Abstract
Background
Herpes simplex virus (HSV)-1 and HSV-2 are common infections affecting the global population, with HSV-1 estimated to affect 67% of the global population. HSV can have rare but severe manifestations, such as encephalitis and neonatal herpes, necessitating the use of reliable and accurate diagnostic tools for the detection of the viruses. Currently used HSV diagnostic tools require highly specialized skills and availability of a laboratory setting but may lack sensitivity. The numerous recently developed HSV diagnostic tools need to be identified and compared in a systematic way to make the best decision about which diagnostic tool to use. The diagnosis of HSV is essential for prompt treatment with antivirals. To select the best test for a patient, knowledge of the performance and limitations of each test is critical.
Objective
This systematic review has summarized recent studies evaluating HSV-1 and HSV-2 diagnostic tools.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, selection criteria, data extraction, and data analysis were determined before the commencement of the study. Studies assessing the specificity/sensitivity of HSV-1 or HSV-2 diagnostic tools published between 2012 and 2018 were included. Quality assessment of included studies was performed using the quality assessment of diagnostic accuracy studies (QUADAS-2) tool.
Results
Searches of the PubMed database yielded 264 studies; 11 studies included 11 molecular assays, and 8 studies included 19 different serological assays for the detection of HSV-1, HSV-2, or both. A greater proportion of molecular assay–based tools are being developed by commercial entities. Studies that tested molecular assays mostly focused on cutaneous and mucosal HSV infections (n=13); 2 studies focused on ocular disease, whereas only 1 study focused on the central nervous system manifestations. The Simplexa HSV 1 & 2 Direct is currently the only Food and Drug Administration–approved device for use on cerebrospinal fluid. No tools focused on prenatal screening. We also present performance metrics of tests for benchmarking of future technology. Most of the included studies had a high risk of bias rating in half of the QUADAS-2 tool risk of bias domains.
Conclusions
The use of serologic tests to diagnose genital lesions is inappropriate because positive results may be due to chronic infection, whereas negative results may overlook recent infection. The incidence of acute infections is rising. As these infections present the greatest risk to fetuses, work needs to be done to prevent vertical transfer. Prenatal screening for primary infection and subsequent medical intervention will assist in lowering the rate of neonatal herpes. In conclusion, HSV diagnosis is moving away from culture-based methods to serology-based or polymerase chain reaction–based methods. Sensitive, rapid, and efficient HSV diagnostic tools should be adopted for the prevention of acute infections and neonatal herpes.
Keywords: diagnostic techniques and procedures, herpes simplex, diagnosis
Introduction
Herpes simplex virus (HSV)-1 and HSV-2 are DNA viruses that belong to Alphaherpesvirinae, a subfamily of the Herpesviridae family [1]. They are the causative agents in a wide range of human diseases that include oral and genital mucocutaneous lesions and some rare but life-threatening conditions such as fulminate encephalitis [2].
HSV is a very common condition, with HSV-1 and HSV-2 infection estimated to affect 67% and 11% of the global population, respectively [2]. The prevalence of HSV can be even higher in low- and middle-income countries or among certain patient subpopulations. For instance, HSV-2 prevalence in sub-Saharan countries is estimated to be as high as 53.7% among individuals aged 15 to 25 years [3]. The prevalence of HSV-2 can be used as a biomarker for the HIV epidemic because of its high association among patients with an HSV-2 infection [4].
One of the most severe manifestations of HSV is encephalitis, which can have a mortality rate up to 97% [5]. Encephalitis is a rare manifestation, which could result in about 4 to 5 cases/million population/year in developed countries [6,7]. HSV infections are very common among women of reproductive age, which can increase the risk of virus transmission from the mother to the child during birth, resulting in neonatal herpes [4]. Neonatal herpes is another severe manifestation of the disease and can cause long-term health complications requiring appropriate and reliable identification of the disease.
In addition to the prevalence and potential complications, lesions caused by HSV-1 and/or HSV-2 are nonspecific and have variable presentations. Chronic carriage necessitates the use of different laboratory testing methods appropriate to each case [8]. Currently, viral culture is the most commonly used method of diagnosis, and it is considered the gold standard method [8]. This method is limited, however, by the need of a laboratory setting, aseptic technique, and variable accuracy dependent on disease stage [8]. It also takes up to 7 days to get the viral culture results, during which time the infection may have developed further. The viral culture will have a lowered sensitivity after the first 48 hours of appearance of the symptoms and is best if administered as soon as the symptoms appear [9]. Although antigens specific to HSV-1 and HSV-2 can also be readily detected by direct immunofluorescence assay, these methods have been found to lack sensitivity in nonsymptomatic patients [8].
Other methods for HSV diagnosis include serological tests, which use blood sample to check for the disease antibodies [9]. These tests can take between 1 day and 3 weeks [9]. Serological tests are suitable to detect the subtype of the HSV virus (HSV-1 or HSV-2) and can detect asymptomatic patients [10]. Serology-based tests can also be used to confirm a clinical diagnosis of HSV because it is a more reliable method than clinical diagnosis [10]. It has been argued that serology-based tests are more direct and economical compared with viral cultures [10].
More modern methods used for detection of infection include techniques that detect viral DNA such as polymerase chain reaction (PCR). These methods use molecular-based assays and are widely used for the detection of infectious diseases [11]. Such methods have been found to be more sensitive and rapid and require less stringent conditions in terms of collection data [11,12]. Type-specific diagnosis is also possible using antigen detection techniques such as enzyme immunoassays targeting the glycoprotein G antigen [8].
A thorough knowledge of the performance and limitations of available tests is critical to select the most appropriate test for a patient as well as inform clinical decision making and the development of future technologies. Previous systematic reviews regarding HSV diagnostic tools reviewed only commercially available tests used to diagnose only one type of the virus (HSV-2) and only reviewed tests used in sub-Saharan Africa [13]. Another review looked at HSV diagnostic tools but limited its population only to pregnant women and neonates [14]. This systematic review summarizes recent studies evaluating HSV-1 and HSV-2 diagnostic tools. We will evaluate the study characteristics, the performance of the various tests included in the studies, and finally discuss their limitations and strengths. We will then suggest areas with unmet medical needs.
Methods
Selection criteria, data extraction, and methods of analysis were decided before the study, in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15].
Eligibility Criteria
We selected only original articles published between 2012 and 2018. We used the Population, Intervention, Comparator, Outcome (PICO) framework to highlight the review’s inclusion and exclusion criteria (Textbox 1). The population included both animal and human populations of all sex and age groups. Patients with multimorbidities were also included, for example, patients with hepatitis and/or HIV because of the possibility that HSV coexists with diseases such as HIV and hepatitis in some patient populations [16,17]. The intervention under study included diagnostic tools to detect all types of HSV-1 or HSV-2 infections including oral, genital, ocular, or central nervous system (CNS)–related infections. The elements in the PICO framework were determined as a result of a preliminary search in PubMed before the formulation of the review’s eligibility criteria. To help compare studies and their outcomes, we only included studies that reported sensitivity and/or specificity as performance indicators of the diagnostic tools [18]. We excluded book chapters, reviews and reports, and articles not in the English language. To capture the most up to date evidence, we restricted the search to the years 2012 to 2018 (Multimedia Appendix 1).
Review inclusion and exclusion criteria reported using the Population, Intervention, Comparator, Outcome framework.
Population
All animal and human populations, including individuals of all ages and both sexes such as neonates, infants, children, adolescents, and pregnant women.
Individuals with multimorbidity will also be included such as patient coinfected with herpes simplex virus (HSV) and HIV, or HSV and hepatitis C.
Intervention
All diagnostic tests for HSV-1 or HSV-2 used for any type of the infection such as oral, anogenital, ocular, or central nervous system infections.
Diagnostic tests that detect other viruses in addition to HSV-1 and HSV-2 will not be included.
Comparator
Not specified.
Outcome
Sensitivity and/or specificity as performance measured of the diagnostic test.
Dates
Database searches will be limited to the dates April 17, 2012 to December 31, 2018.
Search Strategy and Sources of Information
The complete search strategy used to identify studies is detailed in Multimedia Appendix 1. PubMed (MEDLINE) was searched from January 1, 2012 to December 31, 2018. We only searched the PubMed database because most of the studies concerning diagnostic tools are indexed in MEDLINE [19]. We also searched the bibliographies of relevant articles and reviews that we identified in our initial scoping searches.
Study Selection
Studies were selected first by removing duplicates from the initially identified studies, then manually screening the titles and abstracts of the studies against the eligibility criteria, and finally performing full-text reading of eligible studies. ZA and AA completed study screenings. Study records were managed using Mendeley Desktop (v1.16.3) software.
Data Extraction
Data extraction sheets were developed before data extraction. Data extraction was completed separately by the study authors ZA and AA to increase accuracy. Items collected in the data extraction sheets were identified and finalized through iterative discussions with the study authors.
The data extraction sheets collected information on the following:
General study information including country of the study, HSV subtype, sample size, study design (retrospective or prospective), the name of the diagnostic test under study, as well as the comparison tools.
Methodological details including sample collection method, transportation/storage method, study aims, and conclusions.
