Abstract
Coats disease is an idiopathic retinal vascular disorder with retinal telangiectasia with intraretinal and/or subretinal exudation without appreciable retinal or vitreal traction. The condition is sporadic with no associated systemic abnormalities. Unilateral involvement in young males is the typical presentation with most cases being diagnosed in the first and second decade of life. Younger the patient, more severe is the presentation and poorer the visual outcome. The management varies with the stage of the disease. Over the years, we have shifted from enucleation to a more conservative approach for the treatment of Coats disease with laser photocoagulation, cryotherapy and surgery for retinal detachment achieving good outcomes. The anti-VEGF agents have come into the scene as important form of adjuvant treatment along with the traditional management options. This article describes the clinical features, underlying pathology, classification and staging, the complications and the management of Coats disease and gives an overview of the changing trends in treatment and outcomes spanning across five decades.
Keywords: Coats disease, exudation, retina, telangiectasia
Coats disease, a clinical entity known from early 1900s, remains a diagnostic challenge because of its varied presentation and resemblance to other vascular and exudative retinopathies. With understanding of the classical clinical features, one can confidently diagnose it in most cases and ancillary investigations are helpful to confirm diagnosis.
George Coats first described this condition in 1908 as unilateral retinal vascular abnormality with exudation occurring in young males.[1] Subsequently, Leber reported another entity characterized by multiple retinal aneurysms, associated with retinal degeneration.[2] In 1955, Reese described the similarities between Coats disease and Leber miliary aneurysms and concluded that they represent a spectrum of the same disease with the underlying pathology being retinal telangiectasia leading to progressive exudation and retinal detachment.[3]
Coats disease or Coats “response” has been inappropriately used as a blanket term for fundus changes seen in a variety of exudative retinopathies.[4,5] These include branch retinal vein occlusion,[6] retinitis pigmentosa,[7,8,9,10,11,12] ocular toxoplasmosis,[13] morning glory anomaly,[14] retinal macroaneurysms, retinal capillary hemangiomatosis, familial exudative retinopathy and retinal vasoproliferative tumors.[15] It has also been used for retinal findings in some systemic syndromes like muscular dystrophy,[16,17,18] Turner syndrome,[19] epidermal nevus syndrome,[20] Cornelia de Lange syndrome,[21] Alport syndrome,[22] Senor--Loken syndrome,[23] and Hallermann--Streiff syndrome.[24] It is, therefore, imperative to differentiate these exudative retinopathies from true Coats disease in order to reduce the prevailing confusion regarding the disease.
Shields et al., in the 2000 Sanford Gifford Memorial Lecture, described the clinical features, natural course, and complications of Coats disease.[15] The authors used strict guidelines to diagnose Coats disease and differentiate from other simulating conditions in their large series of 150 cases. They defined Coats disease as idiopathic retinal telangiectasia with intraretinal and/or subretinal exudation without appreciable retinal or vitreal traction. Retinal telangiectasia was defined as dilated, irregular caliber, small- to medium-sized retinal blood vessels. The term exudative retinopathy was used for yellow exudation in the sensory retina or subretinal space.
In this review, we elucidate the clinical features, natural history, and complications of Coats disease with an insight into newer treatment modalities and advancements in recent years.
Clinical Features
Coats disease typically occurs in young males and is diagnosed in the first or second decades of life. Coats disease can also occur in adults with findings being similar to those in children, but often less advanced. We caution that other causes of exudative retinopathy should be excluded before diagnosing Coats disease in adults. Most authorities believe that older patients with Coats disease likely represent cases which were asymptomatic in childhood and, therefore, diagnosed at an older age.
Coats disease is typically unilateral (95--100%).[15,25,26] True cases of bilateral Coats disease are rare, with the other eye being asymptomatic and showing subtle peripheral telangiectatic vessels. Male predominance and unilaterality are consistent features in the literature with no specific explanation.[25,26,27,28,29,30] In a recent large analysis on 351 consecutive cases of Coats disease from a single center, 295 (84%) were male and 351 (100%) were unilateral.[26] Using strict definition of Coats disease, this condition is sporadic and nonhereditary with no associated systemic abnormalities or racial predilection.
Patients can present with an array of findings including vision loss, strabismus, xanthocoria, nystagmus or pain. Despite this being primarily a retinal vascular abnormality, anterior segment findings can be noted including corneal edema, megalocornea, shallow anterior chamber, anterior chamber cholesterolosis, neovascularization of iris and resultant iris heterochromia, and cataract. In some cases, this condition can remain asymptomatic and is diagnosed on fundus examination, especially in older patients. Dalvin et al. reviewed clinical features by patient age (≤ 3 vs. > 3-10 vs. >10 years) in 351 cases (105 vs. 122 vs. 124) and noted the mean age of presentation (2 vs. 6 vs. 27 years) and unilaterality (100% in each group).[25] The youngest patients were more commonly referred as possible retinoblastoma (29% vs. 15% vs. 0%, P < 0.001), had worse presenting visual acuity (<20/200: 80% vs. 67% vs. 31%, P < 0.001) and more anterior segment signs like xanthocoria (68% vs. 44% vs. 6%, P < 0.001), strabismus (50% vs. 36% vs. 11%, P < 0.001), and iris neovascularization (12% vs. 76% vs. 3%, P = 0.02).[25] The youngest age group also had more advanced stage of Coats (stage 3B: 65% vs. 38% vs. 10%, P < 0.001) with greater clock hours of telangiectasia (7 vs. 5 vs. 4, P < 0.001), light bulb aneurysms (7 vs. 4 vs. 3, P < 0.001), exudation (10 vs. 7 vs. 5, P < 0.001), and subretinal fluid (10 vs. 7 vs. 4, P < 0.001).[25] Other studies done in United Kingdom have shown similar results with younger patients having more severe disease, poorer visual outcome, and more commonly requiring primary enucleation.[31,32]
Visual acuity can vary from 20/20 to no perception of light with most patients suffering from severe visual loss. At presentation, Shields et al. documented visual acuity in 351 cases and noted the presenting verbal visual acuity to be 20/40 (22%), 20/50--20/200 (30%) or <20/200 (49%).[26] The main causes of poor vision in Coats relate to presence of subfoveal fluid or exudation, subfoveal fibrosis or hemorrhage, macular edema, development of epiretinal membrane, or optic atrophy.[33]
The gold standard for diagnosis of Coats disease is examination of the fundus by indirect ophthalmoscopy. Retinal telangiectasia is found in all cases. These have been described as “light bulb telangiectasias” because of the bulbous terminal configuration and associated extensive yellow exudation.[34] These abnormal vessels are usually found in the peripheral zones between the equator to ora serrata and are fusiform in shape.[33] The zone between the equator and vascular arcades are affected in about one-third cases.[15] Macular telangiectatic vessels are rare. The inferior and temporal quadrants are most commonly involved.[15] In some patients, more than one zone or quadrant is affected with a diffuse distribution.[15] Intraretinal exudation is present in almost all cases and can be widespread and away from the telangiectasia. The exudation tends to gravitate toward the macula.[27,28,29,30] A dense macular exudate or a fibroglial nodule predicts poor visual prognosis despite adequate treatment.[33] Other finding in the posterior pole includes subtotal or total exudative retinal detachment. In a third of the patients, more than six clock hours of retinal detachment is present.[15] The extent of retinal detachment has been observed to be more in younger children, the worse being in those less than 3 years of age.[15,25] Retinal hemorrhage, although unusual, may be found close to the vascular abnormality. Retinal macrocysts, vasoproliferative tumor, and vitreous hemorrhage are complications resulting from long standing retinal detachment.[15]
Pathogenesis
There are two factors which contribute to the development of features that are described in this condition. First is the breakdown of blood retinal barrier due to changes at the endothelial level of the retinal vasculature. Plasma leaks into the vessel wall resulting in thickening and “sausage-like” shape of vessels[35] [Fig. 1]. The second is the presence of abnormal pericytes, which along with the damaged endothelium causes bulging of the vessels and the characteristic telangiectasia.[36] The damaged vasculature often rests in a bed of relative retinal ischemia. Exudation of lipids from these vessels is responsible for retinal detachment, retinal thickening, and retinal cyst formation.[36,37]
Figure 1.
