15:379–85 [DOI] [PubMed] [Google Scholar]
- 76.Vaduganathan M. Modern drug development which patients should come first?. JAMA 2014; 312:2619–20 [DOI] [PubMed] [Google Scholar]
- 77.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004; 22:4626–31 [DOI] [PubMed] [Google Scholar]
- 78.Hayes MJ, Kaestner V, Mailankody S, Prasad V. Most medical practices are not parachutes: a citation analysis of practices felt by biomedical authors to be analogous to parachutes. CMAJ Open 2018; 6:E31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marquart J, Chen EY, Prasad V. Estimation of the percentage of US patients with cancer who benefit from genome-driven oncology. JAMA Oncol 2018; 4:1093–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gill J, Obley AJ, Prasad V. Direct-to-consumer genetic testing: the implications of the US FDA's first marketing authorization for BRCA mutation testing. JAMA 2018; 319:2377–8 [DOI] [PubMed] [Google Scholar]
- 81.The online gene test finds a dangerous mutation. It may well be wrong, https://www.nytimes.com/2018/07/02/health/gene-testing-disease-nyt.html (2018, accessed 31 October 2018)
- 82.Kaiser J. Is genome-guided cancer treatment hyped?. Science 2018; 360:365. [DOI] [PubMed] [Google Scholar]
- 83.Moscow JA, Fojo T, Schilsky RL. The evidence framework for precision cancer medicine. Nat Rev Clin Oncol 2018; 15:183–92 [DOI] [PubMed] [Google Scholar]
- 84.Prasad V. Perspective: the precision-oncology illusion. Nature 2016; 537:S63. [DOI] [PubMed] [Google Scholar]
- 85.Garber K. Blood test may predict cancer immunotherapy benefit. Science 2018; 360:1387. [DOI] [PubMed] [Google Scholar]
- 86.Parikh RB, Prasad V. Blood-based screening for colon cancer: a disruptive innovation or simply a disruption?. JAMA 2016; 315:2519–20 [DOI] [PubMed] [Google Scholar]
- 87.Rixe O, Fojo T. Is cell death a critical end point for anticancer therapies or is cytostasis sufficient?. Clin Cancer Res 2007; 13:7280–7 [DOI] [PubMed] [Google Scholar]
- 88.Stewart DJ, Stewart AA, Wheatley-Price P, Batist G, Kantarjian HM, Schiller J, Clemons M, Bradford JP, Gillespie L, Kurzrock R. The importance of greater speed in drug development for advanced malignancies. Cancer Med 2018; 7:1824–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prasad V, Massey PR, Fojo T. Oral anticancer drugs: how limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol 2014; 32:1620–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Armstrong K, Anderson ME, Carethers JM, Loscalzo J, Parmacek MS, Wachter RM, Zeidel ML. International exchange and American medicine. N Engl J Med 2017; 376:e40. [DOI] [PubMed] [Google Scholar]
- 91.Burotto M, Prasad V, Fojo T. Non-inferiority trials: why oncologists must remain wary. Lancet Oncol 2015; 16:364–6 [DOI] [PubMed] [Google Scholar]
- 92.Lu E, Shatzel J, Shin F, Prasad V. What constitutes an "unmet medical need" in oncology? An empirical evaluation of author usage in the biomedical literature. Semin Oncol 2017; 44:8–12 [DOI] [PubMed] [Google Scholar]
- 93.Prasad V, Kumar H, Mailankody S. Ethics of clinical trials in low-resource settings: lessons from recent trials in cancer medicine. J Glob Oncol 2015; 2:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aggarwal A, Unger-Saldaña K, Lewison G, Sullivan R. The challenge of cancer in middle-income countries with an ageing population: Mexico as a case study. ecancermedicalscience 2015; 9:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mailankody S, Prasad V. Thinking systematically about the off-label use of cancer drugs and combinations for patients who have exhausted proven therapies. Oncologist 2016; 21:1031–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009–13. BMJ 2017; 359:j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lammers A, Wang R, Cetnar J, Prasad V. Time from US Food and Drug Administration approval to publication of data for cancer drugs: a comparison of first and subsequent approvals. Blood Cancer J 2017; 7:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aggarwal A, Fojo T, Chamberlain C, Davis C, Sullivan R. Do patient access schemes for high-cost cancer drugs deliver value to society?- lessons from the NHS Cancer Drugs Fund. Ann Oncol 2017; 28:1738–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cifu AS, Prasad VK. Medical debates and medical reversal. J Gen Intern Med 2015; 30:1729–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blagoev KB, Wilkerson J, Fojo T. Hazard ratios in cancer clinical trials – a primer. Nat Rev Clin Oncol 2012; 9:178–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ross JS. Clinical trials – we get what we pay for. JAMA Intern Med 2018;178:1457 [DOI] [PubMed]
- 102.Fojo T, Giannakakou P. Decade in review – funding in cancer research: National Cancer Institute awards – a work in progress. Nat Rev Clin Oncol 2014; 11:634–6 [DOI] [PubMed] [Google Scholar]
- 103.Drugbank, https://www.drugbank.ca/drugs (2016, accessed 2 November 2018)
- 104.STAT. FDA approves Lilly pill for common advanced breast cancer. https://www.statnews.com/2017/09/29/fda-verzenio-breast-cancer/ (2017, accessed 2 November 2018)
- 105.Prostate.net. What is the cost of zytiga? https://prostate.net/articles/cost-of-zytiga (2013, accessed 2 November 2018)
- 106.Medical News Today. Adcetris may cost $4,500 per vial, or over $100,000 for a course of lymphoma treatment, https://www.medicalnewstoday.com/articles/233141.php (2011, accessed 2 November 2018)
- 107.Pharmacy Times. Ado-trastuzumab Emtansine (Kadcyla), https://www.pharmacytimes.com/publications/health-system-edition/2013/july2013/ado-trastuzumab-emtansine-kadcyla (2013, accessed 2 November 2018).