After identification of the diagnostic test under study, the tests were categorized as follows: serology-based HSV detection assays, molecular assays for detection of HSV, or culture-based methods. Tables for each method were then created that summarized the performance of each test. Although sensitivity and specificity were our primary performance metrics, agreement (total, positive, and negative), kappa, area under receiver operator curve, negative predictive value, and positive predictive value were also recorded. Cost and time to run the test were also extracted. In addition, the Food and Drug Administration and European Medicines Agency status of each included diagnostic tool were ascertained through search of the respective organizations websites [20,21]. For studies that reported analytical performance and clinical performance, only the clinical performance results were reported in this review.
Risk of Bias in Studies
The risk of bias of included studies was assessed using the QUADAS-2 tool [22].
Results
Search Results and Study Inclusion
Following PRISMA guidelines [23], we identified 264 records from the PubMed search and an additional 30 through the bibliography search. There were no duplicates in the records identified; hence, all 294 record titles and abstracts were screened for eligibility; 235 articles were excluded based on the eligibility criteria. Full-text reading of the 59 screened articles resulted in a total of 19 full-text studies for inclusion in this systematic review (Multimedia Appendix 2). We screened the abstracts of 264 articles for relevance and found 59 records that could not be excluded based purely on reviewing the abstracts and titles and these were assessed as full texts. We subsequently excluded 40 articles if, after deeper examination, we found that there was no performance metric measured (n=8), the article did not include a diagnostic tool (n=9), study described viruses other than HSV-1 or HSV-2 (n=11), sensitivity and/or specificity were not measured (n=8), or the article was a review (n=4).
Study Characteristics
Summaries of the 19 included studies can be found in Tables 1 and 2. The majority of studies were prospective in nature (n=12; fewer studies (n=7) relied on archived samples (Table 1). The number of studies over the last 6 years has grown, reaching its highest in 2017: from 5 studies in 2012 to 11 in 2017 (Table 1). This growth declined in 2018, with only 1 study found to be focused on HSV-1 or 2 diagnostic tools. Although the search was completed on December 31, 2018, there may be other 2018 studies that were yet to be published on the day of the search. Sample sizes within studies ranged from 60 in Al‐Shobaili et al [24] and Loughman et al [25] to 3408 in Patel et al. [26] with a median sample size being of 179 patients (Table 1). Studies investigated diagnostic tools for HSV-1/2 (n=10), HSV-2 only (n=7), or HSV-1 only (n=2), and in 1 study, the viral subtype could not be ascertained [27], so it was labelled as HSV-1/2. In addition, 58% of tests were molecular assays, whereas the remainder used serology-based HSV detection methods. No study used culture-based method as their primary method; studies did use them as a comparison method (Table 1). In terms of disease state, 2 studies investigated patients with ocular disease [27,28], 1 for CNS infection [29], and the remainder for patients with suspected mucosal and/or cutaneous lesions (Table 1). Given the heterogeneous nature of the studies included within this review in terms of sample type, collection, and patient demographics, it was not possible to draw statistical inferences or perform a meta-analysis.
Table 1.
Summary of included studies. Table summarizes study location, herpes subtype, sample size, study design (retrospective or prospective), and the name of the diagnostic test under study as well as the tools it will be compared with.
| Study ID | Country | Study design | HSVa-1/2 | Sample size | Disease | Test | Test type | Comparison |
| Al‐Shobaili et al [24] | Saudi Arabia | Prospective | HSV-2 | 60: 35 male and 25 female | Genital ulcer disease (genital) | Herpe Select Express Rapid Test (Focus Technologies Inc, Cypress, CA) | Immunochromatographic (serology-based HSV detection assay) | HerpeSelect 2 IgGELISA (Focus Technologies Inc, Cypress, CA); Kalon HSV-2 IgGb ELISAc assay (Kalon Biological Ltd, Guilford, United Kingdom); MAb-EIAd |
| Barrado et al [28] | Spain | Prospective | HSV-1 | 188 | Suspicion of herpetic epithelial keratitis and nonherpetic corneal ulcer (ocular) | Real-time singleplex PCRe | Molecular assays for detection of HSV | Cell Culture- csPCR, csCC, cswPCR, csWCC |
| Burton et al [30] | United States | Retrospective | HSV-2 | 84 | Veterans with hepatitis C and HSV-2 coinfection | Focus HerpeSelect Hsv-2 IgG (Focus Technologies Inc, Cypress, CA) | Serology-based HSV detection assay | Biokit HSV-2 rapid assay (Biokit United States, Lexington, MA) |
| Gitman et al [31] | United States | Prospective | HSV-1, HSV-2 | 171 | Dermal, genital, ocular, mouth | Simplexa HSV 1 & 2 PCR (Focus Diagnostics, Cypress, CA) | Molecular assays for the detection of HSV | Cell culture (Diagnostic Hybrids, Athens, OH); Cytospin-enhanced DFAf; Real-time TaqMan PCR (LDTg HSV PCR) |
| Granato et al [32] | United States | Prospective | HSV-1, HSV-2 | 1351 | Cutaneous and mucocutaneous herpes infection (cutaneous) | AmpliVue HSV-1 & 2 assay (Quidel, San Diego, CA) | Molecular assays for detection of HSV | ELVIS HSV ID and D3 Typing System (Quidel DHI, Athens, OH) |
| Hobbs et al [33] | Kenya | Retrospective | HSV-2 | 198 | Not specified | HerpeSelect 2 (Focus Diagnostics, Cypress, CA); Kelon ELISA (Kalon Biological, Guildford, United Kingdom) | Serology-based HSV detection assay | Same tests but with serum sample |
| Lang et al [34] | Canada | Prospective | HSV-1, HSV-2 | 276 | Anogenital, oral | Viper HSV-Qx assay (BD Molecular Diagnostics) | Molecular assays for detection of HSV | LightCycler 2.0 platform (HSV-LC) (Roche Diagnostics, Basel, Switzerland) |
| Lee et al [35] | Singapore | Retrospective | HSV-1, HSV-2 | 117 | Not specified | Luminex ARIES HSV-1 & 2 assay (Luminex Corp, Austen, TX) | Molecular assays for detection of HSV | Förster resonance energy transfer-based PCR assay and FTD Neuro 9 assay (Fast Track Diagnostics, Junglinster, Luxembourg) |
| Liermann et al [36] | Germany | Retrospective | HSV-1, HSV-2 | 263 | Not specified | Orgentec ELISA Anti-HSV-2 IgM (Mainz, Germany); Orgentec ELISA Anti-HSV-1 IgM (Mainz, Germany); Orgentec ELISA anti-HSV-1/2 IgM (Mainz, Germany); Serion ELISA classic HSV-2 IgM IgM (Würzburg, Germany); Serion ELISA classic HSV-1 IgM (Würzburg, Germany); Serion ELISA classic HSV-1 + 2 IgM IgM (Würzburg, Germany); Orgentec ELISA Anti-HSV-2 IgG (Mainz, Germany); Orgentec ELISA anti-HSV-1 IgG (Mainz, Germany); Serion ELISA classic HSV-2 IgG IgM (Würzburg, Germany); Serion ELISA classic HSV-1 IgG IgM (Würzburg, Germany); Serion ELISA classic HSV-1 + 2 IgG IgM (Würzburg, Germany) | Serology-based HSV detection assay | HerpeSelect 1 ELISA IgG (Focus Diagnostics, Cypress, CA); HerpeSelect 2 ELISA IgG (Focus Diagnostics, Cypress, CA); Immunoblot assay recomLine HSV-1 & 2 IgG (Mikrogen, Neuried, Germany) |
| Liu et al [37] | China | Prospective | HSV-2 | 318 | Not specified | gG321580His ELISA test | Serology-based HSV detection assay | HerpeSelect 2 ELISA IgG (Focus Diagnostics, Cypress, CA) |
| Loughman et al [25] | France | Retrospective | HSV-2 | 60: 42 female and 18 male | Not specified | HSV-2 biochip test using reagents from the HerpeSelect® 2 IgG kit (Focus Diagnostics, CA; product EL0920G) | Serology-based HSV-2 immunoassay with biochip | LIAISON HSV-2 type specific IgG chemiluminescent immunoassay test (DiaSorin, MN) |
| Binnicker et al [29] | United States | Prospective | HSV-1, HSV-2 | 100 | Central nervous system infection due to herpes simplex virus (CNS) | Simplexa HSV 1 & 2 Direct (Focus Diagnostics, Cypress, CA) | Molecular assays for detection of HSV | Roche HSV-1/2 ASR (Roche Diagnostics, Indianapolis) |
| Miller et al [38] | United States | Prospective | HSV-1, HSV-2 | 179 | Genital herpes | IDbox HSV-1/2 assay (GenturaDx) | Molecular assays for detection of HSV | Culture; MultiCode HSV PCR |
| Parra-Sánchez et al [39] | Spain | Prospective | HSV-1, HSV-2 | 283 | Various mucosal and cutaneous lesions, CSF, urinary | HSV OligoGen kit (Operon-Immuno&Molecular Diagnostics, Zaragoza, Spain) | Molecular assays for detection of HSV | Roche LightCycler HSVóQual Kit assay (Roche Diagnostics, Basel, Switzerland) |
| Patel et al [26] | South Africa and Zambia | Retrospective | HSV-2 | 3408 | HSV-2/HIV-1 coinfected individuals and HIV-uninfected heterosexual partners | Kalon HerpeSimplex virus type 2 IgG ELISA (Kalon Biological Ltd, Surrey, United Kingdom) | Serology-based HSV detection assay | Washington HSV western blot |
| Van Der Pol et al [40] | United States | Prospective | HSV-1, HSV-2 | 508 | Anogenital infections (anogenital) | BD ProbeTec HSV-Qx (HSVQx) system (BD Diagnostics, Sparks, MD) | Molecular assays for detection of HSV | Elvis culture system (San Diego); Laboratory-developed PCR assay |
| Shevlin and Morrow [41] | United States | Retrospective | HSV-2 | 100 | Not specified | Uni-Gold HSV-2 rapid (Trinity Biotech, Ireland) | Serology-based HSV detection assay | Washington HSV western blot |
| Shoji et al [27] | Japan | Prospective | Viral subtype could not be ascertained | 59 + 23 healthy volunteers | Herpes simplex keratitis (ocular) | HSV DNA (by PCR) and HSV specific sIgA antibody levels (ELIZA) in tears | Serology-based HSV detection assay and molecular assays for detection of HSV | —h Control patients? |
| Tong et al [42] | United States | Prospective | HSV-1, HSV-2 | 176 | Genital and oral lesions (oral and genital) | IsoGlow HSV typing assay (BioHelix Corp, Beverly, MA) | Molecular assays for detection of HSV | IsoAmp HSV (BioHelix Corp, Beverly, MA); ELVIS Shell Vial Assay (Diagnostic Hybrids, Athens, OH) |
aHSV: herpes simplex virus.