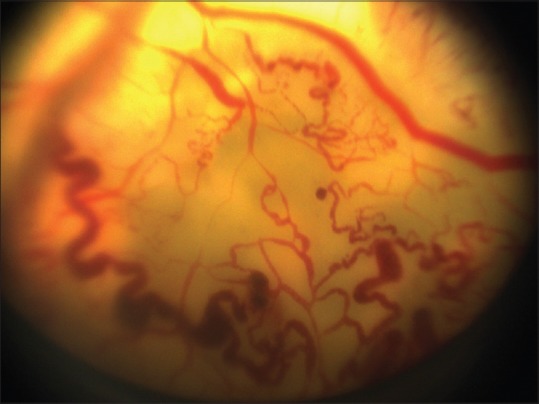
Retinal telangiectasia and subretinal exudation characteristic of Coats disease
Complications
Most of the complications are secondary to chronic retinal detachment. Patients can have neovascular glaucoma with iris and angle neovascularization. These patients may present with a painful blind eye and often require primary enucleation. Anterior chamber cholesterolosis is an unusual complication which develops most likely as a result of migration of cholesterol crystals from the exudates in the subretinal space into the anterior chamber possibly through a retinal dialysis.[38] Fluorescein angiography has shown capillary nonperfusion areas around the telangiectatic vessels. Despite the ischemic nature of the disease, disc and retinal neovascularization are uncommon. This can also explain the low incidence of retinal and vitreous hemorrhage as complications of the disease (3%)[15] [Fig. 2]. Retinal macrocysts are degenerative changes. Longer the duration of retinal detachment, more likely is the finding of retinal macrocysts. Vasoproliferative tumor is a vascular mass in the fundus developing in response to chronic retinal detachment.
Figure 2.
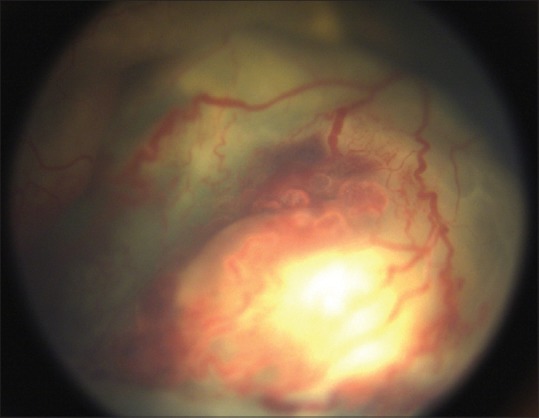
Advanced Coats with total exudative retinal detachment and intraretinal hemorrhage around peripheral telangiectatic vessels
Differential Diagnosis
About half of the cases of Coats disease are initially diagnosed as a different pathology, mostly retinoblastoma. In an analysis of 2,781 patients referred as retinoblastoma, 22% had pseudoretinoblastoma.[39] Among these, 40% had Coats disease, 28% were cases of persistent fetal vasculature, and 5% had vitreous hemorrhage. The most important differential is retinoblastoma with serious clinical implications.[39] Although retinoblastoma is the most common intraocular malignancy in children, it is also the most common misdiagnosis leading to enucleation in a case of Coats disease. Coats disease has no positive family history which may be relevant in a familial retinoblastoma. Leukocoria in Coats disease is because of exudative retinal detachment. This may reach up to the posterior capsule of lens and has a bright yellow or yellow--orange hue (xanthocoria) with characteristic overlying dilated blood vessel [Fig. 3]. This is different from the grey--white appearance at the pupil (leukocoria) in a case of retinoblastoma. On ultrasound, linear echo indicating retinal detachment can be seen in advanced cases. The subretinal fluid in Coats is acoustically clear but medium reflective vitreous echoes can be there. These are because of diffuse subretinal cholesterolosis and should not be misread as calcification, which are dense echoes on A scan. A solid mass with orbital shadowing is almost never seen in Coats.[40] In advanced cases, osseous metaplasia of retinal pigment epithelium can simulate calcium in retinoblastoma due to the high echogenicity. But calcium in retinoblastoma is randomly distributed while in Coats, it has a curvilinear pattern.
Figure 3.
