Abstract
Purpose:
To compare the outcomes of conventional laser photocoagulation versus additional posterior barrage laser in advanced stage 3 retinopathy of prematurity (ROP).
Methods:
A total of 20 infants with bilateral symmetric zone 2 stage 3 advancing ROP were treated with conventional laser treatment followed by randomization of one eye to receive additional posterior retinal laser treatment. Disc–fovea and inter-arcade distance was measured. The patients were followed up prospectively for 3 months. Structural and functional outcomes and safety profile were analyzed.
Results:
18/20 (90%) eyes in the study group and 19/20 (95%) eyes in the control group achieved regression of disease. Faster and complete regression was observed at 4 weeks after posterior laser compared to the control group (P = 0.024). Disc–fovea and inter-arcade distance was comparable in both groups.
Conclusion:
Additional posterior barrage laser is a safe technique that led to faster and more complete regression in eyes with advancing ROP. Final regression profile was comparable in both treatment modalities.
Keywords: Laser barrage, laser treatment, retinopathy of prematurity
Retinopathy of prematurity (ROP) is a potentially blinding proliferative retinopathy. Conventionally, treatment of ROP entails laser ablation of the avascular retina peripheral to the vascular ridge. Most cases, especially with zone 2 disease, respond very well to laser treatment. Certain cases show progression or develop sequelae requiring surgical intervention with eventual suboptimal anatomic and functional outcomes.
Additional laser application to the posterior vascular retina (barrage laser) has been reported in the literature.[1,2,3,4,5] However, most of these reports are limited to retrospective series with no definitive treatment criteria and treatment was performed in cases with progressive disease unresponsive to conventional laser treatment.
Emerging angiographic evidence suggests that occurrence of significant nonperfusion areas posterior to the ridge,[6] and treating them via posterior laser barrage can lead to faster and more complete regression. Moreover, in advancing cases, laser-induced chorioretinal adhesions can anchor retina and prevent progressive posterior retinal detachment and foveal involvement. The authors believed that as severe progressive ROP gives a very short time window for any form of treatment, a timely barrage laser may prevent further progression to tractional retinal detachment which may require surgical intervention. When used in selected zone 2 cases, the additional rows of laser are not expected to further the refractive or visual field changes. Recent reports have also expanded on this concept adding merit to this underutilized technique.[4]
Nevertheless, there remains a lack of quality data regarding indications for treatment, efficacy, and complications compared with conventional laser treatment. We report results of this prospective randomized study conducted to examine the potential benefits, safety profile and possible limitations of barrage laser technique.
Methods
A randomized interventional study was conducted after approval from the Institutional Ethics Committee with strict adherence to the Declaration of Helsinki at a tertiary care eye hospital in North India. Informed consent was obtained from parents/legal guardians before recruitment.
All premature infants with gestational age <34 weeks and birth weight <2000 g were screened for ROP over a 1-year period (October 2012 to October 2013). Infants with bilateral symmetric type 1 ROP (in zone 2 with stage 3 and “plus” disease) of more than four clock hours of temporal ridge with developing traction and high risk of progression to stage 4 were recruited. Both treatment naïve and recently laser treated cases (<3 weeks duration) that met the inclusion criteria were included, provided the postmenstrual age (PMA) was <45 weeks. Exclusion criteria were babies with zone 1 or zone 3 disease, advanced stages (4 and 5) of ROP, and presence of media opacity.
Relevant antenatal, perinatal, postnatal, and maternal history was documented. A baseline anterior segment and dilated fundus examination using binocular indirect ophthalmoscope was performed along with Retcam 3 (Clarity Medical Systems, Inc., Pleasanton, CA, USA) widefield fundus imaging. Disease was classified based on International Classification of ROP.[7] A baseline refraction was performed before the laser procedure.