bIgG: immunoglobulin G.
cELISA: enzyme-linked immunosorbent assay.
dMAb-EIA: monoclonal antibody enzyme immunoassay.
ePCR: polymerase chain reaction.
fDFA: direct fluorescent antibody.
gLDT: laboratory developed test.
hNot applicable.
Table 2.
Methodological characteristics of included studies, summarizing study aims, conclusions, and demographic data.
| Study ID | Patient demographic data | Aims/rationale | Conclusions |
| Al‐Shobaili et al [24] | 58% male; mean age 37 (SD 13.6) years | To evaluate a point-of-care test (HerpeSelect Express Rapid Test) for more rapid turnaround of results in a nonlaboratory setting. | The HerpeSelect Express Rapid Test has adequate sensitivity and specificity for confirming HSVa-2 infection in patients with genitourinary disease. The test is good at diagnosing high-risk individuals. |
| Barrado et al [28] | 52.2% male; mean age 56.9 (SD 19.7) years | Assess if conjunctival swab samples were equivalent to corneal scrapings to diagnose herpetic keratitis. | Conjunctival swabs may serve as supplemental method for the diagnosis of typical HKb despite limited sensitivity when the collection of corneal scrapings would not be feasible. PCRc must be considered the gold standard for diagnosis of typical HK. |
| Burton et al [30] | 92% male; 67% African American; 33% white; 97% heterosexual | To assess type-specific tests for HSV-2 in patients with chronic hepatitis C infection | In veterans with chronic hepatitis C infection, HerpeSelect 1 HSV-2 index values between 1.1 and 2.89 should be confirmed with an alternate test for HSV-2 infection. Only effective within certain range. |
| Gitman et al [31] | 26.4% male; 148 adult; 23 pediatric | Compare the performance, time to result, and cost of the Simplexa HSV 1 & 2 Direct PCR with those of conventional | Simplexa HSV 1 & 2 Direct PCR is expensive but required the least training, had the lowest hands-on time and fastest assay time (75 min vs 3 hours by LDTd HSV PCR), detected most positives, considered an internal control, and provided the HSV type. |
| Granato et al [32] | —e | To evaluate the performance of Amplivue HSV-1 + 2 assay compared with ELVIS HSV IDD3 in detecting HSV-1-2 from clinical specimens. | The results of this study show that the AmpliVue HSV-1/2 assay was more sensitive than ELVIS culture for detecting HSV-1 and HSV-2 in a wide variety of cutaneous and mucocutaneous specimens |
| Hobbs et al [33] | 198 adult male and female; 18 samples used for Kalon assay; 178 samples used for the Focus assay | To evaluate and validate the use of Focus and Kalon ELISAsf in detecting HSV-2 antibodies from dried blood sample as part of an HIV prevention trial. | The use of dried blood spots can reduce time and provide an effective way to test for HSV-2 in resource-limited settings. The study found that the use of dried blood spots with the Kalon assay did not perform well and that the use of dried blood spots with the Focus assay resulted in comparable sensitivity and specificity to the use of serum sample. |
| Lang et al [34] | — | Compare HSV-Q and real-time HSV PCR in terms of accuracy and cost. Device approved for anogenital infections; this study investigates its use on lesions from other anatomical locations. | The HSV-Qx assay was found to be highly sensitive and accurate. Gray zone may be required for specimens with values falling between 50 and 800 maximum relative fluorescence units. HSV-Qx has a lower cost per specimen ($22) compared with that of HSV-LC ($34). Samples lying near the positivity cut off should be retested. |
| Lee at al [35] | — | To evaluate the analytical and clinical performance of the Luminex ARIES assay in detecting HSV-1 and HSV-2 compared with an approved assay for the HSV testing for the diagnosis of meningitis/encephalitis. | The analytical performance results of the assay showed that the assay had lower sensitivity than the comparator. On the other hand, the clinical performance results of the assay showed that it had comparable performance to the routinely used assay. Compared with 2 other routinely used assays, the ARIES assay required the shortest amount of hands-on and assay time and was the least labor-intensive. The study concluded that Luminex ARIES assay can be used for successful detection of HSV-1 and HSV-2 for skin and genital infections, meningitis, and encephalitis. |
| Liermann et al [36] | Healthy children aged between 5 months and 3 years, healthy voluntary blood donors aged 18 to 65 years, or hospitalized patients aged between 14 and 70 years | Evaluate the HSV type-common and type-specific IgGg and IgMh enzyme-linked immunosorbent assays for the diagnosis of acute and latent HSV infections. | HSV type-common IgM ELISAs can be useful to confirm acute newly acquired HSV infections; the use of single-type IgM ELISAs on the basis of whole-virus antigen is dispensable. |
| Liu et al [37] | 64% male; mean age 35.9 (SD 4.52) years for males; mean age 30.7 (SD 4.65) years for females | Need for a convenient, high-quality, rapid, and inexpensive domestic serodiagnostic kit that differentiates between HSV-1 and HSV-2. | The study indicates that gG321–580H has a high diagnostic potential for HSV-2 virus serodiagnosis in humans. |
| Loughman et al [25] | 30% male; median age of 40.5 (range: 15-85) | To validate the HSV-2 biochip assay as a point-of-care test to be able to use it in resource-limited settings. | Membrane-touch biochip requires some improvements such as expanded calibration before it can be used as a rapid diagnosis tool. The tool was validated and performs comparable to other standard diagnostic methods. The study concluded that the assay could work potentially as a rapid, point-of-care test for HSV-2. |
| Binnicker et al [29] | — | Investigates a more rapid, cheaper method of detecting HSV in CSFi fluid that only uses a small amount of fluid. This makes it suitable for use in neonates. | The Simplexa HSV-1/2 assays demonstrated a combined sensitivity and specificity of 96.2% and 97.9%, respectively. Assay does not require nucleic acid extraction. Results are available in 60 min. The Simplexa assay requires only 50 mL of CSF. |
| Miller et al [38] | — | New system designed to maximize sensitivity. This study assesses whether this is achieved and is fully automated. | Assay shows acceptable clinical performance characteristics and demonstrates promise for further development of this fully automated platform for detection of pathogen nucleic acid in clinical laboratories |
| Parra-Sánchez et al [39] | 42.2% male; median age 30.5 (Q1-Q3 24.0-39.0) years | Evaluate a new assay, the HSV OligoGen Kit for the detection of HSV in several types of clinical samples | Detection of HSV-1 and HSV-2 in clinical samples by HOKj was not significantly different from detection by LCHK assay (P ≥.8, t test). Statistical data obtained in this study confirm the usefulness and reliable results of this new assay from a variety of specimens. |
| Patel et al [26] | 51% male; median age (IQR) of 32.0 (27-39); HIV-positive: 50% | Evaluate an onsite HSV tool in a population of HIV infected and noninfected patients and to evaluate the precision of the Kalon HSV-2 IgG ELISA. | In populations with optimal diagnostic accuracy, Kalon is a reliable stand-alone method for on-site HSV-2 IgG antibody detection. Kalon can be utilized in resource-limited settings, enhancing the feasibility to monitor the epidemic and assess intervention efforts. |
| Van Der Pol et al [40] | 34% male; median age: 25 years (range: 17-71) | Results of a multicenter study evaluating the performance of a recently FDAk-approved, commercially available, type-specific nucleic acid amplification test that allows type-specific HSV discrimination. | Assay performs as well as the other assays on a fully automated system that provides results within a few hours rather than many days. No differences in test performance based on gender, clinic type, location of the lesion, or type of lesion were observed. |
| Shevlin and Morrow [41] | 35% male (estimate); Pediatric sera (age <18 years) were not included | Evaluation of a point-of-care and inexpensive device to detect anti-HSV anybody in serum or blood. | UHR is a reliable, low-cost alternative to other point-of-care HSV-2 diagnostic tests. Showed both sensitivity and specificity in a small group of adults. |
| Shoji et al [27] | 51% male | Assesses whether the combined utilization of IgAantibody (HSV-sIgA) levels in tears and real-time PCR can improve diagnostic ability. | The combination of laboratory detection of HSV DNA by real-time PCR and of HSV-sIgA by ELISA using tear samples enables higher reliability in diagnosing the subgroups of HSKl. |
| Tong et al [42] | — | Substantial market need for a low-cost, point-of-care HSV typing assay. Such a device is assessed within this study. | Both formats of the IsoGlow HSV typing assay had sensitivities comparable to that of the FDA-cleared IsoAmp HSV test and specificity for the 2 types of HSV comparable with that of ELVIS HSV turnaround time of around 1 hour. |
aHSV: herpes simplex virus.
bHK: Herpes Keratitis.
cPCR: polymerase chain reaction.
dLDT: laboratory developed test.
eNot applicable.
fELISA: enzyme-linked immunosorbent assay.
gIgG: immunoglobulin G.
hIgM: immunoglobulin M.
iCSF: cerebrospinal fluid.
jHOK: HSV OligoGen kit.
kFDA: Food and Drug Administration.
lHSK: Herpes Simplex Keratitis.