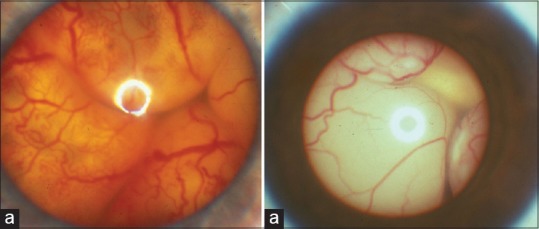
Pupillary reflex in Coats and retinoblastoma. (a) Xanthocoria in a child with Coats disease. (b) leukocoria in a child with endophytic retinoblastoma
There is no history of prematurity or family history and vitreoretinal and retinal drag are not seen except in rare, untreated cases. This helps in differentiating Coats from other exudative retinopathies in children like retinopathy of prematurity, familial exudative retinopathy, and Norrie disease. Other differentials include other causes of leukocoria in children---retinal hemorrhage, hemangioblastoma, toxocariasis, persistent fetal vasculature, choroidal hemangioma, coloboma, endophthalmitis, cytomegalovirus retinitis, and toxoplasmosis.[15]
The three classical features that clinches the diagnosis of Coats are exudative retinal detachment, irregularly-dilated telangiectatic vessels, and peripheral nonperfusion.[39,41]
Investigations
There are several methods to distinguish Coats disease from simulating conditions.[39] Ultrasound features have been described above and are helpful in differentiating Coats disease from retinoblastoma. Presence and extent of exudative retinal detachment, retinal elevation, and subretinal exudation are also seen on ultrasound. Fluorescein angiography shows hyperfluorescence of the retinal telangiectasia in the venous phase, areas of capillary dropouts, early hypofluorescence in the areas of retinal exudation with late staining and late leakage from the abnormal vessels, macular edema and neovascularization of disc retina and iris.[26] Optical coherence tomography (OCT) helps in documentation of cystoid macular edema, epiretinal membrane, vitreomacular traction, subretinal/intraretinal fluid, central macular thickness, and subfoveal choroidal thickness[26] [Fig. 4]. Computed tomography (CT) is useful in ruling out retinoblastoma which would have a solid tumor with calcification, but it becomes confounding in cases of retinoblastoma without calcification.[42] In advanced cases of Coats, bone formation along vessel walls and calcified submacular nodule can show up as areas of calcification in a CT scan in up to 20% of the cases.[43,44,45] Magnetic resonance imaging (MRI) is more useful in aiding diagnosis in advanced Coats. The exudate is hyperintense on both T1 and T2 whereas in retinoblastoma the tumor is hyperintense on T1 but hypointense on T2 and shows enhancement with gadolinium contrast.
Figure 4.
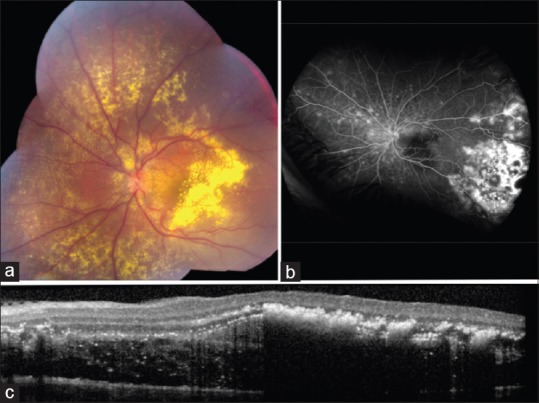
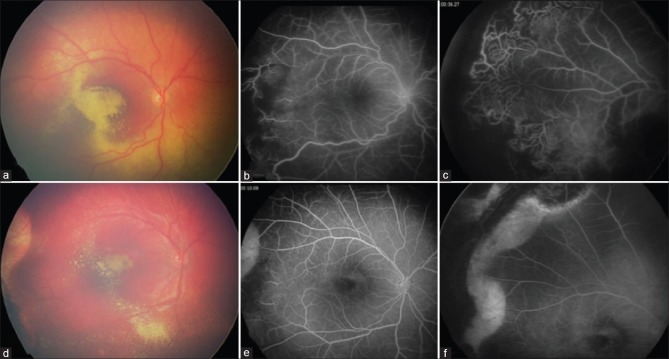
Stage 3A2 Coats disease with (a) foveal exudation, (b) peripheral telangiectasia and leakage on fluorescein angiography, and (c) subfoveal fluid and exudation on macular optical coherence tomography
The analysis of subretinal fluid collected during posterior sclerotomy for treatment of retinal detachment can aid in diagnosis by demonstrating lipid laden macrophages and cholesterol crystals.[15] Fine needle aspiration biopsy (FNAB) of the subretinal fluid through the anterior chamber is not recommended when the diagnosis can be made with noninvasive tests and if retinoblastoma is being considered in the presence of total retinal detachment. If it is not possible to rule out retinoblastoma with noninvasive tests, then enucleation seems to be a better option than FNAC.
Classification
Shields et al. proposed a staging classification of Coats disease based on their observations with a retrospective review of 150 cases of Coats treated by various modalities[33] [Table 1 and Figs. 5–8]. An updated classification has been suggested with stage 2B being subdivided into stage 2B1 without subfoveal nodule and stage 2B2 with subfoveal nodule since presence of a subfoveal nodule significantly affects the visual outcome.[31]
Table 1.
Classification of Coats disease
| Stage | Fundus features |
|---|---|
| 1 | Retinal telangiectasia only |
| 2 | Telangiectasia and exudation |
| 2A | Extrafoveal exudation |
| 2B | Foveal exudation |
| 3 | Exudative retinal detachment |
| 3A | Subtotal detachment |
| 3A1 | Extrafoveal |
| 3A2 | Foveal |
| 3B | Total retinal detachment |
| 4 | Total retinal detachment and glaucoma |
| 5 | Advanced end-stage disease |
From Shields JA, Shields CL, Honavar S, Demirci H. Classification and management of Coats disease: The 2000 Proctor Lecture. Am J Ophthalmol 2001;131:572-83
Figure 5.
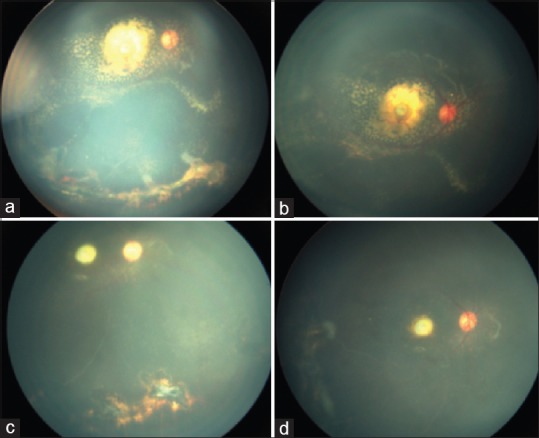
Stage 2B Coats disease (a and b) before and (c and d) after treatment with laser photocoagulation. (a) Peripheral telangiectasia with exudation (b) dense organised intra- and subretinal exudation at the macula. After treatment with diode laser photocoagulation (c) resolution of telangiectasia and minimal residual peripheral exudation (d) drastic reduction of posterior pole exudation
Figure 8.
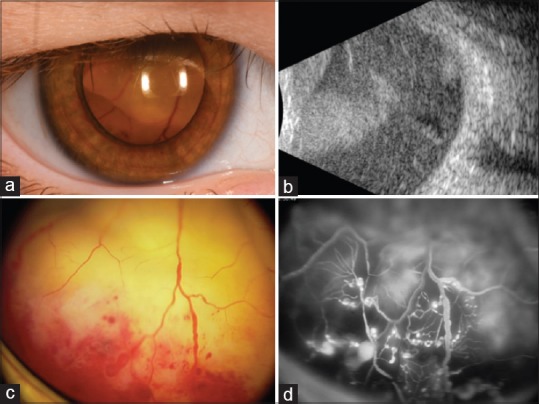
Stage 4 Coats disease showing bullous exudative retinal detachment and irregular, telangiectatic vessels in right eye of a 27 month old male child, treated with enucleation
Figure 7.
