Abstract
The androgen receptor (AR) is often activated in prostate cancer patients undergoing androgen-ablative therapy because of the activation of cellular pathways that stimulate the AR despite low androgen levels. In many of these tumors, the cAMP-dependent protein kinase A (PKA) pathway is activated. Previous studies have shown that PKA can synergize with low levels of androgen to enhance androgen signaling and consequent cell proliferation, leading to castration-resistant prostate cancer. However, the mechanism by which PKA causes AR stimulation in the presence of low/no androgen is not established yet. Here, using immunofluorescence immunoblotting assays, co-immunoprecipitation, siRNA-mediated gene silencing, and reporter gene assays, we demonstrate that PKA activation is necessary for the phosphorylation of heat shock protein (HSP90) that binds to unliganded AR in the cytoplasm, restricting its entry into the nucleus. We also found that PKA-mediated phosphorylation of the Thr89 residue in HSP90 releases AR from HSP90, enabling AR binding to HSP27 and its migration into the nucleus. Substitution of the Thr89 in HSP90 prevented its phosphorylation by PKA and significantly reduced AR transactivation and cellular proliferation. We further observed that the transcription of AR target genes, such as prostate-specific antigen (PSA), is also lowered in the HSP90 Thr89 variant. These results suggest that using a small-molecule inhibitor against the HSP90 Thr89 residue in conjunction with existing androgen-ablative therapy may be more effective than androgen-ablative therapy alone in the treatment of prostate cancer patients.
Keywords: protein kinase A (PKA), androgen receptor, heat shock protein 90 (HSP90), androgen, prostate cancer, castration-resistant prostate cancer (CRPC), cell signaling, kinase signaling, hormone-responsive tumor, endocrine disorder
Introduction
The androgens, acting via the androgen receptor (AR),2 play a central role in the development and maintenance of all male organs, including the prostate gland (1). Their role in the emergence and progression of prostate cancer was demonstrated by seminal studies of Huggins and Hodges (2) several decades ago. Since then, endocrine therapies aiming to physically or chemically inhibit androgen signaling, by reducing circulating androgen levels and/or blocking the AR activation, constitute the primary treatment option for prostate cancer patients (3). Although these therapies result in a temporary shrinkage of tumor mass, the cancer inevitably reappears in a more aggressive, androgen-independent form known as androgen-independent (AI) or castration-resistant prostate cancer (CRPC). Despite decades of intense basic and clinical research, no treatment options are available for AI prostate cancer.
Studies reveal that despite low circulating androgen levels, the AR is activated and often expressed at higher levels in CRPC patients (4). The transition from androgen-dependent to androgen-independent phenotype is attributed to a complex interplay of signaling molecules that ultimately result in aberrant androgen signaling and proliferation of tumor cells. In the absence of androgens, stimulation of tumor growth may be caused by various growth factors, such as epidermal growth factor, interleukin-6, or neuropeptides, such as bombesin- and gastrin-releasing peptides (5). The AI stage is often associated with an increased expression of G protein–coupled receptors (GPCRs), mediators of diverse cellular responses to extracellular molecules (6). Further, the expression of GPCR ligands, such as follicle-stimulating hormone, endothelin 1, and neuropeptides, is also higher in the prostate tumor. The higher expression of both the GPCRs and their ligands in the tumor samples indicates that probably the receptors are in the “on” state in these cells and somehow contribute to the initiation or progression of the disease. Activated GPCRs mediate their signals via G proteins that belong to four groups: Gs, Gi, Gq, and G12. Whereas Gq regulates the activity of phospholipases and intracellular Calcium levels, G12 regulates low-molecular weight effectors, such as the Rho GTPases. The Gs and Gi primarily regulate adenylyl cyclases that synthesize cAMP, which in turn activates protein kinase A (PKA) (7). PKA consists of two regulatory (R) and two catalytic kinase subunits (C) (8). In the inactive state, the R subunits dimerize and bind to the C subunits, whereas binding of cAMP to the R subunits causes a conformational change, releasing the free and active C subunit that phosphorylates numerous cellular targets.
Studies reveal that PKA/cAMP plays a significant role in prostate cell growth and proliferation (7). PKA I is overexpressed in prostate adenocarcinoma cells, and in patients with advanced prostate cancer, PKA RI overexpression was observed in 17.5% of the men and correlated significantly with poor patient outcome. Further, PKA RI overexpression predicted distal metastasis and biochemical failure in prostate cancer patients, highlighting a relationship between PKA signaling and progression to CRPC (9).
The AR is a nuclear receptor that mediates the actions of androgens, such as testosterone and dihydrotestosterone. Ligand binding to the AR leads to its dissociation from heat shock proteins in the cytosol and translocation into the nucleus, where it binds to specific androgen response elements (AREs) upstream of target genes and modulates their transcription (10, 11).
Several studies have demonstrated that the AR can be stimulated by high cAMP levels. AR transactivation by androgens could be enhanced by forskolin and other activators of adenylate cyclase in AR-transfected monkey kidney CV-1 cells or PC3 cells (1, 12). The increase in AR transcription was caused by enhanced AR–DNA binding and was not due to an increase in AR protein expression (1). Further, forskolin-induced PKA activation resulted in enhanced PSA expression in the presence of a functional AR (13). The activated α subunit of Gs protein could also activate AR in prostate cancer cells and synergize with low concentrations of androgen to completely activate the AR through PKA (6). Although these studies indicate a definite cross-talk between the two pathways, the exact role of PKA in AR activation is still unexplored.
In the present study, we demonstrate that activation of PKA is essential for nuclear translocation of AR and is a prerequisite for classical AR signaling. The unstimulated AR is bound to HSP90 and HSP70 in the cytosol, and phosphorylation of HSP90 protein at the Thr89 residue by PKA results in its disassociation from the AR, allowing the AR to bind to HSP27 and migrate into the nucleus.
Results
PKA activation is necessary for AR transactivation
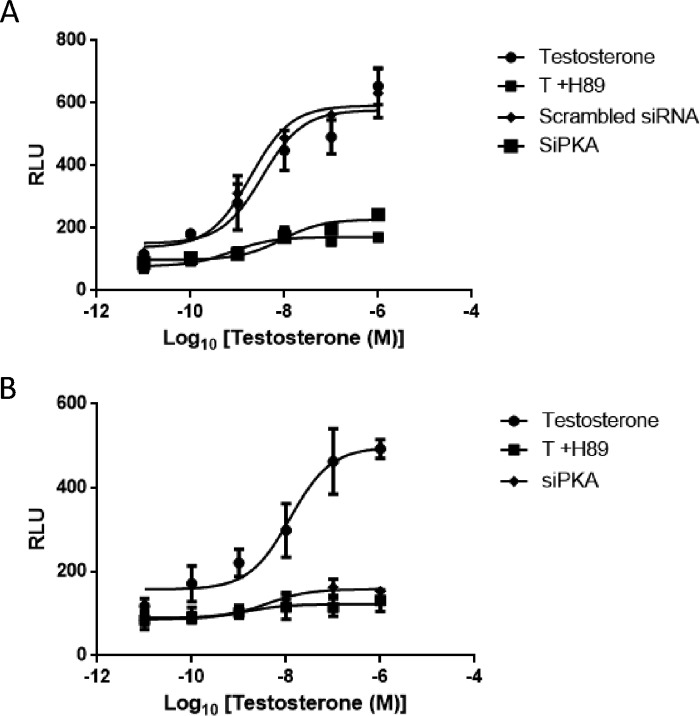
Aberrant activation of AR is the primary cause of transition from androgen dependence to androgen independence observed in patients with advanced prostate cancer (14). The cAMP/PKA pathway can induce AR in the presence of low/no androgens and remains activated in prostate cancer cells (1). In fact, androgens themselves are reported to activate PKA in prostate cells via a membrane GPCR (15). To evaluate whether activation of PKA is necessary for nuclear AR transactivation, androgen-induced ARE-regulated reporter activity was monitored in LNCaP cells in the presence and absence of PKA inhibitor H89 (30 μm, isoquinoline) or PKA-specific siRNA (PKA-siRNA) (120 nm) (Fig. 1A). Pretreatment of LNCaP cells with H89 or transfection with PKA-siRNA resulted in a significant decrease in testosterone (T)-induced AR activity (Fig. 1A). Similarly, in human embryonic kidney 293 (HEK293) cells, transfected with AR and ARE-luciferase, pretreatment with H89 or transfection with PKA-siRNA resulted in a marked decrease in testosterone-induced reporter activity (Fig. 1B), indicating that PKA activation is critical for optimal AR transactivation. Cells overexpressing scrambled siRNA did not exhibit any change in reporter activity (Fig. 1, A and B).