The methodological characteristics of included studies are presented in Table 2. Basic demographic information was reported in 13 studies. In studies that reported participant gender (n=11), between 30% and 92% of study participants were male. Participants’ age ranged from 5 months to 85 years (Table 2).
Diagnostic Test Performance
Molecular Assays
Molecular assays for detection of HSV are summarized in Table 3. A total of 11 diagnostic tools were investigated within the 11 studies (including 1 study that used both molecular and serological methods in conjunction [27], out of which 6 have received FDA approval; Table 3). Some FDA-approved tools had an approval for only some components of the test or certain uses of the test only. For example, the Viper HSV-Qx assay [34] and the BD ProbeTec HSV-Qx (HSVQx) system [40] were only approved for anogenital lesions, Luminex ARIES HSV-1 & 2 Assay [35] was approved for cutaneous of mucocutaneous lesions only; 8 study samples were collected using mucosal or cutaneous swabs (Table 3); in the remaining 3 studies, 1 collected samples through corneal scrapings or conjunctival swabs, 1 collected CSF, and 1 tears (for ocular manifestations; Multimedia Appendix 3).
Table 3.
Molecular assays for the detection of herpes simplex virus, summarizing the different molecular assays studied within the 10 included studies. Information includes regulatory status, collection/storage/transport method, and performance.
| Test | Study ID | Manufacturer | FDAa status | EMAb status | Sample type | Collection method |
| Real-time singleplex PCRc | Barrado et al [28] | University Hospital Madrid, Spain (academic) | No | No | Corneal scrapings and conjunctival swabs | Corneal scrapings by a platinum Kimura spatula using as viral transport medium UTM (Universal transport medium, Copan Diagnostic, Inc). Conjunctival swabs were obtained by polyester swabs. |
| Simplexa HSV 1 & 2 Direct | Gitman et al [31] | Focus Diagnostics, Cypress, CA | Yes | Unclear | Dermal, ocular, mouth | Swabs were collected from local clinics, doctors’ offices, and inpatient wards in 3 mL of M4 viral transport medium (Remel, Lenexa, KS) |
| Simplexa HSV 1 & 2 Direct | Binnicker et al [29] | Focus Diagnostics, Cypress, CA | Yes | Unclear | CSFd | Unknown how CSF collected. |
| AmpliVue HSV-1 & 2 assay | Granato et al [32] | Quidel, San Diego, CA | Yes | Unclear | Cutaneous and mucocutaneous swabs | All specimens were collected on swabs, transported to the laboratory in viral transport medium |
| Viper HSV-Qx assay | Lang et al [34] | BD Diagnostics, Sparks, MD | Yes (only for anogenital lesions) | Unclear | Anogenital and oral swabs | —e |
| Luminex ARIES HSV-1 & 2 assay | Lee et al [35] | Luminex Corp, Austen, TX | Yes (only for cutaneous or mucocutaneous lesion samples) | Unclear | Lesion swab samples | Lesion swab samples were collected in clinic and deidentified for the study |
| BD ProbeTec HSV-Qx (HSVQx) system | Van Der Pol et al [40] | BD Diagnostics, Sparks, MD | Yes (only for anogenital lesions) | — | Anogenital swabs | Sampled using a polyester swab, which was placed directly into universal viral transport medium (Becton, Dickinson [BD], Sparks, MD), and then using a second, foam-tipped swab, which was placed directly into the BDQx liquid wet-swab transport medium (Qx WS). |
| IDbox HSV-1/2 assay | Miller et al [38] | GenturaDx, Hayward, CA | No | No | Genital swabs | Swabs collected in 3 mL of universal transport medium, transported to labs, aliquoted samples stored in −70°C |
| HSV OligoGen kit | Parra-Sánchez et al [39] | Operon-Immuno&Molecular Diagnostics, Zaragoza, Spain | No | No | Various swabs; 110 ulcer specimens, 48 urine, 48 endocervical, 43 CSFs, 4 urethral, and 3 pharyngeal swabs | Cobas PCR media urine sample kit or cobas PCR media female swab sample kit (Roche Diagnostics GmbH, Mannheim, Germany); 110 ulcer specimens, 48 urine, 48 endocervical, 43 CSF, 4 urethral, and 3 pharyngeal swabs |
| HSV DNA (by PCR) and HSV specific sIgA antibody levels (ELIZA) | Shoji et al [27] | School of Medicine, Tokyo, Japan (academic) | No | No | Tears | Using Schirmer strips (Schirmer Tear Production Measuring Strips; Showa- Yakuhinkako, Tokyo, Japan) |
| IsoGlow HSV typing assay | Tong et al [42] | BioHelix Corp, Beverly, MA | No | Unclear | Swabs | Clinical swabs suspended in vial transport medium were collected from the Cleveland Clinic (Cleveland, OH). The samples were shipped on ice for overnight delivery and were aliquoted on receipt. Some were placed at −80°C for long-term storage, and some were placed at −20°C for short-term storage or near-term testing. |
aFDA: Food and Drug Administration.
bEMA: European Medicines Agency.
cPCR: polymerase chain reaction.
dCSF: cerebrospinal fluid.
eNot applicable.
The performance metrics of the various molecular tests are summarized for comparison with future technologies summarized in Figures 1 and 2. One study [35] reported positive percent agreement and negative percent agreement instead of sensitivity and specificity, which is equivalent to the sensitivity and specificity measures in the absence of a standard [43]. Sensitivities for molecular methods ranged from 76.9% (27) to 100% (for a number of tools) (Figures 1 and 2). The study by Shoji et al [27] was the single study that used both a serological and molecular technique in conjunction using tears as a sample material. The study reported the lowest specificity of 82.6% using their ELIZA and PCR techniques. The second worst sensitivity was indicated by a real-time singleplex PCR tested in an academic center by Barrado et al [28]. Here, a sensitivity of 77.8% using corneal scrapings was found [28]. All other values were above 90% (Multimedia Appendix 3; Figures 1 and 2). Gitman et al [31] aimed to compare cell culture, DFA, and a laboratory-developed real-time TaqMan PCR (LDT HSV PCR) for the detection of HSV in dermal, genital, ocular, mouth, or other swab samples [31]. Conventional culture was found to have a sensitivity and specificity of 87.9 (0.768-0.943) and 99.1 (0.945-1.000), respectively. The difference when using direct immunofluorescence, TaqMan PCR, or Simplexa Direct PCR did not improve these metrics [31].
Figure 1.
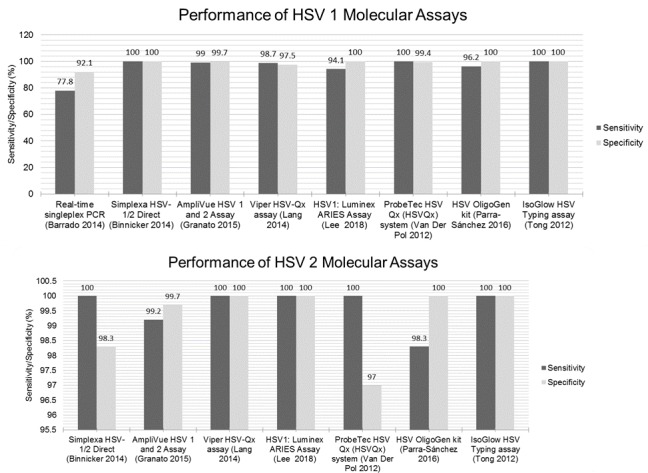
Sensitivity and specificity of molecular-based diagnostics. Because of the heterogeneous nature of studies included in this systematic review, it is not possible to draw strict conclusions with regard to performance metrics. This figure, therefore, aims solely to act as a means of visually displaying the sensitivity and specificity results of each study. HSV: herpes simplex viruses.
Figure 2.