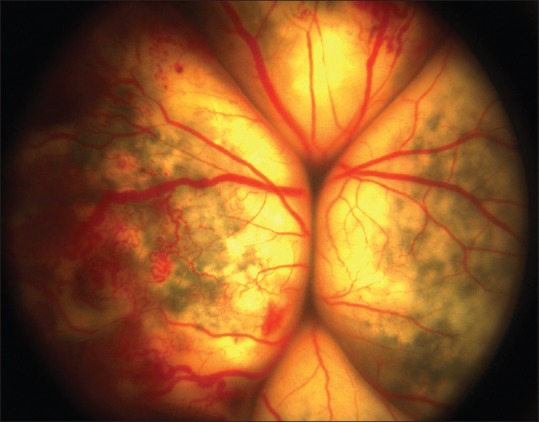
Stage 3B Coats disease with (a) massive retinal detachment up to the crystalline lens, (b) total detachment on ultrasonography, (c) peripheral confluent telangiectasia with intraretinal hemorrhage overlying subretinal exudation, and (d) irregularly dilated retinal vessels and light bulb telangiectasia with leakage on fluorescein angiography
Management
The aim of management in Coats disease is to address and eradicate the telangiectatic vessels in order to control and cause resolution of intra- and subretinal exudates and ultimately salvage the globe with preservation of vision. Patients with only telangiectasia with little or no exudation, not threatening the vision can be observed. Treatment must be initiated if progression is noted. Laser photocoagulation is indicated in early Coats (stage 1--3a) with exudation but no or shallow retinal detachment. Argon laser cauterizes the vessels, although complete ablation requires multiple sittings of laser. There should be a gap of at least 3 months between two sessions of laser as resolution of exudation occurs slowly [Fig. 5].
Retinal detachment where laser cannot work but approximation of cryoprobe to the telangiectatic vessels is still possible with scleral indentation can be treated with double freeze--thaw cryotherapy. Peripheral lesions, stage 3A and 3B are all amenable to cryotherapy. Cryotherapy also requires multiple sittings and can cause a transient increase in subretinal exudation and retinal detachment (ablatio fugax). The recommended safe schedule is cryotherapy of up to two quadrants at a time with a gap of four weeks between sessions. Treatment to the same area should be undertaken after 3 months, like in the case of laser, to allow time for resolution[33] [Fig. 6].
Figure 6.
Stage 3A2 Coats disease (a-c) before and (d-f) after treatment with cryotherapy and laser photocoagulation. (a) Macular exudative retinopathy with (b) fluorescein angiography demonstrating temporal macular leakage and disc staining and (c) peripheral nonperfusion with telangiectasia. At 6 months following treatment, there was resolution of (d) macular exudation, (e) vascular and disc staining, and (f) peripheral telangiectasia. Visual acuity increased from 20/400 initially to 20/40 at 6 months
In advanced cases, stage 3 with extensive retinal detachment and some cases of stage 4, vitreoretinal surgery with silicone oil or intravitreal gas injection can be attempted if there is reasonable chance of reattaching the retina. Simultaneous laser photocoagulation or cryotherapy to the abnormal vasculature after the retina is attached creates a chorioretinal scar. Presence of elevated intraocular pressure and iris neovascularization are the main predictors of enucleation in Coats [Fig. 8]. A painful blind eye is best treated with enucleation. In stage 3B or 5, with blind eye and the absence of pain, mere observation is recommended. The eye at this stage has total retinal detachment and may be phthisical. Intravitreal triamcinolone is useful as an adjuvant therapy. It causes a decrease in the central retinal thickness with resolution of exudates with subsequent improvement in visual acuity. However, complications like cataract and glaucoma are well known with the use of steroids.
Anti-VEGF are the newest additions to the armamentarium for management of Coats. At present, there are only limited case reports and small case series highlighting the utility of these agents as adjunctive treatment modality along with the traditional treatment options [Table 2]. They lead to the regression of abnormal vessels, resolution of macular edema, and exudation and stabilization or improvement of vision.[45] But it is important to remember that even though VEGF levels are increased in Coats disease, they are not involved in the pathogenesis. Therefore, these agents can only aid in the management but not treat the underlying condition. Table 3 summarizes the various treatment modalities preferred for the different stages of the disease.[45]
Table 2.
Studies showing the use of anti-VEGF in the management of Coats disease
| Author | Year | Type of Study | Study population | Age | Stage of disease | Treatment | Follow up | Results |
|---|---|---|---|---|---|---|---|---|
| Venkatesh et al.[46] | 2008 | Case series | 2 | 14, 16 y | 2 | Bevacizumab 1.25 mg, laser photocoagulation | 6 m | Improvement in VA Resolution of subfoveal serous detachment and exudative detachment No recurrence |
| Lin et al.[47] | 2010 | Case series | 3 | 10 y, 6 m, 12 y | 2B-3B | Bevacizumab 2.5 mg, laser photocoagulation | 12 m | Improvement in VA Resolution of exudation No recurrence |
| Ramasubramanian et al.[48] | 2011 | Case series | 8 | 88 m (mean) | 2-3 B | Bevacizumab 1.25 mg (8), cryotherapy (8), laser photocoagulation (4), triamcinolone (1) | 8.5 m | Reduction of exudation Vitreoretinal fibrosis (4) RD (4) |
| Zhao et al.[49] | 2011 | Case report | 1 | 3 | 3B | Bevacizumab 1.25 mg | 15 w | Improvement in VA Decrease in subretinal exudate and dilated vessels Resolution of RD |
| Bohm et al.[50] | 2011 | Case report | 1 | 26 y | 2 | Bevacizumab 1.25 mg×3 triamcinolone 2 mg | 87 weeks | Significant improvement in VA Significant decrease in CRT No recurrence |
| Ray et al.[51] | 2012 | Case series, comparative | 10 treated with bevacizumab, 10 control | 4.88 y, 4.64 y | 2B-3B | Bevacizumab, laser or cryotherapy (10) Laser or cryotherapy (10) | 9 m | Bevacizumab group require treatment over longer period. failures in control group |
| Zheng et al.[52] | 2013 | Case series | 19 | Pediatric- 6.9 y Adults 33.6 y | 2-3 B | Bevacizumab 1.25 mg | 9.1 m, 10.6 m | 1. Pediatric- significant improvement in VA. Resolution of SRF and exudates in all 2. Adults- no significant change in VA. Resolution of subretinal fluid and exudation all 3. vitreoretinal fibrosis in 2 |
| Kodama et al.[53] | 2014 | Case series | 2 | 15 y, 11 y | 3 A | Bevacizumab Laser photocoagulation | 2 y, 1 y | Improvement in VA Decreased exudation |
| Bhat et al.[54] | 2016 | Case series | 5 | 34 m (mean) | 3 B | Bevacizumab, SRF drainage with cryotherapy, laser photocoagulation | 19 m | 75% developed TRD |
| Zhang et al.[55] | 2018 | Case series | 28 | 3 or more | Laser photocoagulation Ranibizumab or conbercept 0.5 mg/0.05 ml | 24.3 months | 1. Significant improvement in VA 2. No significant adverse effects 3. 7% recurrence |
Anti-VEGF=Anti Vascular endothelial growth factor, VA=Visual acuity, RD=Retinal detachment, CRT=Central retinal thickness, SRF=subretinal fluid, TRD=tractional retinal detachment
Table 3.
