Abstract
The roles of factor XIIIa–specific cross-links in thrombus formation, regression, or probability for embolization are largely unknown. A molecular understanding of fibrin architecture at the level of these cross-links could inform the development of therapeutic strategies to prevent the sequelae of thromboembolism. Here, we present an MS-based method to map native factor XIIIa cross-links in the insoluble matrix component of whole-blood or plasma-fibrin clots and in in vivo thrombi. Using a chaotrope-insoluble digestion method and quantitative cross-linking MS, we identified the previously mapped fibrinogen peptides that are responsible for covalent D-dimer association, as well as dozens of novel cross-links in the αC region of fibrinogen α. Our findings expand the known native cross-linked species from one to over 100 and suggest distinct antiparallel registries for interprotofibril association and covalent attachment of serpins that regulate clot dissolution.
Keywords: protein cross-linking, fibrin, transglutaminase, mass spectrometry (MS), thrombosis, fibrinolysis, chemical digestion, factor XIII
Introduction
Although clot formation is critical for hemostasis, pathological thrombosis results in ischemic heart disease, stroke, and venous thromboembolism, which is responsible for at least one in four deaths globally (1). Despite a well-established understanding of the molecular cascades regulating coagulation, limited molecular information is available about the resulting insoluble fibrin matrix that helps block life-threatening hemorrhage or gives rise to deadly intravascular thrombi. A key step in clot formation is the generation of fibrin cross-links, a process that is catalyzed by the transglutaminase activity of factor XIIIa (FXIIIa).2
The αC domain of fibrinogen A (FIBA) (UniProtKB accession number P02671, residues 392–610) has been implicated as a key determinate of the stability of a fibrin clot (2). Indeed, FIBA is susceptible to limited cleavage by intracellular and extracellular proteolytic enzymes (3), is required for clot stabilization via FXIIIa (4), and is the site for cross-linking with key regulatory proteins such as α2-antiplasmin (A2AP) (5–7). The structure of human fibrinogen has been solved via X-ray crystallography (PDB code 3GHG). However, this structure is missing the disordered αC domain (Fig. 1B). This leaves the model for fibrin polymerization relatively unknown (Fig. 1, C and D). Consistently, patients with FXIII deficiency can form clots, but they are easily degraded (8). Likewise, FXIIIa activity is a critical determinant of venous thrombus composition, size, and subsequent tissue repair (4), highlighting the importance of fibrin cross-linking to regulate both the clot architecture and regulation by sequestration of plasmin and inhibitors that will influence matrix remodeling kinetics. Previous studies applied MS to identify substrates of FXIIIa (Ref. 9 and several more reviewed in Ref. 10) and to compare the reactivity of glutamine residues in the αC domain of fibrinogen A (11). Mass spectrometry has yet to be applied to identify native protein cross-link sites in a fibrin clot.
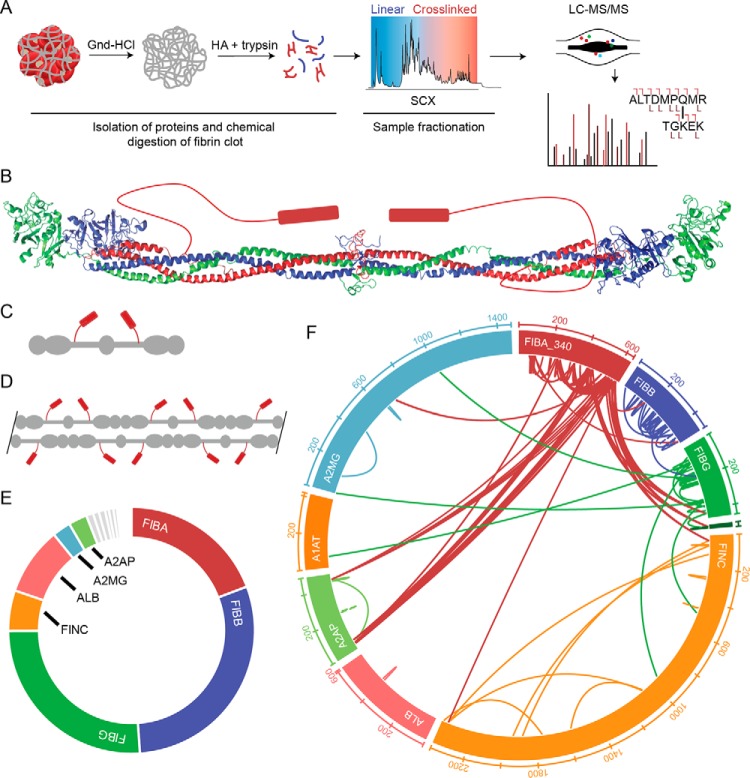
Figure 1.
