Abstract
We report a case of non-familial, sporadic fetal retinoblastoma (RB) that was accidently detected at 39 weeks of gestation on pre-natal ultrasonography in left eye (OS). Post-natal examination revealed Group A and, Group D RB in right eye (OD) and OS, respectively. At 35 days, selective ophthalmic artery intra-arterial chemotherapy (IAC) was performed in OS and laser for OD. Pre-natal ultrasound and its application in RB are limited to those cases with a strong genetic predisposition. Our case was accidently detected at late gestation with no familial or genetic predisposition. In addition, this was the youngest reported case that received IAC on literature review.
Keywords: Fetal retinoblastoma, intra-arterial chemotherapy neonatal retinoblastoma, pre-natal ultrasonography, retinoblastoma
Retinoblastoma (RB) commonly presenting as white reflex and strabismus is often detected in late infancy. Being a hereditary cancer syndrome with associated RB1 gene mutation, prenatal diagnosis of RB is done in the presence of strong familial predisposition. Non-hereditary sporadic RB comprises of 60%. Fetal ultrasound as a routine is an accurate tool in detecting fetal anomalies.[1] Indications of ocular screening as a part of fetal ultrasound is yet to be determined, often limited as pre-natal screening in high-risk scenarios.[2] We report a case of non-familial, sporadic RB accidently detected with fetal ultrasonography and successfully received intra-arterial chemotherapy at 35 days of birth.
Case Report
A 28-year-old G1P1A0, presented at 39 weeks of gestation for routine follow up. Pre-natal ultrasonography showed singleton fetus with normal growth parameters for the gestational age. It also detected a hyper-echoic intraocular lesion in the left eye (OS) measuring 15 × 15 × 12 mm in size that was suspected to be retinoblastoma (RB). Right eye (OD) was found to be normal [Fig. 1a]. After an uncomplicated caesarean section next day, a healthy male baby weighing 3300 grams was delivered. Immediately after birth an underlying intra-ocular tumor was noticed in OS [Fig. 1b]. Subsequently was referred to us for further evaluation and management at 4 weeks post-natal.
Figure 1.
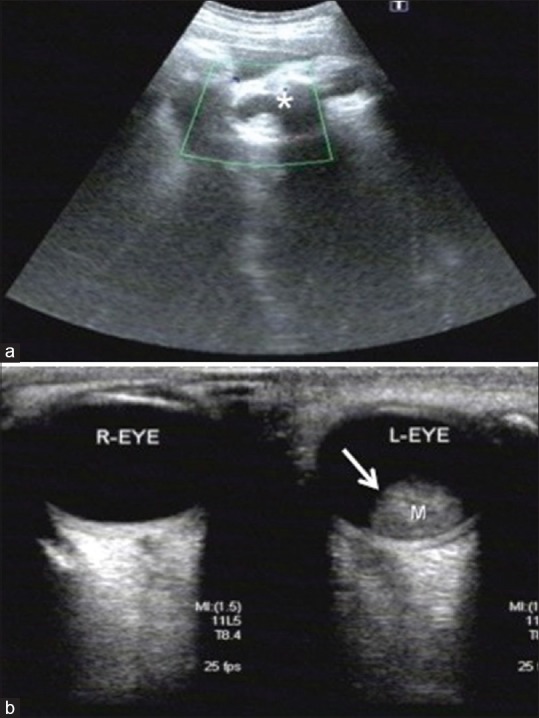
(a) Obstetric ultrasound scan at 39 weeks gestation; coronal image of fetal orbit shows a hyperechoic intraocular lesion on the left side located in the retina measuring 15 × 15 × 12 mm. (white astrix) No lesions are detected in the right orbit. (b) Ultrasound B-scan of right and left eye at birth. There is a solitary dome-shaped elevated mass in the posterior pole with high internal reflectivity and corresponds to the finding in fetal ultrasound intrauterine. (white arrow) Vitreous cavity is normal with dot like echogenicity adjacent to the intraocular mass
Detailed examination under anesthesia was performed. Intraocular pressure was measured normal. Horizontal corneal diameter was 10 mm both eyes (OU) with normal anterior segment. Fundus evaluation revealed bilateral RB. Right eye had normal optic disc and fovea with multiple small equatorial tumors measuring 1.5 × 1.5 × 1 mm and 1 × 1 × 1 mm [Fig. 2a]. A large exophytic solitary white mass with surface vascularization and feeder vessels occupying the macula and, overhanging the optic disc, measured 15 × 12 × 11.5 mm in OS with exudative retinal detachment and diffuse sub-retinal seeds [Fig. 2b]. Contrast enhanced Magnetic Resonance Imaging (MRI) of orbit and brain confirmed intraocular mass in OS with normal optic nerve and intact ocular coats and, brain was normal. Further it was classified as Group A in OD and Group D in OS; TNMH classification (AJCC 8th edition) was cT1a and cT2b in OD and OS respectively with M0, N0, HX status.