Preferred modalities of treatment of Coats disease based on the disease stage
| Stage | Treatment |
|---|---|
| Stage 1, 2 | Laser photocoagulation or Cryotherapy |
| Stage 3 | Laser photocoagulation or Cryotherapy, External drainage of total retinal detachment can be beneficial |
| Stage 4 | External drainage of total retinal detachment, vitreoretinal surgery, or glaucoma surgery may be necessary. Occasionally, observation is advised |
| Advanced end-stage (Stage 5), asymptomatic | Observation |
| Advanced end-stage (Stage 5), with painful eye | Enucleation |
| Adjuvant therapy | Intravitreal or periocular triamcinolone, Anti-VEGF |
Adapted from Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of Coats disease: A review of the literature. Ophthalmologica 2012;227:175-82
Visual outcome varies with the stage of the disease, the more advanced stages demonstrating poor visual acuity both at presentation and at the time of final visit.[56] Factors predictive of poor vision (<20/200) include retinal telangiectasia involving the superior quadrant, diffuse or postequatorial zonal involvement, non-Caucasian race, failure of exudation to resolve after treatment and the presence of retinal macrocysts. In one analysis of 351 case, visual acuity following treatment was 20/20--20/40 (18%), 20/50--20/200 (22%), and 20/400 or worse (59%) and a trend toward better vision in recent years was documented, believed to be related to earlier recognition of the condition and more effective therapies.[26] Recurrence of Coats disease has been observed in approximately 3--7% of cases over 10 years.[26,33] Long term follow-up of these patients is thus recommended even after complete resolution.
Management and visual outcome based on stage of disease
The visual outcome following treatment of Coats disease is dependent on disease stage. Shields et al. classified Coats disease in 351 consecutive cases of which Snellen visual acuity before and after treatment was available for 160 cases [stage 1 (n = 2) vs. stage 2A (n = 17) vs. stage 2B (n = 22) vs. stage 3A1 (n = 26) vs. stage 3A2 (n = 40) vs. stage 3B (n = 42) vs. stage 4 (n = 9) vs. stage 5 (n = 2)].[56] They excluded patients treated elsewhere and preverbal patients. In this study, they found that the more advanced stage of the disease was associated with poor visual acuity at presentation (<20/200) (0% vs. 0% vs. 50% vs. 35% vs. 38% vs. 83% vs. 100% vs. 100%, P < 0.001), higher mean intraocular pressure (17 vs. 15 vs. 15 vs. 15 vs. 15 vs. 15 vs. 37 vs. 26, P < 0.001), greater frequency of xanthocoria (0% vs. 18% vs. 9% vs. 15% vs. 10% vs. 55% vs. 56% vs. 50%, P < 0.001), corneal edema (0% vs. 0% vs. 0% vs. 0% vs 0% vs. 0% vs. 22% vs 50%, P = 0.001), and iris neovascularization (0% vs. 0% vs. 0% vs. 4% vs. 0% vs. 7% vs. 67% vs. 100%, P < 0.001).[56] In terms of posterior segment findings, more advanced the stage, more were the number of clock hours of telangiectasia (1 vs. 4 vs. 4 vs. 4 vs. 5 vs. 7 vs. 9 vs. not available (NA), P < 0.001), light bulb aneurysms (1 vs 3 vs. 2 vs. 3 vs. 4 vs. 7 vs. 9 vs. NA, P < 0.001), exudation (0 vs. 4 vs. 5 vs. 6 vs. 6 vs. 9 vs. 10, P < 0.001) and subretinal fluid (0 vs. 1 vs. 2 vs. 5 vs. 5 vs. 10 vs. 12, P < 0.001).[56]
The Shields classification system is useful in guiding the treatment and predicting ocular and visual outcomes [Table 4] with more advanced stage demonstrating the need for primary enucleation (P < 0.001) and worse final visual acuity (<20/200) (P < 0.001). Stage 4 eyes were more likely to require enucleation than stage 2A eyes (P < 0.001), stage 2B eyes (P < 0.001), stage 3A1 eyes (P < 0.001), stage 3A2 eyes (P < 0.001), and stage 3B eyes (P = 0.001); stage 3B eyes were more likely to require enucleation than stage 3A1 eyes (P = 0.04). There was no difference in disease recurrence by stage.[56] Poor visual acuity in advanced stages can be attributed mainly to persistent retinal detachment (0% vs. 25% vs. 38% vs. 6% vs. 51% vs. 100% vs. 100%, P < 0.001) rather than macular scar alone (NA vs. 50% vs. 75% vs. 38% vs. 78% vs. 49% vs. 0% vs 0%, P < 0.001). There was no difference in visual acuity loss ≥ 3 lines, total number of lines of visual acuity loss, visual acuity gain ≥ 2 lines, or total number of lines of visual acuity gain by stage. For stages 2B, 3A1, and3A2, the main reason for visual acuity gain ≥ 2 lines was resolution of foveal exudation (P < 0.001) and for 3A1 3A2, and 3B, the main reason for visual acuity gain was resolution of subretinal fluid (P < 0.001).[56] For those managed conservatively, less advanced disease was more likely to be treated with argon laser photocoagulation than cryotherapy. In terms of outcome, more advanced disease showed less frequent resolution of disease, subretinal fluid, and exudation [Table 4].[56]
Table 4.
