Abstract
Purpose:
To study the clinical and microbiological profile, treatment modalities, and anatomical and functional outcomes among children and adolescents with endogenous endophthalmitis (EE) at a tertiary eye care centre in India.
Methods:
Medical records of subjects <18 years, presenting with EE from 1997 to 2007 were reviewed. Cases where the causative organism was identified were included. Treatment regimen included systemic antibiotics, vitrectomy, intravitreal antibiotics, and enucleation. Systemic evaluation to identify the source of infection was done by an internist. Microbiological analysis of blood, urine, and ocular specimens was done. The favorable anatomical outcome was defined as the attached retina, with controlled intraocular pressure and clear media at the last follow up. The favorable functional outcome was defined as vision >3/60 on the final follow up. Univariate regression analysis was done to identify factors predicting functional outcome.
Results:
Thirty eyes of 30 subjects (23 (77%) males) were studied. The mean age at presentation was 6.8 years (range=1–16 years). Fever was evident in four (13%) and blood culture was negative in all cases. Gram-positive organisms were identified in 11 (37%) eyes, fungi in 3 (10%), and toxocara in 8 (27%) eyes. Twenty-three (77%) eyes underwent vitrectomy. Favorable functional and anatomical outcomes were achieved in 9 (30%) and 12 (40%) eyes, respectively. Eyes undergoing vitrectomy showed significant correlation with good functional outcome (P = 0.05).
Conclusion:
EE is under-reported and not well studied in children. The absence of systemic features may be evident in a developing country with over the counter availability of antibiotics. Gram-positive infections are common and vitrectomy is a beneficial modality of treatment.
Keywords: Children, endogenous endophthalmitis, vitrectomy
Endophthalmitis is a potentially devastating condition with poor anatomical and functional outcomes.[1,2,3,4] Endogenous endophthalmitis (EE) is rare and accounts for 2%–8% cases of endophthalmitis in general.[3,4,5] Pediatric EE has been reported to account for only 0.1%–4% of all cases; the highest incidence of cases reported being from India and the lowest from the USA.[6,7]
Children also show differences in presentation as compared with adults. Late presentation, variable systemic features, malnourishment, and impaired immunological status, with a lack of established guidelines for management of EE further confound this relatively unaddressed condition.
Relatively few cases of EE among children and adolescents have been reported in the literature. The last major review by Greenwald et al. in 1986 on EE included 24 of 72 patients, aged <20 years.[8] In a 17-year St Thomas’ prospective case series of 19 patients, there was only 1 child with EE.[9] Another retrospective series on EE reported only 1 of the 27 patients who was <18 years of age over a 4-year study period in an East Asian set up.[10] Isolated case reports of EE in children with culture-positive organisms ranging from Pseudomonas species, Neisseria species, and of fungal causes have been reported.[11,12,13]
To the best of our knowledge, this is the largest study in the literature reporting and analyzing the outcomes of EE in the pediatric age group.