Workflow and validation of clot proteomic method. A, outline of the clot processing and analysis workflow. After formation, the clot is washed extensively with the strong chaotrope guanidine (Gnd) HCl to remove components that are not covalently attached to the fibrin structure. The resulting pellet is subjected to chemical digestion with hydroxylamine (NH2OH; HA) followed by enzymatic digestion with trypsin. The resulting peptides are fractionated by SCX chromatography. Fractionated samples are analyzed by tandem MS. B, crystal structure of human fibrinogen with cartoon representation of the αC domain (PDB code 3GHG). C, cartoon model of fibrinogen structure shown in B. D, cartoon model of protofibril. E, area under the curve estimation of protein abundance in whole-blood clot. Proteomic characterization revealed 125 protein identifications at 1% FDR with the majority of signal arising from fibrin (70% total spectral counts, >80% AUC signal intensity). F, wheel diagram showing identifications and position within proteins of cross-links identified in whole-blood clot with expectation (E) value greater than 1.0E−5. A2MG, α2-macroglobulin; ALB, albumin; A1AT, α1-antitrypsin.
Cross-linking MS (CL-MS) has quickly become a widely used method to map protein–protein interactions and provide distance constraints to elucidate topology in multiprotein complexes using chemical cross-linking reagents (12, 13). This approach has been primarily used to map cross-links in isolated proteins and recently in cell culture and tissues to obtain more global information (14). The limited molecular understanding of clot structure, especially with respect to the cross-linking post-translation modifications, is in part explained by the limited availability of tools to directly analyze the insoluble fraction of protein matrices. Here, we develop an approach for the characterization of fibrin clots to obtain molecular detail regarding the specificity of FXIIIa cross-link sites and substrates.
Methods and results
Based on proteomic methods to characterize insoluble extracellular matrices (15–17) and chemical cross-links (18, 19), we developed an analytical method to characterize FXIIIa cross-links in ex vivo generated fibrin clots (Fig. 1A; detailed analytical methods are provided in the supporting methods). For development, human blood from healthy volunteer donors was allowed to clot (contact activation), and the insoluble material was isolated with strong chaotrope washes. The remaining clot was subjected to chemical digestion with hydroxylamine followed by further digestion with trypsin and fractionated by strong cation exchange (SCX) chromatography. The fractions were thus analyzed by tandem MS and bioinformatics analysis (Fig. 1A). From 125 proteins found in the insoluble fraction, we observed 61 and 193 unique intermolecular and intramolecular cross-linked peptides, respectively (Fig. 1, E and F, and supporting File 1). When the same approach was applied to plasma from the same blood sample, the chromatographic UV signal and identified cross-links were highly similar, consistent with the well-established role of plasma-derived protein components in the formation and architecture of the resulting clot matrix (Plasma Clot 1 (Plsm1) sample; supporting File 1 and Fig. 3, A and D).
Figure 3.