- 108.Pignata M, Chouaid C, Le Lay K, Luciani L, McConnachie C, Gordon J, Roze S. Evaluating the cost-effectiveness of afatinib after platinum-based therapy for the treatment of squamous non-small-cell lung cancer in France. CEOR 2017; 9:655–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pink Sheet. Chiron/cetus' proleukin (IL-2) therapy will cost $6,000-$8,000 per course, https://pink.pharmaintelligence.informa.com/PS020819/CHIRONCETUS-PROLEUKIN-IL2-THERAPY-WILL-COST-60008000-PER-COURSE (1992, accessed 2 November 2018)
- 110.National Institute for Health and Care Excellence. Technology appraisal guidance-alectinib for untreated ALK-positive advanced non-small-cell lung cancer, https://www.nice.org.uk/guidance/ta536/documents/final-appraisal-determination-document%20https://prescriptionhope.com/alecensa-alectinib (2018, accessed 16 October 2018)
- 111.Drug Topics. Apalutimide for prostate cancer: what to know, http://www.drugtopics.com/new-products/apalutimide-prostate-cancer-what-know (2018, accessed 2 November 2018)
- 112.pan-Canadian Oncology Drug Review. pan-Canadian oncology drug review final economic guidance report Arsenic trioxide (trisenox) for acute promyelocytic leukemia, https://www.cadth.ca/sites/default/files/pcodr/pcodr-trisenox-apl-fn-egr.pdf (2014, accessed 2 November 2018)
- 113.International Medical Products Price Guide, Management Sciences for Health. Asparaginase, http://mshpriceguide.org/en/single-drug-information/?DMFId=69&searchYear=2014 (2016, accessed 2 November 2018)
- 114.Lash A. Xconomy, in immuno-oncology’s latest win, FDA OK’s Genentech Drug, https://xconomy.com/san-francisco/2016/05/18/in-immuno-oncologys-latest-win-fda-oks-genentech-drug/ (2016, accessed 2 November 2018).
- 115.Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation, https://www.cadth.ca/sites/default/files/pcodr/pcodr_avelumab_bavencio_mcc_fn_rec.pdf (2018, accessed 2 November 2018)
- 116.Healio, Immuno-oncology. UK panel rejects CAR T-cell therapy due to cost, https://www.healio.com/hematology-oncology/lymphoma/news/online/%7Bd32cc851-3898-4caf-81e9-0e8cc841e2f2%7D/uk-panel-rejects-car-t-cell-therapy-due-to-cost (2018, accessed 2 November 2018)
- 117.UKMi, London Cancer New Drugs Group. Axitinib as an option for second line advanced renal cell carcinoma with progression after tyrosine kinase inhibitor, https://www.medicinesresources.nhs.uk/upload/documents/Evidence/Drug%20Specific%20Reviews/Axitinib%20as%20an%20option%20for%20second%20line%20advanced%20renal.pdf (2013, accessed 2 November 2018)
- 118.Ontario Public Drug Programs, Ministry of Health and Long-Term Care. Azacitidine, http://www.health.gov.on.ca/en/pro/programs/drugs/ced/pdf/vdaza.pdf (2011, accessed 2 November 2018)
- 119.Leong H, Bonk ME. Bendamustine (treanda) for chronic lymphocytic leukemia: a brief overview. P T 2009; 34:73–6 [PMC free article] [PubMed] [Google Scholar]
- 120.News-Medical, Life Science. Avastin (bevacizumab) price, https://www.news-medical.net/health/Avastin-(Bevacizumab)-Price.aspx (2018, accessed 2 November 2018)
- 121.Geskin L, Malone DC. An exploratory cost-effectiveness analysis of systemic treatments for cutaneous T-cell lymphoma. J Dermatolog Treat 2018; 29:522–30 [DOI] [PubMed] [Google Scholar]
- 122.CADTH, pan-Canadian oncology drug review final economic guidance report. Blinatumomab (Blincyto) for pediatric acute lymphoblastic leukemia, https://www.cadth.ca/sites/default/files/pcodr/pcodr_blinatumomab_blincyto_all_pediatric_fn_egr.pdf (2017, accessed 2 November 2018)
- 123.Durie B, Binder G, Pashos C, Khan Z, Hussein M, Borrello I. Total cost comparison in relapsed/refractory multiple myeloma. J Med Econ 2013; 16:614–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.National Institute for Health and Care Excellence. Final appraisal determination – bosutinib for previously treated chronic myeloid leukaemia, https://www.nice.org.uk/guidance/ta299/documents/chronic-myeloid-leukaemia-bosutinib-final-appraisal-determination-document2 (2013, accessed 16 October 2018)
- 125.Berger K, Schopohl D, Rieger C, Ostermann H. Economic and clinical aspects of intravenous versus oral busulfan in adult patients for conditioning prior to HSCT. Support Care Cancer 2015; 23:3447–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elliott W, Chan J. Relias Media, the trusted source for healthcare information and continuing education. Pharmacology update, Brigatinib Tablets (Alunbrig), https://www.reliasmedia.com/articles/140851-brigatinib-tablets-alunbrig (2017, accessed 16 October 2018)
- 127.National Institute for Health and Clinical Excellence. Final appraisal determination-Cabazitaxel for hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen, https://www.nice.org.uk/guidance/ta255/documents/prostate-cancer-cabazitaxel-final-appraisal-determination-document2 (2012, accessed 16 October 2018)
- 128.Meng J, Lister J, Vataire AL, Casciano R, Dinet J. Cost-effectiveness comparison of cabozantinib with everolimus, axitinib, and nivolumab in the treatment of advanced renal cell carcinoma following the failure of prior therapy in England. CEOR 2018; 10:243–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grullich C. Cabozantinib: a MET, RET, and VEGFR2 tyrosine kinase inhibitor. Recent Results Cancer Res 2014; 201:207–14 [DOI] [PubMed] [Google Scholar]
- 130.Managed Markets Network Journals, Clinical Care Targeted Communications Group. LLC. How much will Amgen's carfilzomib for multiple myeloma cost, https://www.ajmc.com/newsroom/how-much-will-amgens-carfilzomib-combination-treatment-for-multiple-myeloma-cost (2018, accessed 16 October 2018)