Methods
This study was a retrospective analysis of children and adolescents (age <18 years) presenting with EE diagnosed at a tertiary eye care center in south India over a period of 13 years from 1997 to 2007. Only subjects where a definite causative organism was identified were further analyzed in the study. Prior ethics committee approval was obtained and the study was carried out in accordance with the tenets of the Declaration of Helsinki. All patients with a clinical diagnosis of EE based on clinical presentation with no recent history of ocular trauma, ocular surgery, or previous inflammation were included. A systemic history regarding any febrile illness or previous hospitalization was recorded. A detailed history was also taken to rule out noninfectious panuveitis. Ocular fluid like vitreous and/or aqueous was submitted for microbiological testing. Aqueous tap was done whenever possible; the otherwise undiluted vitreous sample was collected during the planned therapeutic vitrectomy. In patients undergoing evisceration, the eviscerated specimen was subjected to microbiological and histopathological analyses. The obtained samples were initially studied with Gram's stain, 10% wet KOH mount, Giemsa's stain and Ziehl–Neelsen stain to identify bacterial or fungal etiology. Samples of aqueous and vitreous were directly inoculated on blood agar, chocolate agar, Sabouraud's dextrose agar, thioglycolate medium, brain–heart infusion agar, and Lowenstein–Jensen agar. Polymerase chain reaction (PCR) test was done on the aqueous and vitreous samples in selected cases based on clinical suspicion. A positive culture was defined as the growth of the same organism on two or more liquid media or confluent growth on one solid medium. In all cases, blood and urine were collected and sent for microbiological culture and patients were evaluated by the internist for a systemic focus of infection. Blood and urine samples showing culture growth or high antibody titers of organisms were also considered significant. Treatment regimen ranged from systemic antibiotics in all children to vitrectomy, intravitreal antibiotics injection, and evisceration depending on the clinical presentation. Systemic evaluation to find out the cause/source of metastasis of infection was also performed by an internist. Subjects whose all microbiological investigations were negative, but the clinical picture was suggestive of endophthalmitis, were not included in further analysis. All such children were also evaluated by a uveitis expert to rule out the noninfectious cause of ocular inflammation. Initial treatment with intravitreal antibiotics was based on the staining results. Further treatment was based on culture and PCR results of systemic and ocular specimens. Patients were started on systemic cefotaxime and gentamycin according to body weight in cases of bacterial infection to cover Gram-positive and Gram-negative bacteria and systemic fluconazole in fungal infections.
Age and sex, medical conditions predisposing to infection, ocular features, extraocular manifestations of infection, treatment details, and final outcome in terms of visual acuity (VA) and anatomy were studied. Modalities of treatment and follow-up in terms of outcome and complications were recorded. The favorable anatomical outcome was defined as an attached retina, with controlled intraocular pressure and a clear media. The favorable functional outcome was defined as best corrected visual acuity (BCVA) >3/60 (ambulatory vision) at the final follow-up. Statistical analysis was performed using descriptive statistics and χ2 test for univariate analysis for potential factors associated with good functional outcome. The software used in the analysis was SPSS 14.
Results
Of the total 214 children with endophthalmitis who presented to a tertiary eye care center in India during the study period, 62 eyes of 62 children, all under the age of 18 years, presented with EE. Of these, a causative organism could be isolated in 30 eyes. The mean age of these 30 subjects in the study was 6.8 years ± 3.8 (range 1–16 years) and 23 (77%) were males. Table 1 summarizes the ocular manifestations among the subjects in the study group.
Table 1.
Endogenous endophthalmitis in children and adolescents: Presenting ocular features
| Clinical features | Number | (%) |
|---|---|---|
| AC cells with flare | 30 | (100) |
| Vitritis | 30 | (100) |
| Yellow glow with no fundus details seen | 20 | (67) |
| Vitreous exudates | 7 | (23) |
| Retinal detachment | 3 | (10) |
| Hypopyon | 8 | (26.7) |
| Complicated cataract | 4 | (13.3) |
| AC exudates | 8 | (26.7) |
| Posterior synechiae | 3 | (10) |
| Retrolenticular membrane | 3 | (10) |
| Granulomas in posterior segment | 3 | (10) |
| Hyphema | 1 | (3.3) |
| Subretinal exudates | 1 | (3.3) |
AC: anterior chamber, ELISA: enzyme-linked immunosorbent assay, Ig: immunoglobulin
At presentation, 27 (90%) eyes had VA <3/60 with 4 eyes (13%) being PL negative. Overall, 11 (37%) eyes showed improvement in VA at final visit compared with baseline with 4 (13%) eyes having final VA >6/18. Nine (30%) eyes showed favorable functional outcome.
The outcomes of microbiological investigations are shown in Table 2 with microorganisms detected on any of the following procedures. The vitreous specimen was taken in 21 of 30 eyes and AC tap in 4 eyes. Blood and urine cultures were sent for all subjects, while special investigations like PCR (1 eye), IgM (toxocara) in vitreous (2 eyes), and serum (6 eyes) were done in selected eyes based on the clinical picture.
Table 2.
