Abstract
The androgen receptor (AR) is tightly linked to prostate cancer, but the mechanisms by which AR transactivation is dysregulated during cancer progression are not fully explored. Dagar et al. examined AR translocation to the nucleus to identify a link between heat shock protein 90 (HSP90) and protein kinase A (PKA). Their findings provide a potential mechanism of the initiation of AR transactivation and potential targets for developing and refining treatments for prostate cancer.
Introduction
Androgens such as dihydrotestosterone bind to the androgen receptor (AR),2 a transcription factor, initiating its transit to the nucleus, where AR regulates expression of genes involved in organ development and fertility. Androgens and AR signaling also contribute to the development and progression of prostate cancer. Current prostate cancer treatments, therefore, involve androgen deprivation therapy, which is effective when a patient's cancer depends upon androgen. However, these patients will eventually develop castration-resistant prostate cancer (CRPC), in which AR transcriptional activity may become independent of androgen. Despite many efforts to investigate AR signaling, we still do not understand all of the steps involved, limiting efforts to identify treatment strategies for these vulnerable patients. Dagar et al. (1) now try to connect known protein–protein interactions, phosphorylation sites, and activating cofactors into a model for AR activation, to aid in new directions to tackle CRPC.
The work from Dagar et al. begins with three critical pieces of information. First, in vitro AR transactivation is stimulated by cAMP-dependent protein kinase A (PKA) (2). Specifically, stimulation of PKA activity in androgen-deprived prostate cancer cells leads to an increase in nuclear AR protein with concomitant induction of AR-driven reporter genes and endogenous prostate-specific antigen (PSA) gene expression (3) by mechanisms not clearly understood but may involve phosphorylation of MED1 (mediator complex subunit 1) that is essential for AR transcriptional activity or other targets such as phosphorylation of CREB1 (cAMP-responsive element-binding protein), which is required for its transcriptional activity. Phospho-CREB1 regulates expression of many genes, including AR (4) and, in combination with androgen, the AR-target gene, PSA. CREB1 is a driver of survival, cell-cycle, and metabolic transcription programs and co-localizes with FoxA1 on the cistrome in prostate cancer cells (5). Gene expression analyses reveal that CREB1/FoxA1 target genes are predictive of prostate cancer recurrence. Previous work has also shown that levels of both regulatory (R) and catalytic (C) subunits of PKA are elevated in prostate cancer, and, for the regulatory subunit, these elevated levels are significantly related to poor patient outcome (2, 6), suggesting that a PKA-dependent mechanism may be highly relevant to cancer progression.
Second, the molecular chaperone heat shock protein 90 (HSP90) plays a critical role in AR signaling. In the absence of androgen, AR is localized in the cytoplasm in a complex with HSP90 and other factors. HSP90 interaction stabilizes AR in a conformation with better affinity for androgen. Once androgen binds AR, it dissociates from HSP90 and is able to translocate into the nucleus. Increased levels of HSP90 are detected in prostate cancer cells.
Third, both AR and HSP90 are substrates for PKA phosphorylation: AR at Ser-650 in the hinge region, which is important for nuclear import and transcriptional activity (7), and HSP90 at Thr-89. Moreover, multiple other molecules are involved in the initiation of AR transactivation, so whether these pathways and processes intersect to mediate nuclear translocation of AR, and whether through a direct or indirect manner, were unclear.
