Abstract
Purpose:
To study the efficacy of intravitreal Aflibercept, an anti Vascular Endothelial Growth factor, (anti-VEGF) in Retinopathy of Prematurity (ROP).
Methods:
This was a retrospective, interventional consecutive case series of 46 Indian eyes which received intravitreal injection of Aflibercept for High risk Prethreshold ROP, Threshold ROP, and Aggressive-Posterior ROP (AP-ROP).
Results:
Aflibercept was effective in achieving the primary endpoint, namely regression of ROP following the injection in all 46 eyes (100%) at one week following the injection. 32.6% (15/46) of eyes achieved secondary endpoint namely complete vascularization, with no recurrence of ROP at varying time intervals: as early as 15 weeks to as late as 29 weeks after injection, at intervals ranging from 49 to 64 weeks PCA.
Conclusion:
Intravitreal Aflibercept was effective in inducing complete regression of all types of ROP in all the eyes in our series. In addition, 32.6% of cases did not need a secondary intervention, with no recurrence of ROP and complete vascularization of the retina. In 81.8% of Zone I ROP eyes, Aflibercept facilitated continuation of retinal vascular development following regression of ROP, resulting in less extensive laser during treatment of ROP recurrence. This is the largest series of Aflibercept in ROP, till date as per MEDLINE search.
Keywords: Aflibercept, prematurity, retinopathy, VEGF
Intravitreal injections of Anti- VEGF (Vascular Endothelial Growth factor) are increasingly being employed in eyes with Retinopathy of Prematurity (ROP), ever since the BEAT-ROP study highlighted the efficacy of intravitreal Bevacizumab in the management of stage III plus ROP.[1,2] Of all the anti-VEGF agents, Bevacizumab has been employed the maximum in ROP with several studies including comparison with laser photocoagulation which is the standard of care, available in the literature.[3,4] Aflibercept (EYLEA; Regeneron Pharmaceutical Inc, Tarrytown, NY, USA and Bayer, Basel, Switzerland), also named VEGF Trap-eye, has been employed in ROP, previously as monotherapy in 26 eyes.[5] We hereby report our experience with Aflibercept in ROP.
Methods
The clinical data of 46 consecutive Indian eyes referred to a tertiary eye care centre in South India, which received 0.025 ml (1 mg) of Aflibercept for High risk Prethreshold ROP, Threshold ROP, Aggressive-Posterior ROP (AP-ROP) was retrospectively analyzed. Informed consent was taken from the parents of all the babies, prior to the procedure. Both eyes of 23 babies with ROP (46 eyes) received the injection sequentially in the same sitting.
Primary endpoint was defined as regression of ROP following the injection. Secondary endpoint was defined as complete retinal vascularization without recurrence of ROP. The initial and subsequent serial ROP screenings was done by indirect ophthalmoscopy after pupillary dilatation by a single pediatric retina specialist (author, VV). Complete vascularization was deemed to have been achieved after the retinal vessels were seen reaching the ora by indirect ophthalmoscopy. Fluorescein Angiography (FA) was not done.
Results
Aflibercept was effective in achieving the primary endpoint, namely regression of ROP following the injection in all 46 eyes (100%) at 1 week following the injection. Hence, rescue treatments for worsening of ROP despite the injection were not carried out in our cohort. Table 1 is the consolidated data of all the 46 eyes of 23 babies. Figs. 1–4 are the original fundus photographs before and 1 week after intravitreal Aflibercept in right and left eye of a baby with threshold ROP, showing regression of plus disease and new vessels as well as decrease in ridge height, following the injection.
Table 1.