Investigative procedures for identification of the causative organism
| Investigation | Positive culture (n) | (%) |
|---|---|---|
| Vitreous | 23 | (76.7) |
| Vitreous culture | 20 | (66.7) |
| Vitreous PCR | 1 | (3.3) |
| Vitreous IgM antibody (toxocara) | 2 | (6.7) |
| Aqueous culture* | 4 | (13.3) |
| Urine culture | 2 | (6.7) |
| Blood | ||
| Serum IgM (Toxocara) | 6 | (20) |
| Blood ELISA IgG (Cysticercus) | 1 | (3.3) |
| Blood culture | 0 |
*Including eyes with positive vitreous culture. AC: anterior chamber, ELISA: enzyme-linked immunosorbent assay, Ig: immunoglobulin, PCR: polymerase chain reaction
Underlying systemic features included fever in four patients (13%) and bronchopneumonia and diarrhea in one patient each (3.3%). All patients had received systemic antibiotics before reporting to our centre. Table 3 summarizes the various organisms isolated from culture. Organisms identified included Gram-positive bacteria (11 eyes (36.7%)), Gram-negative bacteria (7 (23.3%)), toxocara (8 (26.7%)), fungi (3 (6%)), and cysticercus (1 (3.3%)) eye.
Table 3.
Identification of organisms isolated among the study eyes
| Organism | Number of eyes | Medium |
|---|---|---|
| Staphylococcus epidermidis | 3 | Vitreous |
| Streptococcus pyogenes | 3 | Vitreous |
| Staphylococcus aureus | 2 | Vitreous |
| Pseudomonas | 2 | Vitreous |
| Alkaligenes dentiferous | 2 | Vitreous |
| Fungi (Candida albicans) | 2 | Vitreous |
| Streptococcus viridans | 1 | Vitreous |
| Moraxella | 1 | Vitreous |
| Enterococcus faecalis | 1 | Vitreous |
| Micrococcus | 1 | Vitreous |
| Fungi (Aspergillus) | 1 | Vitreous |
| Toxocara (IgM) | 1 | Vitreous IgM |
| Escherichia coli | 2 | Urine |
| Toxocara (IgM) | 7 | Blood |
| Cysticercus (ELISA) | 1 | Blood |
ELISA: enzyme-linked-immunosorbent serologic assay, Ig: immunoglobulin
As noted before, all 30 patients received intravenous antibiotics. Twenty-three (77%) eyes underwent vitrectomy with or without other surgical procedures. All eyes undergoing vitrectomy received intravitreal antibiotics/amphotericin B at the conclusion of the procedure with or without dexamethasone, depending on clinical and microbiological information at that stage. Intravitreal antibiotics were additionally administered in 11 (36.7%) eyes, with (n = 8) or without (n = 3) intravitreal dexamethasone, while intravitreal amphotericin B was given to 2 (6.7%) eyes with fungal endophthalmitis. The number of these intravitreal injections ranged from 1 to 4. Enucleation was performed in two eyes with clinical suspicion of retinoblastoma, but on microbiological analysis of vitreous samples, one case returned positive for Toxocara IgM antibody and another for panfungal genome on PCR and KOH stain for aspergillus.
The good functional outcome with a vision of >3/60 was achieved in 9 (30%) eyes and a favorable anatomical outcome was achieved in 14 (47%) eyes at a final mean follow-up of 35.5 ± 55.1 months (range 6–208 months). Of the 16 (53%) eyes that had an unfavorable anatomical outcome, 5 (16.7%) eyes had phthisis bulbi, 3 (10%) had a retinal detachment, 7 (23.3%) had unresolved vitreous condensations, and 2 (6.7%) eyes were enucleated.
Table 4 summarizes the univariate analysis results evaluating the correlation of variables like Gram staining, type of organism, presence of underlying systemic illness, vitrectomy, use of systemic steroids, and relatively clear media at presentation (i.e. eyes with relatively mild vitritis allowing visualization of first-order retinal vessels) with functional outcome. Eyes that had undergone vitrectomy had a statistically significant correlation with good functional outcome (P = 0.05). No correlation with the functional outcome was observed with the use of systemic steroids, Gram staining profile, clear media at presentation, and with presence or absence of underlying systemic illness. Also, children <5 years of age had a higher proportion of Gram-negative EE (seven of nine eyes with Gram stain positivity) compared with children >5 years (one of nine eyes) (P = 0.001).