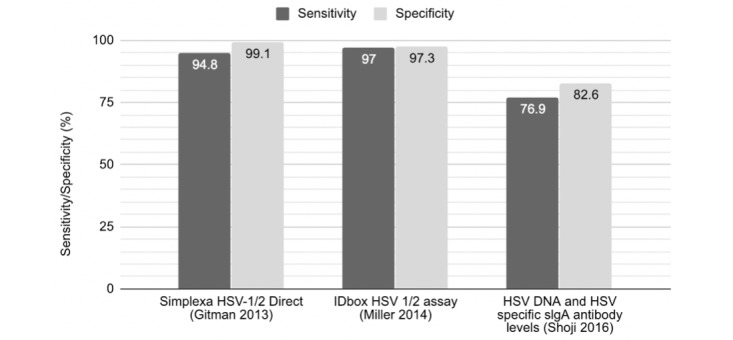
Sensitivity and specificity of molecular-based diagnostics. Because of the heterogeneous nature of studies included in this systematic review, it is not possible to draw strict conclusions with regard to performance metrics. This figure, therefore, aims solely to act as a means of visually displaying the sensitivity and specificity results of each study. HSV: herpes simplex viruses.
Serological Assays
Serological assays for the detection of HSV are summarized in Multimedia Appendix 3. A total of 19 serological diagnostic tools were investigated within 8 studies (plus 1 study [27] that used both molecular and serological methods in conjunction—detailed in Table 3); 4 of the serological diagnostic tools have received FDA approval (Multimedia Appendix 3) of which HerpeSelect 2 [33] was approved for use with serum only, and the test used by Loughman et al [25] only used reagents from the FDA-approved HerpeSelect 2 IgG kit. All of the studies in this category used serum samples, and only 1 study also tested the use of dried blood spots instead [33]. The performance metrics of the various tests are summarized for comparison to future technologies (Multimedia Appendix 3 and Figure 3). Sensitivity for serological tools ranged from 39% in hepatitis-coinfected individuals using the Herpeselect Hsv-2 IgG [30] tool to 100% (for a number of tools; Figure 4). All others were equal to or above 80% (Multimedia Appendix 3 and Figure 3). The study by Liermann et al [36] investigated the detection of primary infection and concluded that its HSV IgM serology tool should not be used to make decisions for antiviral treatment in HSV.
Figure 3.
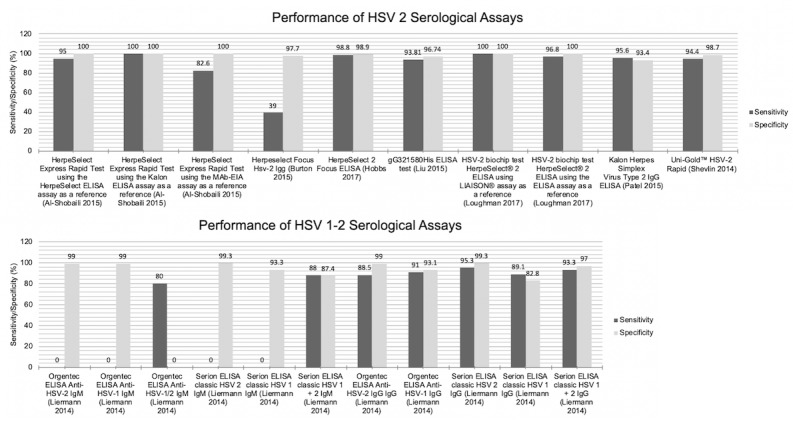
Sensitivity and specificity of molecular-based diagnostics. Because of the heterogeneous nature of studies included in this systematic review, it is not possible to draw strict conclusions with regard to performance metrics. This figure, therefore, aims solely to act as a means of visually displaying the sensitivity and specificity results of each study. HSV: herpes simplex viruses.
Figure 4.
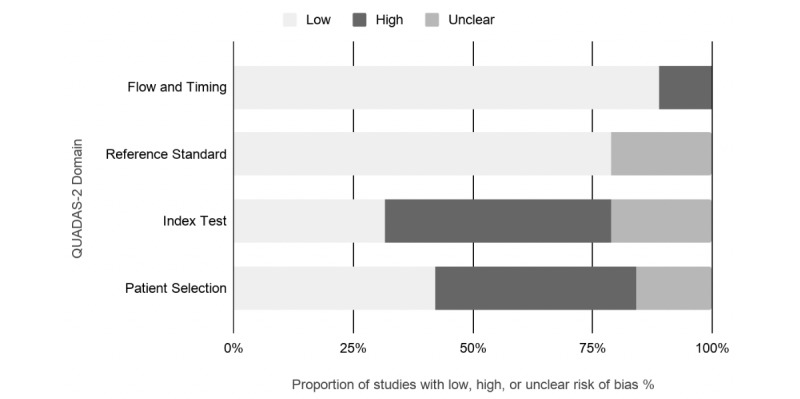
Graphical display for the Quality Assessment of Diagnostic Accuracy Studies-2 tool results. QUADAS: quality assessment of diagnostic accuracy studies.
Cost
Gitman et al [31] carried out the most comprehensive cost analysis, comparing the performance, time to result, and cost of the Simplexa HSV 1 & 2 Direct PCR with those of conventional cell culture, DFA, and a laboratory-developed real-time TaqMan PCR (LDT HSV PCR). The results of this are displayed in Table 4 (table was taken directly from the corresponding article [31]).
Table 4.
Comparison of estimated costs per reportable result and expertise required. Table adapted from Gitman et al [31].
| Test | Assay time | Frequency of testing | Cost ($) | Expertise | ||
|
|
|
|
Materials and reagents | Labor | Total |
|
| Cytospin-DFAa | 90 min | On demand | 5.14 | 8.78 | 13.92 | Mid-high |
| Conventional culture | 1-7 days | Examined once a day | 5.4 | 11.94 | 17.94 | Mid-high |
| Simplexa Direct PCR | 75 min | Once a day | 39.54 | 2.69 | 42.23 | Mid |
| LDTb HSVc PCRd with extraction | 3 h | Once a day | 19.96 | 5.07 | 25.03 | High |
aDFA: direct fluorescent antibody.
bLDT: laboratory developed test.
cHSV: herpes simplex virus.
dPCR: polymerase chain reaction.
Gitman et al [31] concluded that the Simplexa HSV 1 & 2 Direct PCR was the most expensive but required the least training of the assays used, had the lowest hands-on time, and fastest assay time (75 min, vs 3 hours by LDT HSV PCR), and provided the HSV type. The Simplexa Direct PCR is an FDA-approved device (Table 3), and such could be used to benchmark developing technologies.
The cost-to-run per specimen was also compared between the Viper platform and real-time PRC in the study by Lang et al [34]. Compared with the HSV-LC system, the Viper instrument was found to be $12 cheaper to run per specimen ($22 vs $34). In addition, the platform is fully automated [34]. Although they did not carry out a formal cost analysis, the study by Binnicker et al [29] compared time with run for real-time PCR assay and the commercially available Simplexa HSV 1 & 2 Direct (Focus Diagnostics, Cypress, CA) using CSF samples. In addition, Lee et al [35] has reported that the automated ARIES assay takes only 2 hours/12 samples and has the fastest turnaround time compared with other in-house assays. In addition, to avoiding the need for nucleic acid extraction, the Simplexa devices run time was quoted as 60 min in comparison to 4 hours by conventional methods. A separate tool, the HSV-Qx assay, was found to produce results in a few hours compared with many days for conventional culture techniques [40]. Tong et al [42] also demonstrated a turnaround time of 1 hour using their portable device, although it was not fully automated (requires hands on time of 5 min).
Sample Type and Patient Subpopulations
Binnicker et al [29] found that the Simplexa HSV-1/2 assays demonstrated a combined HSV-1 and HSV-2 sensitivity and specificity of 96.2% and 97.9%, respectively. Critically, the results are available in 60 min, and the test only requires 50 µL of CSF [29].
Risk of Bias in Individual Studies
Most of the studies received high risk of bias in the patient selection and in the index test domains of the QUADAS tool (Table 5; Figure 4). High risk of bias in the patient selection domain was mostly because of the lack of randomization in recruiting patients and the use of case-control design in selecting participants and/or recruited patients with confirmed diagnosis. Only few studies included patients with suspected disease and no pretested samples [40,41] or recruited a random sample [26]. High risk of bias in the index test category was mostly because of the index test results being interpreted with the knowledge of the reference test results; most of the studies used the reference test first, and after knowing the results, performed the index test. Because all of the studies used the gold standard or an approved and validated reference tests, the reference standard mostly received low risk of bias (Figure 4). Studies that received high bias rating in the flow and timing domain used different reference standards for different patient groups and did not include all patients or samples in the analysis [27,36]. Studies that received low risk of bias rating in general recruited a random sample [26], blinded study conductors to the results of the reference and index tests [26,29,36], deidentified previously collected samples [41], or conducted reference and index tests simultaneously [34]. All of the studies received low risk of bias in the applicability concerns domains of the QUADAS tool because of the reviews’ inclusion criteria being inclusive of a wide range of patients, index tests, and references standards (Table 5).
Table 5.