Figure 2.
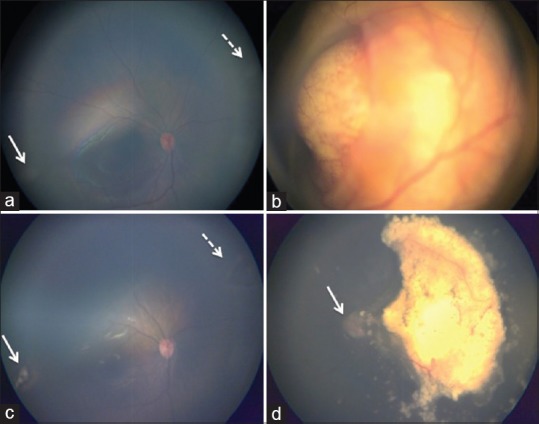
Color fundus photograph of both eyes pre-treatment and post-treatment. Pre- treatment a and b, (a) RB Group A - Shows multiple small retinal lesions in the inferotemporal (white arrow) and superonasal equator (white dotted arrow). Optic disc and fovea is within normal limits. (b) RB Group D- Large solitary whitish retinal mass overhanging the optic disc located at the macula. There is total retinal detachment with diffuse subretinal seeds. Post-treatment c and d, (c) shows complete regression of inferotemporal (white arrow) and superonasal (white dotted arrow) tumors with focal laser therapy. (d) following 2 sessions of selective ophthalmic artery intra-arterial chemotherapy, tumor was completely regressed (Type-1). Subretinal fluid has completely resolved and the optic disc is visualized. (white arrow)
After discussion on various management options with the parent, OD received focal laser and IAC was performed in OS at 35 days post-natal with melphalan (3.5 mg) and alternate carboplatin (20 mg) and topotecan (0.5 mg) in the first and second session, respectively. The tumor regressed (Type 1) after 1st session with complete resolution of exudative retinal detachment after the 2nd session [Fig. 2c]. Following 2 months of focal treatment, OD developed a new tumor that received laser [Fig. 2d]. At 14 months there was no recurrence OU.
Discussion
Retinoblastoma although arising from the embryonal cells, remains undetected until birth to present as white-reflex and strabismus. Prenatal diagnosis of RB is usually done in high-risk scenarios as in the presence of strong familial predisposition, prenatal positive RB1 mutation and, mutation detected in the parent or sibling.[1] These comprises of 40% cases reported worldwide and, are often bilateral with multiple tumors. Rest of the 60% is non- familial, nevertheless 20% of this can have germline mutation and present early.[2] These often go unnoticed, to present as advanced tumors in neonates that pose risk to life and eye salvage. Neonatal RB was reported as 7–10%.[3,4]
Fetal ultrasound is an accurate tool for evaluating intra-uterine formative period and, detecting fetal facial anomalies and congenital malformations. Ocular abnormalities detected during pre-natal ultrasound are congenital cataract, teratoma, and RB. Maat-Kievit et al. reported the first case of fetal RB presenting as large facial tumor at 21 weeks of gestation with termination of pregnancy.[5] Salim et al. reported a case presenting as orbital mass at 38 weeks of gestation.[6] Indications of ocular screening as a part of fetal ultrasound is yet to be determined. Lehman detected RB in a preterm neonate at 36 weeks that went undetected in pre-natal ultrasonography.[7] The ability to detect tumors is dependent on size, smaller tumors may often go undetected as in OD of our patient. However, RB is reported to have a doubling time of 15 days.[8] Therefore, in a fetus it grows to attain a large size where eye salvage is hindered by the time it is clinically diagnosed.
Post- natal eye screening of neonates is limited to premature born babies and there are no universal guidelines. In a pilot study from India, 1021 term neonates with birth weight more than 2000 grams (gms) within 3 days from birth were screened and found one RB (0.10%).[8] Similarly, Li et al. screened 3573 healthy full-term Chinese neonates weighing atleast 2500 gms within a week after birth. Of this 2 (0.06%) were suspected to have RB or astrocytic hamartoma.[9] Neonatal RB screening is again limited to those that have a family history. But, sporadic variant is not rare, which can present early and often goes undetected unless they enlarge to a size that is visible as white reflex. Kivela et al. reported 4 out of 11 (36%) neonates with sporadic RB.[4] Since retinal development is known to have a prominent centro-peripheral gradient, it is often foveal in location progressing to involve the entire entire macula. Late detection challenges vision salvage. Although majority is unilateral to begin with, it is not uncommon to find new tumors in contralateral eye subsequently. The disease severity can also be asymmetrical as in our case.