Table 4.
Univariate analysis of possible factors associated with the favorable functional outcome
| Variable | Favorable functional outcome, (n=9) (30%) | Unfavorable functional outcome, (n=21) (70%) | P | |
|---|---|---|---|---|
| Gram staining (n=18) | Positive (n=11) | 2 (18%) | 9 (82%) | 0.33 |
| Negative (n=7) | 3 (43%) | 4 (57%) | ||
| Systemic illness (n=6) | Yes | 1 (17%) | 5 (83%) | 1 |
| No | 8 (33%) | 16 (67%) | ||
| Systemic steroids (n=8) | Yes | 3 (37.5%) | 5 (62.5%) | 0.90 |
| No | 6 (27%) | 16 (73%) | ||
| Type of organism | Bacterial (n=18) | 5 (28%) | 13 (72%) | 0.94 |
| Nonbacterial (n=12) | 4 (33%) | 8 (67%) | ||
| Media clarity | No | 6 (27%) | 16 (73%) | 0.92 |
| Yes (n=8) | 3 (37.5%) | 5 (62.5%) | ||
| Vitrectomy (n=23) | Vitrectomy | 9 (39%) | 14 (61%) | 0.05 |
| No vitrectomy | 0 | 7 (100%) | ||
Discussion
There have been no large case series of EE focusing exclusively on the younger (up to 18 years) age group. The review of literature was performed to compare our results with the published literature.
Demography
Though the incidence of pediatric EE is considered rare, our experience shows that in the Indian subcontinent, the incidence is relatively higher affecting 62 (28.9%) out of 214 children with endophthalmitis in our case series. The reason for the higher incidence could be hypothesized to be general malnutrition among the children in this part of the world which reduces the immunity, thus making children more prone to latent infections to become manifest.[7]
The mean age at presentation in our series was 6.8 years (range 1–16 years). Though the published literature has reported cases of EE in very young children, including a small case series from India reporting neonatal EE secondary to neonatal sepsis,[14] the mean age in our series is higher.
The reason for the lack of reporting in the infant and lower age group in this part of the world could be higher infant mortality rate which is about 47.57 per 1,000 live births compared with 4–5 deaths per 1,000 live births in western countries and lack of referral facilities to a tertiary care hospital.[15] Causative organisms implicated in EE include pseudomonas and other Gram-negative bacteria [Table 4]. In our study, we also found a significant association between Gram-negative infection and age of the child; with children <5 years of age having more Gram-negative EE compared with children above 5 years age (P = 0.001).
A male preponderance (23 vs 7 patients) was observed in our study group, similar to that observed in the previous reviews.[8,9,10] The reason for such preponderance was not clear though. In our study, the left (n = 16) and right eyes (n = 14) were nearly equally affected with EE, though Greenwald et al.[8] reported the right eye to be twice likely to be involved and postulated that it was because of the direct arterial blood flow to the right carotid. Later studies done by Wong et al., St. Thomas eye study, and others showed no such difference in laterality.[9,10]
Clinical features: Ocular and systemic
Four (13.3%) eyes presented with nil perception of light. Of these, one eye was prephthisical. Most of the children presented with features similar to the presentation in adults with pain, redness, chemosis, lid edema, anterior chamber cells and flare (100%), vitritis (100%), and vitreous exudates (23%) [Fig. 1]. Some of the children presented with rare and unusual features like granulomas in the posterior segment (10%). Ultrasound of the eyes in all children showed vitreous opacities with or without membranes and increased choroidal thickness.
Figure 1.
