Abstract
Mutations in the genes encoding telomerase reverse transcriptase (TERT) and telomerase's RNA components as well as shortened telomeres are risk factors for idiopathic pulmonary fibrosis, where repetitive injury to the alveolar epithelium is considered a key factor in pathogenesis. Given the importance of TERT in stem cells, we hypothesized that TERT plays an important role in epithelial repair and that its deficiency results in exacerbation of fibrosis by impairing this repair/regenerative process. To evaluate the role of TERT in epithelial cells, we generated type II alveolar epithelial cell (AECII)–specific TERT conditional knockout (SPC-Tert cKO) mice by crossing floxed Tert mice with inducible SPC-driven Cre mice. SPC-Tert cKO mice did not develop pulmonary fibrosis spontaneously up to 9 months of TERT deficiency. However, upon bleomycin treatment, they exhibited enhanced lung injury, inflammation, and fibrosis compared with control mice, accompanied by increased pro-fibrogenic cytokine expression but without a significant effect on AECII telomere length. Moreover, selective TERT deficiency in AECII diminished their proliferation and induced cellular senescence. These findings suggest that AECII-specific TERT deficiency enhances pulmonary fibrosis by heightening susceptibility to bleomycin-induced epithelial injury and diminishing epithelial regenerative capacity because of increased cellular senescence. We confirmed evidence for increased AECII senescence in idiopathic pulmonary fibrosis lungs, suggesting potential clinical relevance of the findings from our animal model. Our results suggest that TERT has a protective role in AECII, unlike its pro-fibrotic activity, observed previously in fibroblasts, indicating that TERT's role in pulmonary fibrosis is cell type–specific.
Keywords: telomerase, telomerase reverse transcriptase (TERT), epithelial cell, senescence, fibrosis, inflammation, idiopathic pulmonary fibrosis (IPF), lung disease, pulmonary dysfunction
Introduction
Telomere length shortening with or without telomerase gene mutations or polymorphisms have been reported in many disorders, but their precise pathogenetic significance remains unclear (1–5). Telomerase is a ribonucleoprotein complex and consists of a catalytic component, telomerase reverse transcriptase (TERT),4 and an RNA template (TR). Telomerase adds a telomere repeat sequence to the 3′ end of telomeres on chromosomal ends, which are protective structures for chromosome stabilization (6–8). Additionally telomeres are protected by a shelterin complex composed of six subunit proteins: TRF1, TRF2, TIN2, RAP1, TPP1, and POT1 (9). In addition to participation in telomere length regulation, this complex prevents recognition of the telomere ends as double-strand breaks, which can incite an inappropriate DNA damage response (10). Deficiency or loss of one of these subunits causes different aspects of telomere dysfunction via nonidentical mechanisms (11). For example, both TRF1 and TRF2 negatively regulate telomere length and prevent chromosome end-to-end fusions in vivo, but only TRF1 appears to be associated with mitotic spindle assembly, whereas TRF2 plays key roles in t-loop formation and suppression of ataxia telangiectasia-mutated (ATM) activation (9, 12–14). In addition to its telomere maintenance role, extratelomeric roles of TERT in regulation of cellular proliferation, apoptosis, differentiation, and senescence have been reported (15–18). Moreover TERT is present in mitochondria, where it is implicated in reduction of reactive oxygen species and mitochondrial DNA (19). Thus, the role of TERT likely involves both telomeric and/or extratelomeric mechanisms affecting a multiplicity of cell functions.
Mutant TERT, TR, and/or shortened telomeres in peripheral blood leukocytes are amply documented as risk factors for idiopathic pulmonary fibrosis (IPF) (3, 4, 20, 21). Induced telomerase and TERT expression is noted in lung fibroblasts from patients with fibrotic interstitial lung disease, including IPF, and in models of lung injury and fibrosis without effect on telomere length (22). Given the different and sometimes opposing roles of the cell types involved in fibrosis, the pathogenic significance of TERT in these different cell populations might reflect these differences. TERT in fibroblasts is essential for pulmonary fibrosis because fibroblast-specific TERT deficiency reduces bleomycin (BLM)-induced pulmonary fibrosis (23, 24). However selective deletion of the shelterin components TRF1 or TRF2 in type II alveolar epithelial cells (AECII) causes cellular senescence, regeneration defects, and increased susceptibility to injury or fibrosis with or without telomere shortening (25–27). Moreover, in Tert-null mice, intravenous administration of a TERT-expressing viral vector reconstitutes its expression predominantly, but not exclusively, in AECII and reduces BLM-induced pulmonary inflammation and fibrosis (28). This reduction in fibrosis is associated with some lengthening of telomeres with reduction in the proportion of shortened telomeres.
IPF is an age-associated chronic progressive lung fibrosis with a fatal outcome (29–31). It is currently proposed to be an epithelial fibroblast disease (32, 33) in which repetitive epithelial cell injury and defective/deficient regeneration cause release of mediators that initiate interstitial fibroblast recruitment, propagation, and activation to constitute fibrotic foci (32, 34–36). However, the precise mechanisms, especially the role of TERT in AECII, have not been fully elucidated, although cell proliferation/regeneration, senescence, and apoptosis have been implicated in the pathogenesis of IPF (37–39).
In this study, the role of TERT in AECII was investigated using AECII-specific TERT conditional knockout mice (23, 40, 41). The results showed that AECII-specific TERT-deficient mice did not spontaneously develop pulmonary fibrosis or inflammation or induced cellular senescence in AECII. However, this selective TERT deficiency in AECII caused significant enhancement of BLM-induced pulmonary injury, inflammation, and fibrosis, accompanied by inhibition of cellular proliferation and induction of senescence in AECII. Elevated levels of AECII cellular senescence were also observed in human IPF lung tissue.
Results
Generation of AECII-specific TERT-deficient mice
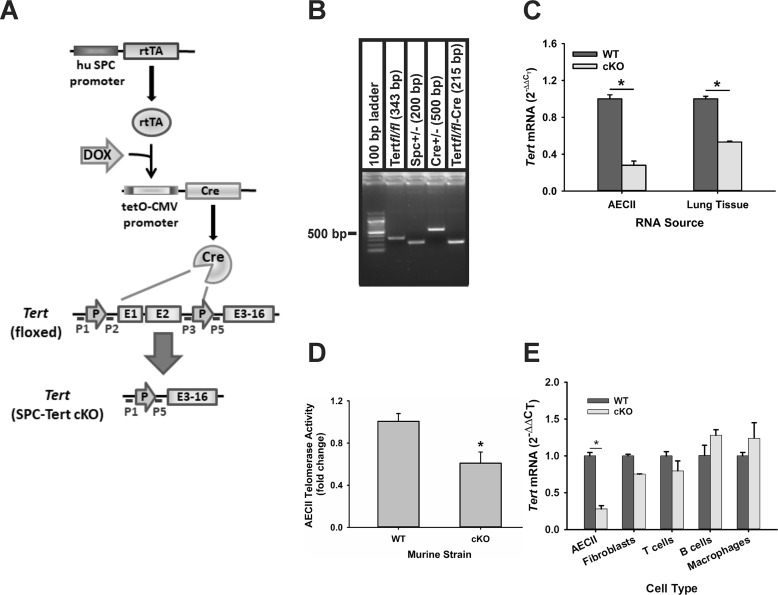
Triple-transgenic AECII-specific TERT-deficient mice were generated by breeding floxed Tert mice (23) with doxycycline-induced Cre mice under control of the human surfactant protein C (SPC) promoter as described under “Experimental procedures” (Fig. 1A). The genotype of Tertfl/fl/Spc+/−, Cre+/− mice was confirmed by PCR, which showed presence (Fig. 1B) of the PCR fragments for Tertfl/fl (343 bp) using primers P3 and P5, Spc (200 bp), Cre (500 bp), and a Tert-null allele (215 bp) using primers P1 and P5 (primer locations are diagrammed in Fig. 1A). To induce selective TERT deficiency in AECII, triple-transgenic mice were treated with doxycycline for 10 consecutive days and are referred to as SPC-Tert cKO mice. The controls (WT) were double-transgenic (Spc+/−, Cre+/−) mice, which were similarly treated with doxycycline. Treatment with doxycycline up to 9 months had no significant effects on body weight gain/growth, food intake, and lung histology in both SPC-Tert cKO and WT mice (data not shown). In SPC-Tert cKO mice, TERT mRNA was significantly reduced by ∼50% in isolated AECII after 4 days of doxycycline treatment (data not shown), which was further reduced to ∼30% (or ∼70% reduction) of control (WT AECII) levels on day 10 of treatment (Fig. 1C). In lung tissue, TERT mRNA was reduced by 46.8% in the cKO mice (Fig. 1C). A significant reduction of 39.1% was also observed for telomerase activity in Tert cKO AECII (Fig. 1D), which was less than that observed for TERT mRNA. The lower decrease in protein versus mRNA levels could be due to some combination of contaminating non-AECII cells (routinely <10%), which would have intact TERT expression, and/or residual TERT protein and enzyme activity after 7 days of doxycycline treatment, i.e. the half-life for protein could be longer or more stable than the mRNA. In contrast, TERT mRNA was not significantly altered in mouse lung fibroblasts (MLF), macrophages, T cells, and B cells from cKO mice (Fig. 1E). These results confirmed the selective ablation of TERT in AECII.