Consolidated data of RAVE study 1
| S. no. of baby | GA (weeks) | B Wt. (Gms) | Type of ROP | PCA at time of 1st treatment (IV Aflibercept) | Zone at time of 1st treatment (IV Aflibercept) | Primary outcome (regression of ROP) and time | Nature of 2nd treatment | PCA in weeks and Zone at 2nd treatment | PCA, Zone and Nature of 3rd treatment | Secondary outcome (complete retinal vascularisation with no ROP Recurrence) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | 660 | HR Prethreshold | 34 | 2 | Yes, 1 week after injection | Laser | 45 (LE) 48(RE). Zone II | No | |
| 2 | 29 | 1050 | Threshold | 36 | 2 | Yes, 1 week after injection | Laser | 43 (LE) 45 (RE); Zone II | No | |
| 3 | 29 | 900 | AP ROP | 36 | 1 | Yes, 1 week after injection | None | Yes | ||
| 4 | 27 | 580 | AP ROP | 33 | 2 | Yes, 1 week after injection | Laser | 41, Zone II | No | |
| 5 | 31 | 1020 | AP ROP | 35 | 2 | Yes, 1 week after injection | None | Yes | ||
| 6 | 27 | 680 | AP ROP | 33 | 1 | Yes, 1 week after injection | Laser | 46, Zone II | No | |
| 7 | 28 | 906 | HR Prethreshold | 34 | 2 | Yes, 1 week after injection | None | Yes | ||
| 8 | 27 | 740 | Threshold | 33 | 2 | Yes, 1 week after injection | Laser | 40, Zone II | No | |
| 9 | 29 | 915 | AP ROP | 37 | 2 | Yes, 1 week after injection | None | Yes | ||
| 10 | 27 | 534 | HR Prethreshold | 35 | 2 | Yes, 1 week after injection | None | Yes | ||
| 11 | 26 | 670 | AP ROP | 35 | 1 | Yes, 1 week after injection | Laser | 48, Zone II | No | |
| 12 | 29 | 740 | AP ROP | 37 | 1 | Yes, 1 week after injection | IV Bevacizumab | 44, Zone I | 68 weeks Z1 Laser | No |
| 13 | 28 | 980 | AP ROP | 34 | 2 | Yes, 1 week after injection | IV Ranibizumab | 48, Zone II | 60 weeks Z2 Laser | No |
| 14 | 35 | 1400 | HR Prethreshold | 39 | 2 | Yes, 1 week after injection | None | Yes | ||
| 15 | 29 | 1050 | Threshold | 36 | 1 | Yes, 1 week after injection | Laser | 44, Zone II | No | |
| 16 | 33 | 1520 | AP ROP | 45 | 1 | Yes, 1 week after injection | Laser | 52, Zone II | No | |
| 17 | 26 | 725 | HR Prethreshold | 35 | 1 | Yes, 1 week after injection | Laser | 48, Zone II | No | |
| 18 | 28 | 675 | AP ROP NVE | 35 | 1 | Yes, 1 week after injection | IV Aflibercept | 44 (RE) 46 (LE); Zone I | 54 weeks Z2 Laser | No |
| 19 | 21 | 980 | AP ROP | 27 | 1 | Yes, 1 week after injection | IV ranibizumab | 48 (RE) 49 (LE); Zone II | No | |
| 20 | 31 | 1220 | HR Prethreshold | 35 | 1 | Yes, 1 week after injection | Laser | 40; Zone II | No | |
| 21 | 30 | 600 | HR Prethreshold | 36 | 2 | Yes, 1 week after injection | Laser (LE) None (RE) | 49, Zone II | No (LE) Yes (RE) |
|
| 22 | 30 | 1180 | AP ROP with NVE | 34 | 1 | Yes, 1 week after injection | None | Yes | ||
| 23 | 26 | 880 | Threshold ROP | 33 | 2 | Yes, 1 week after injection | Laser | 47, Zone II | No |
Figure 1.