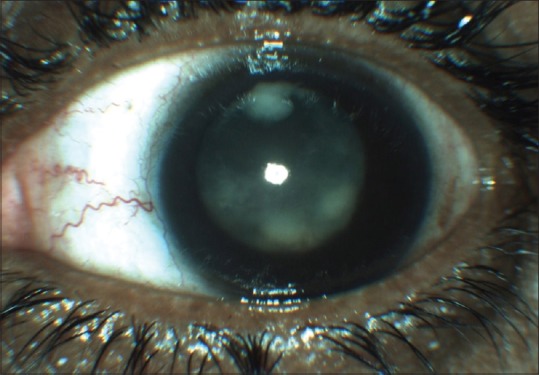
Slit-lamp photograph showing diffuse posterior endogenous endophthalmitis with vitreous exudates seen in anterior vitreous behind the lens. The fundus details were obscured by the vitreous reaction. Stapylococcus aureus was identified from the vitreous culture in this case. Note the relatively quiet anterior chamber
It is known that most of the patients with EE typically present with systemic symptoms and an underlying systemic disease, namely diabetes mellitus, liver abscess, pneumonia, and urinary tract infection.[8] In this study, there were few systemic features; fever being the most common (13%) followed by broncho pneumonia and diarrhea (3.3% each). The lack of systemic features also points to the immunocompromised state of the affected subjects, with the most likely reason for such a presentation being protein-energy malnutrition (PEM). According to UN estimates, 2.1 million Indian children die before reaching the age of 5 every year (4 every minute) due to malnutrition.[16] Most of the subjects in our series were systemically asymptomatic with only ocular complaints as the presenting feature. It is noteworthy that 9 of these cases (30%) were due to parasitic endophthalmitis (toxocara n = 8, cysticercosis n = 1). Subjects were simultaneously referred to internist for their systemic evaluation.
While the clinical features are of help in identifying patients with EE, the most commonly reported misdiagnosis in children is retinoblastoma.[17,18,19]
Two eyes in our series were enucleated with suspicion of retinoblastoma. Both cases (1 and 9 years age, respectively) presented with redness, leukocoria, PL+ vision, with ultrasonography suggesting a retinochoroidal focal mass lesion with a moderate number of vitreous echoes. Additionally, the first case had clump exudates in the anterior chamber, while the second case had eye pain due to raised intraocular pressure, shallow anterior chamber, posterior synechiae, and retrolental neovascularization. However, histopathology and culture of vitreous samples isolated Aspergillus and Toxocara in the two samples, respectively.
Investigations
In reports from Greenwald et al., Wong et al., and St Thomas case series, blood cultures proved to be a very significant source for positive culture growth, with three-quarters of the blood cultures being positive for microbial growth.[6,8,9,10,20,21] In the present case series, none of the blood cultures isolated any organisms. One of the reasons for such a difference could be that all the patients received prior systemic antibiotics before presenting at our tertiary care center. Second, we could not undertake serial blood cultures for the patients and subject the blood for culture only once which could also give falsely negative results. The two most common methods of obtaining an intraocular specimen are anterior chamber (AC) paracentesis and removal of a vitreous sample using a vitreous-cutter. In our series, vitreous samples were obtained in 21 (33%) eyes and AC tap in 4 (13.3%) eyes. Diagnosis of toxocara endophthalmitis was confirmed by ELISA for antitoxocara IgM antibodies in serum of six (20%) and vitreous sample of two (6.7%) subjects. PCR was also helpful in the diagnosis of one case with aspergillus endophthalmitis and is being increasingly utilized in the diagnosis of endophthalmitis.[22,23,24,25,26]
Organisms isolated
Etiology of endophthalmitis identified in our series included Gram-positive bacteria (11 eyes (36.7%)), Gram-negative bacteria (7 (23.3%)), toxocara (8 (26.7%)), fungi (3 (10%)), and cysticercus (1 (3.3%)) eye. In the study by Wong et al. and St Thomas case series, endogenous bacterial endophthalmitis was frequently caused by Gram-negative bacteria.[9,10] Klebsiella has been reported to be the most common organism in EE in adults having diabetes mellitus and liver abscess.[27] Organisms reported to be commonly causing endophthalmitis in children are Pseudomonas aeruginosa, Neisseria meningitides, and Gram-positive bacteria.[8,17] Although in our series, Gram-negative organisms were not the most frequent cause, among the Gram-negative bacteria, both pseudomonas and E. coli contributed equally. An interesting observation in our series was the presence of uncommon less virulent organisms causing endophthalmitis like Alkaligenes spp, Staphylococcus epidermidis, Moraxella, and Micrococci. Enterococcus faecalis reported in our study is also a rare organism causing EE.[28]