Figure 1.
Generation of doxycycline-induced SPC-Tert cKO mice. A, the strategy for generation of SPC-Tert cKO. Double-transgenic mice bearing the SPC-rtTA and (tetO) 7-CMV-Cre transgenes were bred to mice bearing the floxed Tert to generate triple-transgenic progeny. In the presence of doxycycline (DOX), the activated rtTA bound to (tetO) 7-CMV led to activation of Cre recombinase, which then excised the floxed Tert sequence (part of the promoter and exons 1 and 2) in AECII. B, PCR genotyping using mouse tail DNA. The PCR fragments for Tertfl/fl (343 bp) using primers P1 and P2, Spc (200 bp), Cre (500 bp), and Cre-excised SPC-Tert cKO (215 bp) using primers P1 and P5 are shown. C, Tert ablation in AECII and lung tissue. AECII and lung tissue total RNA were isolated from WT or SPC-Tert cKO mice (cKO) and analyzed for TERT (Tert) mRNA expression by qPCR. TERT mRNA levels were calculated as 2−ΔΔCT and shown as -fold change over WT controls. D, cell lysates were collected from AECII isolated from mice 7 days after doxycycline treatment and analyzed for telomerase activity by telomerase PCR ELISA and are shown as -fold change over WT controls. E, Tert levels in the indicated cell types were analyzed as described in C. *, p < 0.05 between the two indicated groups, with n = 3 for AECII, fibroblasts, T and B cells, and macrophages and n = 5 mice for lung tissue analyses.
TERT deficiency enhanced susceptibility to BLM-induced lung injury
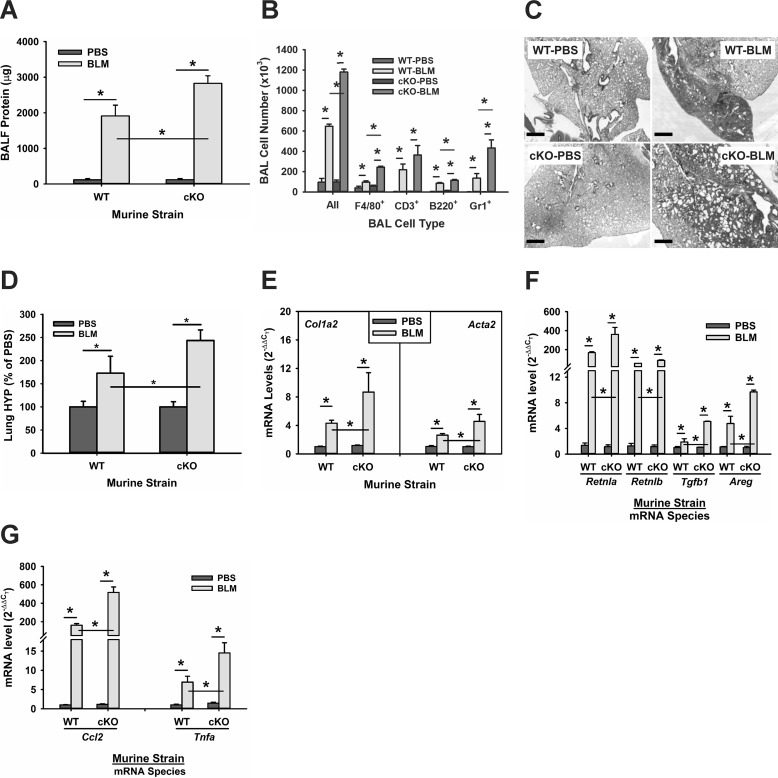
Telomerase deficiency is suggested to cause alveolar stem cell replicative senescence and impaired epithelial regeneration (42, 43), which could diminish epithelial repair and exacerbate injury. To evaluate the effect of TERT deficiency on BLM-induced lung alveolar epithelial injury, bronchoalveolar lavage (BAL) was performed with PBS 7 days after BLM treatment to evaluate protein leak and inflammation. BLM treatment in WT mice caused the expected significant injury, as reflected by a 19-fold increase in protein recovery in the BAL fluid, which was increased significantly to 28-fold in SPC-Tert cKO mice (Fig. 2A). This indication of increased BLM-induced injury in cKO mice was also reflected in an increased inflammatory response. An elevated total number of BAL cells was observed, as expected, in BLM-injured WT lungs (∼ 6-fold over PBS controls), which was increased further to 10-fold over PBS controls in SPC-Tert cKO lungs after BLM treatment (Fig. 2B). Further analysis of the differential counts revealed a significantly greater increase in the number of inflammatory/immune cells, including alveolar F4/80+ macrophages, B220+ B cells, and Gr-1+ cells in cKO BAL fluid than in WT controls, whereas a slight increase in CD3+ T cells from BLM-treated cKO mice was not statistically significant. BAL protein level and cell number in PBS-treated SPC-Tert cKO mice were not significantly different from those in PBS-treated WT mice. These findings suggested that AECII TERT deficiency rendered the alveolar epithelium more susceptible to the BLM-induced injury but had no significant effects without BLM treatment.
Figure 2.
TERT deficiency enhanced alveolar epithelial damage and pulmonary fibrosis in SPC-Tert cKO mice. A, WT and SPC-Tert cKO mouse lungs were lavaged on day 7 after PBS or BLM treatment. Total protein was measured using a BCA protein assay kit. BALF, bronchoalevolar lavage fluid. B, total BAL cells were counted in BAL fluids, and the numbers of F4/80+, CD3+, B220+, and Gr1+ BAL cells were determined using flow cytometry. C, representative H&E-stained lung tissue sections are shown. Original magnification, ×20. Scale bars = 500 μm. D, lung tissues from WT or SPC-Tert cKO mice were homogenized, and the lung collagen content was measured by HYP assay in whole-lung homogenates. E, lung tissue RNA was analyzed for fibrotic marker type I collagen (Col1a2) and α-SMA (Acta2) expression at the mRNA level by qPCR analysis. F and G, lung tissue RNA was also analyzed for the profibrotic cytokines FIZZ1 (Retnla), FIZZ2 (Retnlb), TGFβ1 (Tgfb1), and amphiregulin (Areg) (F) and the inflammatory cytokines MCP1 (Ccl2) and TNFα (Tnfa) (G). *, p < 0.05 between the two indicated groups; n = 3 in A and B and n = 5 mice in C and E–G; in D, n = 11 for WT-PBS, n = 13 for WT-BLM, n = 12 for cKO-PBS, and n = 13 for cKO-BLM.