Classification of Coats Disease and its Relevance to Management and Outcome in 160 eyes of 160 patients
| Stage | Conservative therapy* (%) | Argon laser photocoagulation (%) | Cryotherapy (%) | Sub-Tenon’s corticosteroid injection | Intravitreal corticosteroid injection | Anti- VEGF | Primary enucleation (%) | Poor visual outcome ≤20/200 (%) | Resolution of disease (%) | Disease recurrence (%) | Resolution of leaking telangiectasia (%) | Resolution of subretinal fluid (%) | Resolution of foveal exudation (%) | Enucleation outcome (%)# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 (100) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 2 (100) | NA | NA | 0 |
| 2 | ||||||||||||||
| 2A | 15 (88) | 13 (87) | 7 (47) | 3 (20) | 1 (7) | 4 (27) | 0 (0) | 2 (12) | 12 (71) | 1 (6) | 11 (65) | 6 (60) | NA | 0 |
| 2B | 21 (95) | 10 (48) | 17 (81) | 1 (5) | 0 (0) | 2 (10) | 0 (0) | 8 (36) | 14 (64) | 0 (0) | 17 (77) | 9 (69) | 14 (64) | 0 |
| 3 | ||||||||||||||
| 3A | ||||||||||||||
| 3A1 | 26 (100) | 16 (62) | 21 (81) | 5 (19) | 2 (5) | 3 (12) | 0 (0) | 8 (31) | 19 (73) | 0 (0) | 20 (77) | 18 (72) | 3 (19) | 0 |
| 3A2 | 39 (98) | 29 (74) | 32 (82) | 10 (26) | 4 (12) | 9 (23) | 0 (0) | 18 (45) | 31 (78) | 0 (0) (2) | 32 (80) | 32 (80) | 11 (29) | 1 (3) |
| 3B | 34 (81) | 12 (35) | 30 (88) | 5 (15) | 1 (25) | 4 (12) | 2 (5) | 37 (88) | 21 (50) | 1 (2) | 21 (50) | 20 (48) | 16 (40) | 7 (17) |
| 4 | 4 (44) | 0 (0) | 2 (50) | 1 (25) | 0 (0) | 1 (25) | 4 (44) | 9 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (78) |
| 5 | 1 (50) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 1 (50) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | |
| P | <0.001 | <0.001 | 0.001 | 0.50 | 0.63 | 0.60 | <0.001 | <0.001 | <0.001 | 0.70 | <0.001 | <0.001 | 0.01 | <0.001 |
*Laser photocoagulation, cryotherapy, injection, #Primary + secondary, AntiVEGF=Anti Vascular endothelial growth factor, From Shields CL, Udyaver S, Dalvin LA, Lim LAS, Atalay HT, Khoo CTL, Mazloumi M, Shields JA. Visual acuity Outcomes in Coats Disease by Classification Stage in 160 Patients. In press
Trends in management over decades
A recent review of 351 cases of Coats disease over a period of 45 years showed a positive trend in the management and outcome.[26] A comparative evaluation by decade (1970 vs. 1980 vs. 1990 vs. 2000 vs. 2010s) revealed no significant difference in the demographic features, presenting verbal visual acuity, Coats disease stage, location and clock hours of telangiectasia and aneurysms and neovascularization of the optic disc, retina and iris (clinical and on fluorescein angiography).[26] As compared with the 2010s, patients in 1980s presented with greater mean intraocular pressure (P = 0.001), mean clock hours of exudation (P = 0.01), prevalence of four quadrants of exudation (P = 0.01), four quadrants of subretinal fluid (P = 0.02) and total exudative retinal detachment (P < 0.001).
Table 5 shows the change in trend of management and outcomes over five decades. Fewer eyes are now managed by observation or enucleation. Overall, more eyes have complete resolution of disease with greater globe salvage in 2010s.[26]
Table 5.
Changing trends in the treatment and outcome of Coats disease over the years
| Treatment and outcome | 1970s | 1980s | 1990s | 2000s | 2010s | P |
|---|---|---|---|---|---|---|
| Observation | 39% | 21% | 33% | 21% | 11% | 0.002 |
| Total number of treatments | 2.9 | 2.0 | 1.8 | 3.6 | 4.5 | 0.001 |
| Laser photocoagulation | 55% | 33% | 38% | 40% | 72% | <0.001 |
| Intravitreal AntiVEGF | 0% | 4% | 2% | 13% | 18% | 0.003 |
| Primary enucleation | 11% | 16% | 3% | 4% | 1% | 0.001 |
| Complete resolution | 58% | 45% | 37% | 55% | 73% | 0.002 |
| Complete subretinal fluid resolution | 64% | 59% | 38% | 58% | 72% | 0.01 |
| Primary or secondary enucleation | 17% | 27% | 14% | 13% | 6% | 0.04 |
Anti-VEGF=Anti vascular endothelial growth factor, Shields CL, Udyaver S, Dalvin LA, Lim LAS, Atalay HT, Khoo CTL, Mazloumi M, Shields JA. Coats disease in 351 eyes: Analysis of features and outcomes over 45 years (by decade) at a single center. Ind J Ophthalmol 2019;67:in press.
Conclusion
Coats disease is congenital, nonhereditary, idiopathic retinal vascular telangiectasia with intraretinal and subretinal exudation, which presents with a spectrum of clinical features. Majority of the cases present with advanced disease. Conservative management includes laser photocoagulation, cryotherapy, intravitreal steroids, intravitreal anti-VEGF injections and vitreoretinal surgery, with outcome ranging from excellent visual acuity to no perception of light. Symptomatic blind and cosmetically disfigured eyes are typically enucleated. In the recent years, the visual outcome in Coats disease has improved which can be attributed to earlier detection and aggressive treatment.[26,57] Improvements in retinal imaging, diagnostic accuracy, surgical techniques for retinal detachment, lasers and intraocular injections have contributed to successful management. Greater awareness about the typical features, diagnosing the disease at an early stage and initiating appropriate treatment can greatly improve the visual outcomes and reduce the rate of enucleation in Coats disease.