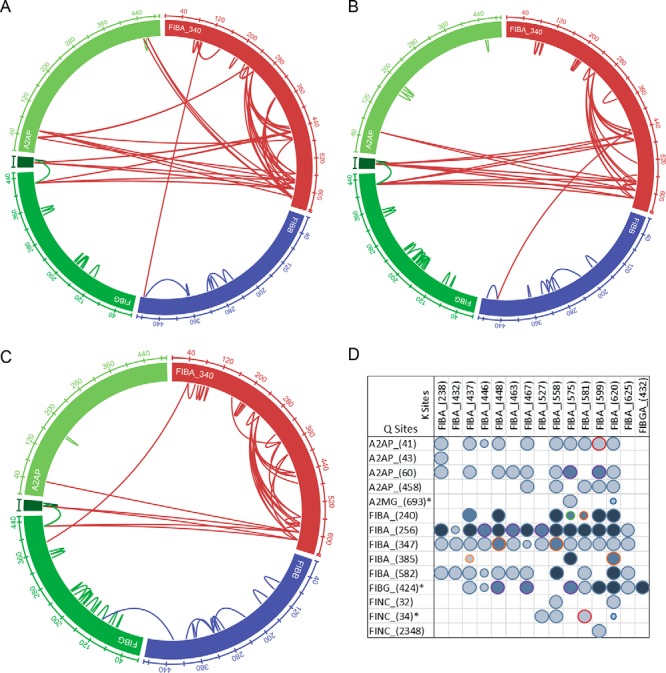
In vivo thrombus and plasma clot analyses. A, wheel diagram showing identified cross-linked peptides within the fibrin and A2AP sequences for Plsm1. B, wheel diagram showing identifications and position within proteins of cross-links identified in Plsm2. C, wheel diagram showing identifications and position within proteins of cross-links identified in the in vivo thrombus (IVT). D, cross-linking profile showing the occurrence of spectral matches for prominent cross-link sites (93 unique cross-links in total). The symbol size indicates the minimum E value for a given cross-link across all data sets: E < 1E−4, large circle; E < 0.001, medium circle; E < 0.01, small circle. The symbol color represents the number of samples in which the cross-link was identified in in-depth analysis: dark blue, in all three clot samples (WB, Plsm2, and IVT); medium blue, in two samples; light blue, in a single sample. The symbol outline represents samples in which the cross-link was identified: purple, WB and Plsm2; dark orange, WB and IVT; green, Plsm2 and IVT; light blue, WB; red, Plsm2; light orange, IVT. A2MG, α2-macroglobulin.
Both spectral matches and other label-free quantification estimates based on area under the curve indicated that fibrin proteins were the most abundant components followed by albumin and a small subset of serum proteins (Fig. 1E). Sequence coverage for the three chains of fibrinogen were almost complete (FIBA, 97%; FIBB, 94%; FIBG 90%). Most of the proteins that have been reported as substrates of FXIIIa using small-molecule tagging approaches (10) were also identified here, including PAI-1, THBS1, and FINC. In addition, abundant erythrocyte proteins (hemoglobin and cytoskeletal components) and key platelet markers (integrins and granule components) were present, highlighting the cellular components that are known to interact with the fibrin clot.
These results were used to generate a sample-specific subset database that was queried for cross-linked peptides. The majority of high-quality cross-links originated from the αC region of FIBA (UniProt P02671-2, FIBA-340) from Gln-240 to Lys-625. As previously reported, FXIIIa appears to be more selective for Gln residues within the disordered region of FIBA with Gln-240, Gln-256, Gln-347, and Gln-582 giving rise to most of the observed cross-links (10, 11). Some prominent Lys residues identified include Lys-437, Lys-448, Lys-558, Lys-575, Lys-599, and Lys-620. The identified cross-linked peptides represent an expansion on previously reported cross-linked proteins such as FIBG–FIBG (20), FIBA–A2AP (5–7), and FIBA–FINC (21, 22) with a level of molecular detail not previously available. The cross-links identified are shown (Fig. 1F) and can be accessed on the ProXL web portal (https://www.yeastrc.org/proxl_public/viewProject.do?project_id=143).3
The C-terminal γ–γ peptide responsible for the covalent attachment of FIBG globular D domains originally reported by Chen and Doolittle (20), the only identified ex vivo cross-link to date, was also identified in all samples here (Fig. 2, A and B). The mono-cross-linked form displayed a more complete fragment ion series as expected. Of interest for clinical diagnostics, these peptides form the basis of the plasmin released D-dimer product used in point-of-care settings for evaluation of thromboembolic disease and many other conditions with increased risk of thrombotic events (cancer, pregnancy, inflammatory diseases, severe renal disease, and recent surgical procedures) (23). In addition, we identified this peptide cross-linked to some of the prime lysine sites in the αC region of FIBA as reported (27). These are interesting with respect to the proposed structural models (24, 25) and represent possible branch points in the fiber architecture and interprotofibril attachment.
Figure 2.