SPC-Tert cKO mice developed enhanced pulmonary fibrosis induced by BLM
BLM-induced lung fibrosis is significantly reduced in mice with TERT deficiency, globally or selectively in bone marrow (24) or in collagen I–expressing (mesenchymal) cells (23, 24). To further examine whether the in vivo role of TERT in lung injury and fibrosis is cell type–specific, lung injury/fibrosis was induced by BLM in WT control and SPC-Tert cKO mice and evaluated on day 7 or 21 after BLM injection. The results showed that selective TERT deficiency in AECII in the PBS-treated group had no significant effects on lung histology and did not cause a significant change in extracellular matrix and cytokine gene expression compared with WT controls (Fig. 2, C–G). However, in contrast to the diminished fibrosis in mice with TERT deficiency in bone marrow or mesenchymal cells (23, 24), upon BLM injury, TERT deficiency in AECII resulted in significant enhancement of pulmonary fibrosis, as shown by more extensive and diffuse fibrosis with cystic changes resembling honeycomb lung (Fig. 2C). Consistently, whole-lung collagen content, as measured by lung hydroxyproline content, was increased >2.4-fold in BLM-treated SPC-Tert cKO mice, which was significantly higher than the 1.7-fold increase in BLM-treated WT mice (Fig. 2D). Thus, TERT deficiency in AECII caused a significant increase of 41.2% in response to BLM treatment. In addition, enhanced lung fibrosis induced by BLM in SPC-Tert cKO mice was evidenced by significantly higher BLM-induced expression of type I collagen and α-smooth muscle actin (α-SMA) than in WT mice at the mRNA level (Fig. 2E). These enhanced BLM-induced fibrotic changes in SPC-Tert cKO mice were accompanied by augmented BLM-induced expression of multiple fibrogenic associated cytokines, including TGFβ1, FIZZ1, FIZZ2, and amphiregulin (Fig. 2F), and the inflammatory cytokines TNFα and MCP1 (Fig. 2G). These findings together indicate that, although AECII-specific TERT-deficient mice failed to exhibit spontaneous development of lung inflammation or fibrosis, they did show significantly enhanced lung injury, inflammation, and fibrosis in response to BLM treatment relative to that seen in WT mice.
TERT deficiency inhibited AECII proliferation in response to BLM-induced injury
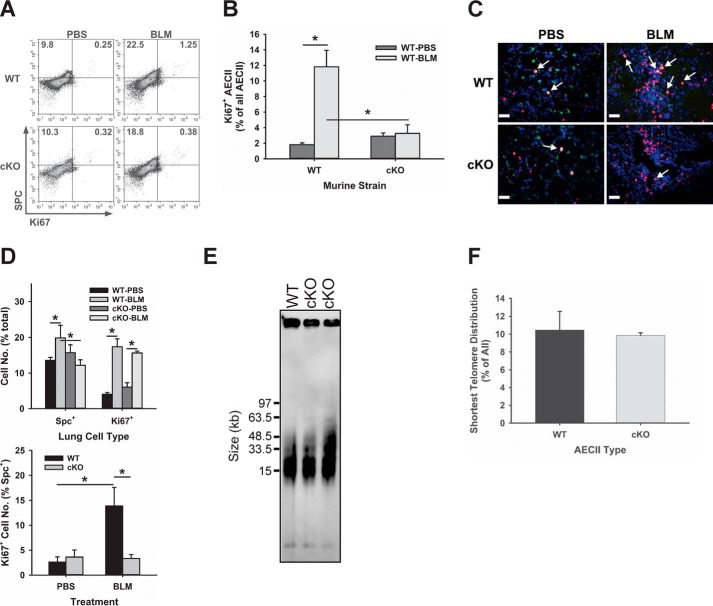
Because epithelial cell proliferation is essential for epithelial regeneration, which is critical for successful lung repair, the effect of TERT deficiency on AECII proliferation was evaluated to see whether its impairment was associated with the enhanced fibrotic response to BLM. Potential impairment of AECII proliferation by TERT deficiency may represent a mechanism underlying the observed enhanced BLM-induced lung injury and fibrosis in SPC-Tert cKO lungs. To assess proliferation in AECII, single-cell suspensions from lung tissue samples were analyzed by flow cytometry for expression of the cell proliferation marker Ki67 and the AECII marker SPC. The results showed that the number of proliferating AECII (double-positive for Ki67 and SPC) was increased by 5-fold in WT lung cells in response to BLM-induced injury (0.25 versus 1.25) but only increased <1.2-fold in AECII (0.32 versus 0.38) from SPC-Tert cKO lungs (Fig. 3A). Compared with WT controls, SPC+ AECII in cKO lungs were decreased from 22.5% to 18.8% after BLM treatment (Fig. 3A). The proportion of proliferating AECII (Ki67/SPC double-positive cells of total SPC+ cells) in BLM-treated WT mouse lungs was also significantly higher than in BLM-treated cKO mice (11.8% versus 3.27%, respectively) (Fig. 3B). This negative effect of TERT deficiency on AECII proliferation was confirmed in lung tissue sections immunostained for Ki67 and SPC (Fig. 3C). Direct cell counting showed that BLM treatment caused increased numbers of SPC+ AECII in the WT, which was significantly diminished in cKO lungs (Fig. 3D, top panel). Although abundant SPC/Ki67 double-positive cells were observed in BLM-treated WT lung tissue sections, they were less frequent in SPC-Tert cKO lungs (Fig. 3D, bottom panel) despite the presence of abundant Ki67 single-positive cells (Fig. 3D, top panel) representing non-AECII cells, such as α-SMA–expressing fibroblasts (data not shown). These findings indicated that TERT deficiency impaired AECII proliferation and, thus, regeneration in response to BLM injury, which might contribute to the observed enhanced injury and fibrosis in SPC-Tert cKO mice. AECII telomere length was not significantly affected by TERT deficiency, and the proportion of short telomeres (<15 kb) was not significantly different between WT and Tert-deficient AECII (Fig. 3, E and F). Thus, the diminished proliferative response in TERT-deficient AECII was not associated with detectable telomere shortening.
Figure 3.
TERT deficiency suppressed AECII proliferation in BLM-induced pulmonary fibrosis. A and B, whole-lung single-cell suspensions were analyzed for SPC- and Ki67-positive cells by flow cytometry. The total SPC-positive population was further analyzed to show the percentage of Ki67-positive cells in the SPC-positive population, which is shown in B. A representative analysis from three separate flow cytometry runs showing similar results is shown in A. Analysis from the combination of all three runs showing the average percentage of proliferated AECII that were SPC+ and Ki67+ in total SPC+ AECII is shown as a bar graph in B. C, SPC and Ki67 double immunofluorescence staining was performed on paraffin-embedded lung tissue sections. SPC signals are shown in green, Ki67 in red, and nuclei in purple-blue (DAPI). Original magnification, ×400. Scale bars = 20 μm. D, results of direct cell counting from the relevant micrographs are expressed as percentages of all lung cells (top panel) or SPC+ cells (bottom panel), with the latter indicating the percentage of proliferating AECII. *, p < 0.05 between the two indicated groups; n = 5 for WT-PBS, WT-BLM, and cKO-BLM and n = 6 for the cKO-PBS group. E, telomere length assay in AECII. Tert cKO and their control mice received doxycycline for 30 days, and AECII were isolated from mice without BLM treatment and then embedded with agarose gel. Telomere length was performed using TRF-Southern blotting. F, the proportion of the shortest telomere signals is shown as the intensity percentage of shortest telomere (<15 kb) of the total telomere signal. n = 3 mice/group.