Figure 1.
Inhibition of PKA activation inhibits the testosterone regulated transactivation of AR. A, LNCaP cells were co-transfected with PSA.61-Luc with or without siPKA (120 nm)/scrambled siRNA (100 nm). B, HEK293 cells were co-transfected with PCMV-AR and PSA.61-Luc plasmids with or without siPKA (120 nm). Transfected cells were pretreated with H89 (30 μm) for 40 min prior to treatment with increasing concentrations of testosterone (10 pm, 0.1 nm, 1 nm, 10 nm, 100 nm, and 1000 nm). Each point represents the mean ± S.E. (error bars) of the normalized luciferase activities obtained from three independent experiments performed in duplicate. RLU, relative Luciferase units.
PKA activation is necessary for nuclear translocation of AR
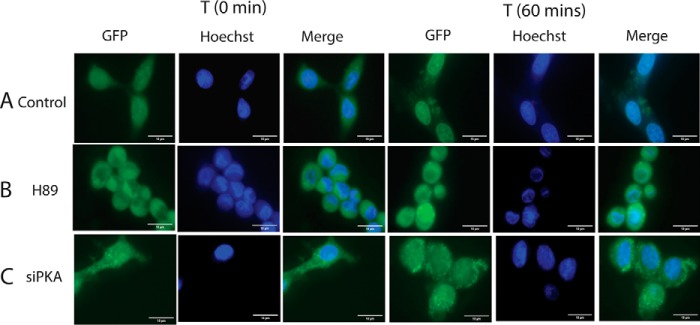
Although PKA activation is necessary for AR activity, the precise role of activated PKA in classical AR signaling needs further investigation. Studies by Kasbohm et al. (6) have indicated that PKA activation may regulate the nuclear translocation of AR, whereas others have indicated that PKA activation may enhance the interaction of AR with transcription factors post-translocation (16). To investigate whether PKA activation plays a role in the nuclear translocation of AR, LNCaP cells were transfected with GFP-tagged AR, and translocation was monitored at different time points (0, 5, 15, and 30 min and 1 and 4 h) post-testosterone treatment, in the presence and absence of PKA inhibitor H89 (Fig. S1). In the absence of testosterone, GFP-AR was mostly localized in the cytoplasm of the cells, and a time-dependent import of GFP-AR into the nucleus was observed following testosterone treatment, as reported earlier (17). In fact, greater than 90% of the GFP-AR had translocated completely within 1 h of treatment (Fig. 2 (T) and Fig. S1A). However, prior treatment of these cells with H89 (Fig. 2 (T + H89) and Fig. S1B) or knocking down PKA expression using PKA-siRNA inhibited nuclear import (Fig. 2 (T + siPKA) and Fig. S1C), indicating the need for PKA activation in the nuclear import process of AR.
Figure 2.
Inhibition of AR translocation using PKA inhibitor, H89, and by siPKA in LNCaP cells. LNCaP cells were transfected with GFP-AR with or without PKA-siRNA (120 nm). Transfected cells were treated or not treated with H89 (30 μm) for 40 min prior to treatment with testosterone (10 nm). A, images at 0- and 60-min time intervals after treatment with testosterone. B, images after treatment with testosterone in the presence of H89. C, images of siPKA-transfected cells after treatment with testosterone. Scale bar, 10 μm (added using ImageJ).
PKA activation invokes the dissociation of AR from AR–HSP90 complex
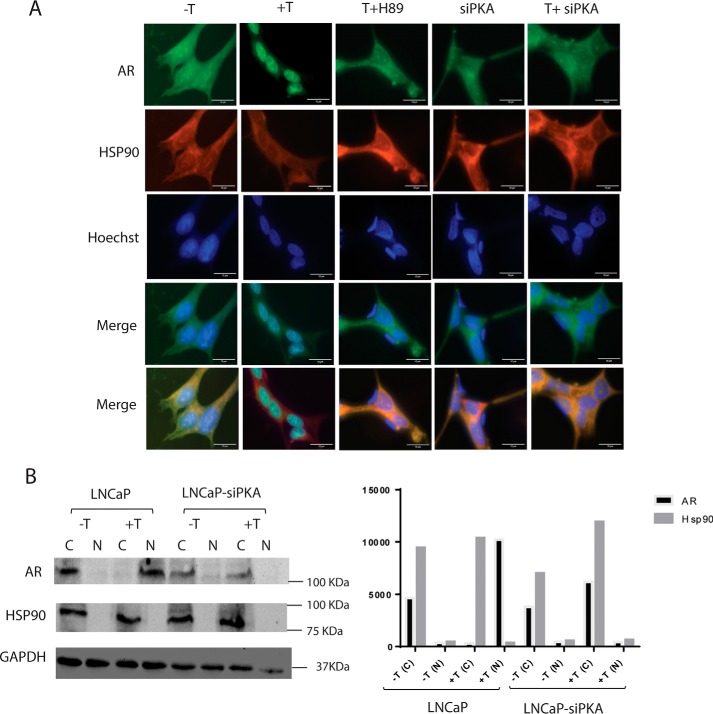
Molecular chaperones such as HSP90 stabilize the AR in the absence of ligand in the cytoplasm (11, 18). The AR in cytoplasm is maintained in an inactive but responsive state as a part of a large heterocomplex composed of heat shock proteins, co-chaperones, and other proteins (1). Binding of ligand leads to a conformational change in the AR, causing its dissociation from the large complex and translocation into the nucleus. Within the nucleus, the AR binds to androgen response elements and recruits coactivators and other factors in the vicinity of target genes to regulate their transcription (19). Studies reveal that androgen-bound AR dissociates from HSP90 but exhibits increased association with another chaperone called HSP27 (19). PKA-mediated phosphorylation could be necessary for the release of AR from heterocomplex or its subsequent migration into the nucleus. We monitored the relative locations of AR and HSP90 in LNCaP cells and assessed the impact of inhibition of PKA on the localization of the two molecules. LNCaP cells transfected with GFP-AR were treated with 10 nm testosterone for 1 h, and immunofluorescence studies were performed using TRITC-labeled anti-HSP90 antibody. In unstimulated cells, both GFP-AR and HSP90 were localized in the cytoplasm, and GFP-AR moved into the nucleus following testosterone treatment, whereas HSP90 remained in the cytosol (Fig. 3, −T and +T). In LNCaP cells pretreated with H89, T treatment did not induce import of GFP-AR into the nucleus, and both HSP90 and GFP-AR fluorescence were confined to the cytoplasm (Fig. 3, T + H89). Similarly, both HSP90 and GFP-AR colocalized predominantly in the cytosol following testosterone treatment in LNCaP cells, overexpressing PKA-siRNA (Fig. 3, siPKA and T + siPKA). Further, the subcellular location of AR and HSP90 in unstimulated (vehicle-treated) and stimulated (testosterone-treated) LNCaP cells and siPKA-transfected LNCaP cells was investigated by Western blotting, post-treatment. In unstimulated LNCaP cells (vehicle-treated), most of the AR and HSP90 was present in the cytoplasmic fraction, whereas in stimulated cells, AR was predominantly present in the nucleus while HSP90 was in the cytoplasmic fraction (Fig. 3B, LNCaP, −T, +T). Similar results were obtained in the case of unstimulated and stimulated LNCaP cells transfected with scrambled siRNA (data not shown). In siPKA-transfected LNCaP cells, AR and HSP90 were both predominantly present in the cytoplasm before and after testosterone treatment (Fig. 3B, siPKA-LNCaP, −T, +T).