Laser treatment was performed using frequency-doubled Nd:YAG (532 nm) green laser with an indirect laser delivery system within 48 hours of presentation. In treatment-naïve cases, conventional laser treatment was first performed in both eyes with near confluent laser spots being applied to all areas of peripheral avascular retina using a 28D (Volk, Mentor, OH) condensing lens. Later, one eye was randomized to receive additional posterior barrage laser. In previously laser treated cases, laser augmentation was first performed to all skip areas, whenever required. Only cases that showed progression even after complete laser treatment were included in the study and one eye was randomized to receive barrage laser. While performing barrage laser, three to four rows of laser spots encompassing all clock hours of traction were applied about one-half disc diameters from the posterior edge of the ridge with interspersed spots sparing the retinal vessels [Fig. 1a], and were made congruent with peripheral laser at the edges of the traction. A 20D (Volk, Mentor, OH) condensing lens was preferred over a 28D lens for better magnification and treatment accuracy.
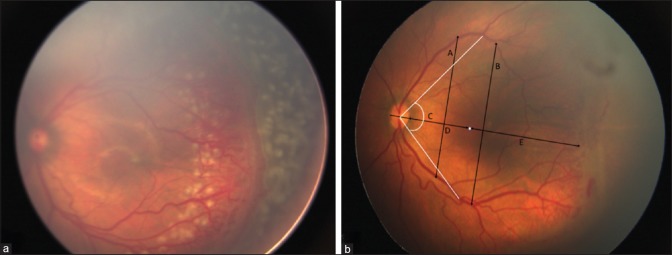
Figure 1.
(a) Technique of barrage laser. Left eye of a 34-week post menstrual age baby showing zone 2 posterior stage 3 ROP with plus disease. Posterior barrage laser spots are visible in between the tortuous vessels. (b) Image analysis: Fovea is represented by the white dot. A - Inter-arcade distance at 2 disc diameter from the temporal edge of disc. B - Inter-arcade distance at 4 disc diameter from the temporal edge of the disc. C – Angle subtended by the temporal vessels from the fovea at the center of the disc. D - Disc–fovea distance. E - Fovea–ridge distance. D + E - Disc–ridge distance
Patients were followed up weekly till resolution of disease and then monthly for 3 months. Photographic documentation was performed at all visits and refraction under cycloplegia was performed at 4 and 12 weeks.
Measurements were performed using Image J software (NIH, USA) by a single observer, though a second observer was reserved for doubtful cases. Only clear and well-focused images were used for analysis. The study parameters included:
Disease regression—defined as normalization of plus disease and disappearance of the ridge. Photographic documentation was performed on predefined follow-up time points at 1, 2, 4, 8, and 12 weeks from the day of posterior barrage laser.
Disc–fovea drag—The disc–fovea distance, fovea–ridge distance, and the disc–ridge distance were measured from the temporal edge of the optic disc on a straight line joining the center of the disc, the center of the fovea, and the vascular ridge [Fig. 1b].
Constriction of temporal vascular arcades— Inter-arcade distance at 2 and 4 disc diameters from the temporal disc edge and the angle subtended by the major vascular arcade at the fovea were used to determine the constriction of arcades [Fig. 1b].
Statistical analysis was performed using SPSS ver. 20 (SPSS Inc., Chicago, IL, USA). Independent samples t-test and paired t-test were used for intergroup and intragroup analysis at baseline and 3 months, respectively. P value of < 0.05 was considered significant.
Results
A total of 562 babies were screened at our ROP clinic during the study period. ROP was diagnosed in 143 babies, and 62 babies were classified as type 1 ROP babies out of which 22 babies were recruited. Two babies were lost to follow-up and were removed from the study.
The mean gestational age of the patients was 28 ± 2.5 weeks (25–34 weeks) and the mean PMA at presentation was 35.9 ± 3.08 weeks (30–42 weeks). Mean birth weight was 1146.65 ± 241 g (825–1580 g). Six (30%) babies were male and 14 (70%) were female; 12 (60%) had vaginal births and 8 (40%) required lower segment cesarean section; 11 (55%) babies were singleton and 9 (45%) were twins. Neonatal risk factors such as presence of septicemia (13) followed by RDS (12) and jaundice (8) were commonly seen.
A total of 14 babies (70%) had earlier received conventional laser treatment from elsewhere and additional barrage laser was performed after a mean duration of 2.7 weeks. Six babies (30%) received both laser treatments together. Three to four clock hours of neovascular ridge and traction were present in 70% (n = 14) cases, 7–9 clock hours in 15% (n = 3), and >9 clock hours in another 15% (n = 3).