Effect of AECII TERT deficiency on cellular senescence
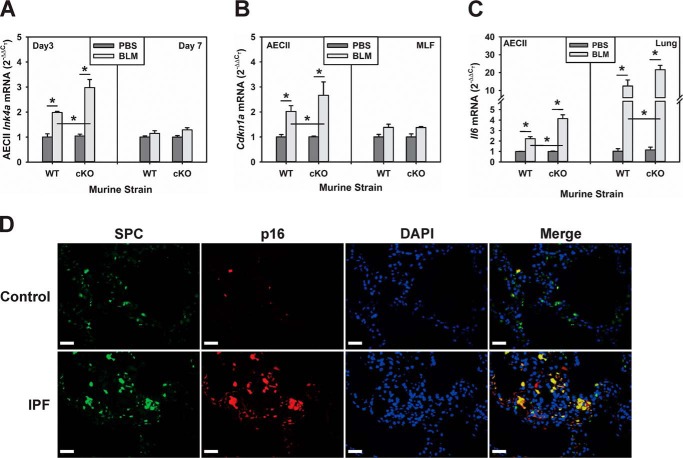
Proliferation arrest is a hallmark of cellular senescence and may represent the basis for the noted proliferative impairment in TERT-deficient AECII. Moreover, Sirt1 suppression of senescence is associated with and depends on induction of TERT expression (18, 25), and derepression of TERT can reverse senescence (44). To evaluate this possibility, expression of the cellular senescence marker genes p16 and p21 was evaluated in AECII isolated from WT and SPC-Tert cKO mice. The results revealed no significant effect of AECII TERT deficiency on p16 or p21 expression in the absence of lung injury (Fig. 4, A and B). BLM treatment caused a significant elevation in AECII expression of both p16 and p21 in WT mice, but the BLM-induced increase was significantly greater in AECII of SPC-Tert cKO mice (Fig. 4, A and B). Induction of p16 in AECII occurred as early as day 3 after BLM treatment and was >2-fold higher in cKO mouse lungs compared with WT lungs at this time point. Induction of p16 diminished by day 7 (Fig. 4A), whereas p21 induction was greater at this time point, with a similar higher level of expression in lungs of cKO mice (Fig. 4B). The level of p21 mRNA was not significantly different between MLF isolated from WT and SPC-Tert cKO mice, although BLM induced slight (∼30%) and comparable induction of p21 mRNA in both WT and cKO lungs (Fig. 4B). Associated with this enhanced expression of senescence markers in TERT-deficient AECII in BLM-injured lungs were parallel alterations in both AECII and lung tissue expression of IL-6, a characteristic component of the senescence-associated secretory phenotype. Thus, BLM induced IL-6 expression was significantly greater in lungs of cKO mice with selective AECII TERT deficiency than in WT mice (Fig. 4C). These findings suggest that selective enhancement of AECII senescence because of TERT deficiency might represent the basis for the noted impaired AECII proliferation in SPC-Tert cKO mice. Interestingly, analysis of human lung tissue sections from IPF patients also revealed a selective increase in p16 expression in AECII. This was undertaken using double immunofluorescence staining for SPC and p16 on lung tissue sections from IPF patients and control subjects to assess AECII-specific senescence in situ. The results revealed that there were increased numbers of cells displaying double-positive SPC and p16 staining in IPF lungs compared with the rare double-positive cells in control lungs (Fig. 4D), indicating that more AECII cells were senescent in IPF lungs.
Figure 4.
TERT deficiency enhanced BLM-induced senescence in lung cells. A, AECII were isolated from WT or SPC-Tert cKO lungs on day 3 and day 7 after PBS or BLM treatment and analyzed for senescence marker p16 (Ink4a) mRNA expression by qPCR. B, the p21 (Cdkn1a) mRNA levels in isolated AECII and MLF on day7 after PBS or BLM treatment were analyzed. C, AECII and lung tissue RNA samples were analyzed for IL-6 (Il6) mRNA. *, p < 0.05 between the two indicated groups; n = 3 for PBS and n = 4 for the BLM group in A–C. D, cellular senescence in IPF. SPC and p16 double immunofluorescence microscopy was performed on paraffin-embedded human lung tissue sections from control or IPF patients. SPC signals are shown in green, p16 in red, and nuclei in purple-blue with DAPI. Representative single and merged images from control and IPF lung sections are shown. Original magnification, ×400. Scale bars = 20 μm.
Discussion
Given that alveolar epithelial injury is associated with pulmonary fibrosis (32), we generated SPC-Tert cKO mice to assess the role of TERT in this lung cell type. AECII-specific TERT deficiency per se had no noticeable effect on lung histology or senescent marker expression. SPC-Tert cKO mice did not develop pulmonary inflammation or fibrosis and exhibited no respiratory distress with normal body weight gain up to 9 months of AECII TERT deficiency. However, selective AECII TERT deficiency enhanced susceptibility to BLM-induced lung injury and fibrosis. The enhanced fibrosis is consistent with the association of TERT mutations with IPF and other fibrotic interstitial lung diseases (3–5). TERT deficiency in AECII enhanced BLM-induced senescence and impaired proliferation, resulting in diminished epithelial repair and regeneration, given the known property of AECII as progenitor cells (45). This is consistent with the observation that AEC injury and failure of regeneration increases susceptibility to pulmonary fibrosis (26, 37). Interestingly, genetic ablation of TERT in hepatocyte progenitors causes a marked increase in stellate cell activation and liver fibrosis (46). In contrast, selective TERT deficiency in collagen-expressing mesenchymal cells protects mice from lung fibrosis, probably by limiting collagen-expressing lung fibroblast proliferation and increasing susceptibility to apoptosis (23). However, global TERT deficiency is reported to either reduce or not alter fibrosis (24, 47). Thus, TERT in epithelial and mesenchymal cells may have opposing effects on the pathogenesis of pulmonary fibrosis, but the net effect of deficiency in all cell types might depend on the variable effect of TERT deficiency in the different cell types involved in fibrosis. This further signifies that somatic TERT gene mutation(s) can have either a pro- or anti-fibrotic effect, depending on which cell type(s) is/are affected. Interestingly, a recent study suggests that telomere shortening primarily affects AECII in pulmonary fibrosis because it is observed in alveolar type 2 cells but not in surrounding cells in patients with familial or sporadic interstitial fibrosis (48), whereas telomere shortening is not detectable in fibrotic lung fibroblasts isolated from human IPF or TR KO mice (22). However telomere shortening in familial IPF with TERT mutation is not tissue-specific because the mutation takes place in germ line cells; indeed, telomere length in blood is found to correlate with that in fibrotic AECII in familial interstitial pneumonias with TERT mutation but not in sporadic IPF (48). The lack of significant telomere shortening in AECII from Tert cKO mice is in part due to the short time frame of TERT deficiency and the fact that mice have much longer telomeres than humans (2, 7). Later generations of telomerase deficiency are required to detect significant telomere shortening (2, 22), which could account for the lack of detectable shortening within the short time frame of the animal model experiments in this study. It may be that a critically short telomere length, such as in IPF with TERT mutation, could be a factor in pathogenesis, but this does not exclude nontelomere-mediated or extratelomeric/noncanonical mechanisms. However, this biological difference in telomere length between species should be considered when interpreting findings from mouse models vis à vis potential relevance to human telomere-related lung diseases.