Figure 3.
Colocalization of HSP90 and AR in presence and absence of H89 and siPKA. LNCaP cells were transfected with GFP-AR (5 μg) with or without siPKA (120 nm). Transfected cells were treated using vehicle or testosterone (10 nm) for 1 h. H89 (30 μm) treatment was done 40 min prior to testosterone treatment. HSP90 localization was determined by immunofluorescence staining. Nuclei were stained using Hoechst. Scale bar, 10 μm (added using ImageJ). B, cytoplasmic and nuclear fractionation of AR and HSP90 in unstimulated (vehicle-treated) and testosterone-stimulated cells in the presence and absence of siPKA. LNCap cells transfected with AR and HSP90 with or without siPKA (120 nm) were treated using vehicle or testosterone (10 nm) for 2 h. Western blotting was done with 50 μg of protein using AR or HSP90 antibody. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control.
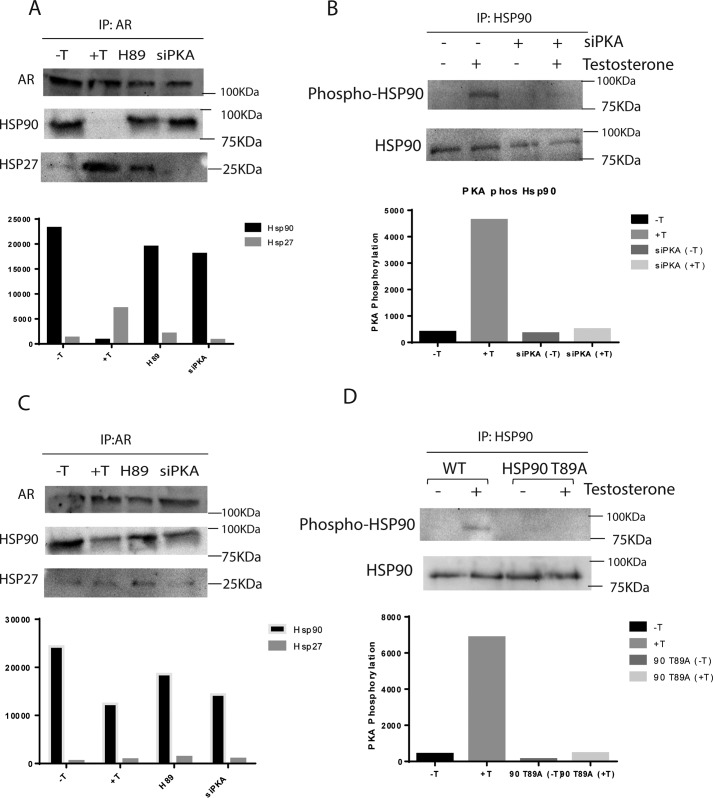
To evaluate whether PKA activation regulates the binding of AR to HSP90, co-immunoprecipitation studies were performed in LNCaP cells treated with testosterone with or without prior H89 treatment. Untreated cells were used as control. In AR-immunoprecipitated complexes, probing with HSP90 antibody revealed AR and HSP90 interaction in untreated cells and dissociation of AR from HSP90 in testosterone-treated cells (Fig. 4A). In H89-treated cells or cells transfected with PKA-siRNA, AR—HSP90 association was intact following testosterone treatment, and AR was not released from HSP90 (Fig. 4A), clearly demonstrating involvement of the PKA activation step in the release of AR from HSP90. Also, HSP27–AR interaction was increased in testosterone-treated cells compared with control; however, HSP27–AR association was much less in LNCaP cells treated with H89 or transfected with PKA-siRNA (Fig. 4A). Significantly, in co-immunoprecipitation experiments, immunoprecipitation with HSP90 antibody and probing with PKA substrate antibody indicated that phospho-HSP90 levels increased after testosterone treatment in LNCaP cells, whereas its levels in H89-treated or PKA-siRNA–transfected cells were the same as in untreated cells, following testosterone treatment (Fig. 4B). From the co-immunoprecipitation studies, it can be concluded that AR—HSP90 interaction is severed following testosterone treatment, due to phosphorylation of HSP90 by testosterone via a PKA-mediated pathway. Also, the inhibition of PKA activation prevents HSP90 phosphorylation and consequent dissociation of AR from HSP90 complex, making AR unsuitable for migration into the nucleus.
Figure 4.
Androgens lead to HSP90 phosphorylation via the PKA signaling pathway. A, inhibition of PKA activation increases HSP90 levels in complex with AR as HSP27 decreases. LNCaP cells were co-transfected with PCMV-AR, pLNCX2-HSP90, and pLEX-HSP27 with or without siPKA (120 nm). Cells were treated using testosterone (10 nm) for 1 h post-transfection. 300 μg of total extract were immunoprecipitated (IP) using AR antibody, and Western blotting was done using HSP90, HSP27, and AR antibodies. B, testosterone promotes PKA-dependent phosphorylation of HSP90. LNCaP cells transfected with or without siPKA (120 nm) were treated or not treated with testosterone (10 nm) for 1 h. 250 μg of total extract were immunoprecipitated with HSP90 antibody, and Western blotting was done using PKA substrate and HSP90 antibody. C, mutated HSP90 T89A increases HSP90 levels in complex with AR as HSP27 decreases. LNCaP cells were co-transfected with PCMV-AR, pLNCX2-HSP90 T89A, and pLEX-HSP27 with or without siPKA (120 nm). After transfection, cells were treated using 10 nm testosterone for 1 h. 300 μg of total extract were immunoprecipitated using AR antibody, and Western blotting was performed using HSP90, HSP27, and AR antibodies. D, PKA phosphorylation does not take place in the presence of HSP90 T89A. LNCaP cells transfected with or without pLNCX2-HSP90 T89A were treated or not treated with testosterone (10 nm) for 1 h. 250 μg of total extract were immunoprecipitated with HSP90 antibody, and Western blotting was done using PKA substrate and HSP90 antibody.