Tabular presentation for the Quality Assessment of Diagnostic Accuracy Studies tool results. Table format adapted from Whiting et al [22].
| Study | Risk of bias | Applicability concerns | |||||
|
|
Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard |
| Al-Shobaili et al [24] | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Barrado et al [28] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Burton et al [30] | High risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Gitman et al [31] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Granato et al [32] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Hobbs et al [33] | High risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lang et al [34] | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lee et al [35] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Liermann et al [36] | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Liu et al [37] | High risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Loughman et al [25] | High risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Binnicker et al [29] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Miller et al [38] | High risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Parra-Sánchez et al [39] | High risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Patel et al [26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Van Der Pol et al [40] | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Low risk |
| Shevlin and Morrow [41] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Shoji et al [27] | High risk | High risk | Unclear risk | High risk | Low risk | Low risk | Low risk |
| Tong et al [42] | Unclear risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Discussion
Overview
To select the most appropriate test for a patient, a thorough knowledge of the performance and limitations of available tests is critical to inform clinical decision making and the development of future technologies that may penetrate innovation gaps. This systematic review aimed to summarize recent study articles evaluating HSV-1 and HSV-2 diagnostic tools. We evaluated the study characteristics and the performance of the various tests included in the studies; here, we conclude by discussing the various strengths and limitations of various tools and how they may relate to the development of future diagnostics.
Performance
It is evident that further work needs to be done with regard to tools that work better with different sample types. It also appears that tools developed in academic centers perform worse. This may be because they are at an earlier stage of the developmental pathways and thus, not nearing approval or commercialization. Overall, tests were more specific than sensitive, and it appears that serological tests perform worse.
Culture- and laboratory-based methods have become the gold standard choice for the diagnosis of HSV-1 and HSV-2 infections. However, translational effort is focused on developing alternate techniques based on serological and molecular assays as evidenced by the absence of novel culture-based methods in this review.
Specimen quality and adequate transport and handling to maintain infectivity are essential while using culture methods. Because of the enveloped nature of the HSV-1 and HSV-2 virus, specimens collected using a swab must be transferred to suitable viral transport media with the addition of antimicrobials. Using standard culture methods, results are usually available within 5 days; although in some cases, the result may take up to 2 weeks. Such issues are largely mitigated when using molecular methods. The use of an antigen detection system such as Herpchek (DuPont, Wilmington, DE) can decrease turnaround time [44]. Shell vial culture also has a decreased average turnaround time, with high sensitivity achieved within 24 hours [45]. In Tong et al [42], the IsoGlow HSV Typing assay (Nucleic acid amplification tests—based technique) was compared with the ELVIS Shell Vial Assay where it displayed similar performance, while having the advantages that it was more rapid, portable, and does not require laboratory equipment. Another limitation of traditional culture methods is that viral subtyping requires the additional step of using HSV-1 and HSV-2 monoclonal antibodies [46,47]. Similarly Lee et al [35], found that the ARIES assay required the least amount of time when compared with 2 other routinely used assays and the least technical skills and knowledge [35]. The enzyme-linked virus-inducible system (ELVIS; Diagnostic Hybrids, Inc, USA) enables faster turnaround than standard culture or shell vial methods, with similar performance characteristics [45]. High sensitivities using culture-based methods are hindered by highly variable specimen quality, dependency on stage of the lesion, as well as primary versus recurrent infection [47].
Turnaround Time and Cost
In HSV infections, especially when it results in CNS-related infections, rapid turnaround is essential for treatment [48,49]. Rapid turnaround is important for providing the treatment to patient as early as possible. In the case of HSV, which has no cure, antivirals are the only treatment available to manage the symptoms of the disease [50]. Antivirals are recommended to be administered within the first 48 to 72 hours of the appearance of symptoms for best results [51], which is a very short time interval that requires a rapid turnaround time in case the provider has to wait for the diagnostic test results before prescribing the medication. Even more important is the throughput time (ie, how many tests can be run using the system within certain amount of time) of a diagnostic test. In most cases, a confirmed diagnostic is necessary to provide antiviral treatment for HSV since the prolonged use of antivirals can lead to antiviral resistance [50]. According to Lee et al [35], the ARIES assay’s throughput time was reported as 12 samples in 2 hours. This throughput time is rapid compared with 3 to 7 days of processing time needed for viral culture [52].
Automation increases turnaround time, improve reproducibility of the test, and reduce the chance of human error [53]. Although not quantified, capital would be saved because of the automated nature and more rapid turnaround of such devices. Automated diagnostic tests may only require minimal technical knowledge reducing the likelihood or economically wasteful misclassification because of limited knowledge of the lab technician [53]. Rapid turnaround time is important for most infectious or viral diseases. For example, for respiratory viruses, diagnostic test should be provided at the point-of-care (possible with results ready in less than 1.6 hours) to ensure the best outcomes for the patient [54].
Sample Type and Patient Subpopulations
The HIV status of an individual does not appear to affect the performance of HSV ELISAs [55]. This is an important point because of the high rate of coinfection and increased risk of transmission and acquisition of HIV by HSV-positive individuals [56,57]. Among other common sexually transmitted diseases, only coinfection with Neisseria gonorrhoeae has been shown to significantly reduce HSV immunoassay’s performance [57]. However, the specificity of immunoassays can differ based on population demographics based on the findings of a meta-analysis conducted on studies from Africa [13].
While HSV can rarely be cultured from the CSF, molecular assays can yield results in a matter of hours, which can be important when dealing with possible HSV encephalitis or meningitis. Currently, the only device approved for use for HSV virus detection in CSF is the Simplexa HSV 1 & 2 Direct (Focus Diagnostics, Cypress, CA) (Table 4). The latter is important for the use of the test in the neonatal population. Neonates immune system has a limited ability in fighting off infections, hence resulting in mortality rates exceeding 80% from HSV infection [58]. A PCR-based HSV detection system from CSF is critical for this patient population. Given this information, it is concerning that greatest incidence of HSV infections occurs in women of reproductive age increasing the risk of maternal transmission of the virus to the fetus or neonate [59,60]. This is a major public health concern in both developed and developing countries: a 3-year study in Canada found a neonatal HSV incidence of 5.9 per 100,000 live births and a case fatality rate of 15.5% [61]. Prevention is challenging given the asymptomatic nature of the disease in its latent phase [61], and current guidelines recommend delivery by cesarean section. This is because 85% of vertical transmissions occur during the vaginal delivery where the fetus comes into contact with infected genital secretions [61]. A 33-year study conducted in the New York, USA, concluded that deaths from HSV have increased over the time period (incidence 0.82 deaths per 100,000 live births), despite reductons in deaths from HIV and syphilis [62]. They stated this is due to an increasing number of pregnant women having no immunity to HSV type 1 putting them at increased risk of contracting the disease during pregnancy [62]. Interestingly, neonatal HSV-1 infection in developing countries is rare with the vast majority of infections being caused by HSV-2, which carries a worse prognosis. In addition, acute infection is associated with a higher transmission rate in part because of the lack of maternal to fetal antibody transfer in the third trimester [60]. A test that can accurately diagnose acute (not just latent infection) is urgently required, as current methods may not detect early infection. The study by Liermann et al [36] was the only study to investigate detection of primary infection, and concluded that its HSV IgM serology tool should not be used to make decisions for antiviral treatment in HSV [36]. Thus, no diagnostic tools included in this systematic review have addressed this unmet medical need.
The use of serologic tests to diagnose suspected genital lesions are inappropriate because positive results may be due to chronic infection, whereas negative results may overlook recent infection.
Risk of Bias of the Included Studies
A common source of bias in diagnostic tool studies occurs when studies recruit patients based on their disease status. This often leads to overestimation of the results and may exaggerate diagnostic accuracy [22]. All of the studies included in this review used sample populations that had a higher than average prevalence of HSV infection. As a result, these sensitivities may be overestimated.
Limitations
The studies included within this systematic review employed heterogeneous methods while also using different patient populations. For this reason, a statistical comparison of results was not conducted, and instead, the results displayed in a qualitative manner to benchmark future technology. Although a PRISMA-compliant search was searching the PubMed database, it is possible that some studies may have been inadvertently missed.
Conclusions
The landscape of diagnostic tools for HSV-1 and HSV-2 infections is rapidly moving away from laboratory-based and culture methods that have long been considered the gold standard technique. A majority of tools study cutaneous and mucosal HSV infections (n=13); 2 tests focused on ocular disease, whereas a single one on CNS manifestations. No diagnostic tools included in our systematic review are currently suitable for use as prenatal tools, however. The incidence of acute infections is rising, and because these infections present the greatest risk to unborn fetuses, further work needs to be done to develop diagnostic tools to detect primary infection in expectant mothers to prevent vertical transfer. This will assist in lowering the rate of neonatal herpes, which can be a life-threatening condition. We believe this can only be achieved through prenatal screening for primary infection and subsequent medical intervention.
Acknowledgments
The authors would like to thank the librarians at Imperial College London for their assistance in the search strategy. This work was funded by the European Institute of Innovation and Technology Health (grant 18654).
Abbreviations
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DFA
direct fluorescent antibody
- ELISA
enzyme-linked immunosorbent assay
- FDA
Food and Drug Administration
- HSV
herpes simplex viruses
- PICO
Population, Intervention, Comparator, Outcome
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS
quality assessment of diagnostic accuracy studies
Search terms.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 flow diagram.