- 131.Dalal AA, Guerin A, Mutebi A, Culver KW. Treatment patterns, clinical and economic outcomes of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer receiving ceritinib: a retrospective observational claims analysis. J Drug Assess 2018; 7:21–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 2009; 101:1044–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Reyes C, Gazauskas G, Becker U, Moreno S, Veenstra DL. Cost-effectiveness analysis of obinutuzumab versus ofatumumab for previously untreated chronic lymphocytic leukemia (CLL). Blood 2014; 124:1324 [Google Scholar]
- 134.National Centre for Pharmacoeconomics. Cost-effectiveness of cobimetinib (Cotellic®) for the treatment of unresectable or advanced metastatic melanoma with a BRAF V600: mutation, only in combination with vemurafenib, http://www.ncpe.ie/wp-content/uploads/2016/04/Web-summary-cobimetinib.pdf (2017, accessed 2 November 2018)
- 135.National Centre for Pharmacoeconomics. Cost effectiveness of crizotinib (Xalkori®) for the treatment of adult patients with previously treated anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC), http://www.ncpe.ie/wp-content/uploads/2013/02/Summary-Crizotinib-20130826-with-additional-info-2.pdf (2013, accessed 2 November 2018)
- 136.Kruse GB, Amonkar MM, Smith G, Skonieczny DC, Stavrakas S. Analysis of costs associated with administration of intravenous single-drug therapies in metastatic breast cancer in a U.S. population. JMCP 2008; 14:844–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuznar W. American Health & Drug Benefits, decitabine more cost-effective than conventional induction in older patients with AML, http://www.ahdbonline.com/issues/2014/february-2014-vol-7-no-1-special-issue-ash-2013-payers-perspectives-in-oncology/1648-decitabine-more-cost-effective-than-conventional-induction-in-older-patients-with-aml (2013, accessed 2 November 2018)
- 138.Novartis, Novartis oncology universal co-pay assistance program, https://www.hcp.novartis.com/products/tafinlar-mekinist/advanced-melanoma/access/ (2018, accessed 2 November 2018)
- 139.Summary of product characteristics. Tafinlar, https://www.ema.europa.eu/documents/product-information/tafinlar-epar-product-information_en.pdf (2018, accessed 2 November 2018)
- 140.Curl P, Vujic I, van 't Veer LJ, Ortiz-Urda S, Kahn JG. Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma. PLoS One 2014; 9:e107255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lertkhachonsuk AA, Hanvoravongchai P. Comparison of cost-effectiveness between actinomycin D versus methotrexate-folinic acid in the treatment of low-risk gestational trophoblastic neoplasia. J Reprod Med 2016; 61:230–4 [PubMed] [Google Scholar]
- 142.Del Rey C, Asano E. Accumulated treatment cost of new therapies in multiple myeloma: comparing the combination of daratumumab, bortezomib and dexamethasone with carfilzomib and dexamethasone for who have received at least one prior therapy in Brazil. Value Health 2017; 20:A876 [Google Scholar]
- 143.Cost effectiveness of crizotinib (Xalkori) for the treatment of adult patients with previously treated anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). National Centre for Pharmacoeconomics, http://www.ncpe.ie/wp-content/uploads/2013/02/Summary-Crizotinib-20130826-with-additional-info-2.pdf (2013, accessed 16 October 2018)
- 144.Wiles S, Kabalan M, Sharma R, Shatzel J, Pang J, Yi D, Alatovic I, Saif S, Narasimha D, LaPenna J, Troitino A, Attwood K, Yin Y, Wetzler M. Decitabine is more cost effective than standard conventional induction therapy in elderly acute myeloid leukemia patients. Blood 2013; 122:2699 [Google Scholar]
- 145.National Institute for Health and Care Excellence. Dasatinib, nilotinib and high-dose imatinib for treating imatinib-resistant or intolerant chronic myeloid leukaemia. Technology appraisal guidance [TA425], https://www.nice.org.uk/guidance/ta425/chapter/2-The-technologies (2016, accessed 16 October 2018)
- 146.Geskin L, Malone DC. An exploratory cost-effectiveness analysis of systemic treatments for cutaneous T-cell lymphoma. J Dermatolog Treat 2018; 29:522–30 [DOI] [PubMed] [Google Scholar]
- 147.National Institute for Health and Care Excellence. Appraisal consultation document. Dinutuximab for treating high-risk neuroblastoma, https://www.nice.org.uk/guidance/gid-tag507/documents/appraisal-consultation-document (2015, accessed 2 November 2018)
- 148.ClaimSecure, Drug Review. Firmagon – new hormone therapy for the treatment of advanced prostate cancer, https://www.claimsecure.com/content/pdfs/en-CA/DrugReviews/DrugReview_Vol9_Issue5_en.pdf (2010, accessed 16 October 2018)
- 149.Ojeda B, de Sande LM, Casado A, Merino P, Casado MA. Cost-minimisation analysis of pegylated liposomal doxorubicin hydrochloride versus topotecan in the treatment of patients with recurrent epithelial ovarian cancer in Spain. Br J Cancer 2003; 89:1002–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.OptumRx, Clinical Services Department. Idhifa (enasidenib) – new orphan drug approval, https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-approvals/drugapprovals_idhifa_2017-0801.pdf (2017, accessed 16 October 2018)
- 151.Prostate.net. What is the cost of xtandi? https://prostate.net/articles/cost-of-xtandi (2013, accessed 2 November 2018)
- 152.Shetty N, Gupta S. Eribulin drug review. South Asian J Cancer 2014; 3:57–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hurtaud A, Donnadieu A, Escalup L, Cottu PH, Baffert S. Costs associated with eribulin treatment for patients with metastatic breast cancer in a comprehensive cancer center in France. Breast 2016; 30:73–9 [DOI] [PubMed] [Google Scholar]