PKA activation phosphorylates the Thr89 residue in HSP90
PKA, being a kinase, could possibly regulate the AR and HSP90 complex formation by phosphorylating the AR, HSP90, or any other protein within the complex. Studies have shown that the only site in AR that is phosphorylated by PKA is Ser650, and mutation of this Ser to Ala, S650A, did not have any significant impact on AR transactivation (6). HSP90 is phosphorylated by PKA at a single site, Thr89, in PAEC cells (20). Mutation of this threonine residue inhibits PKA-mediated phosphorylation of HSP90 and prevents the translocation of HSP90 to the surface of these cells (20). To investigate whether PKA-induced phosphorylation of Thr89 plays a key role in the dissociation of AR–HSP90 complex, co-immunoprecipitation studies were performed in LNCaP cells transfected with HSP90 T89A (a kind gift from Heitian Lei). In cells overexpressing mutant HSP90, immunoprecipitation with AR antibody, followed by immunoblotting with HSP90 antibody, revealed that a significant percentage of AR remained in complex with HSP90 following testosterone treatment unlike in cells expressing WT HSP90, where complete dissociation of AR and HSP90 was observed (Fig. 4C). Because these cells also contain a small percentage of endogenous WT HSP90, a small percentage of AR is dissociated from the HSP90 complex, reflected in the minor difference in intensity of the AR-bound HSP90 product obtained in treated and untreated cells. In the same cells pretreated with H89 or transfected with PKA-siRNA, most of the AR remained in complex with HSP90 upon testosterone treatment, as in the untreated cells (Fig. 4C). An inverse proportionality is usually observed in the binding of HSP90 and HSP27 to AR (20). HSP27 can bind to AR only when it is released from the HSP90 complex. In keeping with this, in LNCaP cells overexpressing HSP90 T89A, only a small fraction of HSP27 was bound to AR following testosterone treatment, as most of the AR was still in complex with mutated HSP90. AR could probably uncouple only from those complexes that were formed by a few molecules of WT HSP90 present in these cell that could be phosphorylated. Co-immunoprecipitation studies using anti-HSP90 antibody, followed by immunoblotting with PKA substrate antibody, indicated that in cells overexpressing HSP90 T89A, negligible levels of phospho-HSP90 product is obtained compared with cells that express WT HSP90 upon testosterone treatment. (Fig. 4D). To confirm that the observed difference in immunoblots was not due to any difference in affinity of the anti-HSP90 antibody for the WT and mutant HSP90 proteins, Western blotting was performed using lysates from LNCaP cells transfected using HSP90, HSP90 T89A, or vector alone. Results demonstrate that the antibody can detect both WT and mutant HSP90 T89A efficiently (Fig. S3).
Nuclear translocation of the HSP90 T89A mutant
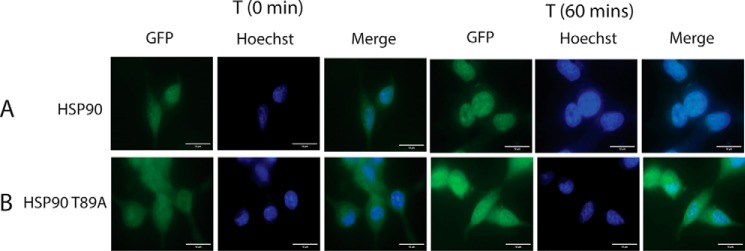
The migration of AR into the nucleus is possible once it dissociates from the HSP90 heterocomplex. In LNCaP cells overexpressing HSP90 T89A and GFP-AR, the translocation of GFP-AR was monitored by a fluorescence microscope at different time intervals following testosterone treatment. Whereas in LNCaP cells expressing WT HSP90, most of the AR translocated into the nucleus by 1 h (Fig. 5 (HSP90) and Fig. S2A), in cells overexpressing the HSP90 mutant, most of the AR remained in the cytoplasm following testosterone treatment for 4 h (Fig. 5 (HSP90 T89A) and Fig. S2B) or more (data not shown).
Figure 5.
Inhibition of AR translocation in HSP90 T89A–transfected LNCaP cells. LNCaP cells were transfected using GFP-AR alone or in combination with HSP90 T89A mutant. Transfected cells were treated with testosterone (10 nm), and images were captured. A, images at 0- and 60-min time intervals after treatment with testosterone (10 nm). B, images captured at 0- and 60-min time intervals post-testosterone treatment in HSP90 T89A–transfected cells. Scale bar, 10 μm (added using ImageJ).
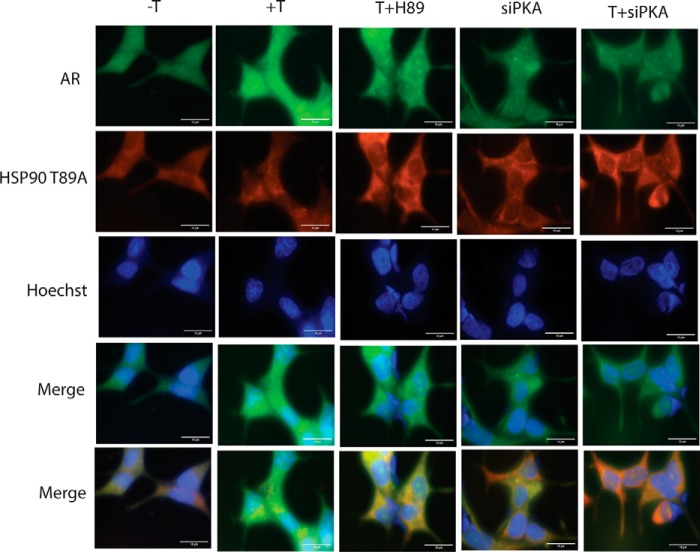
Also, in these cells, GFP-AR and mutant HSP90 colocalize in the cytoplasm before and after testosterone treatment (10 nm, 1 h; Fig. 6, −T and +T). Cells pretreated with H89 or transfected with PKA-siRNA behaved in the same way as untreated and testosterone-treated cells (Fig. 6, T + H89, siPKA, and T + siPKA). Collectively, these results indicate that phosphorylation of HSP90 by PKA at residue Thr89 is essential for the release of AR from the HSP90 complex and its subsequent migration into the nucleus.
Figure 6.
Colocalization of HSP90 T89A and AR in the presence and absence of H89 and siPKA. LNCaP cells were transfected with GFP-AR (5 μg) and HSP90 T89A with or without siPKA. Medium was changed after 24 h of transfection. Transfected cells were treated with testosterone (10 nm) for 1 h. HSP90 localization was determined using immunofluorescence staining. Nuclei were stained with Hoechst. Scale bar, 10 μm (added using ImageJ).
Inhibition of AR transactivation in LNCaP cells expressing HSP90 T89A
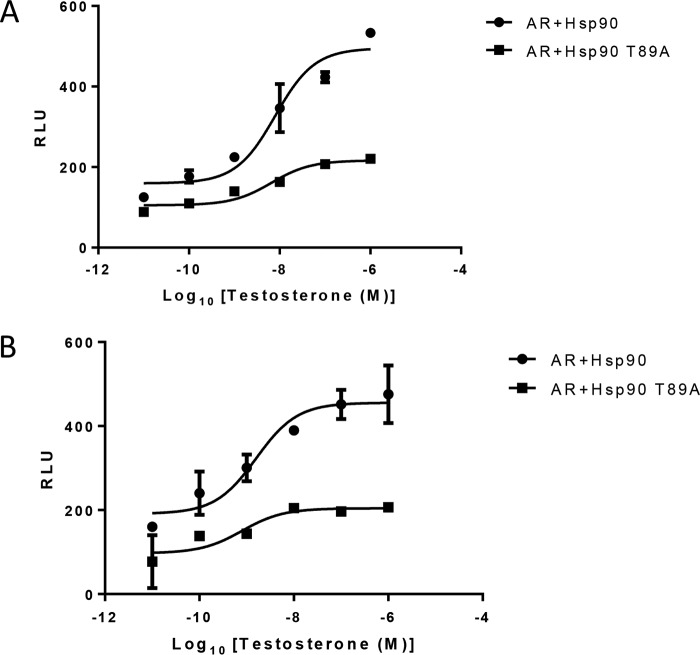
In the cells expressing mutant HSP90, most of the AR was in complex with HSP90 and could not be imported into the nucleus. To evaluate the transcription efficiency of AR in cells overexpressing the mutant HSP90, cells were treated with increasing concentrations of testosterone, and ARE-regulated luciferase activity was measured and compared with that in LNCaP cells expressing WT HSP90. In both LNCaP and HEK293 cells, a significant fall in testosterone-induced reporter activity (58.56% and 56.5%, respectively) was observed in cells overexpressing HSP90 T89A as compared with those expressing WT HSP90 (Fig. 7, A and B). In fact, little increase in reporter activity was observed following testosterone treatment in both of these cell lines (Fig. 7, A and B).