The role of TERT in telomere maintenance and DNA replication accounts for its intimate involvement in cell proliferation, aging, and senescence (49–51). However, its extratelomeric roles in cell proliferation, differentiation, and mitochondrial function (17, 19, 46) should also be considered, especially because the noted effects of TERT deficiency in AECII were unaccompanied by telomere shortening. Furthermore, induction of telomerase and TERT in BLM-induced pulmonary fibrosis and in human lung fibroblasts isolated from patients with IPF is not associated with significant changes in telomere length (22, 52). In this study, the enhanced susceptibility of SPC-Tert cKO mice to BLM-induced lung injury and fibrosis is also not correlated with telomere shortening in AECII during the 3-week fibrotic period, and telomere shortening is not observed in the lungs of mice after BLM treatment (22, 25, 28). Thus, the noted enhancement of lung injury and fibrosis because of AECII TERT deficiency does not appear to depend on loss of the telomeric function of TERT, unlike the noted telomere dysfunction in TRF1 or TRF2 deficiency. The data in mouse cells from another study also suggest that telomere length is not a primary determinant of senescence/crisis in mouse cells because embryonic fibroblasts from late generation (G1-G6) TR knockout mice exhibit similar efficiency in colony-forming ability as cells derived from WT mice (2). Although quantitative analysis of the proportion of short telomeres (<15 kb) from Southern blots showed that TERT deficiency did not significantly affect the distribution of short telomeres in AECII, it does not rule out potential shortening in select individual chromosomes. Hence, the findings cannot completely exclude potential telomere dysfunction from loss of TERT expression, which might induce a DNA damage response.
Although TRF1/2 deficiency promptly caused cellular senescence associated with uncapping of telomere ends and inflammation, TERT deficiency caused increased susceptibility to development of cellular senescence, inflammation, and fibrosis, which was manifested only upon exposure to stressful or injurious stimuli, e.g. BLM treatment. This manifestation of susceptibility upon stress resembles the two-hit model of human IPF, which postulates the requirement for a “second hit” to expose an underlying susceptibility because of impaired AECII function or regenerative capacity (27, 53). TERT deficiency negatively affects AECII proliferation, resulting in impaired epithelial regeneration, thus contributing to enhanced pulmonary fibrosis. These findings are consistent with a recent report showing that, in G2 Tert KO mice, reconstitution of TERT expression by i.v. injection of a TERT-expressing viral vector preferentially rescues AECII from cellular senescence and increases proliferation. This effect on AECII results in higher regeneration potential, which is associated with diminished pulmonary fibrosis induced by low-dose (0.5 mg/kg) BLM (28). Thus, these studies, using complementary approaches on TERT expression in AECII, result in a similar conclusion regarding the protective role of TERT in lung injury and fibrosis.
Aging is considered a risk factor for IPF, and recent evidence suggests the importance of cellular senescence, a characteristic of organismal aging, as a potential contributor to susceptibility to development of IPF, although the basis of this relationship is unclear (29, 54–56). Lung cells from IPF patients exhibit a reduction in Sirt1 expression (37, 57) and evidence of cellular senescence, as manifested by induction of p53, p21, and/or p16, whose levels of expression correlate with the severity of fibrotic lung disease (58). Selective senescence in AECII because of TERT deficiency diminishes epithelial cell proliferation, which could negatively affect regeneration, resulting in enhancement of fibrosis. Interestingly decreased AECII senescence because of miR-34a deficiency in aged mice affords protection from BLM-induced pulmonary fibrosis but has the opposite effect in young mice, probably because of a greater influence on reduced senescence in fibroblasts (relative to AECII), resulting in increased fibroproliferation (57). In IPF, accumulation of senescent cells is seen primarily in the alveolar epithelium and less frequently in fibroblasts and is usually observed in areas of active fibrosis concomitant to the aberrant secretory pattern of the lung epithelium (54). This dichotomy between the degree of senescence in AECII versus fibroblasts was also observed in this study in the BLM model (Fig. 5). Moreover, TERT deficiency in fibroblasts makes them more susceptible to apoptosis in BLM-induced pulmonary fibrosis (23) but fails to induce AECII apoptosis in SPC-Tert cKO mice (data not shown). This difference in responsiveness to TERT deficiency could account for the lesser degree of senescence in lung fibroblasts than AECII in BLM-induced lung fibrosis. Taken together, the role of TERT during pulmonary fibrosis may be cell type–specific, reflecting the net effect on the lung of the predominant active cell cycle affected by TERT deficiency. Thus, AECII versus fibroblasts at the early versus late stage of fibrosis in young or old animals might be differentially affected by TERT deficiency, resulting in the differential effects on fibrosis. The precise molecular mechanisms by which TERT protects BLM-treated AECII in a telomere-independent manner requires further elucidation.
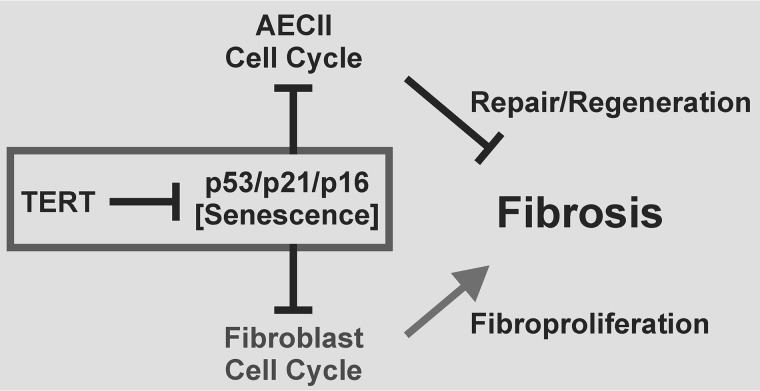
Figure 5.
Schematic of the cell type–specific role of TERT in pulmonary fibrosis. TERT is shown as a suppressor of senescence, which, in turn, interrupts the cell cycle in AECII and fibroblasts by derepression of p53/p21/p16. This, in turn, will suppress epithelial cell repair/regeneration, a critical factor in limiting development and progression of pulmonary fibrosis. Thus, the effect of TERT deficiency in AECII is to enhance fibrosis, as demonstrated in this study. In contrast, suppression of fibroproliferation in the case of senescence in fibroblasts should limit fibrosis, as noted previously (57).
Experimental procedures
Mice and BLM model of pulmonary fibrosis
Floxed Tert mice were generated as before (23). To generate AECII-specific TERT conditional knockout mice, the floxed Tert mice (Tert fl/fl) were crossed with SPC-rtTA/tetO-CMV-Cre mice bearing a doxycycline-inducible Cre recombinase where (tetO)-CMV-driven Cre expression is driven by the doxycycline-induced reverse tetracycline transactivator (rtTA) under control of SPC promoter (40, 41). The genotype of Tert fl/fl/Spc+/−, Cre+/− mice were confirmed by PCR, and the resulting triple-transgenic AECII-specific conditional knockout mice on a C57BL/6J background were used as described (Fig. 1, A and B). Spc+/−, Cre+/− mice (SPC-rtTA/tetO-CMV-Cre) were used as relevant controls and are referred to as WT controls for simplicity.