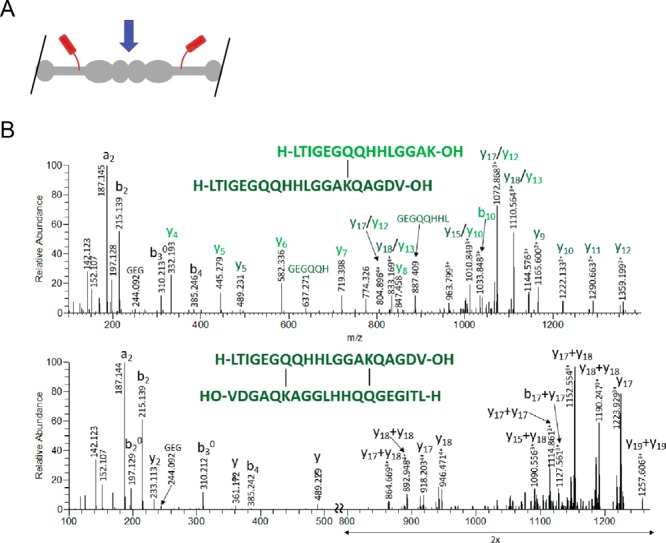
Identification of D-dimer cross-link. A, cartoon model of predicted D-dimer. The blue arrow indicates the D-dimer interface. B, the human FIBG-A isoform (P02769-2) C-terminal cross-linking sites shown for Gln-424–Lys-432 that correspond to the C-terminal γ–γ cross-linking site. Fragment ions between the cross-linked sites (di-cross-linked form; lower panel) are absent from the fragmentation spectra; these would require a minimum of two fragmentation events, which is highly unlikely.
To determine whether the method could be used to study banked clinical plasma samples, we also tested clots generated from frozen plasma in the presence of EDTA or citrate (37–54 cross-linked sites; 5% FDR). Based on more cross-links identified from citrated plasma in nonfractionated samples, we performed SCX fractionation and analysis as described for the whole-blood sample for a clot prepared from previously frozen plasma. The 125 identified cross-links were a subset of those found in the whole-blood samples, supporting the use of this technology to retrospectively analyze and identify clot mechanisms associated with clinical parameters and outcomes (Plasma Clot 2 (Plsm2); Fig. 3, B and D, and Fig. S1).
This technique can be directly applied to thrombi formed in vivo from patients to provide insight into the effect of wound and pathological environments on clot structure. We retrieved and analyzed a thrombus aspirated from a mediastinal wound during active hemorrhage immediately following cardiac surgery. Analysis of the linear peptides revealed the presence of 731 proteins. Despite the increased proteome complexity, 55 cross-links were still identified. Again, a small number of FIBA αC-region glutamine residues account for the majority of FXIIIa cross-link sites (Fig. 3, C and D), especially those with high-confidence identification. These and the corresponding most readily cross-linked lysine residues are reported (Fig. 3D). Again, these data show that the αC region provides docking for FXIIIa and covalent attachment of protease inhibitors to regulate proteolytic degradation of the resulting clot (fibrinolysis).
An estimate of the stoichiometry can be calculated by dividing the area under the curve (AUC) of a given cross-linked peptide by the sum of AUCs for the cross-link and the linear peptides it contains. This likely produces a lower-limit estimate based on the general observation of improved ionization efficiency of shorter (linear) peptides. Using the quantitative CL-MS approach (26), the C-terminal γ–γ peptide (UniProt accession number P02679-2, FIBG-A, intermolecular Gln-424–Lys-432) cross-linking was determined to be higher in the in vivo thrombus compared with the whole-blood clot (82 versus 48%). Of note, elevated levels of C-terminal γ–γ peptide cross-linking has been reported to be consistent with a stiffer clot (27). This experiment demonstrates the utility of the method for analysis of complex clinical samples where insights should lead to transformational understanding of thrombus formation and stability.