- 154.National Institute for Health and Clinical Excellence. Final appraisal determination – erlotinib for the treatment of non-small-cell lung cancer, https://www.nice.org.uk/guidance/ta162/documents/lung-cancer-nonsmallcell-erlotinib-final-appraisal-determination2 (2008, accessed 16 October 2018)
- 155.Doyle JJ, Dezii CM, Sadana A. A pharmacoeconomic evaluation of cisplatin in combination with either etoposide or etoposide phosphate in small cell lung cancer. Semin Oncol 1996; 23:51–60 [PubMed] [Google Scholar]
- 156.Ontario Public Drug Programs, Ministry of Health and Long-Term Care. Everolimus, http://www.health.gov.on.ca/en/pro/programs/drugs/ced/pdf/afinitor.pdf (2011, accessed 16 October 2018)
- 157.Ontario Public Drug Programs, Ministry of Health and Long-Term Care. Exemestane (for early breast cancer), http://www.health.gov.on.ca/en/pro/programs/drugs/ced/pdf/exemestane.pdf (2008, accessed 2 November 2018)
- 158.Tilleul P, Jacot W, Emery C, Lafuma A, Gourmelen J. Management and cost analysis of cancer patients treated with G-CSF: a cohort study based on the French national healthcare insurance database. J Med Econ 2017; 20:1261–7 [DOI] [PubMed] [Google Scholar]
- 159.Suh DC, Powers CA, Barone JA, Shin H, Kwon J, Goodin S. Full costs of dispensing and administering fluorouracil chemotherapy for outpatients: a microcosting study. Res Social Adm Pharm 2010; 6:246–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoon J, Phibbs CS, Chow A, Weinstock MA, Veterans affairs keratinocyte carcinoma chemoprevention Trial Group. Impact of topical fluorouracil cream on costs of treating keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis. J Am Acad Dermatol 2018; 79:501–7.e2 [DOI] [PubMed] [Google Scholar]
- 161.Kuznar W. Value based cancer care, cost-effectiveness comparison: FOLFIRI versus FOLFOX, http://www.valuebasedcancer.com/issue-archive/2011/february-2011-vol-2-no-1/vbcc-448/ (2011, accessed 2 November 2018)
- 162.Attard CL, Brown S, Alloul K, Moore MJ. Cost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancer. Curr Oncol 2014; 21:e41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.National Institute for Health and Clinical Excellence. Final appraisal determination. Fulvestrant for the treatment of locally advanced or metastatic breast cancer, https://www.nice.org.uk/guidance/ta239/documents/breast-cancer-metastatic-fulvestrant-final-appraisal-determination-document2 (2011, accessed 2 November 2018)
- 164.In The Journals Plus. HemOncToday Osimertinib not cost-effective for EGFR-mutated lung cancer, https://www.healio.com/hematology-oncology/lung-cancer/news/in-the-journals/%7B0c4e2499-4e39-408d-baf6-43618fe00a0c%7D/osimertinib-not-cost-effective-for-egfr–mutated-lung-cancer (2018, accessed 16 October 2018)
- 165.Goldstein DA, Krishna K, Flowers CR, El-Rayes BF, Bekaii-Saab T, Noonan AM. Cost description of chemotherapy regimens for the treatment of metastatic pancreas cancer. Med Oncol 2016; 33:48. [DOI] [PubMed] [Google Scholar]
- 166.Scott JR, Zhou Y, Cheng C, Ward DA, Swanson HD, Molinelli AR, Stewart CF, Navid F, Jeha S, Relling MV, Crews KR. Comparable efficacy with varying dosages of glucarpidase in pediatric oncology patients. Pediatr Blood Cancer 2015; 62:1518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Keat CH, Ghani NA. Cost-effectiveness analysis of granisetron-based versus standard antiemetic regimens in low-emetogenic chemotherapy: a hospital-based perspective from Malaysia. Asian Pac J Cancer Prev 2013; 14:7701–6 [DOI] [PubMed] [Google Scholar]
- 168.Prasad M, Ben-Porat L, Hoppe B, Aghajanian C, Sabbatini P, Chi DS, Hensley ML. Costs of treatment and outcomes associated with second-line therapy and greater for relapsed ovarian cancer. Gynecol Oncol 2004; 93:223–8 [DOI] [PubMed] [Google Scholar]
- 169.Warren E, Ward S, Gordois A, Scuffham P. Cost-utility analysis of imatinib mesylate for the treatment of chronic myelogenous leukemia in the chronic phase. Clin Ther 2004; 26:1924–33 [DOI] [PubMed] [Google Scholar]
- 170.Shanafelt TD, Borah BJ, Finnes HD, Chaffee KG, Ding W, Leis JF, Chanan-Khan AA, Parikh SA, Slager SL, Kay NE, Call TG. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract 2015; 11:252–8 [DOI] [PubMed] [Google Scholar]
- 171.Sorensen SV, Peng S, Dorman E, Cote S, Tambour M, Pan F, Sengupta N. The cost-effectiveness of ibrutinib in treatment of relapsed or refractory chronic lymphocytic leukemia. Health Econ Outcome Res 2016; 2:2 [Google Scholar]
- 172.Scottish Medicines Consortium. Ibritumomab tiuxetan (Zevalin®). Schering Health Care Ltd, https://www.scottishmedicines.org.uk/files/ibritumomab_tiuxetan__Zevalin__Resubmission_FINAL_June_2007_for_website.pdf (2007, accessed 2 November 2018)
- 173.Kuznar W. American Health & Drug Benefits, once off patent, imatinib will be the most cost-effective treatment for newly diagnosed CML, http://www.ahdbonline.com/issues/2015/february-2015-vol-8-special-issue-payers-perspectives-in-oncology/1905-once-off-patent-imatinib-will-be-the-most-cost-effective-treatment-for-newly-diagnosed-cml (2015, accessed 16 October 2018)
- 174.Arits AH, Spoorenberg E, Mosterd K, Nelemans P, Kelleners-Smeets NW, Essers BA. Cost-effectiveness of topical imiquimod and fluorouracil vs. photodynamic therapy for treatment of superficial basal-cell carcinoma. Br J Dermatol 2014; 171:1501–7 [DOI] [PubMed] [Google Scholar]