The study from Dagar et al. (1) is a start to providing a link between PKA signaling, HSP90 function, and AR transactivation. The authors first confirmed that PKA not only stimulates AR translocation, but is necessary for robust stimulation, finding a marked difference in an AR-mediated readout in the presence of the PKA inhibitor H89 or a PKA-directed siRNA sequence. It has been postulated that PKA could induce transactivation of AR by preventing AR interaction with one of its co-repressors, SMRT (silencing mediator for retinoic acid and thyroid hormone receptor) (8). However, Dagar et al. observed that the H89 inhibitor or siRNA inhibited nuclear import of AR, providing another potential mechanism. The authors then performed co-immunoprecipitation and immunofluorescence staining experiments with an antibody to AR or HSP90 to confirm the interaction between AR and HSP90 in transfected LNCaP cells in the absence of androgen and the loss of this interaction in the presence of androgen. Application of H89 or the PKA-targeted siRNA blocked the dissociation of AR and HSP90 in the presence of androgen. Finally, the authors wanted to examine whether phosphorylated HSP90 was involved and therefore tested a mutant of a phosphorylation site on HSP90. When the mutant HSP90 T89A was used, co-localization of HSP90 mutant and AR was observed in the cytoplasm in the presence of androgen, and AR transactivation was less compared with using WT HSP90 in the presence of androgen. The authors conclude that these observations were due to the inability of PKA to phosphorylate HSP90. Moreover, the authors demonstrated that cells in which PKA phosphorylation of HSP90 was blocked (via the H89 inhibitor, the PKA-targeted siRNA, or the HSP90 mutant) showed lower growth rates, providing a possible mechanistic link to the poor cancer prognosis.
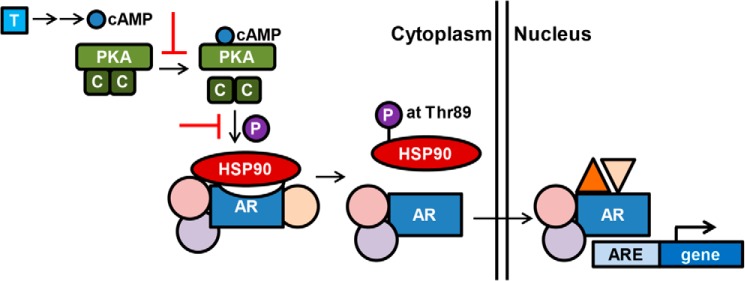
The findings from Dagar and colleagues provide a potential mechanism of nuclear translocation of AR: PKA activation leads to phosphorylation at Thr-89 on HSP90, stimulating the dissociation of the AR–HSP90 complex and allowing nuclear translocation of AR (Fig. 1). More details need to be investigated to fully elucidate PKA and the complexity of AR nuclear translocation and transactivation in the context of other essential components, such as importin, MED1, and CREB1, that are known to be phosphorylated to regulate nuclear import of cargo and are involved in transcriptional activity. Drawing upon transcriptome analyses reveals that only a very small subset (<3%) of genes are commonly regulated by androgen and the PKA pathways in human prostate cancer cells (9), thereby suggesting additional mechanisms beyond nuclear translocation and loss of interaction with a co-repressor to achieve global changes in androgen-regulated gene expression. Nevertheless, PKA and HSP90 may be potential candidates for developing therapeutic targets in combination with AR inhibitors for CRPC patients.
Figure 1.
Nuclear translocation and activation of AR through cross-talk with cAMP/PKA signaling. Multiple molecules (unlabeled circles and triangles) form complexes with AR and associate with AR inactivation or activation. Targeting PKA signaling transduction or the HSP90 chaperone (red T bars) may be potential therapeutic strategies for CRPC patients. C, catalytic units of PKA, released from the PKA oligomer upon cAMP binding to act on target proteins; P, phosphorylation; T, androgens; ARE, androgen response element.
HSP90 inhibitors have been developed for cancer treatments. In May 2019, at least 48 clinical trials with HSP90 inhibitors were listed on the ClinicalTrials database. For prostate cancer, one trial testing monotherapy (HSP90 inhibitor AT13387) or combination therapy with an inhibitor of androgen biosynthesis (abiraterone acetate) was carried out. However, currently, there is no Food and Drug Administration–approved HSP90 inhibitor for cancer treatments. Moreover, there are multiple isoforms of HSP90 (e.g. HSP90α and HSP90β). A novel inhibitor specifically targeting HSP90β was suggested to show anti-proliferative effect in prostate cancer (10). Thus, an HSP90 isoform-specific inhibitor may be more beneficial. Specific inhibition of the entry keys for AR translocation may add a novel approach to the repertoire of therapeutics to block AR transactivation.