To induce selective TERT deficiency in AECII, triple-transgenic mice (8 to 10 weeks old) were treated with doxycycline in both food (1.6–2.7 mg/3–5 g of food) and drinking water (2 mg/liter) for 10 consecutive days with three replacements per week. WT controls were also treated with doxycycline. SPC-Tert cKO and WT mice were treated with BLM (Blenoxane, Mead Johnson) dissolved in sterile PBS by endotracheal instillation on day 0 at a dose of 1.9 units/kg body weight as before (59). Control mice received a PBS injection alone. Doxycycline only in the drinking water was continued for another 14 days after BLM injection. Where indicated, BAL was performed using 1 ml of PBS and repeated five times for collection of BAL fluid and cells. Total BAL cells were counted using a hemocytometer, and the differential cell counts were analyzed by flow cytometry. Total protein was measured with the Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL).
Lung cell isolation and separation
Primary MLF were isolated and maintained as before (60) and used at passages 2–5 in the indicated experiments. Primary AEC II were isolated as described previously (61). Briefly, lungs were instilled with dispase II (Roche Diagnostics) followed by low-melt agarose (Sigma) and digested for 45 min. Lungs were then dissected and treated with DNase. The cell suspensions were negatively selected for CD16/32- and CD45-expressing cells by magnetic-activated cell sorting separation system (Miltenyi Biotec Inc., San Diego, CA), followed by further negative selection for nonadherent cells by incubation on Petri dishes. The AECII were then plated and cultured for 2 days on plates coated with 200 μg/ml Matrigel (BD Biosciences) before use. Lung macrophages and T and B lymphocytes were separated using a magnetic-activated cell sorting separation system with F4/80, pan-CD90.2, and B220 microbeads (Miltenyi Biotec Inc.), respectively.
Telomerase activity assay and telomere length measurement
Telomerase activity was assayed using the TeloTAGGG Telomerase PCR ELISA kit (Roche, catalog no. 11854666910) in accordance with the manufacturer's protocol. Cell lysates heated to 85 °C for 15 min were used as negative controls. Telomere length was analyzed in the AECII isolated from doxycycline-treated WT or Tert cKO mice as described above. Analysis was performed using terminal restriction fragment (TRF) Southern blotting with the Telo TAGGG telomere length assay kit (Roche) as described previously (22). The enzyme-digested AECII DNA plug (CHEF Genomic DNA Plug Kit, Bio-Rad) was separated on agarose gel with pulsed field electrophoresis followed by Southern blotting. Carestream MI SE (Carestream Health, Inc., Rochester, NY) was used for analysis of the proportion of the shortest telomere signals.
Real-time quantitative RT-PCR
For real-time quantitative RT-PCR analysis, TaqMan primers for type I collagen (Col1a2), α-SMA (Acta2), TERT (Tert), TGFβ1 (Tgfb1), FIZZ1 (Retnla), FIZZ2 (Retnlb), amphiregulin (Areg), TNFα (Tnfa), MCP1 (Ccl2), p16 (p16), p21 (p21), IL-6 (Il6), and 18s RNA were purchased from Thermo Fisher Scientific. The 18S RNA was used as internal control or reference for normalization. One-step RT-PCR was performed as before (60), using a GeneAmp 7500 sequence detection system (Applied Biosystems, Rockford, IL). Results were expressed as 2−ΔΔCT using the indicated control group as a calibrator (62).
Hydroxyproline assay
Lung collagen content was determined by measuring the hydroxyproline (HYP) content from lung homogenates as described previously (60, 63). The results were expressed as microgram of HYP per lung.
Histology and immunofluorescence staining
The paraffin-embedded lung sections were stained with H&E and Masson's trichrome for routine evaluation of histopathology. For immunofluorescence staining, paraffin-embedded mouse or human lung tissue sections (human sections were obtained from the Lung Tissue Research Consortium, NHLBI, National Institute of Health) were antigen-retrieved in 10 mmol/liter sodium citrate (pH 6.0) using a microwave for 20 min. Anti-mouse Ki67 (ab16667, Abcam, Cambridge, MA, 1:500), anti-mouse SPC (sc-7706, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, 1:300), mouse anti-human p16 (ab117443, Abcam, 1:200), and rabbit anti-human SPC (ab90716, Abcam, 1:200) were used as primary antibodies for mouse and human, respectively. Isotype IgGs were used as controls for the primary antibodies. Anti-mouse/rabbit IgG-NL493/NL557 (NL004, NL-006, and NL007, R&D Systems) or anti-goat IgG-Alexa Flour 488 (ab150129, Abcam) were used as secondary antibodies for Ki67 and SPC, respectively. Mounting medium containing DAPI (Vector, Burlingame, CA) was applied, and the sections were examined with a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) using the Nuance 3.0.2 multispectral imaging system (PerkinElmer Life Sciences). For quantitation of cell counting, cells positive for Spc, KI67, or both were counted from at least five high-power fields, and 1,000 or more total cells (DAPI+) were counted for each group.
Flow cytometry
This was undertaken as before (60). The lung single-cell suspension was first stained with the viability dye eFluor 506 (65-0866-14, eBioscience, San Diego, CA, 1:100 dilution) and then fixed/permeabilized (BD Cytofix/Cytoperm, BD Biosciences) prior to staining with anti-mouse Ki67 (ab15580, Abcam, 1:100) and anti-SPC-Alexa Fluor 647 (bs-10067R-A647, Bioss Antibodies, Woburn, MA, 1:50). BAL cells were stained with a combination of anti-mouse F4/80-Alexa Fluor 647 (122609, 1:200), CD3-phosphatidylethanolamine-Cy7 (100200, 1:100), CD45R/B220-allophycocyanin-Cy7 (103223, BioLegend, San Diego, CA, 1:100), and Gr1-phosphatidylethanolamine (553128, BD Biosciences, 1:50). The cells were also stained with isotype controls conjugated with the same fluorochrome as their respective antibodies and each single-color antibody that was included in the antibody mixes. The data were acquired with a NovoCyte flow cytometer and analyzed by NovoExpress software (Acea Biosciences, Inc., San Diego, CA). The gating for each antibody was based on the isotype controls, and only live cells were analyzed.
Statistical analysis
All data were expressed as mean ± S.D. unless otherwise indicated. Differences between means of various treatment and control groups were assessed for statistical significance by analysis of variance, followed by post hoc analysis using Scheffé's test. p < 0.05 was considered to indicate statistical significance.
Animal and human studies
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Michigan. All human studies were reviewed and approved by the institutional review board at the University of Michigan and abide by the Declaration of Helsinki principles.
Author contributions
T. L. and S. H. P. conceptualization; T. L., F. G. D. L. S., K. K. K., and S. H. P. formal analysis; T. L., F. G. D. L. S., Y. Z., Z. W., and A. E. R. methodology; T. L. and S. H. P. writing-original draft; T. L. and S. H. P. project administration; T. L. and S. H. P. writing-review and editing; S. H. P. supervision; S. H. P. funding acquisition.
Acknowledgments
We thank Lisa Riggs for excellent technical assistance with lung tissue section preparation and H&E and Masson's trichrome staining.
This work was supported by National Institutes of Health Grants HL 052285, HL 112880, and HL 138417 (to S. H. P). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TERT
- telomerase reverse transcriptase
- TR
- telomerase RNA template
- IPF
- idiopathic pulmonary fibrosis
- BLM
- bleomycin
- AECII
- type II alveolar epithelial cell(s)
- cKO
- conditional knockout
- MLF
- mouse lung fibroblast(s)
- BAL
- bronchoalveolar lavage
- α-SMA
- α-smooth muscle actin
- TGF
- transforming growth factor
- TNF
- tumor necrosis factor
- SPC
- surfactant protein C
- rtTA
- reverse tetracycline transactivator
- TRF
- terminal restriction fragment
- HYP
- hydroxyproline
- qPCR
- quantitative PCR.