Serological assays for detection of herpes simplex virus and summary of the different serological assays studied within the 6 included studies.
Footnotes
Conflicts of Interest: DB is a stockholder in Translation Ventures Ltd (Charlbury, UK), IP Asset Ventures Ltd, and Biolacuna Ltd (Oxford, UK), companies that, among other services, provide biomanufacturing, regulatory, and financial advice to pharmaceutical clients. DB is also subject to the CFA Institute's codes, standards, and guidelines, so he must stress that this piece is provided for academic interest only and must not be construed in any way as an investment recommendation. Additionally, at time of publication, DB and the organizations with which he is affiliated may or may not have agreed to and/or pending funding commitments from the organizations named herein.
References
- 1.Davison A. Encyclopedia of Virology (Third Edition) Oxford: Academic Press; 2008. Herpesviruses: General Features. [Google Scholar]
- 2.World Health Organization. 2017. [2019-05-07]. Herpes simplex virus https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus .
- 3.Torrone EA, Morrison CS, Chen P, Kwok C, Francis SC, Hayes RJ, Looker KJ, McCormack S, McGrath N, van de Wijgert JH, Watson-Jones D, Low N, Gottlieb SL, STIMA Working Group Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med. 2018 Dec;15(2):e1002511. doi: 10.1371/journal.pmed.1002511. http://dx.plos.org/10.1371/journal.pmed.1002511 .PMEDICINE-D-17-03449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouyoumjian SP, Heijnen M, Chaabna K, Mumtaz GR, Omori R, Vickerman P, Abu-Raddad LJ. Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS. 2018 Jun 19;32(10):1343–1352. doi: 10.1097/QAD.0000000000001828. http://europepmc.org/abstract/MED/29794495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016 Dec;13(3):493–508. doi: 10.1007/s13311-016-0433-7. http://europepmc.org/abstract/MED/27106239 .10.1007/s13311-016-0433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jørgensen LK, Dalgaard LS, Østergaard LJ, Nørgaard M, Mogensen TH. Incidence and mortality of herpes simplex encephalitis in Denmark: a nationwide registry-based cohort study. J Infect. 2017 Dec;74(1):42–49. doi: 10.1016/j.jinf.2016.09.004.S0163-4453(16)30243-2 [DOI] [PubMed] [Google Scholar]
- 7.Granerod J, Cousens S, Davies NW, Crowcroft NS, Thomas SL. New estimates of incidence of encephalitis in England. Emerg Infect Dis. 2013;19(9) doi: 10.3201/eid1909.130064. doi: 10.3201/eid1909.130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014 May 12;11:83. doi: 10.1186/1743-422X-11-83. https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-11-83 .1743-422X-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WebMD. 2005. [2019-05-07]. Herpes Test: What You Should Know https://www.webmd.com/genital-herpes/herpes-tests-what-you-should-know .
- 10.Wald A, Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis. 2002 Oct 15;35(Suppl 2):S173–82. doi: 10.1086/342104.CID020042 [DOI] [PubMed] [Google Scholar]
- 11.Burd EM. Validation of laboratory-developed molecular assays for infectious diseases. Clin Microbiol Rev. 2010 Jul;23(3):550–76. doi: 10.1128/CMR.00074-09. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=20610823 .23/3/550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald A, Huang M, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003 Nov 01;188(9):1345–51. doi: 10.1086/379043.JID30698 [DOI] [PubMed] [Google Scholar]
- 13.Biraro S, Mayaud P, Morrow RA, Grosskurth H, Weiss HA. Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Dis. 2011 Feb;38(2):140–7. doi: 10.1097/OLQ.0b013e3181f0bafb. [DOI] [PubMed] [Google Scholar]
- 14.Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, Pietropaolo V. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009 Apr 06;6:40. doi: 10.1186/1743-422X-6-40. https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-6-40 .1743-422X-6-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Oct;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. http://linkinghub.elsevier.com/retrieve/pii/S0895-4356(09)00180-2 .S0895-4356(09)00180-2 [DOI] [PubMed] [Google Scholar]
- 16.Burton MJ, Penman A, Adams-McAlpine G, Overman TL, Hook EW. Prevalence and characteristics of herpes simplex virus type-2 coinfection in veterans with hepatitis C. Am J Med Sci. 2012 Dec;344(6):436–40. doi: 10.1097/MAJ.0b013e31824585bd.S0002-9629(15)30782-5 [DOI] [PubMed] [Google Scholar]
- 17.Looker. Elmes JA, Gottlieb SL, Schiffer JT, Vickerman P, Turner KM, Boily MC. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017 Dec;17(12):1303–1316. doi: 10.1016/S1473-3099(17)30405-X. https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(17)30405-X .S1473-3099(17)30405-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM. Diagnostic tests. 1: sensitivity and specificity. Br Med J. 1994 Jun 11;308(6943):1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mckibbon KA, Marks S. Searching for the best evidence. Part 2: searching CINAHL and Medline. Evid Based Nurs. 1998 Oct 01;1(4):105–107. doi: 10.1136/ebn.1.4.105. [DOI] [Google Scholar]
- 20.US Food & Drug Administration. [2019-05-07]. https://www.fda.gov/
- 21.European Medicines Agency. [2019-05-07]. https://www.ema.europa.eu/en .
- 22.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009.155/8/529 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. http://dx.plos.org/10.1371/journal.pmed.1000097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Shobaili H, Hassanein KM, Mostafa MS, Al Duways AS. Evaluation of the HerpeSelect Express rapid test in the detection of herpes simplex virus type 2 antibodies in patients with genital ulcer disease. J Clin Lab Anal. 2015 Jan;29(1):43–6. doi: 10.1002/jcla.21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loughman T, Singh B, Seddon B, Noone P, Santhosh P. Validation of a membrane touch biosensor for the qualitative detection of IgG class antibodies to herpes simplex virus type 2. Analyst. 2017 Jul 24;142(15):2725–2734. doi: 10.1039/c7an00666g. [DOI] [PubMed] [Google Scholar]
- 26.Patel EU, Manucci J, Kahle EM, Lingappa JR, Morrow RA, Piwowar-Manning E, James A, Maluzi KF, Cheeba MM, Gray G, Kosloff B, Delany-Moretlwe S, Inambao M, Vwalika B, Quinn TC, Laeyendecker O. Precision of the Kalon Herpes Simplex Virus Type 2 IgG ELISA: an international inter-laboratory assessment. BMC Infect Dis. 2015 Dec 30;15:398. doi: 10.1186/s12879-015-1130-6. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-015-1130-6 .10.1186/s12879-015-1130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoji J, Sakimoto T, Inada N, Kamei Y, Matsubara M, Takamura E, Sawa M. A diagnostic method for herpes simplex keratitis by simultaneous measurement of viral DNA and virus-specific secretory IgA in tears: an evaluation. Jpn J Ophthalmol. 2016 Jul;60(4):294–301. doi: 10.1007/s10384-016-0448-y.10.1007/s10384-016-0448-y [DOI] [PubMed] [Google Scholar]
- 28.Barrado L, Suarez MJ, Pérez-Blázquez E, Otero JR, Folgueira MD. Could polymerase chain reaction tests on conjunctival swabs be useful to diagnose herpetic keratitis? Enferm Infecc Microbiol Clin. 2014 Jan;32(1):28–30. doi: 10.1016/j.eimc.2013.07.005.S0213-005X(13)00211-5 [DOI] [PubMed] [Google Scholar]
- 29.Binnicker MJ, Espy MJ, Irish CL. Rapid and direct detection of herpes simplex virus in cerebrospinal fluid by use of a commercial real-time PCR assay. J Clin Microbiol. 2014 Dec;52(12):4361–2. doi: 10.1128/JCM.02623-14. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=25274992 .JCM.02623-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton M, Van Wagoner NJ, Sunesara I, Penman A, Swiatlo E, Hook EW. Evaluating the performance of the focus HerpeSelect® HSV-2 IgG in veterans with chronic hepatitis C infection. J Med Virol. 2015 Aug;87(8):1377–81. doi: 10.1002/jmv.24195. [DOI] [PubMed] [Google Scholar]