Figure 7.
Inhibition of AR transcription in the presence of HSP90 mutant. A, LNCaP cells were transfected with PSA.61-Luc and pLNCX2-HSP90/pLNCX2-HSP90 T89A. B, HEK293 cells were transfected with PCMV-AR, PSA.61-Luc, and pLNCX2-HSP90/pLNCX2-HSP90 T89A. Transfected cells were treated with increasing concentrations of testosterone (10 pm, 0.1 nm, 1 nm, 10 nm, 100 nm, and 1000 nm). Each point represents the mean ± S.E. (error bars) of the normalized luciferase activities obtained from three independent experiments performed in duplicate.
Reduced cell proliferation and PSA expression in the absence of activated PKA
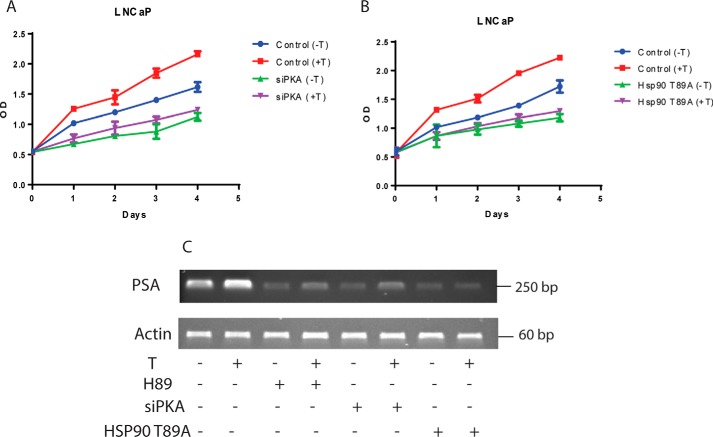
Androgens play an important role in the survival and maintenance of prostate cells. Androgens also stimulate PKA activation to promote androgen signaling (15). We therefore examined whether activation of PKA is required for the proliferative effects induced by androgens in prostate cells. To confirm this, LNCaP cells were grown in testosterone-deficient (control) medium or testosterone-supplemented medium, in the presence or absence of PKA-siRNA. The proliferation of cells grown in control medium was 2.9-fold in 4 days, and proliferation of cells grown in testosterone was 25% more than that in control in 4 days (Fig. 8A). In cells overexpressing the PKA-siRNA, net proliferation was 2-fold in 4 days in control medium and increased by an insignificant margin following testosterone treatment (Fig. 8A). LNCaP cells transfected using scrambled siRNA proliferated in the same manner as nontransfected LNCaP cells (data not shown). In LNCaP cells overexpressing HSP90 T89A mutant, cell proliferation increased by only 2-fold in both control and testosterone-treated medium compared with HSP90-transfected LNCaP cells that proliferated 2.9-fold in control medium or 3.9-fold in testosterone-containing medium (Fig. 8B). We further evaluated PSA gene expression as a prototype androgen-responsive gene in LNCaP cells, under normal conditions (Fig. 8C, lanes 1 and 2), in the presence and absence of H89 (Fig. 8C, lanes 3 and 4), upon overexpression of PKA-siRNA (Fig. 8C, lanes 5 and 6), and in LNCaP cells overexpressing mutant HSP90 T89A (Fig. 8C, lanes 7 and 8). Inhibition of PKA activation in LNCaP cells by H89 or overexpressing PKA-siRNA or by overexpression of HSP90 T89A mutant caused an overall reduction in PSA expression in testosterone-treated cells (Fig. 8C). In H89-treated or PKA-siRNA–transfected cells, a modest increase in PSA expression could be observed upon testosterone treatment; however, an increase in expression was not observed in cells overexpressing HSP90 T89A mutant, demonstrating the pivotal role of androgen-induced PKA activation and subsequent HSP90 phosphorylation in AR-mediated gene transcription and survival of prostate cells.
Figure 8.
A, androgen-induced LNCaP cell proliferation and PSA expression requires PKA activation. LNCaP cells that were transfected or not transfected with siPKA (120 nm) were treated with testosterone (10 nm) for 24, 48, 72, and 96 h, and cell growth rates were determined by an MTT assay. B, transfection of HSP90 T89A mutant inhibits LNCaP cell proliferation. HSP90 T89A–transfected and mock-transfected cells were treated with testosterone (10 nm) for 24, 48, 72, and 96 h, and cell growth rates were determined by an MTT assay. C, testosterone-induced PSA expression in LNCaP cells requires PKA activation. LNCaP cells transfected or not transfected with siPKA/HSP90 T89A were treated with testosterone (10 nm) or vehicle alone for 24 h. Total RNA was isolated, and expression of PSA, an AR target gene, was measured by RT-PCR. β-Actin was used as control. Error bars, S.E.
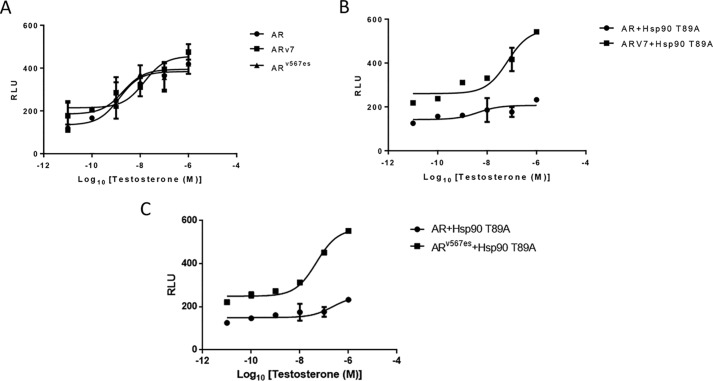
AR-V7 and ARv567es can induce AR signaling independent of PKA-induced HSP90 phosphorylation
Expression of WT HSP90 is required to stabilize the AR ligand-binding domain (LBD) in a high-affinity androgen-binding state, and the loss of HSP90 function significantly reduces the binding affinity of AR for androgens (21). However, in clinical samples, AR variants such as AR-V7 and ARv567es have been identified that lack the LBD of AR. They localize to the nucleus and cause ligand-independent transcription to induce castration-resistant growth in patients. As these variants lack LBD, they probably do not require association with HSP90 complexes for optimal hormone binding (22). However, whether HSP90 is required for initial folding of these proteins or subsequent AR transcription is not clear. We investigated the effect of overexpression of HSP90 T89A mutant on the transcription of these AR splice variants. As expected, in the presence of WT HSP90, the ARE-regulated transcription of AR, AR-V7, and ARv567es were comparable (Fig. 9A). When HSP90 T89A mutant was overexpressed in these cells, the transcription of AR was reduced significantly, whereas the transcription of AR-V7 or ARv567es was not affected (Fig. 9, B and C). These results clearly reveal that activation of PKA and subsequent HSP90 phosphorylation at residue Thr89 is critical for the transcription of WT AR, but not mandatory for the transcription of AR splice variants AR-V7 or ARv567es. Also, these results suggest that phosphorylation of HSP90 probably does not play a major role in the folding and subsequent function of these AR variants, as transcription occurs unhindered in the presence of HSP90 T89A mutant.
Figure 9.