References
- 1. Mitchell J. R., Wood E., and Collins K. (1999) A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 10.1038/990141 [DOI] [PubMed] [Google Scholar]
- 2. Blasco M. A., Lee H. W., Hande M. P., Samper E., Lansdorp P. M., DePinho R. A., and Greider C. W. (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 10.1016/S0092-8674(01)80006-4 [DOI] [PubMed] [Google Scholar]
- 3. Alder J. K., Chen J. J., Lancaster L., Danoff S., Su S. C., Cogan J. D., Vulto I., Xie M., Qi X., Tuder R. M., Phillips J. A. 3rd, Lansdorp P. M., Loyd J. E., and Armanios M. Y. (2008) Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. U.S.A. 105, 13051–13056 10.1073/pnas.0804280105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armanios M. Y., Chen J. J., Cogan J. D., Alder J. K., Ingersoll R. G., Markin C., Lawson W. E., Xie M., Vulto I., Phillips J. A. 3rd, Lansdorp P. M., Greider C. W., and Loyd J. E. (2007) Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 10.1056/NEJMoa066157 [DOI] [PubMed] [Google Scholar]
- 5. Chibbar R., Gjevre J. A., Shih F., Neufeld H., Lemire E. G., Fladeland D. A., and Cockcroft D. W. (2010) Familial interstitial pulmonary fibrosis: a large family with atypical clinical features. Can. Respir. J. 17, 269–274 10.1155/2010/591523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackburn E. H. (2001) Switching and signaling at the telomere. Cell 106, 661–673 10.1016/S0092-8674(01)00492-5 [DOI] [PubMed] [Google Scholar]
- 7. Greider C. W. (1996) Telomere length regulation. Annu. Rev. Biochem. 65, 337–365 10.1146/annurev.bi.65.070196.002005 [DOI] [PubMed] [Google Scholar]
- 8. Nakamura T. M., Morin G. B., Chapman K. B., Weinrich S. L., Andrews W. H., Lingner J., Harley C. B., and Cech T. R. (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science 277, 955–959 10.1126/science.277.5328.955 [DOI] [PubMed] [Google Scholar]
- 9. de Lange T. (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- 10. Erdel F., Kratz K., Willcox S., Griffith J. D., Greene E. C., and de Lange T. (2017) Telomere recognition and assembly mechanism of mammalian shelterin. Cell Rep. 18, 41–53 10.1016/j.celrep.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H., Li F., He Q., Deng T., Xu J., Jin F., Coarfa C., Putluri N., Liu D., and Songyang Z. (2017) Systematic analysis of human telomeric dysfunction using inducible telosome/shelterin CRISPR/Cas9 knockout cells. Cell Discov. 3, 17034 10.1038/celldisc.2017.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim C. J., Zaug A. J., Kim H. J., and Cech T. R. (2017) Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat. Commun. 8, 1075 10.1038/s41467-017-01313-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muñoz P., Blanco R., de Carcer G., Schoeftner S., Benetti R., Flores J. M., Malumbres M., and Blasco M. A. (2009) TRF1 controls telomere length and mitotic fidelity in epithelial homeostasis. Mol. Cell. Biol. 29, 1608–1625 10.1128/MCB.01339-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karlseder J., Broccoli D., Dai Y., Hardy S., and de Lange T. (1999) p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283, 1321–1325 10.1126/science.283.5406.1321 [DOI] [PubMed] [Google Scholar]
- 15. Collado M., Blasco M. A., and Serrano M. (2007) Cellular senescence in cancer and aging. Cell 130, 223–233 10.1016/j.cell.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 16. Liu T., Hu B., Chung M. J., Ullenbruch M., Jin H., and Phan S. H. (2006) Telomerase regulation of myofibroblast differentiation. Am. J. Respir. Cell Mol. Biol. 34, 625–633 10.1165/rcmb.2005-0252OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi J., Southworth L. K., Sarin K. Y., Venteicher A. S., Ma W., Chang W., Cheung P., Jun S., Artandi M. K., Shah N., Kim S. K., and Artandi S. E. (2008) TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 4, e10 10.1371/journal.pgen.0040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamashita S., Ogawa K., Ikei T., Udono M., Fujiki T., and Katakura Y. (2012) SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem. Biophys. Res. Commun. 417, 630–634 10.1016/j.bbrc.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 19. Santos J. H., Meyer J. N., Skorvaga M., Annab L. A., and Van Houten B. (2004) Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3, 399–411 10.1111/j.1474-9728.2004.00124.x [DOI] [PubMed] [Google Scholar]
- 20. Cronkhite J. T., Xing C., Raghu G., Chin K. M., Torres F., Rosenblatt R. L., and Garcia C. K. (2008) Telomere shortening in familial and sporadic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 178, 729–737 10.1164/rccm.200804-550OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsakiri K. D., Cronkhite J. T., Kuan P. J., Xing C., Raghu G., Weissler J. C., Rosenblatt R. L., Shay J. W., and Garcia C. K. (2007) Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl. Acad. Sci. U.S.A. 104, 7552–7557 10.1073/pnas.0701009104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu T., Ullenbruch M., Young Choi Y., Yu H., Ding L., Xaubet A., Pereda J., Feghali-Bostwick C. A., Bitterman P. B., Henke C. A., Pardo A., Selman M., and Phan S. H. (2013) Telomerase and telomere length in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 49, 260–268 10.1165/rcmb.2012-0514OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu T., Yu H., Ding L., Wu Z., Gonzalez De Los Santos F., Liu J., Ullenbruch M., Hu B., Martins V., and Phan S. H. (2015) Conditional knockout of telomerase reverse transcriptase in mesenchymal cells impairs mouse pulmonary fibrosis. PLoS ONE 10, e0142547 10.1371/journal.pone.0142547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu T., Chung M. J., Ullenbruch M., Yu H., Jin H., Hu B., Choi Y. Y., Ishikawa F., and Phan S. H. (2007) Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J. Clin. Invest. 117, 3800–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Povedano J. M., Martinez P., Flores J. M., Mulero F., and Blasco M. A. (2015) Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep. 12, 286–299 10.1016/j.celrep.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 26. Naikawadi R. P., Disayabutr S., Mallavia B., Donne M. L., Green G., La J. L., Rock J. R., Looney M. R., and Wolters P. J. (2016) Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1, e86704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alder J. K., Barkauskas C. E., Limjunyawong N., Stanley S. E., Kembou F., Tuder R. M., Hogan B. L., Mitzner W., and Armanios M. (2015) Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. U.S.A. 112, 5099–5104 10.1073/pnas.1504780112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Povedano J. M., Martinez P., Serrano R., Tejera Á., Gómez-López G., Bobadilla M., Flores J. M., Bosch F., and Blasco M. A. (2018) Therapeutic effects of telomerase in mice with pulmonary fibrosis induced by damage to the lungs and short telomeres. Elife 7, e31299 10.7554/eLife.31299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spagnolo P., Maher T. M., and Richeldi L. (2015) Idiopathic pulmonary fibrosis: recent advances on pharmacological therapy. Pharmacol. Ther. 152, 18–27 10.1016/j.pharmthera.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 30. Pardo A., and Selman M. (2016) Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 13, S417–S421 10.1513/AnnalsATS.201605-341AW [DOI] [PubMed] [Google Scholar]