Management of neonatal RB is challenging in terms of treatment and parental psychological stress. They often have tumor relapses, mandating frequent, and long-term follow-up. It is best managed in retinoblastoma centers under a multidisciplinary team considering the complexities. Systemic intravenous chemotherapy (IVC) in bilateral RB is effective and can possibly be protective in the development of pinealoblastoma, although controversial. Intra-arterial chemotherapy is advantageous with negligible systemic side effects, but often preferred in children >6 months of age. The major concerns in neonates include the catheterization of small arterial branches that can cause difficulty in catheterization. Magan et al. reported a 2-month-old infant who underwent IAC.[11] We successfully performed selective ophthalmic artery chemotherapy at 35 days, the youngest reported, with excellent outcome.
Conclusion
Pre-natal ultrasound was able to detect a large fetal RB in our case that was non-familial and sporadic. This emphasizes that including facial and ocular examination during fetal ultrasound may help in early detection of fetal RB, a curable eye cancer. A dilated pediatric eye screening during routine neonate and infant examinations may also aid in early detection. We also found that IAC at 4th weeks of age was safe and effective with excellent outcome. However, it requires well-trained multidisciplinary team to avoid unforeseen complications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Paquette LB, Miller D, Jackson HA, Lee T, Randolph L, Murphree AL, et al. In utero detection of retinoblastoma with fetal magnetic resonance and ultrasound: Initial experience. AJP Rep. 2012;2:55–62. doi: 10.1055/s-0032-1316465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dommering CJ, Heneman L, Van Der Hout AH, Jonker MA, Tops CM, van den Ouweland AM, et al. Uptake of prenatal diagnostic testing for retinoblastoma compared to other hereditary cancer syndrome in the Netherlands. Fam Cancer. 2017;16:271–7. doi: 10.1007/s10689-016-9943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson DH, Du TT, Beaverson KL. (Neonatal) retinoblastoma in the first month of life. Arch Ophthalmol. 2002;120:738–42. doi: 10.1001/archopht.120.6.738. [DOI] [PubMed] [Google Scholar]
- 4.Kivelä TT, Hadjistilianou T. Neonatal retinoblastoma. Asia Pac J Oncol Nurs. 2017;4:197–204. doi: 10.4103/apjon.apjon_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maat-Kievit JA, Oepkes D, Hartwig NG, Vermeij-Keers C, van Kamp IL, van de Kamp JJ. A large retinoblastoma detected in a fetus at 21 weeks of gestation. Prenat Diagn. 1993;13:377–84. doi: 10.1002/pd.1970130510. [DOI] [PubMed] [Google Scholar]
- 6.Salim A, Wiknjosastro GH, Danukusumo D, Barnas B, Zalud I. Fetal retinoblastoma. J Ultrasound Med. 1998;17:717–20. doi: 10.7863/jum.1998.17.11.717. [DOI] [PubMed] [Google Scholar]
- 7.Lehman SS. Antenatal ophthalmology. JAAPOS. 2003;7:428–9. doi: 10.1016/j.jaapos.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shah PK, Narendran V, Kalpana N. In vivo growth of retinoblastoma in a newborn infant. Indian J Ophthalmol. 2010;58:421–3. doi: 10.4103/0301-4738.67066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinekar A, Govindraj I, Jayadev C, Kumar AK, Sharma P, Mangalesh S, et al. Universal ocular screening of 1021 term infants using wide-field digital imaging in a single public hospital in India- A pilot study. Acta Ophthalmol. 2015;93:e317–6. doi: 10.1111/aos.12685. [DOI] [PubMed] [Google Scholar]
- 10.Li LH, Li N, Zhao JY, Fei P, Zhang GM, Mao JB, et al. Findings of perinatal ocular examination performed on 3573, healthy full- term newborns. Br J Ophthalmol. 2013;97:588–91. doi: 10.1136/bjophthalmol-2012-302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magan T, Khoo CT, Jabbour PM, Shields CL. Intraarterial chemotherapy for retinoblastoma in 2-month-old infant. Retin Cases Brief Rep. 2017;11:24–6. doi: 10.1097/ICB.0000000000000279. [DOI] [PubMed] [Google Scholar]