Treatment
There has been considerable debate on the treatment of EE. Prompt administration of intravenous antibiotic therapy is of utmost importance in the acute management of EE.[9] However, systemic antibiotics do not reach therapeutic levels within the vitreous and this may explain why patients could develop endogenous bacterial endophthalmitis even while on appropriate systemic antibiotics and despite therapeutic blood levels.[29,30] Wong et al. and Greenwald et al. showed that intravitreal injections did not improve the functional outcomes though in most of these case series including the recent one from St Thomas’ Hospital, 82% of patients underwent a vitreous biopsy and 81% received intravitreal antibiotics.[8,9,10] In contrast, 23 of 30 eyes (77%) in our study underwent vitrectomy with antibiotic and/or steroid injection. The literature review also suggests that eyes that underwent vitrectomy were almost three times more likely to retain useful vision and less likely require enucleation/evisceration.[6,10,20,21] The theoretical advantages of vitrectomy include removal of the infecting organisms, endotoxins, exotoxins, and vitreous membranes that could lead to less tissue damage and better tissue penetration of intravitreally administered antibiotics.[31] In our case series, all the eyes presented with a diffuse posterior variety of endophthalmitis with negative blood cultures in all cases. So early vitrectomy with intravitreal antibiotics was considered the treatment of choice along with systemic antibiotics.
Favorable visual outcome was seen in 9 (35%) of the 23 vitrectomized eyes compared with nonvitrectomized eyes where none of the eyes had a favorable visual outcome. Gram staining features, microbiological profile, and relative media clarity at presentation did not appear to affect the functional outcomes in our study, although these results need to be interpreted with caution in view of relatively small sample size. Intravitreal steroids were given in 8 (27%) of the 30 eyes. The review of literature suggests that eyes treated with intravitreal steroids were more than four times more likely to retain useful (count fingers or better) vision that those that did not.[32] We were not able to compare the visual outcomes in eyes that received intravitreal steroids and those that did not because of small sample size and the concurrent vitrectomy in all these eyes, although better visual outcomes were seen in these eyes. Animal models suggest that intravitreal dexamethasone helps to preserve retinal structure and function.[33]
Another important aspect to ponder is the dosages of intravitreal antibiotics and systemic antibiotics administered to subjects <18 years of age. Since no specific guidelines exist in this regard, we used adult dosage for intravitreal antibiotics.
Outcomes
Anatomical success, with a clear media and attached retina, was achieved in 40% of our patients. Thirty percent had a fairly good visual outcome with vision better than counting fingers from a distance of 3 m, 40% had vision better than hand movements, 30% had nil perception of light, and 2 eyes (6.7%) underwent enucleation. The review of literature from 1976 to 1985 showed that 41% of patients had count fingers vision or better, 26% were with no perception of light, and 29% required evisceration or enucleation.[8] Similar figures were reported over the preceding 30 years. The review of literature since 1986 also indicates an unfavorable outcome, with equivalent figures of 32%, 44%, and 25%, respectively.[9,10] The visual outcomes have not changed much though, despite a significant improvement in management aspects and outcomes are similar irrespective of the difference in age groups. Previous studies have investigated various factors which could affect the visual outcome. These included delay in diagnosis,[17,34] use of inappropriate antibiotics,[34] diffuse infection of the vitreous and retina, or panophthalmitis,[8] infection with virulent organisms, and Gram-negative infection.[10]