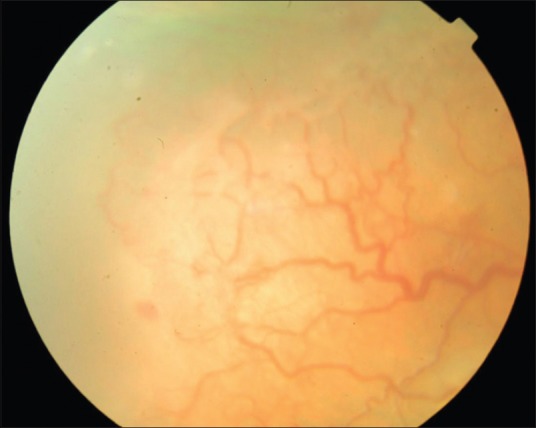
Pre injection fundus photograph of the right eye of a baby with threshold ROP
Figure 4.
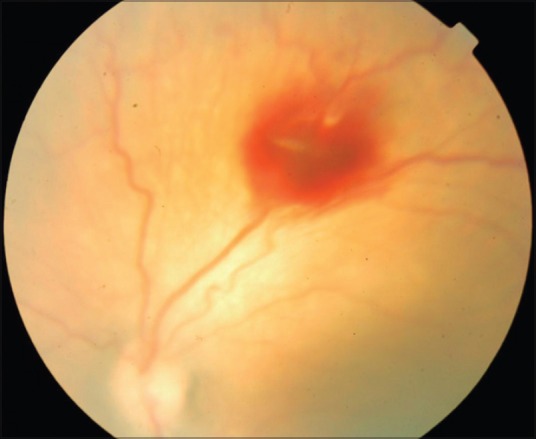
Post injection fundus photograph of the left eye of a baby with threshold ROP, showing regression of new vessels and plus disease as well as reduced height of the ridge
Figure 2.
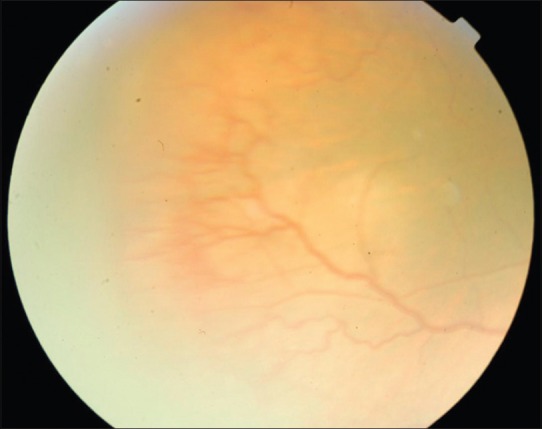
Post injection fundus photograph of the right eye of a baby with threshold ROP, showing regression of new vessels and plus disease as well as reduced ridge height
Figure 3.
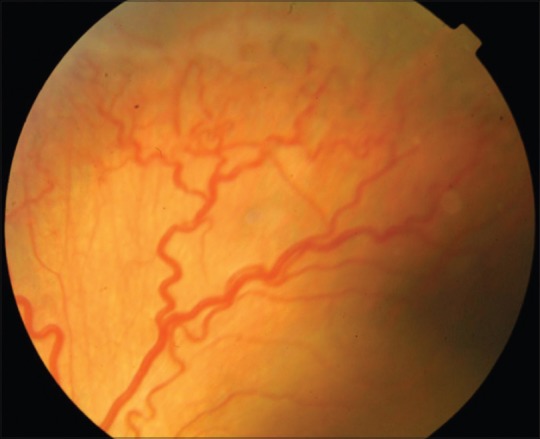
Pre injection fundus photograph of the left eye of a baby with threshold ROP
Number of eyes achieving secondary endpoint namely complete vascularization, with no recurrence of ROP was noted, [Table 2]. In our series, only 32.6% (15/46) achieved the secondary outcome, at varying time intervals: as early as 15 weeks to as late as 29 weeks after injection, at intervals ranging from 49 to 64 weeks PCA. Number and characteristics of eyes that failed to achieve the secondary endpoint and had recurrence of ROP with need for secondary intervention in the form of repeat anti-VEGF injection/laser, was also noted. No complications directly attributable to the injection or the procedure was noted in any of the babies. No eye progressed to stages IV or V in our series.