Regression of ridge in both height and width was seen in 18 eyes (90%) in the barrage laser group and in 19 eyes (95%) in the conventional laser group (P = 0.995). The time taken to disease regression was 4.77 ± 2.48 (n = 18) in the barrage laser group and 5.37 ± 2.83 weeks (n = 19) in the conventional laser group (P = 0.441). However, more eyes achieved a faster regression at 2 weeks (P = 0.184) and 4 weeks (P = 0.024) time points in the barrage laser group [Fig. 2]. In regressed cases, two eyes in the conventional laser group showed residual ridge elements at the last follow-up, which was not seen in the barrage laser group [Fig. 3].
Figure 2.
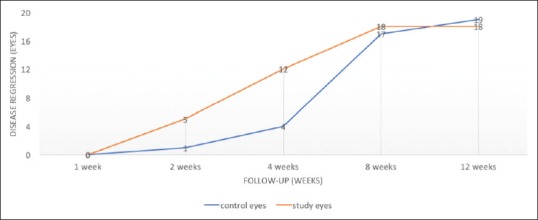
Number of eyes achieving regression in both groups. More eyes achieved regression in barrage laser group at 2 weeks (P = 0.184) and 4 weeks (P = 0.024) compared to standard laser group
Figure 3.
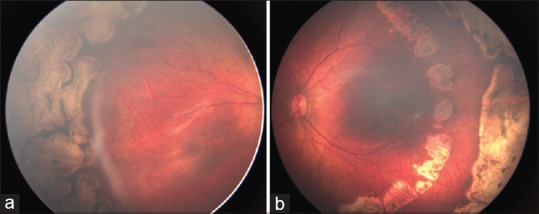
(a) At 3 months follow-up, ridge remnants persist in the right eye. (b) The left eye shows barrage laser scars with complete ridge regression
Two eyes from one patient and one eye from another patient showed disease progression to stage 4A ROP; however, in the barrage laser group the peripheral retinal detachment was limited by chorioretinal adhesions posterior to the ridge [Fig. 4].
Figure 4.
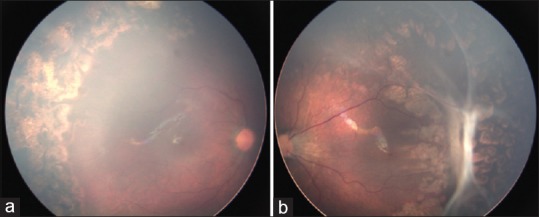
(a) 3 months after laser, right eye shows uneventful regression; (b) Left eye has developed tractional detachment that is limited by barrage laser scars. Significant constriction of temporal arcades and foveal drag denoted by increased disc fovea distance is also noted
The disc–fovea distance, fovea–ridge distance, and the disc–ridge did not show significant change from baseline in either group till the last follow-up. However, compared to baseline, significant constriction of arcades was seen in both groups at 3 months [Table 1].
Table 1.
Comparison of baseline and 3-months post laser data between groups using Independent samples t-test and intragroup using student t-test
| Parameter | Baseline (mm; mean±SD) | 3-months follow-up (mm; mean±SD) | Intragroup comparison: Baseline vs. 3 months | |||||
|---|---|---|---|---|---|---|---|---|
| Barrage laser (n=19) | Standard laser (n=19) | P | Barrage laser (n=19) | Standard laser (n=19) | P | Barrage Laser P (n=19) | Standard laser P (n=19) | |
| Disc fovea distance | 4.532±0.591 | 4.392±0.696 | 0.421 | 4.602±0.632 | 4.274±0.616 | 0.113 | 0.415 | 0.317 |
| Fovea ridge distance | 8.158±1.972 | 9.192±1.64 | 0.087 | 8.484±2.02 | 9.137±1.753 | 0.294 | 0.098 | 0.801 |
| Disc ridge distance | 12.726±2.18 | 13.585±1.965 | 0.21 | 13.086±2.37 | 13.421±2.05 | 0.645 | 0.125 | 0.585 |
| IAD at 2DD | 7.049±1.26 | 7.188±1.054 | 0.714 | 6.439±1.41 | 7.111±1.085 | 0.109 | 0.001 | 0.449 |
| IAD at 4 DD | 8.297±1.39 | 8.486±1.145 | 0.65 | 7.49±1.56 | 7.724±1.244 | 0.613 | 0.0004 | 0.0001 |
| IAA at fovea* (degrees) | 94.89±13.52 | 96.561±7.784 | 0.643 | 89.282±16.59 | 94.588±8.876 | 0.227 | 0.004 | 0.041 |
IAD – Inter-arcade distance, IAA – Inter-arcade angle, DD – Disc diameters; *Inter-arcade angle measured in degrees, rest measurements in millimeters. Data analyzed using Independent samples t-test and student t test
Both the treatment modalities showed similar levels of myopic shift at 3 months follow-up (study group = 2.68 ± 0.744D; control group = 2.53 ± 0.497D, P = 0.837). There were no systemic adverse events in any case.