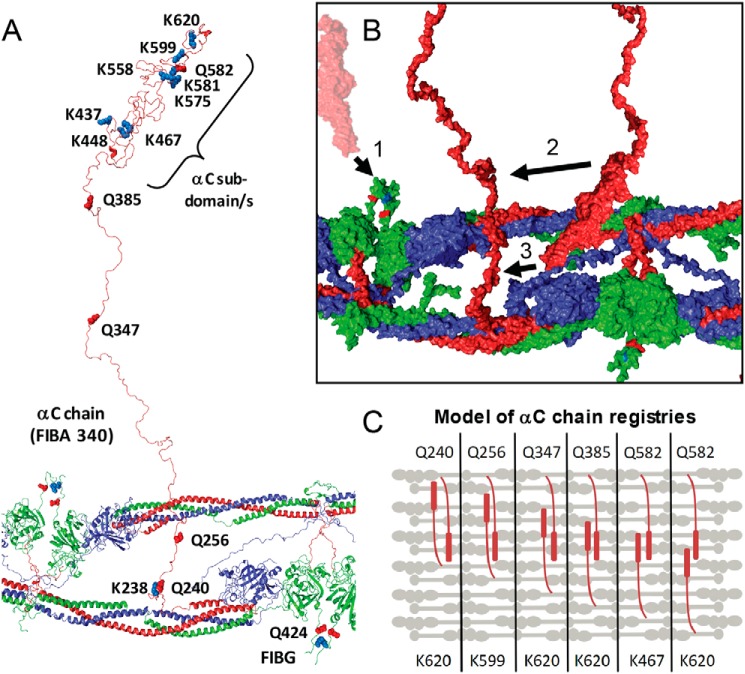
Sets of the identified cross-links are consistent with an antiparallel orientation of neighboring αC domains in specific registries (Fig. 4, B and C). These are likely to be critical for lateral aggregation of neighboring double-stranded fibrin oligomers/protofibrils. It is also likely that these are found in nonperpendicular orientations with respect to the core fibrin polymer as well (not shown). The identified cross-links will lead to increased mechanical integrity of the resulting clots due to the extended covalent polymer generated. These findings support a model of interfibrin cross-linking that depends on interactions established during fibrin protofibril assembly processes. Ultimately, the specific cross-link profile will be important for establishing biomechanical integrity and will likely regulate degradation. Quantitative comparison of these cross-links will be critical for the study of clot properties in disease states.
Figure 4.
Structural representation of FXIIIa fibrin cross-links. A, a modeled fibrin protofibril with αC chains used from Zhmurov et al. (S1.FP_9+10_AaBb.PDB PDB) (24) is shown with the αC linker and subdomain(s) labeled, consistent with the biochemical studies performed by Medved and co-workers (28). B, surface plot (using S1.FP_9+10_AaBb.PDB) showing the αC region from neighboring protofibrils with orientations for FIBG Gln-424–FIBA Lys-620 (1) cross-linking, the αC subdomain N terminus cross-linked to the C terminus of the γ-chain (2), and the αC subdomain cross-linking to the N-terminal side of the αC chain (3). C, simplified model showing multiple registries of antiparallel αC regions cross-linked with perpendicular orientation with respect to the fibrin fiber. Each panel represents a potential orientation of two αC regions spanning across several fibrin fibers.
Future efforts aimed at improved quantification through the implementation of labeling strategies and recombinant labeled standards to achieve more accurate quantification are needed. In addition, further work is required to elucidate intra- versus interprotein cross-linking. These technical developments will enable work aimed at determining the relationship between the cross-linking sites and meso-scale characteristics such as fiber diameter, density, and branching that have been correlated with clinical outcomes (4).
Conclusion
The combination of chaotrope-insoluble digestion methods with CL-MS described herein can be used to map native cross-links formed by FXIIIa in ex vivo generated blood clots and in vivo thrombi. Although numerous protein residues have been mapped as substrates of FXIIIa using small-molecule substrates, peptides, and purified proteins, this approach has expanded the known native cross-linked species from one to over 100. Through further development and future application to patient samples, opportunities exist to expand our understanding of venous and arterial thrombus structure at the protein level and should allow for the identification of novel diagnostic indicators of disordered coagulation and possibly personalized anticoagulant approaches or to predict risk of recurrence. By connecting structure to function, this technique will lead to a better understanding of hemostasis and clot formation and provide the molecular detail to improve models and understanding of thrombosis across multiple-length scales.
Author contributions
L. R. S., R. H., and A. B. data curation; L. R. S., A. B., Z. D., and A. I. formal analysis; L. R. S., R. H., and A. B. methodology; L. R. S. and A. D. writing-review and editing; R. H., Z. D., A. I., and K. C. H. visualization; Z. D. validation; A. D., N. C., and K. C. H. conceptualization; A. D., N. C., and K. C. H. supervision; N. C. and K. C. H. resources; N. C. and K. C. H. writing-original draft; K. C. H. funding acquisition; K. C. H. project administration.