A, increase in transcription of truncated AR variants (ARV7 and ARv567es) following testosterone treatment in cells expressing WT HSP90 or HSP90 T89A. HEK293 cells transfected with PSA.61-Luc and PCMV-AR/pCDNA-ARV7/pCDNA-ARv567es plasmids were treated with increasing concentrations of testosterone (10 pm, 0.1 nm, 1 nm, 10 nm, 100 nm, and 1000 nm). Each point represents the mean ± S.E. (error bars) of the normalized luciferase activities obtained from three independent experiments performed in duplicate. B and C, mutated HSP90 does not affect the transcription of truncated AR variants (ARV7 and ARv567es). HEK293 cells transfected with PSA.61-Luc, PCMV-AR/pCDNA-ARV7/pCDNA-ARv567es, and HSP90/HSP90 T89A plasmids were treated with increasing concentrations of testosterone (10 pm, 0.1 nm, 1 nm, 10 nm, 100 nm, and 1000 nm). Each point represents the mean ± S.E. of the normalized luciferase activities obtained from three independent experiments performed in duplicate.
Discussion
The importance of PKA activation for AR signaling has been demonstrated by several studies. However, the specific role of PKA and its contribution to androgen signaling remains elusive. Here we demonstrate the existence of a tripartite cross-talk between PKA, HSP90, and AR that enables dissociation of ligand-bound AR from HSP90 and its subsequent translocation into the nucleus.
We began by investigating whether the activation of PKA is required for AR-transactivation in prostate cells. ARE-regulated transcriptional activity was significantly lowered when PKA activation was inhibited, either by an inhibitor such as H89 or by overexpressing PKA-siRNA, establishing the need for normal PKA activation for AR signaling.
Nuclear translocation is a prerequisite step for the function of the AR (11). Next, we investigated whether PKA activation was necessary for pre-translocation events or post-translocation events in the AR transcription pathway. Immunofluorescence studies performed to monitor the localization of GFP-tagged AR revealed that inhibition of PKA impeded AR nuclear import, suggesting that PKA activation was an essential event prior to or during nuclear translocation.
Before ligand binding, the AR is held in cytoplasm in a heterocomplex involving HSP90 and several other proteins that present the AR in a high-affinity conformation poised for optimal ligand binding (11, 21). Ligand binding causes a conformational change in the AR that allows release and exchange of chaperones and import proteins for efficient nuclear translocation (23, 24). Studies by Zoubeidi et al. (19) suggest that subsequent to androgen treatment, the association of HSP27 with AR increases, whereas the levels of HSP90-associated AR fall, suggesting that replacement of HSP90 by HSP27 is required for smooth transition of AR from the cytoplasm into the nucleus. Our co-immunoprecipitation studies support this hypothesis, and we further observe that inhibition of PKA activation either by H89 or PKA-siRNA prevents the uncoupling of AR from HSP90 and subsequent association with HSP27. The inability to dissociate from HSP90 and bind to HSP27 possibly hinders the migration of AR into the nucleus. Binding to HSP27 is required not only for AR translocation but also for interaction with AR coactivators STAT3 and ARA55 and the formation of the AR transcription complex (19).
How does PKA activation cause the dissociation of AR from HSP90? PKA, being a kinase, is known to execute the phosphorylation of multiple cellular proteins, including both the AR and HSP90 (20, 25). Because mutation of the sole PKA target sequence in AR does not have an impact on AR transcriptional activity (6), we focused on the phosphorylation of HSP90. In fact, the HSP90 T89A mutant, in which the PKA phosphorylation site is mutated, could support neither AR nuclear translocation efficiently nor AR transactivation. Nuclear translocation of testosterone-treated GFP-AR was similar to that in untreated cells. The ARE-regulated transcription levels observed in HSP90 T89A–overexpressing cells treated with the highest concentration of testosterone were similar to transcription observed in unstimulated cells expressing WT HSP90, indicating that the basal level of AR transcription decreases if PKA activation is blocked.
Our results show that androgen treatment causes phosphorylation of HSP90 in LNCaP cells, the dissociation of AR from HSP90, and its association with HSP27. Inhibition of PKA activation by H89 or PKA-siRNA reduces HSP90 phosphorylation almost to levels observed in unstimulated cells and prevents its consequent dissociation from the AR. In cells overexpressing the HSP90 T89A mutant, negligible levels of the phospho-HSP90 product are visualized as compared with cells expressing WT HSP90, as expected. As a result, much of the AR is still associated with HSP90, unlike cells expressing the WT HSP90, where complete uncoupling of AR and HSP90 is observed by immunoprecipitation studies. However, some dissociation of AR from HSP90 seems to take place in these cells, probably due to the phosphorylation of a few molecules of endogenous WT HSP90 present in them. HSP90 is phosphorylated at various residues, and phosphorylation is a means of regulating HSP90 activity (26). PKA-induced phosphorylation of HSP90 at Thr89 could bring about a conformational switch in HSP90, resulting in the dissociation of AR from HSP90.
It is interesting to note that the transcriptional activity of AR variants ARV7 and ARv567es are similar in cells expressing HSP90 or HSP90 T89A. These two AR variants are often identified in CRPC patients (22), and HSP90 inhibitors, such as geldanamycin, have little or no impact on these variants. These variants do not have the LBD of AR and hence do not need HSP90 for ligand binding. However, whether HSP90 is required at initial stages for proper folding of these proteins or at later stages during transcription was not established (22). Because no change in ARE-regulated transcription of these splice variants was observed in cells expressing WT or mutant HSP90, it seems that phosphorylated HSP90 does not play a role in the maturation or transcriptional activity of these splice variants.
Studies in which simultaneous inhibition of PKA and androgen signaling was administered resulted in reduced prostate tumor cell proliferation as compared with AR inhibition alone (27). However, our studies indicate that instead of inhibiting the PKA pathway (using a PKA inhibitor), which may be important for the function of several proteins, the same can be achieved by mutating a single residue in HSP90. Most of the HSP90 inhibitors developed earlier targeted the ATP-binding domain of HSP90; however, newly developed HSP90 inhibitors, such as AUY922 and STA-9090, with higher specificity and less toxicity are now in clinical trials (28, 29). A small-molecule inhibitor against the PKA phosphorylation site in HSP90 Thr89 may be used in combination with AR inhibitors to minimize the proliferation of tumor cells in patients with advanced prostate cancer. HSP90 is a client chaperone for a variety of proteins involved in different pathways, and the targeted inhibition of HSP90 along with androgen inhibition should have restricted impact on other cellular pathways while achieving maximum impact on androgen signaling and tumor cell proliferation.
Experimental procedures
Plasmids and reagents
The plasmids were kindly provided as follows: human GFP-AR and PSA.61-Luc, Prof. Rakesh K. Tyagi (Special Centre for Molecular Medicine, JNU, Delhi, India); PCMV-AR, Elizabeth Wilson (Reproductive Biology Laboratory, University of North Carolina); pLNCX2-HSP90 and pLNCX2-HSP90 T89A, Hetian Lei (Schepens Eye Research Institute and Department of Ophthalmology, Harvard Medical School, Boston, MA); and pLEX-HSP27, Goupei Zheng (Cancer Hospital and Institute of Guangzhou Medical University, China). Testosterone and H89 (PKA inhibitor) were purchased from Sigma and Cayman, respectively. Antibodies AR-441, HSP90α, and HSP27 were from Santa Cruz Biotechnology, Inc.; PKA substrate antibody was from CST; anti-mouse and anti-rabbit horseradish peroxidase–conjugated secondary antibody and femtoLUCENT were from G Biosciences; and Protein A–agarose was from ABT.
Cell culture
Androgen-dependent LNCaP and COS1 cells were obtained from the National Centre for Cell Science (Pune, India) and were cultured in RPMI 1640 and Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 1% streptomycin (10 mg/ml) respectively.