- 175.pan-Canadian Oncology Drug Review. Initial economic guidance report Inotuzumab ozogamicin (besponsa) for acute lymphoblastic leukemia, https://cadth.ca/sites/default/files/pcodr/pcodr_inotuzumab_besponsa_ALL_in_egr.pdf (2018, accessed 2 November 2018)
- 176.Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T 2012; 37:503–30 [PMC free article] [PubMed] [Google Scholar]
- 177.Egerton N. Ixabepilone (ixempra), a therapeutic option for locally advanced or metastatic breast cancer. P T 2008; 33:523–31 [PMC free article] [PubMed] [Google Scholar]
- 178.Ayyagari R, Neary M, Li S, Rokito A, Yang H, Xie J, Benson AB 3rd. Comparing the cost of treatment with octreotide long-acting release versus lanreotide in patients with metastatic gastrointestinal neuroendocrine tumors. Am Health Drug Benefits 2017; 10:408–15 [PMC free article] [PubMed] [Google Scholar]
- 179.National Institute for Health and Care Excellence. Final appraisal determination – Lapatinib for the treatment of women with previously treated advanced or metastatic breast cancer, https://www.nice.org.uk/guidance/gid-tag387/documents/breast-cancer-advanced-or-metastatic-lapatinib-final-appraisal-determination6 (2009, accessed 16 October 2018)
- 180.Goss TF, Szende A, Schaefer C, Totten PJ, Knight R, Jädersten M, Hellström-Lindberg E, List AF. Cost effectiveness of lenalidomide in the treatment of transfusion-dependent myelodysplastic syndromes in the United States. Cancer Control 2006; 13:17–25 [DOI] [PubMed] [Google Scholar]
- 181.Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, Verma A. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol 2009; 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Takeuchi S, Shiga T, Hirata K, Taguchi J, Magota K, Ariga S, Gouda T, Ohhara Y, Homma R, Shimizu Y, Kinoshita I, Tsuji Y, Homma A, Iijima H, Tamaki N, Dosaka-Akita H. Early prediction of lenvatinib treatment efficacy by using (18)F-FDG PET/CT in patients with unresectable or advanced thyroid carcinoma that is refractory to radioiodine treatment: a protocol for a non-randomized single-arm multicenter observational study. BMJ Open 2018; 8:e021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Drugs.com. Lupron depot prices, coupons and patient assistance programs, https://www.drugs.com/price-guide/lupron-depot (2018, accessed 2 November 2018)
- 184.Marsh T. GoodRx, why was there a 500% price increase on cancer treatment lomustine?, https://www.goodrx.com/blog/why-was-there-a-500-increase-on-cancer-treatment-lomustine/ (2017, accessed 2 November 2018)
- 185.Reuters. FDA clears radioactive drug for cancer that killed Steve Jobs, https://in.reuters.com/article/advanced-accelerator-fda/fda-clears-radioactive-drug-for-cancer-that-killed-steve-jobs-idINKBN1FF2RA (2018, accessed 2 November 2018)
- 186.Gulbrandsen N, Wisloff F, Nord E, Lenhoff S, Hjorth M, Westin J; Nordic Myeloma Study Group. Cost-utility analysis of high-dose melphalan with autologous blood stem cell support vs. melphalan plus prednisone in patients younger than 60 years with multiple myeloma. Eur J Haematol 2001; 66:328–36 [DOI] [PubMed] [Google Scholar]
- 187.Reuters. U.S. FDA approves Novartis' leukemia treatment, https://www.reuters.com/article/us-novartis-fda-idUSKBN17U246 (2017, accessed 2 November 2018)
- 188.Bladder cancer: diagnosis and management London: National Institute for Health and Care Excellence, 2015. https://www.ncbi.nlm.nih.gov/books/NBK356297/ (2015, accessed 2 November 2018) [PubMed]
- 189.Helwick C. Will necitumumab be cost-effective?. Am Health Drug Benefits 2015; 8:11. [PMC free article] [PubMed] [Google Scholar]
- 190.Prior authorization approval criteria, Arranon (nelarabine). file:///C:/Users/Kirshan/Downloads/Arranon_nelarabine.pdf.pdf (2006, accessed 2 November 2018)
- 191.Forbes. Could a cancer drug reverse Parkinson's disease?, https://www.forbes.com/sites/emilymullin/2015/10/21/could-a-cancer-drug-reverse-parkinsons-disease/#1c524ed7f6b5 (2015, accessed 16 October 2018)
- 192.Wolford JE, Bai J, Minion LE, Keller R, Eskander RN, Chan JK, Monk BJ, Tewari KS. Cost-effectiveness of maintenance therapy in advanced ovarian cancer: paclitaxel, bevacizumab, niraparib, rucaparib, olaparib, and pembrolizumab. J Clin Oncol 2018; 36:5508 [Google Scholar]
- 193.Pappas AL. Pharmacy times, Obinutuzumab (Gazyva), https://www.pharmacytimes.com/publications/health-system-edition/2014/may2014/obinutuzumab-gazyva (2014, accessed 2 November 2018)
- 194.Hatswell AJ, Thompson GJ, Maroudas PA, Sofrygin O, Delea TE. Estimating outcomes and cost effectiveness using a single-arm clinical trial: ofatumumab for double-refractory chronic lymphocytic leukemia. Cost Eff Resour Alloc 2017; 15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bath C. The ASCO post, PARP inhibitors have ‘clear benefit’ for patients with ovarian cancer and BRCA mutations, but when and at what cost, http://www.ascopost.com/issues/may-25-2015/parp-inhibitors-have-clear-benefit-for-patients-with-ovarian-cancer-and-brca-mutations-but-when-and-at-what-cost/ (2015, accessed 16 October 2018)
- 196.Vu T, Ellard S, Speers CH, Taylor SC, de Lemos ML, Hu F, Kuik K, Olivotto IA. Survival outcome and cost-effectiveness with docetaxel and paclitaxel in patients with metastatic breast cancer: a population-based evaluation. Ann Oncol 2008; 19:461–4 [DOI] [PubMed] [Google Scholar]
- 197.Mamiya H, Tahara RK, Tolaney SM, Choudhry NK, Najafzadeh M. Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol 2017; 28:1825–1831 [DOI] [PubMed] [Google Scholar]
- 198.Peck P. Medpage today, FDA approves vectibix (panitumumab) for advanced colorectal cancer, https://www.medpagetoday.com/gastroenterology/coloncancer/4199 (2006, accessed 16 October 2018)