Conclusion
To conclude, the differences in endophthalmitis among children and adolescents from that in adults include the relative lack of systemic features and lack of underlying systemic disorders, a high incidence of Gram-positive bacterial etiology and toxocara infection of the eye as well as potential misdiagnosis as masquerade syndrome (retinoblastoma). EE is likely caused by low virulent and less commonly isolated organisms. The advantages of early intervention in the form of vitrectomy in these cases could result in good functional outcomes in a limited few patients although visual rehabilitation remains another challenge in younger children.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hassan IJ, Mac Gowan AP, Cook SD. Endophthalmitis at the Bristol Eye Hospital: An 11-year review of 47 patients. J Hosp Infect. 1992;22:271–8. doi: 10.1016/0195-6701(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 2.Irvine WD, Flynn HW, Miller D, Pflugfelder SC. Endophthalmitis caused by gram negative organisms. Arch Ophthalmol. 1992;110:1450–4. doi: 10.1001/archopht.1992.01080220112031. [DOI] [PubMed] [Google Scholar]
- 3.Shrader SK, Band JD, Lauter CB, Murphy P. The clinical spectrum of endophthalmitis: Incidence, predisposing factors, and features influencing outcome. J Infect Dis. 1990;162:115–20. doi: 10.1093/infdis/162.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Stonecipher KG, Ainbinder DJ, Maxwell DP. Infectious endophthalmitis: A review of 100 cases. Ann Ophthalmol Glaucoma. 1994;26:108–15. [Google Scholar]
- 5.Koul S, Philipson A, Philipson BT. Incidence of endophthalmitis in Sweden. Acta Ophthalmologica. 1989;67:499–503. doi: 10.1111/j.1755-3768.1989.tb04099.x. [DOI] [PubMed] [Google Scholar]
- 6.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: Report of a ten-year retrospective study. Ophthalmology. 1994;101:832–8. [PubMed] [Google Scholar]
- 7.Garg SP, Talwar D, Verma LK. Metastatic endophthalmitis: A reappraisal. Ann Ophthalmol. 1991;23:74–8. [PubMed] [Google Scholar]
- 8.Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: A contemporary reappraisal. Surv Ophthalmol. 1986;31:81–101. doi: 10.1016/0039-6257(86)90076-7. [DOI] [PubMed] [Google Scholar]
- 9.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Opthalmol. 2003;48:403–23. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 10.Wong JS, Chan TK, Lee HM, Chee SP. Endogenous bacterial endophthalmitis: An East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology. 2000;107:1483–91. doi: 10.1016/s0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 11.Reedy JS, Wood KE. Endogenous Pseudomonas endophthalmitis a case report and literature review. Intensive Care Med. 2000;26:1386–9. doi: 10.1007/s001340000623. [DOI] [PubMed] [Google Scholar]
- 12.Balaskas K, Potamitou D. Endogenous Endophthalmitis secondary to bacterial meningitis from Neisseria Meningitidis –a case report and review of literature. Cases J. 2009;2:149. doi: 10.1186/1757-1626-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinch TE, Duker JS, Eagle RC, Jr, Calhoun JH, Augsburger JJ, Fischer DH. Infantile endogenous Candida endophthalmitis presenting as a cataract. Surv Ophthalmol. 1989;34:107–12. doi: 10.1016/0039-6257(89)90038-6. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: A report of six cases. Pediatrics. 2013;131:e1292–7. doi: 10.1542/peds.2011-3391. [DOI] [PubMed] [Google Scholar]
- 15.SRS Bulletin. Sample Registration System. Registrar General, India. 2011 Dec;46 No. 1. Available from: http://pib.nic.in/archieve/others/2012/feb/d2012020102.pdf . [Google Scholar]
- 16.World Bank Report on Malnutrition in India. http://siteresources. worldbank.org/NUTRITION/Resources/281846-1271963823772/India.pdf .