Discussion
The principle of laser treatment in ROP is ablation of avascular retina that serves as a source of angiogenic mediators. This treatment has been so successful that to date it remains the gold standard for treating ROP. However, final anatomic and functional outcome depends on the stage and severity of disease.[8]
In our study, 95% eyes in the conventional laser group and 90% eyes in the barrage laser group achieved complete disease regression. Two eyes in barrage laser group developed tractional detachment which was limited by the barrage laser scars, sparing the macula, unlike the conventional laser treated eye where detachment extended posteriorly. However, severe vascular arcade constriction and foveal drag occurred in all these cases highlighting the inability of barrage laser in preventing tractional sequelae.
Our results are consistent with previous reports in the literature. O’Keefe et al.[1] described barrage laser as a rescue measure in their retrospective series which included six eyes from four infants with stage 4A and two eyes from one infant in advancing stage 3+ disease. Six of these eyes had total regression and two showed residual peripheral traction, but all eyes had flat maculae and vision was maintained. Similar success rates were reported by Axer-Siegel et al.[2] and Arvas et al.[5] in their retrospective series. However, none of these reports delved into treatment guidelines; the decision to perform the technique was at the discretion of the treating surgeon.
Ells et al.[4] advocated barrage laser in treatment-naïve cases with more than four clock hours of thick neovascular ridge, two clock hours of temporal “plus” disease, subretinal fluid associated with neovascular ridge, minimal temporal traction, and a minimum safe distance of 3000 microns from the macula. For secondary treatment, disease progression after at least 2 weeks from primary laser was added as a criterion. This study, however, was also retrospective in nature and lacked a control group.
In our study, we noted that all eyes with four to six clock hours of ridge with traction regressed uneventfully in both treatment groups. More so, 9 of 12 eyes (five in conventional group and four in barrage group) that had more than seven clock hours of thick neovascular ridge and traction underwent uneventful regression. Thus, it seems difficult to identify the ideal case for barrage laser based on clock hours of neovascular ridge and severity of plus disease. The technique may actually be helpful in early stage 4 cases, as it did prevent macular detachment. However, such cases present a potential risk of laser-induced retinal break formation at the base of tractional detachment and were thus not included in the study.
Ells et al.[4] also noted a trend toward faster ridge regression in eyes with barrage laser within one week of laser that was defined as decrease in thickness and vascularity of the ridge and severity of plus disease. However, they found persistent atrophic membranes up to 4 weeks after laser. In our study, the mean time to regression between the two groups was not found to be statistically significant (P = 0.441), although, at 4-week follow-up, more eyes had achieved regression in the barrage laser group compared to conventionally treated eyes (P = 0.024) and any treatment aiding in faster regression can be a huge benefit in cases with rapidly progressing ROP. Also, the presence of fibrovascular ridge remnants was found only in two eyes in the conventional laser group which may signify a more complete regression pattern in the barrage laser-treated eyes. These membranes may cause persistent traction on the retina and could lead to the development of late-onset retinal detachments.[9] Complete regression may also help prevent future complications such as retinal break formation.[10]
The vascularized retina adjacent to the ridge is a region of high metabolic activity and is a potential source of vascular endothelial growth factor (VEGF). Performing laser photocoagulation at this region could lead to decrease in VEGF levels. Laser also causes increased local oxygenation that might help further in decreasing the VEGF load.[11] These biochemical changes may explain the faster regression seen with barrage laser in our series. Ells et al.[4] have also hypothesized different mechanisms to explain this phenomenon that include damage to retinal pigment epithelial barrier leading to resorption of subretinal fluid, photocoagulation destroying vasogenic cells and decreasing VEGF production, and destruction of posterior capillary nonperfusion areas acting as a source of VEGF. Fluorescein angiographic studies have demonstrated frequent occurrence of nonperfusion areas posterior to the ridge in vascular retina[6]; therefore, laser treatment of these areas is expected to lead to faster and more complete regression.