- 31. Meltzer E. B., and Noble P. W. (2008) Idiopathic pulmonary fibrosis. Orphanet J. Rare Dis. 3, 8 10.1186/1750-1172-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Selman M., King T. E., Pardo A., American Thoracic Society, European Respiratory Society, and American College of Chest Physicians (2001) Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151 10.7326/0003-4819-134-2-200101160-00015 [DOI] [PubMed] [Google Scholar]
- 33. King T. E. Jr., Pardo A., and Selman M. (2011) Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 10.1016/S0140-6736(11)60052-4 [DOI] [PubMed] [Google Scholar]
- 34. Selman M., and Pardo A. (2002) Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir. Res. 3, 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gross T. J., and Hunninghake G. W. (2001) Idiopathic pulmonary fibrosis. N. Engl. J. Med. 345, 517–525 10.1056/NEJMra003200 [DOI] [PubMed] [Google Scholar]
- 36. Hinz B., Phan S. H., Thannickal V. J., Galli A., Bochaton-Piallat M. L., and Gabbiani G. (2007) The myofibroblast: one function, multiple origins. Am. J. Pathol. 170, 1807–1816 10.2353/ajpath.2007.070112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cui H., Ge J., Xie N., Banerjee S., Zhou Y., Antony V. B., Thannickal V. J., and Liu G. (2017) miR-34a Inhibits lung fibrosis by inducing lung fibroblast senescence. Am. J. Respir. Cell Mol. Biol. 56, 168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barbas-Filho J. V., Ferreira M. A., Sesso A., Kairalla R. A., Carvalho C. R., and Capelozzi V. L. (2001) Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J. Clin. Pathol. 54, 132–138 10.1136/jcp.54.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Im J., Kim K., Hergert P., and Nho R. S. (2016) Idiopathic pulmonary fibrosis fibroblasts become resistant to Fas ligand-dependent apoptosis via the alteration of decoy receptor 3. J. Pathol. 240, 25–37 10.1002/path.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perl A. K., Wert S. E., Nagy A., Lobe C. G., and Whitsett J. A. (2002) Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. U.S.A. 99, 10482–10487 10.1073/pnas.152238499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tichelaar J. W., Lu W., and Whitsett J. A. (2000) Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275, 11858–11864 10.1074/jbc.275.16.11858 [DOI] [PubMed] [Google Scholar]
- 42. Chen R., Zhang K., Chen H., Zhao X., Wang J., Li L., Cong Y., Ju Z., Xu D., Williams B. R., Jia J., and Liu J. P. (2015) Telomerase deficiency causes alveolar stem cell senescence-associated low-grade inflammation in lungs. J. Biol. Chem. 290, 30813–30829 10.1074/jbc.M115.681619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flores I., Cayuela M. L., and Blasco M. A. (2005) Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256 10.1126/science.1115025 [DOI] [PubMed] [Google Scholar]
- 44. Patel P. L., Suram A., Mirani N., Bischof O., and Herbig U. (2016) Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence. Proc. Natl. Acad. Sci. U.S.A. 113, E5024–E5033 10.1073/pnas.1602379113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Desai T. J., Brownfield D. G., and Krasnow M. A. (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 10.1038/nature12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin S., Nascimento E. M., Gajera C. R., Chen L., Neuhöfer P., Garbuzov A., Wang S., and Artandi S. E. (2018) Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 556, 244–248 10.1038/s41586-018-0004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Degryse A. L., Xu X. C., Newman J. L., Mitchell D. B., Tanjore H., Polosukhin V. V., Jones B. R., McMahon F. B., Gleaves L. A., Phillips J. A. 3rd, Cogan J. D., Blackwell T. S., and Lawson W. E. (2012) Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Exp. Lung Res. 38, 124–134 10.3109/01902148.2012.658148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snetselaar R., van Batenburg A. A., van Oosterhout M. F. M., Kazemier K. M., Roothaan S. M., Peeters T., van der Vis J. J., Goldschmeding R., Grutters J. C., and van Moorsel C. H. M. (2017) Short telomere length in IPF lung associates with fibrotic lesions and predicts survival. PLoS ONE 12, e0189467 10.1371/journal.pone.0189467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cao K., Blair C. D., Faddah D. A., Kieckhaefer J. E., Olive M., Erdos M. R., Nabel E. G., and Collins F. S. (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 121, 2833–2844 10.1172/JCI43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. González-Suárez E., Samper E., Ramírez A., Flores J. M., Martín-Caballero J., Jorcano J. L., and Blasco M. A. (2001) Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 20, 2619–2630 10.1093/emboj/20.11.2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rudolph K. L., Chang S., Lee H. W., Blasco M., Gottlieb G. J., Greider C., and DePinho R. A. (1999) Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 10.1016/S0092-8674(00)80580-2 [DOI] [PubMed] [Google Scholar]
- 52. Nozaki Y., Liu T., Hatano K., Gharaee-Kermani M., and Phan S. H. (2000) Induction of telomerase activity in fibroblasts from bleomycin-injured lungs. Am. J. Respir. Cell Mol. Biol. 23, 460–465 10.1165/ajrcmb.23.4.3958 [DOI] [PubMed] [Google Scholar]
- 53. Kropski J. A., Lawson W. E., and Blackwell T. S. (2012) Right place, right time: the evolving role of herpesvirus infection as a “second hit” in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L441–444 10.1152/ajplung.00335.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pardo A., and Selman M. (2017) Fibroblast senescence and apoptosis: “one-two punch” to slow down lung fibrosis? Am. J. Respir. Cell Mol. Biol. 56, 145–146 [DOI] [PubMed] [Google Scholar]
- 55. Campisi J. (2013) Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Selman M., and Pardo A. (2014) Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis: an integral model. Am. J. Respir. Crit. Care Med. 189, 1161–1172 10.1164/rccm.201312-2221PP [DOI] [PubMed] [Google Scholar]
- 57. Cui H., Ge J., Xie N., Banerjee S., Zhou Y., Liu R. M., Thannickal V. J., and Liu G. (2017) miR-34a promotes fibrosis in aged lungs by inducing alveolar epithelial dysfunctions. Am. J. Physiol. Lung Cell Mol. Physiol. 312, L415–L424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schafer M. J., White T. A., Iijima K., Haak A. J., Ligresti G., Atkinson E. J., Oberg A. L., Birch J., Salmonowicz H., Zhu Y., Mazula D. L., Brooks R. W., Fuhrmann-Stroissnigg H., Pirtskhalava T., Prakash Y. S., et al. (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 10.1038/ncomms14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phan S. H., Varani J., and Smith D. (1985) Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J. Clin. Invest. 76, 241–247 10.1172/JCI111953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu T., Baek H. A., Yu H., Lee H. J., Park B. H., Ullenbruch M., Liu J., Nakashima T., Choi Y. Y., Wu G. D., Chung M. J., and Phan S. H. (2011) FIZZ2/RELM-β induction and role in pulmonary fibrosis. J. Immunol. 187, 450–461 10.4049/jimmunol.1000964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang J., Wheeler S. E., Velikoff M., Kleaveland K. R., LaFemina M. J., Frank J. A., Chapman H. A., Christensen P. J., and Kim K. K. (2013) Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am. J. Pathol. 183, 1559–1570 10.1016/j.ajpath.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 63. Edwards C. A., and O'Brien W. D. Jr. (1980) Modified assay for determination of hydroxyproline in a tissue hydrolysate. Clin. Chim. Acta 104, 161–167 10.1016/0009-8981(80)90192-8 [DOI] [PubMed] [Google Scholar]