- 199.Amdahl J, Diaz J, Sharma A, Park J, Chandiwana D, Delea TE. Cost-effectiveness of pazopanib versus sunitinib for metastatic renal cell carcinoma in the United Kingdom. PLoS One 2017; 12:e0175920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Andrews A. Treating with checkpoint inhibitors – figure $1 million per patient. Am Health Drug Benefits 2015; 8:9. [PMC free article] [PubMed] [Google Scholar]
- 201.Ontario Public Drug Programs. Ministry of Health and Long-Term Care. Pemetrexed (for non-small cell lung cancer), http://www.health.gov.on.ca/en/pro/programs/drugs/ced/pdf/pemetrexed1.pdf (2008, accessed 16 October 2018)
- 202.Chernew ME, Newcomer LN, Swain SM. Treatment and cost implications of pertuzumab. Am J Manag Care 2012; 18:SP151–3 [PubMed] [Google Scholar]
- 203.Zander T, Aebi S, Pabst T, Renner C, Driessen C. Spotlight on pomalidomide: could less be more?. Leukemia 2017; 31:1987–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.OncLive. Ponatinib (CML) Pricing gets a blast from congress, https://www.onclive.com/web-exclusives/ponatinib-cml-pricing-gets-a-blast-from-congress (2016, accessed 16 October 2018)
- 205.Prostate.net. What is the cost of xofigo? https://prostate.net/articles/cost-of-xofigo (2016, accessed 2 November 2018)
- 206.Zhu S, Liu J, Sun W, Tao L, Xiao D. Cost-effectiveness analysis of regorafenib for third-line metastatic colorectal cancer compared to cetuximab plus irinotecan in China. J Health Med Econ 2018; 4:5 [Google Scholar]
- 207.Strumberg D, Schultheis B. Regorafenib for cancer. Expert Opin Investig Drugs 2012; 21:879–89 [DOI] [PubMed] [Google Scholar]
- 208.Suri G, Mistry R, Young KC, Hettle R, May JR, Brixner D, Oderda G, Biskupiak J, Tang D, Bhattacharyya D, Bhattacharyya S. Cost effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole for the treatment of post-menopausal women with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) advanced or metastatic breast cancer from a US private third-party payer perspective. Value Health 2017; 20:A436 [Google Scholar]
- 209.Consumersunion. Is the cost reasonable? one dose of cancer drug rituxan, https://consumersunion.org/outrageous-health-costs/cancer-drug-markup/ (2018, accessed 2 November 2018)
- 210.Benzinga Clovis' Rubraca Vs. TESARO's Niraparib, https://www.benzinga.com/analyst-ratings/analyst-color/16/12/8825438/clovis-rubraca-vs-tesaros-niraparib (2016, accessed 16 October 2018)
- 211.Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T 2011; 36:197–202 [PMC free article] [PubMed] [Google Scholar]
- 212.Kish T, Corry L. Sonidegib (Odomzo) for the systemic treatment of adults with recurrent, locally advanced basal cell skin cancer. P T 2016; 41:322–5 [PMC free article] [PubMed] [Google Scholar]
- 213.Cancer drug costs for a month of treatment at initial Food and Drug Administration Approval, https://www.mskcc.org/sites/default/files/node/25097/documents/chemo-prices-table-bach-center-health-policy-and-outcomes-6-13-14.pdf (accessed 2 November 2018)
- 214.National Institute for Health and Care Excellence. Sunitinib for the first-line treatment of advanced and/or metastatic renal cell carcinoma, Technology appraisal guidance [TA169], https://www.nice.org.uk/guidance/ta169/chapter/3-the-technology (2009, accessed 16 October 2018)
- 215.Ontario Public Drug Programs, Ministry of Health and Long-Term Care. Temsirolimus, http://www.health.gov.on.ca/en/pro/programs/drugs/ced/pdf/torisel.pdf (2011, accessed 16 October 2018)
- 216.Garrison LP, Jr, Wang ST, Huang H, Ba-Mancini A, Shi H, Chen K, Korves C, Dhawan R, Cakana A, van de Velde H, Corzo D, Duh MS. The cost-effectiveness of initial treatment of multiple myeloma in the U.S. with bortezomib plus melphalan and prednisone versus thalidomide plus melphalan and prednisone or lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment. Oncologist 2013; 18:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, Davis KL, Owens DK, Goldhaber-Fiebert JD. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol 2018;36:3192-3202 [DOI] [PubMed]
- 218.Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) final recommendation, https://www.cadth.ca/sites/default/files/pcodr/pcodr_trabectedin_yondelis_lile_scarcoma_in_rec.pdf (2016, accessed 2 November 2018)
- 219.Nordqvist C. Medical News Today. One year on herceptin for breast cancer ideal, https://www.medicalnewstoday.com/articles/250912.php (2012, accessed 2 November 2018)
- 220.The Medical Letter. The medical letter on drugs and therapeutics. In: Brief: trifluridine/tipiracil (lonsurf) for metastatic colorectal cancer, https://secure.medicalletter.org/w1496f (2016, accessed 16 October 2018)
- 221.Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC) Final Recommendation, https://www.cadth.ca/sites/default/files/pcodr/pcodr_vandetanib_caprelsa_mtc_fn_rec.pdf (2017, accessed 16 October 2018)
- 222.Pan-Canadian Oncology Drug Review. Initial economic guidance report Venetoclax (Venclexta) for chronic lymphocytic leukemia, https://www.cadth.ca/sites/default/files/pcodr/pcodr_venetoclax_venclexta_cll_in_egr.pdf (2017, accessed 2 November 2018)
- 223.Rocky Mountain, uniform pharmacy prior authorization request form. Marqibo (vincristine sulfate liposome injection), https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=20&cad=rja&uact=8&ved=2ahUKEwiL27T5hYneAhVQX30KHYh4BdE4ChAWMAl6BAgCEAI&url=https%3A%2F%2Fwww.rmhp.org%2F%2Fmedia%2FRMHPdotOrg%2FFiles%2FPDF%2FLearning-Center%2FPrior-Auth-Services%2FPA-Forms-Medicaid%2FMarqibo-vincristine-sulfate-liposome-injection.ashx%3Fla%3Den&usg=AOvVaw1GgpHIsyC3PM_IzWWbKznN (2018, accessed 2 November 2018)