Laser to the vascular ridge has also been described for advancing ROP without complications.[12,13] We, however, did not perform laser over the ridge. The vascular nature of the ridge poses inherent risks for significant hemorrhage via direct laser injury and resultant difficulty in completion of laser treatment and/or later possible need for surgery. In fact, we found posterior barrage laser to be a challenging technique as laser spots have to be applied in between the dilated and tortuous vessels near the ridge. The proximity to the macular region in certain cases poses additional difficulties and risk of foveal injury, and hence, posterior laser is not recommended in zone 1/zone 2 posterior disease. We did not encounter any adverse events while performing barrage laser near the ridge. Using a 20D condensing lens in place of a 28D lens was found to be more suited as it provides greater magnification for better accuracy of the additional procedure. While three to four posterior laser rows were applied as a protocol for this study, possibly a larger number of laser rows may be applied in cases with higher strength of tractional force and wider width of extraretinal fibrovascular tissue in more advanced cases. It is suggested that posterior laser barrage be performed by trained experts to avoid inadvertent laser injury to the vascular ridge and vessels and prevent posterior laser extension.
We performed morphometric analysis of RetCam images to objectively assess foveal drag and constriction of temporal vascular arcades. Both these parameters describe disease regression sequelae associated with poor functional outcome in the long run. We did not find significant change in the disc–fovea distance in both the groups at the 3 months follow-up, signifying maintenance of anatomic relationship [Table 1]. Both laser groups also showed significant constriction of angle of insertion of major temporal vessels at last follow-up, although the mean values lay in the acceptable range.[14] Our findings reiterate that constriction of vascular arcades is a normal process in the course of ROP regression and suggest that neither conventional laser nor additional barrage laser induces constriction that is significantly a cause of concern.
Myopia is another common association of ROP.[15] It is attributed to differential development of anterior segment structures rather than axial elongation. Greater lens thickness and shallow anterior chamber depth is commonly seen in preterm babies with the history of ROP than in normal term babies accounting for the myopia.[16] Laser-treated babies tend to develop more myopia compared to babies where disease regressed without intervention.[17] In our study, we did not find significant difference in refractive change between the two groups at 3 months follow-up, possibly because of the small area of additional laser. Similarly, it is not expected that few additional laser rows will cause significant visual field loss in larger zone disease.
Our study is limited by a small sample size, exclusion of cases with early stage 4 disease, a relatively short duration of follow-up, and represents early functional outcomes. Additional effects such as induction of myopia and significance of loss of visual fields, if any, due to additional posterior laser require a long-term evaluation.