Table 2.
Number of eyes vis-a-vis secondary outcome: Recurrence of ROP following Intravitreal Aflibercept
| Type of ROP | Total number of eyes | Number of Eyes that achieved secondary endpoint: No recurrence of ROP after first injection | Number of Eyes that did not achieve secondary endpoint: Recurrence of ROP with second intervention |
|---|---|---|---|
| High risk Prethreshold ROP | 14 | 7 (50%) | 7 (50%) |
| Threshold ROP | 8 | 0 | 8 (100%) |
| AP-ROP | 24 | 8 (33%) | 16 (66%) |
The duration of intraocular action of Aflibercept was extrapolated from the analysis of 31 eyes which had recurrence of ROP to be atleast 6 weeks.
In eyes that did not achieve the secondary endpoint, the percentage of eyes in which continuation of vascularization occurred prior to recurrence of ROP was determined by analysis of eyes with Zone I ROP [Table 3]. In our series of 22 eyes with Zone I ROP, Aflibercept facilitated continuation of retinal vascular development following regression of ROP in 81.8% of eyes (18/22).
Table 3.
Eyes with continued vascularisation after regression of Zone I ROP following Intravitreal Aflibercept
| Type of ROP | Total no. of eyes | Total no. of eyes with zone I disease | No. of eyes with zone I disease requiring second intervention | No. of eyes with Zone I disease that had Zone II vascularisation during second intervention |
|---|---|---|---|---|
| High Risk Prethreshold ROP | 14 | 4 | 4 | 4 (100%) |
| Threshold ROP | 8 | 2 | 2 | 2 (100%) |
| AP-ROP | 24 | 16 | 12 | 8 (8/12: 66%) |
Discussion
There could be several advantages to the use of Intravitreal Aflibercept in comparison to other anti-VEGF agents, in ROP. The binding affinity of aflibercept to VEGF receptor (Kd = 0.49 pmol/L) is almost 100 times higher than that of ranibizumab (Kd = 46 pmol/L) and bevacizumab (Kd = 58 pmol/L). Of importance, Aflibercept alone can inhibit VEGF-A (Vascular Endothelial Growth Factor) as well as PlGF-1 and -2 (Placental Growth factor) and VEGF-B, which have also been implicated in pathological vascular remodeling.[6] The heightened efficacy and increased duration of action of Aflibercept could help in quickly inducing regression of ROP in very severe cases and help to maintain the regression. In our series, the primary endpoint, namely regression of ROP was seen in 100% of cases [Table 1]. In Arambulo et al's series of 16 eyes receiving intravitreal Ranibizumab monotherapy alone, regression of ROP was seen in 14 eyes (87.5%).[4] In Salman et al's series of 26 eyes receiving intravitreal Aflibercept monotherapy for ROP, 25 eyes (96.2%) had complete regression of ROP following a single injection.[5]
Second, the intermediate size of aflibercept (115 kDa compared to 48 kDa for ranibizumab and 148 kDa for bevacizumab) results in an estimated intravitreal half-life of 7.1 days and a duration of clinical action possibly as long as 2.5 months[7,8] which is almost twice the 1-month duration of action of ranibizumab. This could result in need for less frequent injections and possibly less recurrence. However, in our series, only 32.6% (15/46 eyes) achieved the secondary outcome of no recurrence following Aflibercept monotherapy. 31 eyes had recurrence in our series. Interestingly, in Salman et al's series of 26 eyes receiving intravitreal Aflibercept monotherapy for ROP, all the 25 eyes (96.2%) which had regression of ROP had no recurrence following a single injection.[5] In Hwang et al's series on intravitreal Bevacizumab for ROP, recurrence following a single injection was seen in 3 of 22 eyes (14%) of cases.[3]
A third advantage of intravitreal Aflibercept in ROP could be, even in eyes with recurrence, the increased duration of intraocular action of the drug could possibly result in continuation of retinal vascularization into the higher zones, with a consequently reduced need for extensive laser. This is important since, extensive laser to large areas of avascularity in ROP has been suggested to be associated with peripheral field loss and high myopia.[2]
In this series, all the eyes with High risk prethreshold ROP, Threshold ROP and AP-ROP had vascularization in Zone I or very posterior Zone II. The parents were explained the option of anti -VEGF injection in an attempt to avoid refractive errors and compromise of peripheral visual field in their children, following laser. They were also explained that laser photocoagulation was the gold standard and the pros and cons of both the procedures. But the parents of all babies chose intravitreal Aflibercept injection to avoid the side effects due to laser.