- 17.Murugan G, Shah PK, Narendran V. Clinical profile and outcomes of pediatric endogenous endophthalmitis: A report of 11 cases from South India. World J Clin Pediatr. 2016;5:370–3. doi: 10.5409/wjcp.v5.i4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison SA, Bateman JB. Endogenous endophthalmitis caused by Streptococcus mitis. Am J Ophthalmol. 1997;123:260–1. doi: 10.1016/s0002-9394(14)71048-7. [DOI] [PubMed] [Google Scholar]
- 19.Shields JA, Shields CL, Eagle RC, Jr, Barrett J, De Potter P. Endogenous endophthalmitis simulating retinoblastoma. The 1993 David and Mary Seslen Endowment Lecture. Retina. 1995;15:213–9. [PubMed] [Google Scholar]
- 20.Chou FF, Kou HK. Endogenous endophthalmitis associated with pyogenic hepatic abscess. J AmCol Surg. 1996;182:33–6. [PubMed] [Google Scholar]
- 21.Liao HR, Lee HW, Leu HS, Lin BJ, Juang CJ. Endogenous Klebsiella pneumoniae endophthalmitis in diabetic patients. Can J Ophthalmol. 1992;27:143–7. [PubMed] [Google Scholar]
- 22.Okhravi N, Adamson P, Carroll N, Dunlop A, Matheson MM, Towler HM, et al. PCR-based evidence of bacterial involvement in eyes with suspected intraocular infection. Invest Ophthalmol Vis Sci. 2000;41:3474–9. [PubMed] [Google Scholar]
- 23.Rosenbaum PS, Mbekeani JN, Kress Y. Atypical mycobacterial panophthalmitis seen with iris nodules. Arch Ophthalmol. 1998;116:1524–7. doi: 10.1001/archopht.116.11.1524. [DOI] [PubMed] [Google Scholar]
- 24.Rickman LS, Freeman WR, Green WR, Feldman ST, Sullivan J, Russack V, et al. Brief report: Uveitis caused by Tropheryma whipplii (Whipple's bacillus) N Eng J Med. 1995;332:363–6. doi: 10.1056/NEJM199502093320604. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann CP, Gabel VP, Heep M, Linde HJ, Reischl U. Listeria monocytogenes -induced endogenous endophthalmitis in an otherwise healthy individual: Rapid PCR-diagnosis as the basis for effective treatment. Eur J Ophthalmol. 1999;9:53–7. [PubMed] [Google Scholar]
- 26.Van Gelder RN. Applications of the polymerase chain reaction to diagnosis of ophthalmic disease. Surv Ophthalmol. 2001;46:248–58. doi: 10.1016/s0039-6257(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 27.Chang FY, Chou MY, Fan RL, Shaio MF. A clinical study of Klebsiella liver abscess. J Formosan Med Assoc. 1988;87:282–7. [PubMed] [Google Scholar]
- 28.Rishi E, Rishi P, Nandi K, Shroff D, Therese KL. Endophthalmitis caused by enterococcus faecalis. A Case Series. Retina. 2009;29:214–7. doi: 10.1097/IAE.0b013e31818eccc7. [DOI] [PubMed] [Google Scholar]
- 29.Barza M, Kane A, Baum J. Intraocular penetration of gentamicin after subconjunctival and retrobulbar injection. Am J Ophthalmol. 1978;85:541–7. doi: 10.1016/s0002-9394(14)75252-3. [DOI] [PubMed] [Google Scholar]
- 30.Smith KG, Ihle BU, Heriot WJ, Becker GJ. Metastatic endophthalmitis in dialysis patients. Am J Nephrol. 1995;15:78–81. doi: 10.1159/000168805. [DOI] [PubMed] [Google Scholar]
- 31.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479–96. [PubMed] [Google Scholar]
- 32.Maxwell DP, Brent BD, Diamond JG, Wu L. Effect of intravitreal dexamethasone on ocular histopathology in a rabbit model of endophthalmitis. Ophthalmology. 1991;98:1370–5. doi: 10.1016/s0161-6420(91)32108-0. [DOI] [PubMed] [Google Scholar]
- 33.Park SS, Samiy N, Ruoff K, D’Amico DJ, Baker AS. Effect of intravitreal dexamethasone in treatment of pneumococcal endophthalmitis in rabbits. Arch Ophthalmol. 1995;113:1324–9. doi: 10.1001/archopht.1995.01100100112040. [DOI] [PubMed] [Google Scholar]
- 34.Wang LS, Lee FY, Cheng DL, Liu CY, Hinthorn DR, Jost PM. Klebsiella pneumoniae bacteremia: Analysis of 100 episodes. J Formos Med Assoc. 1990;89:756–63. [PubMed] [Google Scholar]