Supplementary Material
Acknowledgments
We acknowledge Dr. Monika Dzieciatkowska from the Proteomics Shared Facility for technical assistance. In addition, we thank Dr. Jorge DiPaola for helpful conversations and review of the manuscript.
This work was supported in part by NCI, National Institutes of Health (NIH) Grant R33CA183685 and NIH Grant S10OD021641. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1, supporting methods, and supporting File 1 (cross-link identifications).
The mass spectrometric raw data and spectral libraries associated with this manuscript are available from ProteomeXchange with the accession number PXD012225.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- FXIIIa
- factor XIIIa
- PDB
- Protein Data Bank
- FIB
- fibrinogen
- A2AP
- α2-antiplasmin
- CL-MS
- cross-linking MS
- SCX
- strong cation exchange
- FINC
- fibronectin
- FDR
- false discovery rate
- AUC
- area under the curve
- E
- expectation
- Plsm1
- Plasma Clot 1
- Plsm2
- Plasma Clot 2
- IVT
- in vivo thrombus
- WB
- whole blood.
References
- 1. ISTH Steering Committee for World Thrombosis Day (2014) Thrombosis: a major contributor to global disease burden. Thromb. Res. 134, 931–938 10.1016/j.thromres.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 2. Collet J.-P., Moen J. L., Veklich Y. I., Gorkun O. V., Lord S. T., Montalescot G., and Weisel J. W. (2005) The αC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood 106, 3824–3830 10.1182/blood-2005-05-2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Litvinov R. I., and Weisel J. W. (2016) What is the biological and clinical relevance of fibrin? Semin. Thromb. Hemost. 42, 333–343 10.1055/s-0036-1571342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrnes J. R., and Wolberg A. S. (2016) Newly-recognized roles of factor XIII in thrombosis. Semin. Thromb. Hemost. 42, 445–454 10.1055/s-0036-1571343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Giezen J. J., Minkema J., Bouma B. N., and Jansen J. W. (1993) Cross-linking of α2-antiplasmin to fibrin is a key factor in regulating blood clot lysis: species differences. Blood Coagul. Fibrinolysis 4, 869–875 10.1097/00001721-199312000-00002 [DOI] [PubMed] [Google Scholar]
- 6. Sakata Y., and Aoki N. (1980) Cross-linking of α2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J. Clin. Investig. 65, 290–297 10.1172/JCI109671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee K. N., Lee C. S., Tae W. C., Jackson K. W., Christiansen V. J., and McKee P. A. (2000) Cross-linking of wild-type and mutant α2-antiplasmins to fibrin by activated factor XIII and by a tissue transglutaminase. J. Biol. Chem. 275, 37382–37389 10.1074/jbc.M003375200 [DOI] [PubMed] [Google Scholar]
- 8. Shen L., and Lorand L. (1983) Contribution of fibrin stabilization to clot strength. Supplementation of factor XIII-deficient plasma with the purified zymogen. J. Clin. Investig. 71, 1336–1341 10.1172/JCI110885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doiphode P. G., Malovichko M. V., Mouapi K. N., and Maurer M. C. (2014) Evaluating factor XIII specificity for glutamine-containing substrates using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay. Anal. Biochem. 457, 74–84 10.1016/j.ab.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson V. R., Cordell P., Standeven K. F., and Carter A. M. (2013) Substrates of factor XIII-A: roles in thrombosis and wound healing. Clin. Sci. 124, 123–137 10.1042/CS20120233 [DOI] [PubMed] [Google Scholar]