Financial support and sponsorship
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS, JAS) and the Lucille Wiedman Fund for Pediatric Cancer, Philadelphia, PA (CLS, JAS).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Coats G. Forms of retinal diseases with massive exudation. Roy Lond Ophthalmol Hosp Rep. 1908;17:440–525. [Google Scholar]
- 2.Leber T. Ueber eine durch Vorkommen multipler Miliaraneurysmen charakterisierteForm von Retinaldegeneration. Albrecht von Graefe's Arch Klin Ophthalmol. 1912;81:1–14. [Google Scholar]
- 3.Reese AB. Telangiectasis of the retina and Coats disease. Am J Ophthalmol. 1956;42:1–8. doi: 10.1016/0002-9394(56)90002-2. [DOI] [PubMed] [Google Scholar]
- 4.Woods AC, Duke JR. Coats disease. I. Review of the literature, diagnostic criteria, clinical findings, and plasma lipid studies. Br J Ophthalmol. 1963;47:385–412. doi: 10.1136/bjo.47.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell FP. Coats disease and congenital vascular retinopathy. Trans Am Ophthalmol Soc. 1977;74:365–424. [PMC free article] [PubMed] [Google Scholar]
- 6.Luckie AP, Hamilton AM. Adult Coats disease in branch retinal vein occlusion. Aust NZ J Ophthalmol. 1994;22:203–6. doi: 10.1111/j.1442-9071.1994.tb01717.x. [DOI] [PubMed] [Google Scholar]
- 7.Lanier JD, McCrary JA, III, Justice J. Autosomal recessive retinitis pigmentosa and Coats disease: A presumed familial incidence. Arch Ophthalmol. 1976;94:1737–42. doi: 10.1001/archopht.1976.03910040511009. [DOI] [PubMed] [Google Scholar]
- 8.Fogle JA, Welch RB, Green WR. Retinitis pigmentosa and exudative retinopathy. Arch Ophthalmol. 1978;96:696–702. doi: 10.1001/archopht.1978.03910050386018. [DOI] [PubMed] [Google Scholar]
- 9.Spallone A, Carlevaro G, Ridling P. Autosomal dominant retinitis pigmentosa and 27.Coats-like disease. Int Ophthalmol. 1985;8:147–51. doi: 10.1007/BF00136491. [DOI] [PubMed] [Google Scholar]
- 10.Arrig PG, Lahav M, Hutchins RK, Weiter JJ. Pigmentary retinal degeneration and Coats disease: A case study. Ophthalmic Surg. 1988;19:432–36. [PubMed] [Google Scholar]
- 11.Khan JA, Ide CH, Strickland MP. Coats-type retinitis pigmentosa. Surv Ophthalmol. 1988;32:317–32. doi: 10.1016/0039-6257(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim RY, Kearney JJ. Coats-type retinitis pigmentosa in a 4-year-old child. Am J Ophthalmol. 1997;124:846–8. doi: 10.1016/s0002-9394(14)71707-6. [DOI] [PubMed] [Google Scholar]
- 13.Frezzotti R, Berengo A, Guerra R, Cavalllini F. Toxoplasmic Coats retinitis. Am J Ophthalmol. 1965;59:1099–102. [PubMed] [Google Scholar]
- 14.Kremer I, Cohen S, Izhak RB, Ben-sira I. An unusual case of congenital unilateral Coats disease associated with morning glory optic disc anomaly. Br J Ophthalmol. 1985;69:32–7. doi: 10.1136/bjo.69.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131:561–71. doi: 10.1016/s0002-9394(00)00883-7. [DOI] [PubMed] [Google Scholar]
- 16.Small RG. Coats disease and muscular dystrophy. Trans Am Acad Ophthalmol Otolaryngol. 1968;71:225–31. [PubMed] [Google Scholar]
- 17.Matsuzaka T, Sakuragawa N, Terasawa K, Kuwabara H. Facioscapulohumeral dystrophy associated with mental retardation, hearing loss, and tortuosity of retinal arterioles. J Child Neurol. 1986;1:218–23. doi: 10.1177/088307388600100308. [DOI] [PubMed] [Google Scholar]
- 18.Desai UR, Sabates FN. Long-term follow up of facioscapulohumeral muscular dystrophy and Coats disease. Am J Ophthalmol. 1990;110:568–9. doi: 10.1016/s0002-9394(14)77885-7. [DOI] [PubMed] [Google Scholar]
- 19.Cameron JD, Yanoff M, Frayer WC. Coats disease and Turner's syndrome. Am J Ophthalmol. 1974;78:852–4. doi: 10.1016/0002-9394(74)90310-9. [DOI] [PubMed] [Google Scholar]
- 20.Burch JV, Leveille AS, Morse PH. Icthyosis hystrix (epidermal nevus syndrome) and Coats disease. Am J Ophthalmol. 1980;89:25–30. doi: 10.1016/0002-9394(80)90225-1. [DOI] [PubMed] [Google Scholar]
- 21.Folk JC, Genovese FN, Biglan AW. Coats disease in a patient with Cornelia de Lange syndrome. Am J Ophthalmol. 1981;91:607–10. doi: 10.1016/0002-9394(81)90059-3. [DOI] [PubMed] [Google Scholar]
- 22.Kondra L, Cangemi FE, Pitta CG. Alport's syndrome and retinal telangiectasia. Ann Ophthalmol. 1983;15:550–1. [PubMed] [Google Scholar]
- 23.Schuman JS, Lieberman KV, Friedman AH, Berger M, Schoeneman MJ. Senior-Loken syndrome (familial renal retinal dystrophy) and Coats disease. Am J Ophthalmol. 1985;100:822–7. doi: 10.1016/s0002-9394(14)73374-4. [DOI] [PubMed] [Google Scholar]
- 24.Newell SW, Hall BD, Anderson CW, Lim ES. Hallermann Streiff syndrome with Coats disease. J Pediatr Ophthalmol Strabismus. 1994;31:123–5. doi: 10.3928/0191-3913-19940301-16. [DOI] [PubMed] [Google Scholar]
- 25.Dalvin L, Udyaver S, Lim LS, Mazloumi M, Atalay H, Khoo C, et al. Coats disease: Clinical features and outcomes by age category in 351 cases. Submitted for publication. doi: 10.3928/01913913-20190716-01. [DOI] [PubMed] [Google Scholar]
- 26.Shields CL, Udyaver S, Dalvin LA, Lim LAS, Atalay HT, Khoo CTL, et al. Coats disease in 351 eyes: Analysis of features and outcomes over 45 years (by decade) at a single center. Indian J Ophthalmol. 2019 doi: 10.4103/ijo.IJO_449_19. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitznas M, Joussen F, Wessing A, Meyer-Schwickerath G. Coats disease. An epidemiologic and fluorescein angiographic study. Graefe's Arch Clin Exp Ophthalmol. 1975;195:241–59. doi: 10.1007/BF00414937. [DOI] [PubMed] [Google Scholar]
- 28.Ridley ME, Shields JA, Brown GC, Tasman W. Coats disease. Evaluation of management. Ophthalmology. 1982;89:1381–7. doi: 10.1016/s0161-6420(82)34634-5. [DOI] [PubMed] [Google Scholar]
- 29.Tarkkanen A, Laatikainen L. Coats disease: Clinical, angiographic, histopathological findings and clinical management. Br J Ophthalmol. 1983;67:766–76. doi: 10.1136/bjo.67.11.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egerer I, Tasman WS, Tomer TL. Coats disease. Arch Ophthalmol. 1974;92:109–12. doi: 10.1001/archopht.1974.01010010115006. [DOI] [PubMed] [Google Scholar]