Conclusion
This is the first prospective study describing the barrage laser technique. Barrage laser showed similar rates of regression as conventional laser treatment and morphometric analysis did not show any additional changes on disc–fovea drag and angle of insertion of vascular arcades compared to conventional laser. In situations where disease progression was noted, barrage laser scars protected the macula from progressive detachment, although, the natural course of disease was not altered once the stage of sequelae sets in. The technique led to faster and more complete regression of disease, which is beneficial in advancing cases and may prevent occurrence of retinal detachment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.O’Keefe M, Burke J, Algawi K, Goggin M. Diode laser photocoagulation to the vascular retina for progressively advancing retinopathy of prematurity. Br J Ophthalmol. 1995;79:1012–4. doi: 10.1136/bjo.79.11.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axer-Siegel R, Snir M, Cotlear D, Maayan A, Frilling R, Rosenbaltt I, et al. Diode laser treatment of posterior retinopathy of prematurity. Br J Ophthalmol. 2000;84:1383–6. doi: 10.1136/bjo.84.12.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axer-Siegel R, Maharshak I, Snir M, Friling R, Ehrlich R, Sherf I, et al. Diode laser treatment of retinopathy of prematurity: Anatomical and refractive outcomes. Retina Phila Pa. 2008;28:839–46. doi: 10.1097/IAE.0b013e318169faee. [DOI] [PubMed] [Google Scholar]
- 4.Ells AL, Gole GA, Lloyd Hildebrand P, Ingram A, Wilson CM, Geoff Williams R. Posterior to the ridge laser treatment for severe stage 3 retinopathy of prematurity. Eye. 2013;27:525–30. doi: 10.1038/eye.2012.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvas S, Sarici AM, Akar S. Diode laser photocoagulation posterior to the ridge in severe stage 3+ threshold retinopathy of prematurity. Cutan Ocul Toxicol. 2014;33:197–200. doi: 10.3109/15569527.2013.832687. [DOI] [PubMed] [Google Scholar]
- 6.Lepore D, Molle F, Pagliara MM, Baldascino A, Angora C, Sammartino M, et al. Atlas of fluorescein angiographic findings in eyes undergoing laser for retinopathy of prematurity. Ophthalmology. 2011;118:168–75. doi: 10.1016/j.ophtha.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 7.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 1960. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 8.Good WV Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48. discussion 248-250. [PMC free article] [PubMed] [Google Scholar]
- 9.Park KH, Hwang J-M, Choi MY, Yu YS, Chung H. Retinal detachment of regressed retinopathy of prematurity in children aged 2 to 15 years. Retina Phila Pa. 2004;24:368–75. doi: 10.1097/00006982-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Chandra P, Tewari R, Salunkhe N, Kumawat D, Kumar V. Giant retinal tear with retinal detachment in regressed aggressive posterior retinopathy of prematurity treated by laser. J Pediatr Ophthalmol Strabismus. 2017;54:e34–6. doi: 10.3928/01913913-20170531-01. [DOI] [PubMed] [Google Scholar]
- 11.Augustin AJ, Keller A, Koch F, Jurklies B, Dick B. [Effect of retinal coagulation status on oxidative metabolite and VEGF in 208 patients with proliferative diabetic retinopathy] Klin Monbl Augenheilkd. 2001;218:89–94. doi: 10.1055/s-2001-12251. [DOI] [PubMed] [Google Scholar]
- 12.Steinmetz RL, Brooks HL. Diode laser photocoagulation to the ridge and avascular retina in threshold retinopathy of prematurity. Retina Phila Pa. 2002;22:48–52. doi: 10.1097/00006982-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Uparkar M, Sen P, Rawal A, Agarwal S, Khan B, Gopal L. Laser photocoagulation (810 nm diode) for threshold retinopathy of prematurity: A prospective randomized pilot study of treatment to ridge and avascular retina versus avascular retina alone. Int Ophthalmol. 2011;31:3–8. doi: 10.1007/s10792-010-9411-y. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C, Theodorou M, Cocker KD, Fielder AR. The temporal retinal vessel angle and infants born preterm. Br J Ophthalmol. 2006;90:702–4. doi: 10.1136/bjo.2005.085019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Valenzuela E, Kaufman LM. High myopia associated with retinopathy of prematurity is primarily lenticular. J AAPOS. 2005;9:121–8. doi: 10.1016/j.jaapos.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Chang SHL, Lee YS, Wu SC, See LC, Chung CC, Yang ML, et al. Anterior chamber angle and anterior segment structure of eyes in children with early stages of retinopathy of prematurity. Am J Ophthalmol. 2017;179:46–54. doi: 10.1016/j.ajo.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Davitt BV, Dobson V, Good WV, Hardy RJ, Quinn GE, Siatkowski RM, et al. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology. 2005;112:1564–8. doi: 10.1016/j.ophtha.2005.03.025. [DOI] [PubMed] [Google Scholar]