Cell transfection and siRNA
For plasmid transfection, cells were cultured in 100-mm Petri dishes up to 60–70% confluence. For single transient transfection, 1 μg of plasmid was transfected with Lipofectamine 2000 transfection reagent (Invitrogen), 10 μl/plate. Medium was changed after 24 h of transfection with complete medium (with 10% FBS). Testosterone treatment was done after 24 h of medium change.
For siRNA transfection, cells were cultured in 100-mm Petri dishes up to 40–50% confluence. siRNA was transfected with Lipofectamine 2000 transfection reagent. The siRNA was used to knock down PKA. It was designed against the RIα subunit of PKA (sense, 5′-CCAUGGAGUCUGGCAGUACdTdT-3′; antisense, 5′-GUACUGCCAGACUCCAUGGdTdT-3′). siRNAs were synthesized by IDT.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay
LNCaP cells were cultured and transfected or mock-transfected with siPKA or HSP90 T89A. After 24 h of transfection, cells (5000 cells/well) were seeded in 96-well plates. Testosterone (10 nm) treatment was done after 24 h of seeding. During a time course of 24, 48, 72, and 96 h, cell proliferation was measured using CellTiter 96 AQueous one solution reagent MTT.
Luciferase assay
Cells were cultured in 100-mm Petri dishes and transfected at 60–70% confluence using Lipofectamine 2000. Transfections were carried out with PSA.61-Luc plasmid (1 μg) along with PCMV-AR plasmid (1 μg). After 24 h of transfection, cells were equally split into 60-mm Petri dishes and allowed to attach. After 24 h, the identical cell population was treated with testosterone (10 nm) and T + H89 (30 μm). Luciferase activities were measured in triplicates by using Luciferase assay reagent (Promega) and were normalized with protein concentrations of samples by the Bradford method (HiMedia).
Fluorescence imaging
Fluorescence imaging of live cells was performed through an upright Olympus optical microscope. Cells cultured in 100-mm Petri dishes with coverslips were transfected with GFP-AR plasmid (1 μg) and treated with testosterone (10 nm) and T + H89 (30 μm). At different time intervals of 5 min, 15 min, 30 min, 1 h, 4 h, and 24 h, cells were visualized under a microscope by mounting the coverslip on a microslide.
Immunofluorescence
LNCaP cells were cultured on coverslips and transfected with GFP-AR (5 μg) along with or without siPKA (120 nm)/pLNCX2-HSP90 T89A (2 μg). Medium was changed after 24 h of transfection with complete medium (with 10% FBS). Cells were treated with T/T + H89 for 1 h. After treatment, cells were fixed with ice-cold 100% methanol for 10 min precooled at −20 °C. Cells were then washed three times with PBS and incubated with 0.2% Triton/PBS for 10 min, followed by PBS washing and 30-min blocking in 3% BSA/PBST. After blocking, cells were incubated overnight in antibody HSP90 (1:100) at 4 °C. Cells were visualized using anti-mouse antibody coupled to rhodamine (1:500, 1 h). Images were captured with an upright Olympus optical microscope with a ×100 objective.
Reverse transcription PCR
Cells were cultured and transfected with siPKA/HSP90 T89A, and after 24 h, medium was changed with complete medium. After 24 h of transfection, cells were treated/nontreated with T/T + H89. The total cell RNA was isolated using a TRIzol-based isolation method. Cells were lysed in TRIzol reagent (1 ml). To this lysate, 200 μl of chloroform was added, shaken vigorously, and incubated at room temperature for 5 min. The solution was centrifuged at 12,000 rpm for 15 min at 4 °C. The top aqueous layer was collected carefully and precipitated by adding 500 μl of isopropyl alcohol, followed by incubation for 10 min at room temperature. The precipitated RNA was collected by centrifugation at 12,000 rpm for 10 min at 4 °C. The ethanol was drained off, and the pellet was air-dried resuspended in 50 μl of RNase-free diethyl pyrocarbonate water. 1 μg of RNA obtained was used for cDNA synthesis using the Bio-Rad Iscript cDNA synthesis kit and used for quantitative RT-PCR analysis for PSA expression.
Co-immunoprecipitation
Cells were harvested for immunoprecipitation. Whole-cell lysate was prepared using cell lysis buffer containing 20 mm Tris/HCl (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 1 mm DTT with protease inhibitors aprotinin (10 μg), phenylmethanesulfonyl fluoride (10 μg), and phosphatase inhibitor mixture (10 μl), and then lysates were centrifuged at 14,000 rpm at 4 °C for 15 min. The pellet was discarded, and supernatant was used as whole-cell lysate for immunoprecipitation. The protein concentrations were measured using a Bradford kit (HiMedia). The proteins in cell lysate were immunoprecipitated with 2 μg of AR antibody (Santa Cruz Biotechnology) overnight at 4 °C. The immune complexes were recovered with Protein A–agarose for 1.5 h with rotation. The resin was pelleted, washed with PBS three times, and submitted to SDS-PAGE, followed by Western blotting.
Immunoblotting
The samples separated by SDS-PAGE were transferred to polyvinylidene difluoride membrane (MDI). The membrane was blocked in TBST (TBS/Tween 20, 20 mm Tris/HCl, pH 7.5, 150 mm NaCl, 0.1% Tween 20) with 5% nonfat dry milk for 1 h at room temperature. The membrane was incubated with primary antibody diluted in TBST with 3% nonfat dry milk overnight at 4 °C. Then membrane was washed three times with TBST for 15 min each and incubated with horseradish peroxidase–conjugated secondary antibody for 1.5 h with rotation at room temperature. Following three washes with TBST, immunoreactive bands were detected by femtoLUCENT (G Biosciences).
Statistical analysis
Experiments were repeated at least three times. Data are expressed as mean ± S.E. Statistical analysis was performed using Student's t test, and graphs were generated using Prism (version 5.0).
Author contributions
R. K. T. resources; G. B. conceptualization; G. B. supervision; G. B. project administration; G. B. writing-review and editing. M. D. performed the bulk of the experiments and conducted data analysis; J. P. S. and G. D. helped with experiments; and R. K. T. reviewed the work.
Supplementary Material
Acknowledgments
We express our sincere gratitude to Dr. Lei Hetian (Schepens Eye Research Institute and Department of Ophthalmology, Harvard Medical School, Boston, MA) for HSP90 and HSP90 mutant plasmid. We also thank Dr. Elizabeth Wilson (Laboratory for Reproductive Biology, University of North Carolina) and Dr. Goupei Zheng (Cancer Hospital and Institute of Guangzhou Medical University) for providing AR and HSP27 plasmids, respectively. We are thankful to Prof. Rajendra Prasad (Amity Institute of Biotechnology, Amity University, Gurugram, India) for the use of Chemi Doc.
This work was supported by funds from the Department of Biotechnology Bio Care Project, New Delhi (to G. B. B.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
This article contains Figs. S1–S3.
- AR
- androgen receptor
- ARE
- androgen response element
- AI
- androgen-independent
- CRPC
- castration-resistant prostate cancer
- GPCR
- G protein–coupled receptor
- PKA
- protein kinase A
- R
- regulatory
- C
- catalytic
- LBD
- ligand-binding domain
- FBS
- fetal bovine serum
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
- TRITC
- tetramethylrhodamine isothiocyanate
- PKA-siRNA or siPKA
- PKA-specific siRNA.