- 31.Daruich AL, Moulin AP, Tran HV, Matet A, Munier FL. Subfoveal nodule in Coats disease: Toward an updated classification predicting visual prognosis. Retina (Philadelphia, Pa) 2017;37:1591. doi: 10.1097/IAE.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: Epidemiology and clinical features at diagnosis. Eye. 2010;24:1797–801. doi: 10.1038/eye.2010.126. [DOI] [PubMed] [Google Scholar]
- 33.Shields JA, Shields CL, Honavar S, Demirci H. Classification and management of Coats disease: The 2000 proctor lecture. Am J Ophthalmol. 2001;131:572–83. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 34.Adam RS, Kertes PJ, Lam WC. Observations on the management of Coats disease: Less is more. Br J Ophthalmol. 2007;91:303–6. doi: 10.1136/bjo.2006.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egbert PR, Chan CC, Winter FC. Flat preparations of the retinal vessels in Coats disease. J Pediatr Ophthalmol. 1976;13:336–9. [PubMed] [Google Scholar]
- 36.Fernandes BF, Odashiro AN, Maloney S, Zajdenweber ME, Lopes AG, Burnier MN., Jr Clinical-histopathological correlation in a case of Coats disease. Diagn Pathol. 2006;1:24. doi: 10.1186/1746-1596-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steidl SM, Hirose T, Sang D, Hartnett ME. Difficulties in excluding the diagnosis of retinoblastoma in cases of advanced Coats disease: A clinicopathologic report. Ophthalmologica. 1996;210:336–40. doi: 10.1159/000310735. [DOI] [PubMed] [Google Scholar]
- 38.Shields JA, Eagle RC, Jr, Fammartino J, Shields CL, De Potter P. Coats disease as a cause of anterior chamber cholesterolosis. Arch Ophthalmol. 1995;113:975–7. doi: 10.1001/archopht.1995.01100080025014. [DOI] [PubMed] [Google Scholar]
- 39.Shields CL, Schoenberg E, Kocher K, Shukla SY, Kaliki S, Shields JA. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases. Ophthalmology. 2013;120:311–6. doi: 10.1016/j.ophtha.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 40.Shields J, Shields C. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2016. Intraocular Tumors: An Atlas and Textbook; pp. 1015–26. [Google Scholar]
- 41.Shields JA, Shields CL. Differentiation of Coats’ disease and retinoblastoma. J Ped Ophthalmol Strabism. 2001;38:262–6. doi: 10.3928/0191-3913-20010901-05. [DOI] [PubMed] [Google Scholar]
- 42.Eisenberg L, Castillo M, Kwock L, Mukherji SK, Wallace DK. Proton MR spectroscopy in Coats disease. AJNR Am J Neuroradiol. 1997;18:727–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Senft SH, Hidayat AA, Cavender JC. Atypical presentation of Coats disease. Retina. 1994;14:36–8. doi: 10.1097/00006982-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Edward DP, Mafee MF, Garcia-Valenzuela E, Weiss RA. Coats disease and persistent hyperplastic primary vitreous. Role of MR imaging and CT. Radiol Clin North Am. 1998;36:1119–31, x. doi: 10.1016/s0033-8389(05)70235-9. [DOI] [PubMed] [Google Scholar]
- 45.Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of Coats disease: A review of the literature. Ophthalmologica. 2012;227:175–82. doi: 10.1159/000336906. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesh P, Mandal S, Garg S. Management of Coats disease with bevacizumab in 2 patients. Can J Ophthalmol. 2008;43:245–6. doi: 10.3129/i08-028. [DOI] [PubMed] [Google Scholar]
- 47.Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina. 2010;30:617–22. doi: 10.1097/IAE.0b013e3181c2e0b7. [DOI] [PubMed] [Google Scholar]
- 48.Ramasubramanian A, Shields CL. Bevacizumab for Coats disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96:356–9. doi: 10.1136/bjophthalmol-2011-300141. [DOI] [PubMed] [Google Scholar]
- 49.Zhao T, Wang K, Ma Y, Jiang YR. Resolution of total retinal detachment in Coats disease with intravitreal injection of bevacizumab. Graefes Arc Clin Exp Ophthalmol. 2011;249:1745–6. doi: 10.1007/s00417-010-1563-y. [DOI] [PubMed] [Google Scholar]
- 50.Böhm MR, Uhlig CE. Use of intravitreal triamcinolone and bevacizumab in Coats disease with central macular edema. Graefes Arch Clin Exp Ophthalmol. 2011;249:1099–101. doi: 10.1007/s00417-011-1629-5. [DOI] [PubMed] [Google Scholar]
- 51.Ray R, Barañano DE, Hubbard GB. Treatment of Coats disease with intravitreal bevacizumab. Br J Ophthalmol. 2013;97:272–7. doi: 10.1136/bjophthalmol-2012-302250. [DOI] [PubMed] [Google Scholar]
- 52.Zheng XX, Jiang YR. The effect of intravitreal bevacizumab injection as the initial treatment for Coats disease. Graefes Arch Clin Exp Ophthalmol. 2014;252:35–42. doi: 10.1007/s00417-013-2409-1. [DOI] [PubMed] [Google Scholar]
- 53.Kodama A, Sugioka K, Kusaka S, Matsumoto C, Shimomura Y. Combined treatment for Coats disease: Retinal laser photocoagulation combined with intravitreal bevacizumab injection was effective in two cases. BMC Ophthalmol. 2014;14:36. doi: 10.1186/1471-2415-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat V, D’Souza P, Shah PK, Narendran V. Risk of tractional retinal detachment following intravitreal bevacizumab along with subretinal fluid drainage and cryotherapy for stage 3B Coats disease. Middle East Afr J Ophthalmol. 2016;23:208–11. doi: 10.4103/0974-9233.175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Ke Y, Wang W, Shi X, Hei K, Li X. The efficacy of conbercept or ranibizumab intravitreal injection combined with laser therapy for Coats disease. Graefes Arch Clin Exp Ophthalmol. 2018;256:1339–46. doi: 10.1007/s00417-018-3949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shields CL, Udyaver S, Dalvin LA, Lim LAS, Atalay HT, Khoo CTL, et al. Visual acuity Outcomes in Coats Disease by Classification Stage in 160 Patients. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2019-314363. [In Press] [DOI] [PubMed] [Google Scholar]
- 57.Ong SS, Buckley EG, McCuen BW, II, Jaffe GJ, Postel EA, Mahmoud TH, et al. Comparison of visual outcomes in Coats disease: A 20-year experience. Ophthalmology. 2017;124:1368–76. doi: 10.1016/j.ophtha.2017.03.051. [DOI] [PubMed] [Google Scholar]