- 11. Mouapi K. N., Bell J. D., Smith K. A., Ariëns R. A., Philippou H., and Maurer M. C. (2016) Ranking reactive glutamines in the fibrinogen αC region that are targeted by blood coagulant factor XIII. Blood 127, 2241–2248 10.1182/blood-2015-09-672303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider M., Belsom A., and Rappsilber J. (2018) Protein tertiary structure by crosslinking/mass spectrometry. Trends Biochem. Sci. 43, 157–169 10.1016/j.tibs.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leitner A., Walzthoeni T., Kahraman A., Herzog F., Rinner O., Beck M., and Aebersold R. (2010) Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol. Cell. Proteomics 9, 1634–1649 10.1074/mcp.R000001-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chavez J. D., Lee C. F., Caudal A., Keller A., Tian R., and Bruce J. E. (2018) Chemical crosslinking mass spectrometry analysis of protein conformations and supercomplexes in heart tissue. Cell Syst. 6, 136–141.e5 10.1016/j.cels.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barrett A. S., Wither M. J., Hill R. C., Dzieciatkowska M., D'Alessandro A., Reisz J. A., and Hansen K. C. (2017) Hydroxylamine chemical digestion for insoluble extracellular matrix characterization. J. Proteome Res. 16, 4177–4184 10.1021/acs.jproteome.7b00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill R. C., Calle E. A., Dzieciatkowska M., Niklason L. E., and Hansen K. C. (2015) Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol. Cell. Proteomics 14, 961–973 10.1074/mcp.M114.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomko L. A., Hill R. C., Barrett A., Szulczewski J. M., Conklin M. W., Eliceiri K. W., Keely P. J., Hansen K. C., and Ponik S. M. (2018) Targeted matrisome analysis identifies thrombospondin-2 and tenascin-C in aligned collagen stroma from invasive breast carcinoma. Sci. Rep. 8, 12941 10.1038/s41598-018-31126-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu W. H., Roemer S. C., Zhou Y., Shen Z.-J., Dennehey B. K., Balsbaugh J. L., Liddle J. C., Nemkov T., Ahn N. G., Hansen K. C., Tyler J. K., and Churchill M. E. (2016) The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. eLife 5, e18023 10.7554/eLife.18023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu F., Rijkers D. T., Post H., and Heck A. J. (2015) Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat. Methods 12, 1179–1184 10.1038/nmeth.3603 [DOI] [PubMed] [Google Scholar]
- 20. Chen R., and Doolittle R. F. (1971) γ–γ cross-linking sites in human and bovine fibrin. Biochemistry 10, 4487–4491 10.1021/bi00800a021 [DOI] [PubMed] [Google Scholar]
- 21. Makogonenko E., Ingham K. C., and Medved L. (2007) Interaction of the fibronectin COOH-terminal Fib-2 regions with fibrin: further characterization and localization of the Fib-2-binding sites. Biochemistry 46, 5418–5426 10.1021/bi7001373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoffmann B. R., Annis D. S., and Mosher D. F. (2011) Reactivity of the N-terminal region of fibronectin protein to transglutaminase 2 and factor XIIIA. J. Biol. Chem. 286, 32220–32230 10.1074/jbc.M111.255562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thachil J., Lippi G., and Favaloro E. J. (2017) D-dimer testing: laboratory aspects and current issues. Methods Mol. Biol. 1646, 91–104 10.1007/978-1-4939-7196-1_7 [DOI] [PubMed] [Google Scholar]
- 24. Zhmurov A., Protopopova A. D., Litvinov R. I., Zhukov P., Weisel J. W., and Barsegov V. (2018) Atomic structural models of fibrin oligomers. Structure 26, 857–868.e4 10.1016/j.str.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Kempen T. H. S., Bogaerds A. C. B., Peters G. W. M., and van de Vosse F. N. (2014) A constitutive model for a maturing fibrin network. Biophys. J. 107, 504–513 10.1016/j.bpj.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Z. A., and Rappsilber J. (2019) Quantitative cross-linking/mass spectrometry to elucidate structural changes in proteins and their complexes. Nat. Protoc. 14, 171–201 10.1038/s41596-018-0089-3 [DOI] [PubMed] [Google Scholar]
- 27. Standeven K. F., Carter A. M., Grant P. J., Weisel J. W., Chernysh I., Masova L., Lord S. T., and Ariëns R. A. (2007) Functional analysis of fibrin γ-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximises clot stiffness. Blood 110, 902–907 10.1182/blood-2007-01-066837 [DOI] [PubMed] [Google Scholar]
- 28. Tsurupa G., Mahid A., Veklich Y., Weisel J. W., and Medved L. (2011) Structure, stability, and interaction of fibrin αC-domain polymers. Biochemistry 50, 8028–8037 10.1021/bi2008189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.