References
- 1. Nazareth L. V., and Weigel N. L. (1996) Activation of the human androgen receptor through a protein kinase A signaling pathway. J. Biol. Chem. 271, 19900–19907 10.1074/jbc.271.33.19900 [DOI] [PubMed] [Google Scholar]
- 2. Huggins C., and Hodges C. V. (1941) Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 [DOI] [PubMed] [Google Scholar]
- 3. Denis L. J., and Griffiths K. (2000) Endocrine treatment in prostate cancer. Semin. Surg. Oncol. 18, 52–74 [DOI] [PubMed] [Google Scholar]
- 4. Lonergan P. E., and Tindall D. J. (2011) Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 10, 20 10.4103/1477-3163.83937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarwar M., and Persson J. L. (2011) The protein kinase A (PKA) intracellular pathway and androgen receptor: a novel mechanism underlying the castration-resistant and metastatic prostate cancer. J. Cancer Sci. Ther. S5, 003 10.4172/1948-5956.S5-003 [DOI] [Google Scholar]
- 6. Kasbohm E. A., Guo R., Yowell C. W., Bagchi G., Kelly P., Arora P., Casey P. J., and Daaka Y. (2005) Androgen receptor activation by Gs signaling in prostate cancer cells. J. Biol. Chem. 280, 11583–11589 10.1074/jbc.M414423200 [DOI] [PubMed] [Google Scholar]
- 7. Merkle D., and Hoffmann R. (2011) Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell. Signal. 23, 507–515 10.1016/j.cellsig.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 8. Chin K. V., Yang W. L., Ravatn R., Kita T., Reitman E., Vettori D., Cvijic M. E., Shin M., and Iacono L. (2002) Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann. N.Y. Acad. Sci. 968, 49–64 10.1111/j.1749-6632.2002.tb04326.x [DOI] [PubMed] [Google Scholar]
- 9. Khor L. Y., Bae K., Al-Saleem T., Hammond E. H., Grignon D. J., Sause W. T., Pilepich M. V., Okunieff P. P., Sandler H. M., and Pollack A. (2008) Protein kinase A RI-α (PKA) predicts prostate cancer outcome: an analysis of radiation therapy oncology group trial 86-10. Int. J. Radiat. Oncol. Biol. Phys. 71, 1309–1315 10.1016/j.ijrobp.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gobinet J., Poujol N., and Sultan C. (2002) Molecular action of androgens. Mol. Cell. Endocrinol. 198, 15–24 10.1016/S0303-7207(02)00364-7 [DOI] [PubMed] [Google Scholar]
- 11. Kumar S., Saradhi M., Chaturvedi N. K., and Tyagi R. K. (2006) Intracellular localization and nucleocytoplasmic trafficking of steroid receptors: an overview. Mol. Cell. Endocrinol. 246, 147–156 10.1016/j.mce.2005.11.028 [DOI] [PubMed] [Google Scholar]
- 12. Ikonen T., Palvimo J. J., Kallio P. J., Reinikainen P., and Jänne O. A. (1994) Stimulation of androgen regulated transactivation by modulators of protein phosphorylation. Endocrinology 135, 1359–1366 10.1210/endo.135.4.7925097 [DOI] [PubMed] [Google Scholar]
- 13. Leung J. K., and Sadar M. D. (2017) Non-genomic actions of the androgen receptor in prostate cancer. Front. Endocrinol. (Lausanne) 8, 2 10.3389/fendo.2017.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadar M. D., Hussain M., and Bruchovsky N. (1999) Prostate cancer: molecular biology of early progression to androgen independence. Endocr. Relat. Cancer 6, 487–502 [DOI] [PubMed] [Google Scholar]
- 15. Bagchi G., Wu J., French J., Kim J., Moniri N. H., and Daaka Y. (2008) Androgens transduce the Gαs-mediated activation of protein kinase A in prostate cells. Cancer Res. 68, 3225–3231 10.1158/0008-5472.CAN-07-5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim W., Spear E. D., and Ng D. T. (2005) Yos9p detects and targets misfolded glycoproteins for ER-associated degradation. Mol. Cell. 19, 753–764 10.1016/j.molcel.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 17. Tyagi R. K., Lavrovsky Y., Ahn S. C., Song C. S., Chatterjee B., and Roy A. K. (2000) Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol. Endocrinol. 14, 1162–1174 10.1210/mend.14.8.0497 [DOI] [PubMed] [Google Scholar]
- 18. Cheung J., and Smith D. F. (2000) Molecular chaperone interactions with steroid receptors: an update. Mol. Endocrinol. 14, 939–946 10.1210/mend.14.7.0489 [DOI] [PubMed] [Google Scholar]
- 19. Zoubeidi A., Zardan A., Beraldi E., Fazli L., Sowery R., Rennie P., Nelson C., and Gleave M. (2007) Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 67, 10455–10465 10.1158/0008-5472.CAN-07-2057 [DOI] [PubMed] [Google Scholar]
- 20. Lei H., Venkatakrishnan A., Yu S., and Kazlauskas A. (2007) Protein kinase A-dependent translocation of HSP90α impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J. Biol. Chem. 282, 9364–9371 10.1074/jbc.M608985200 [DOI] [PubMed] [Google Scholar]
- 21. Fang Y., Fliss A. E., Robins D. M., and Caplan A. J. (1996) HSP90 regulates androgen receptor hormone binding affinity in vivo. J. Biol. Chem. 271, 28697–28702 10.1074/jbc.271.45.28697 [DOI] [PubMed] [Google Scholar]
- 22. Shafi A. A., Cox M. B., and Weigel N. L. (2013) Androgen receptor splice variants are resistant to inhibitors of HSP90 and FKBP52, which alter androgen receptor activity and expression. Steroids 78, 548–554 10.1016/j.steroids.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wurtz J. M., Bourguet W., Renaud J. P., Vivat V., Chambon P., Moras D., and Gronemeyer H. (1996) A canonical structure for the ligand-binding domain of nuclear receptors. Nat. Struct. Biol. 3, 87–94 [DOI] [PubMed] [Google Scholar]
- 24. Kallenberger B. C., Love J. D., Chatterjee V. K., and Schwabe J. W. (2003) A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat. Struct. Biol. 10, 136–140 10.1038/nsb892 [DOI] [PubMed] [Google Scholar]
- 25. Gioeli D., Ficarro S. B., Kwiek J. J., Aaronson D., Hancock M., Catling A. D., White F. M., Christian R. E., Settlage R. E., Shabanowitz J., Hunt D. F., and Weber M. J. (2002) Androgen receptor phosphorylation: regulation and identification of the phosphorylation sites. J. Biol. Chem. 277, 29304–29314 10.1074/jbc.M204131200 [DOI] [PubMed] [Google Scholar]
- 26. Soroka J., Wandinger S. K., Mäusbacher N., Schreiber T., Richter K., Daub H., and Buchner J. (2012) Conformational switching of the molecular chaperone HSP90 via regulated phosphorylation. Mol. Cell. 45, 517–528 10.1016/j.molcel.2011.12.031 [DOI] [PubMed] [Google Scholar]
- 27. Eder I. E., Egger M., Neuwirt H., Seifarth C., Maddalo D., Desiniotis A., Schäfer G., Puhr M., Bektic J., Cato A. C., and Klocker H. (2013) Enhanced inhibition of prostate tumor growth by dual targeting the androgen receptor and the regulatory subunit type Iα of protein kinase A in vivo. Int. J. Mol. Sci. 14, 11942–11962 10.3390/ijms140611942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gartner E. M., Silverman P., Simon M., Flaherty L., Abrams J., Ivy P., and Lorusso P. M. (2012) A phase II study of 17-allylamino-17-demethoxygeldanamycin in metastatic or locally advanced, unresectable breast cancer. Breast Cancer Res. Treat. 131, 933–937 10.1007/s10549-011-1866-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He S., Smith D. L., Sequeira M., Sang J., Bates R. C., and Proia D. A. (2014) The HSP90 inhibitor ganetespib has chemosensitizer and radiosensitizer activity in colorectal cancer. Invest. New Drugs 32, 577–586 10.1007/s10637-014-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.