- 224.Fellner C. Vismodegib (erivedge) for advanced Basal cell carcinoma. P T 2012; 37:670–82 [PMC free article] [PubMed] [Google Scholar]
- 225.Joondeph BC. Retina today, will a new low-cost option join the anti-VEGF fold?, http://retinatoday.com/2016/04/will-a-new-low-cost-option-join-the-anti-vegf-fold (2018, accessed 2 November 2018)
- 226.Healio. Zoledronic acid more cost-effective than denosumab for bone metastases, https://www.healio.com/hematology-oncology/breast-cancer/news/in-the-journals/%7B9758e0f5-093a-4d95-bd8b-d742f9dee199%7D/zoledronic-acid-more-cost-effective-than-denosumab-for-bone-metastases (2017, accessed 2 November 2018)
- 227.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern Med 2018;178:1451-1457 [DOI] [PMC free article] [PubMed]
- 228.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect 2015; 29:139–62 [DOI] [PubMed] [Google Scholar]
- 229.Herper M. The cancer drug market just hit $100 billion and could jump 50% in four years. Forbes, https://www.forbes.com/sites/matthewherper/2015/05/05/cancer-drug-sales-approach-100-billion-and-could-increase-50-by-2018/ (2015, accessed 3 September 2018)
- 230.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol 2015; 1:539–40 [DOI] [PubMed] [Google Scholar]
- 231.Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol 2017; 14:381–90 [DOI] [PubMed] [Google Scholar]
- 232.Prasad V, Fojo T, Brada M. Precision oncology: origins, optimism, and potential. Lancet Oncol 2016; 17:e81–6 [DOI] [PubMed] [Google Scholar]
- 233.Prasad V, Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern Med 2017; 177:1569–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Prasad V, Wang R, Afifi SH, Mailankody S. The rising price of cancer drugs – a new old problem? JAMA Oncol 2017;3:277-278 [DOI] [PubMed]
- 235.Fojo T, Lo AW. Price, value, and the cost of cancer drugs. Lancet Oncol 2016; 17:3–5 [DOI] [PubMed] [Google Scholar]
- 236.Sullivan R, Aggarwal A. Health policy: putting a price on cancer. Nat Rev Clin Oncol 2016; 13:137–8 [DOI] [PubMed] [Google Scholar]
- 237.Mailankody S, Prasad V. Implications of proposed medicare reforms to counteract high cancer drug prices. JAMA 2016; 316:271–2 [DOI] [PubMed] [Google Scholar]
- 238.Prasad V, Mailankody S. How should we assess the value of innovative drugs in oncology? Lessons from cost-effectiveness analyses. Blood 2015; 126:1860–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Galkina Cleary E, Beierlein JM, Khanuja NS, McNamee LM, Ledley FD. Contribution of NIH funding to new drug approvals 2010–2016. Proc Natl Acad Sci U S A 2018; 115:2329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kaestner V, Edmiston JB, Prasad V. The relation between publication rate and financial conflict of interest among physician authors of high-impact oncology publications: an observational study. CMAJ Open 2018; 6:E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wagner J, Marquart J, Ruby J, Lammers A, Mailankody S, Kaestner V, Prasad V. Frequency and level of evidence used in recommendations by the National Comprehensive Cancer Network guidelines beyond approvals of the US Food and Drug Administration: retrospective observational study. BMJ 2018; 360:k668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sinha MS, Najafzadeh M, Rajasingh EK, Love J, Kesselheim AS. Labeling changes and costs for clinical trials performed under the US Food and Drug Administration pediatric exclusivity extension 2007 to 2012. JAMA Intern Med 2018; 178:1458–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Prasad S, Gupta SC, Aggarwal BB. Serendipity in cancer drug discovery: rational or coincidence. Trends Pharmacol Sci 2016; 37:435–50 [DOI] [PubMed] [Google Scholar]
- 244.Padma VV. An overview of targeted cancer therapy. Biomedicine 2015; 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine 2017; 34:14–20 [DOI] [PubMed] [Google Scholar]
- 246.Roy NK, Deka A, Bordoloi D, Mishra S, Kumar AP, Sethi G, Kunnumakkara AB. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett 2016; 377:74–86 [DOI] [PubMed] [Google Scholar]
- 247.Banik K, Harsha C, Bordoloi D, Lalduhsaki Sailo B, Sethi G, Leong HC, Arfuso F, Mishra S, Wang L, Kumar AP, Kunnumakkara AB. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett 2018; 416:75–86 [DOI] [PubMed] [Google Scholar]
- 248.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem 2007; 102:580–92 [DOI] [PubMed] [Google Scholar]
- 249.Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs 2007; 16:1753–73 [DOI] [PubMed] [Google Scholar]
- 250.Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol 2010; 37:258–81 [DOI] [PubMed] [Google Scholar]
- 251.Rahaman ST, Sai PR. A short review on carnivorous plants and recent developments in the field of cancer research. iJP 2018; 5:205–12 [Google Scholar]
- 252.Siddiqui AA, Iram F, Siddiqui S, Sahu K. Role of natural products in drug discovery process. Int J Drug Develop Res 2014; 6:172–204 [Google Scholar]
- 253.Harvey AL. Natural products in drug discovery. Drug Discov Today 2008; 13:894–901 [DOI] [PubMed] [Google Scholar]
- 254.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 2015; 14:111–29 [DOI] [PubMed] [Google Scholar]
- 255.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov 2005; 4:206–20 [DOI] [PubMed] [Google Scholar]
- 256.Vuorelaa P, Leinonenb M, Saikkuc P, Tammelaa P, Rauhad JP, Wennberge T, Vuorela H. Natural products in the process of finding new drug candidates. Curr Med Chem 2004; 11:1375–89 [DOI] [PubMed] [Google Scholar]