Acknowledgments
We acknowledge the many scientists whose research and contributions could not be cited due to limited space.
This work was supported by NCI, National Institutes of Health, Grant R01 CA105304. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- AR
- androgen receptor
- HSP90
- heat shock protein 90
- PKA
- protein kinase A
- CRPC
- castration-resistance prostate cancer
- PSA
- prostate-specific antigen
- MED1
- mediator complex subunit 1
- CREB
- cAMP-responsive element-binding protein.
References
- 1. Dagar M., Singh J. P., Dagar G., Tyagi R. K., and Bagchi G. (2019) Phosphorylation of HSP90 by protein kinase A is essential for the nuclear translocation of androgen receptor. J. Biol. Chem. 294, 8699–8710 10.1074/jbc.RA119.007420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarwar M., Sandberg S., Abrahamsson P. A., and Persson J. L. (2014) Protein kinase A (PKA) pathway is functionally linked to androgen receptor (AR) in the progression of prostate cancer. Urol. Oncol. 32, e1–e12 10.1016/j.urolonc.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 3. Sadar M. D. (1999) Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J. Biol. Chem. 274, 7777–7783 10.1074/jbc.274.12.7777 [DOI] [PubMed] [Google Scholar]
- 4. Lindzey J., Grossmann M., Kumar M. V., and Tindall D. J. (1993) Regulation of the 5′-flanking region of the mouse androgen receptor gene by cAMP and androgen. Mol. Endocrinol. 7, 1530–1540 10.1210/mend.7.12.7511785 [DOI] [PubMed] [Google Scholar]
- 5. Sunkel B., Wu D., Chen Z., Wang C. M., Liu X., Ye Z., Horning A. M., Liu J., Mahalingam D., Lopez-Nicora H., Lin C. L., Goodfellow P. J., Clinton S. K., Jin V. X., Chen C. L., et al. (2016) Integrative analysis identifies targetable CREB1/FoxA1 transcriptional co-regulation as a predictor of prostate cancer recurrence. Nucleic Acids Res. 44, 4105–4122 10.1093/nar/gkv1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moen L. V., Ramberg H., Zhao S., Grytli H. H., Sveen A., Berge V., Skotheim R. I., Tasken K. A., and Skalhegg B. S. (2017) Observed correlation between the expression levels of catalytic subunit, Cbeta2, of cyclic adenosine monophosphate-dependent protein kinase and prostate cancer aggressiveness. Urol. Oncol. 35, 111.e1–111.e8 10.1016/j.urolonc.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 7. Zhou Z. X., Kemppainen J. A., and Wilson E. M. (1995) Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol. Endocrinol. 9, 605–615 10.1210/mend.9.5.7565807 [DOI] [PubMed] [Google Scholar]
- 8. Dotzlaw H., Moehren U., Mink S., Cato A. C., Iñiguez Lluhi J. A., and Baniahmad A. (2002) The amino terminus of the human AR is target for corepressor action and antihormone agonism. Mol. Endocrinol. 16, 661–673 10.1210/mend.16.4.0798 [DOI] [PubMed] [Google Scholar]
- 9. Wang G., Jones S. J., Marra M. A., and Sadar M. D. (2006) Identification of genes targeted by the androgen and PKA signaling pathways in prostate cancer cells. Oncogene 25, 7311–7323 10.1038/sj.onc.1209715 [DOI] [PubMed] [Google Scholar]
- 10. Liu W., Jensen D., Lee E., Gills J., and Holzbeierlein J. M. (2018) New HSP90 selective inhibitors as therapeutic agents for prostate and bladder cancer. J. Clin. Oncol. 36, 285–285 10.1200/JCO.2018.36.6_suppl.285 [DOI] [Google Scholar]