- 31.Gitman MR, Ferguson D, Landry ML. Comparison of Simplexa HSV 1 & 2 PCR with culture, immunofluorescence, and laboratory-developed TaqMan PCR for detection of herpes simplex virus in swab specimens. J Clin Microbiol. 2013 Nov;51(11):3765–9. doi: 10.1128/JCM.01413-13. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=24006008 .JCM.01413-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granato PA, Alkins BR, Yen-Lieberman B, Greene WH, Connolly J, Buchan BW, Ledeboer NA. Comparative evaluation of AmpliVue HSV 1+2 assay with ELVIS culture for detecting herpes simplex virus 1 (HSV-1) and HSV-2 in clinical specimens. J Clin Microbiol. 2015 Dec;53(12):3922–5. doi: 10.1128/JCM.01905-15. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=26468497 .JCM.01905-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs MM, Mwanyumba SW, Luseno WK, Hartman S, Halpern CT, Hallfors DD, Cho H. Evaluation of herpes simplex virus type 2 serological tests for use with dried blood spots in Kenya. Sex Transm Dis. 2017 Dec;44(2):101–103. doi: 10.1097/OLQ.0000000000000557. http://europepmc.org/abstract/MED/28081046 .00007435-201702000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang AL, Roberts C, Mazzulli T, Hatchette TF, LeBlanc JJ. Detection and differentiation of herpes simplex viruses by use of the viper platform: advantages, limitations, and concerns. J Clin Microbiol. 2014 Jun;52(6):2186–8. doi: 10.1128/JCM.03636-13. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=24696023 .JCM.03636-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CK, Chai CN, Capinpin SM, Ang A, Ng SY, Lee PL, Ng CW, Yan G, Lee HK, Chiu LL, Jureen R, Yan B, Loh TP. Evaluation of the Luminex ARIES HSV 1&2 Assay and comparison with the FTD Neuro 9 and in-house real-time PCR assays for detecting herpes simplex viruses. Ann Lab Med. 2018 Sep;38(5):440–445. doi: 10.3343/alm.2018.38.5.440. http://www.annlabmed.org/journal/viewJournal.html?year=2018&vol=38&page=440 .38.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liermann K, Schäfler A, Henke A, Sauerbrei A. Evaluation of commercial herpes simplex virus IgG and IgM enzyme immunoassays. J Virol Methods. 2014 Apr;199:29–34. doi: 10.1016/j.jviromet.2014.01.001.S0166-0934(14)00011-1 [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Liu JF, Yu H, Si GJ, Hu J, Li J. Production of a fragment of glycoprotein G of herpes simplex virus type 2 and evaluation of its diagnostic potential. Singapore Med J. 2015 Jun;56(6):346–52. doi: 10.11622/smedj.2014197. http://europepmc.org/abstract/MED/25532518 .SMJ-56-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller S, Samayoa E, Post L, Wright C, McKinley G, Wood M, Ching J. Development and clinical evaluation of a novel fully automated qualitative PCR assay for the diagnosis of anogenital herpes simplex virus infection. Diagn Microbiol Infect Dis. 2014 Oct;80(2):102–6. doi: 10.1016/j.diagmicrobio.2014.06.013.S0732-8893(14)00258-2 [DOI] [PubMed] [Google Scholar]
- 39.Parra-Sánchez M, Marcuello López A, García-Rey S, Zakariya-Yousef Breval I, Bernal Martínez S, Pueyo Rodríguez I, Martín-Mazuelos E, Palomares Folía JC. Performance of the HSV OligoGen kit for the diagnosis of herpes simplex virus type 1 and 2. Diagn Microbiol Infect Dis. 2016 Jul;85(3):315–317. doi: 10.1016/j.diagmicrobio.2016.04.019.S0732-8893(16)30098-0 [DOI] [PubMed] [Google Scholar]
- 40.Van Der Pol B, Warren T, Taylor SN, Martens M, Jerome KR, Mena L, Lebed J, Ginde S, Fine P, Hook EW. Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test. J Clin Microbiol. 2012 Nov;50(11):3466–71. doi: 10.1128/JCM.01685-12. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=22875892 .JCM.01685-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shevlin E, Morrow RA. Comparative performance of the Uni-Gold™ HSV-2 Rapid: a point-of-care HSV-2 diagnostic test in unselected sera from a reference laboratory. J Clin Virol. 2014 Nov;61(3):378–81. doi: 10.1016/j.jcv.2014.08.012. http://europepmc.org/abstract/MED/25200648 .S1386-6532(14)00312-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong Y, McCarthy K, Kong H, Lemieux B. Development and comparison of a rapid isothermal nucleic acid amplification test for typing of herpes simplex virus types 1 and 2 on a portable fluorescence detector. J Mol Diagn. 2012 Nov;14(6):569–76. doi: 10.1016/j.jmoldx.2012.05.005. https://linkinghub.elsevier.com/retrieve/pii/S1525-1578(12)00182-1 .S1525-1578(12)00182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Food & Drug Administration. 2007. [2019-05-07]. Statistical guidance on reporting results from studies evaluating diagnostic tests - Guidance for industry and FDA staff https://www.fda.gov/RegulatoryInformation/Guidances/ucm071148.htm .
- 44.Espy MJ, Uhl JR, Mitchell PS, Thorvilson JN, Svien KA, Wold AD, Smith TF. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000 Feb;38(2):795–9. doi: 10.1128/jcm.38.2.795-799.2000. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=10655387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proffitt MR, Schindler SA. Rapid detection of HSV with an enzyme-linked virus inducible system (ELVIS) employing a genetically modified cell line. Clin Diagn Virol. 1995 Aug;4(2):175–82. doi: 10.1016/0928-0197(95)00011-v.092801979500011V [DOI] [PubMed] [Google Scholar]
- 46.Langenberg A, Zbanyszek R, Dragavon J, Ashley R, Corey L. Comparison of diploid fibroblast and rabbit kidney tissue cultures and a diploid fibroblast microtiter plate system for the isolation of herpes simplex virus. J Clin Microbiol. 1988 Sep;26(9):1772–4. doi: 10.1128/jcm.26.9.1772-1774.1988. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=2846647 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nerurkar LS, Jacob AJ, Madden DL, Sever JL. Detection of genital herpes simplex infections by a tissue culture-fluorescent-antibody technique with biotin-avidin. J Clin Microbiol. 1983 Jan;17(1):149–54. doi: 10.1128/jcm.17.1.149-154.1983. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=6298272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valenstein P. Laboratory turnaround time. Am J Clin Pathol. 1996 Jun;105(6):676–88. doi: 10.1093/ajcp/105.6.676. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhuri A, Kennedy PG. Diagnosis and treatment of viral encephalitis. Postgrad Med J. 2002 Oct;78(924):575–83. doi: 10.1136/pmj.78.924.575. http://pmj.bmj.com/cgi/pmidlookup?view=long&pmid=12415078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poole CL, James SH. Antiviral therapies for herpesviruses: current agents and new directions. Clin Ther. 2018 Aug;40(8):1282–1298. doi: 10.1016/j.clinthera.2018.07.006.S0149-2918(18)30311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.British Medical Journal Best Practice. 2018. [2019-05-07]. Herpes simplex infection: Treatment algorithm https://bestpractice.bmj.com/topics/en-gb/53 .
- 52.Glass N, Nelson H, Huffman L. Screening for genital herpes simplex: Brief update for the U.S. Preventive Services Task Force. Genital Herpes Infection: Serologic Screening. 2016:2018. https://www.uspreventiveservicestaskforce.org/Home/GetFile/1/733/herpesup/pdf . [Google Scholar]
- 53.Jorgenson J. Automation in clinical microbiology. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 54.Brendish NJ, Malachira AK, Beard KR, Ewings S, Clark TW. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a analysis from a randomised controlled trial. Eur Respir J. 2018 Dec;52(2) doi: 10.1183/13993003.00555-2018.13993003.00555-2018 [DOI] [PubMed] [Google Scholar]
- 55.Lingappa J, Nakku-Joloba E, Magaret A, Friedrich D, Dragavon J, Kambugu F, Joloba M, Whalen C, Coombs R, Celum C, Morrow RA. Sensitivity and specificity of herpes simplex virus-2 serological assays among HIV-infected and uninfected urban Ugandans. Int J STD AIDS. 2010 Sep;21(9):611–6. doi: 10.1258/ijsa.2009.008477. http://europepmc.org/abstract/MED/21097732 .21/9/611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004 Apr 15;35(5):435–45. doi: 10.1097/00126334-200404150-00001.00126334-200404150-00001 [DOI] [PubMed] [Google Scholar]
- 57.Summerton J, Riedesel M, Laeyendecker O, Gaydos C, Maldeis NE, Hardick A, Morrow RA, Quinn TC. Effect of sexually transmitted disease (STD) coinfections on performance of three commercially available immunosorbent assays used for detection of herpes simplex virus type 2-specific antibody in men attending Baltimore, Maryland, STD clinics. Clin Vaccine Immunol. 2007 Dec;14(12):1545–9. doi: 10.1128/CVI.00120-07. http://cvi.asm.org/cgi/pmidlookup?view=long&pmid=17913866 .CVI.00120-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitley R, Gnann W. Mucocutaneous manifestations of viral diseases: An illustrated guide to diagnosis and management. Boca Raton, FL: CRC Press; 2016. [Google Scholar]
- 59.Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 2: Varicella-zoster virus infections. Med Microbiol Immunol. 2007 Jun;196(2):95–102. doi: 10.1007/s00430-006-0032-z. [DOI] [PubMed] [Google Scholar]
- 60.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. J Am Med Assoc. 2003 Jan 08;289(2):203–9. doi: 10.1001/jama.289.2.203.joc20645 [DOI] [PubMed] [Google Scholar]
- 61.Kropp RY, Wong T, Cormier L, Ringrose A, Burton S, Embree JE, Steben M. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006 Jun;117(6):1955–62. doi: 10.1542/peds.2005-1778.117/6/1955 [DOI] [PubMed] [Google Scholar]
- 62.Sampath A, Maduro G, Schillinger JA. Infant deaths due to herpes simplex virus, congenital syphilis, and HIV in New York City. Pediatrics. 2016 Dec;137(4) doi: 10.1542/peds.2015-2387. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=26933212 .peds.2015-2387 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 flow diagram.
Serological assays for detection of herpes simplex virus and summary of the different serological assays studied within the 6 included studies.