- 257.Fu RG, Sun Y, Sheng WB, Liao DF. Designing multi-targeted agents: an emerging anticancer drug discovery paradigm. Eur J Med Chem 2017; 136:195–211 [DOI] [PubMed] [Google Scholar]
- 258.Fojo T. Commentary: novel therapies for cancer: why dirty might be better. Oncologist 2008; 13:277–83 [DOI] [PubMed] [Google Scholar]
- 259.Jimeno A, Hidalgo M. Multitargeted therapy: can promiscuity be praised in an era of political correctness. Crit Rev Oncol Hematol 2006; 59:150–8 [DOI] [PubMed] [Google Scholar]
- 260.Kunnumakkara AB, Banik K, Bordoloi D, Harsha C, Sailo BL, Padmavathi G, Roy NK, Gupta SC, Aggarwal BB. Googling the Guggul (Commiphora and Boswellia) for prevention of chronic diseases. Front Pharmacol 2018; 9:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev 2009; 35:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zheng W, Zhao Y, Luo Q, Zhang Y, Wu K, Wang F. Multi-targeted anticancer agents. Curr Top Med Chem 2017; 17:3084–98 [DOI] [PubMed] [Google Scholar]
- 263.Padmavathi G, Rathnakaram SR, Monisha J, Bordoloi D, Roy NK, Kunnumakkara AB. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine 2015; 22:1163–71 [DOI] [PubMed] [Google Scholar]
- 264.Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci 2017; 131:1781–99 [DOI] [PubMed] [Google Scholar]
- 265.Bordoloi D, Roy NK, Monisha J, Padmavathi G, Kunnumakkara AB. Multi-targeted agents in cancer cell chemosensitization: what we learnt from curcumin thus far. Recent Pat Anticancer Drug Discov 2016; 11:67–97 [DOI] [PubMed] [Google Scholar]
- 266.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 2017; 174:1325–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett 2008; 269:352–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Li Y, Li PK, Roberts MJ, Arend RC, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett 2014; 349:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sailo BL, Banik K, Padmavathi G, Javadi M, Bordoloi D, Kunnumakkara AB. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res 2018; 130:259–72 [DOI] [PubMed] [Google Scholar]
- 270.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG, Guha S, Krishnan S, Aggarwal BB. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res 2010; 70:8695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jackson K, Nahata MC. Rising cost of anticancer medications in the United States. Ann Pharmacother 2017; 51:706–10 [DOI] [PubMed] [Google Scholar]
- 272.Steensma DP, Kantarjian HM. Impact of cancer research bureaucracy on innovation, costs, and patient care. J Clin Oncol 2014; 32:376–8 [DOI] [PubMed] [Google Scholar]
- 273.Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics – the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg 2014; 140:1225–36 [DOI] [PubMed] [Google Scholar]
- 274.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin 2018; 68:153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, Khayat D, Boyle P, Autier P, Tannock IF, Fojo T, Siderov J, Williamson S, Camporesi S, McVie JG, Purushotham AD, Naredi P, Eggermont A, Brennan MF, Steinberg ML, De Ridder M, McCloskey SA, Verellen D, Roberts T, Storme G, Hicks RJ, Ell PJ, Hirsch BR, Carbone DP, Schulman KA, Catchpole P, Taylor D, Geissler J, Brinker NG, Meltzer D, Kerr D, Aapro M. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011; 12:933–80 [DOI] [PubMed] [Google Scholar]
- 276.Kantarjian HM, Fojo T, Mathisen M, Zwelling LA. Cancer drugs in the United States: Justum Pretium – the just price. J Clin Oncol 2013; 31:3600–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lopes G, Vulto A, Wilking N, van Harten W, Meier K, Simoens S. Potential solutions for sustaining the costs of cancer drugs. Eur Oncol Haematol 2017; 13:102–7 [Google Scholar]
- 278.Mertens S, de Jongh FE. [Lower costs for anticancer drugs by safety margin around calculated dose and by fine-tuning on ampoule strength]. Ned Tijdschr Geneeskd 2009; 153:B162. [PubMed] [Google Scholar]
- 279.Zafar MZ. Pharmacological study and overcome the cardiotoxicity associated with anticancer drug doxorubicin. QualPrim Care 2017; 25:368–71 [Google Scholar]
- 280.Griggs JJ. Reducing the toxicity of anticancer therapy: new strategies. Leuk Res 1998; 22:S27–33 [DOI] [PubMed] [Google Scholar]
- 281.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25:2097–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Divisi D, Di Tommaso S, Salvemini S. Diet and cancer. Acta Biomed 2006; 77:118–23 [PubMed] [Google Scholar]
- 283.Monisha J, Padmavathi G, Roy NK, Deka A, Bordoloi D, Anip A, Kunnumakkara AB. NF-κB blockers gifted by Mother Nature: prospectives in cancer cell chemosensitization. Curr Pharm Des 2016; 22:4173–200 [DOI] [PubMed] [Google Scholar]
- 284.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 1996; 96:1027–39 [DOI] [PubMed] [Google Scholar]
- 285.Schilsky RL. Implementing personalized cancer care. Nat Rev Clin Oncol 2014; 11:432–8 [DOI] [PubMed] [Google Scholar]
- 286.Ogden A, Rida PC, Aneja R. Let's huddle to prevent a muddle: centrosome declustering as an attractive anticancer strategy. Cell Death Differ 2012; 19:1255–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Beer TM, Prasad V. Taking care of our friends and neighbors: DeVita's the death of cancer and the challenge of letting go. JAMA Oncol 2017; 3:16–7 [DOI] [PubMed] [Google Scholar]
- 288.Patwardhan K. Governance of higher education in Indian systems of medicine: issues, concerns, and challenges. Perspectives on governance of higher education, Bharati Vidyapeeth Deemed University Pune, India, 2010, pp.127–39