In our series of 22 eyes with Zone I disease, 18 eyes had recurrence [Table 3]. Of these, in 14 eyes, vascularization had proceeded to Zone II following regression of ROP. Four eyes had complete vascularization of the retina following a single injection of Aflibercept [Table 2]. Thus, in our series of Zone I ROP, 81.8% of eyes, (18/22), Aflibercept facilitated continuation of retinal vascular development following regression of ROP. Of note, in Arambulo et al's series of 16 eyes receiving intravitreal Ranibizumab monotherapy, retina remained incompletely vascularized upto 6 months in all the 14 eyes showing regression of ROP.[4] In contrast, in Salman et al's series of 26 eyes receiving intravitreal Aflibercept monotherapy for ROP, complete vascularization was achieved in all the 25 eyes (96.2%) that had ROP regression, 6–8 weeks following the injection.[5]
In our series of 46 eyes, only 32.6% (15/46) achieved the secondary outcome, namely complete vascularization of the retina with no recurrence of ROP following a single injection of Aflibercept; (Although we did not do fluorescein angiography, we could clearly follow the dichotomously branching vessels to the ora serrata, in these cases). The recurrence of ROP in the majority of cases could be a reflection of the severity of the antecedent ROP and the systemic factors in the neonatal population. Amongst the 31 eyes with secondary intervention for recurrence of ROP, the recurrence occurred earlier than 2 months in 9 eyes (9/31, 29%), (recurrence in 5 weeks in 2 eyes and 7 weeks in 7 eyes); (the two months duration was analyzed as it is the duration of action of Aflibercept in adult eyes).[7] Hence, we can safely extrapolate that Aflibercept could be expected to maintain its duration of intraocular action for at least 6 weeks following injection in eyes with ROP, this could have implications in follow up care of these babies following regression of ROP.
Fourth, there have been increasing concerns on the systemic effects of intravitreally injected anti-VEGF agents in preterm babies. The systemic half-life of unbound aflibercept is 1.5 days, much lesser than that of bevacizumab (20 days) and closer to that of ranibizumab (6 hours). A recent publication has shown that there was a more pronounced suppression of systemic VEGF in ROP babies treated with Intravitreal Bevacizumab than Aflibercept.[9] Moreover, Bevacizumab is not approved for intraocular use. With all these factors in mind, we had advised intravitreal Aflibercept to our patients with ROP.
We acknowledge that only the short-term outcomes following intravitreal Aflibercept in ROP are presented in this study and an analysis of long-term neurodevelopmental, refractive and structural outcomes in these eyes are necessary to throw more light on the role of intravitreal aflibercept in ROP.
Conclusion
In conclusion, Intravitreal Aflibercept was effective in inducing complete regression of all types of ROP in all the eyes in our series. In addition, 32.6% of cases did not need a secondary intervention, with no recurrence of ROP and complete vascularization of the retina. In 81.8% of Zone I ROP eyes, Aflibercept facilitated continuation of retinal vascular development following regression of ROP, resulting in less extensive laser during treatment of ROP recurrence. This is the largest series of Aflibercept in ROP, till date as per MEDLINE search.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Patel JR, Ranjan SS, Wasserman BN. Antivascular endothelial growth factor in the treatment of retinopathy of prematurity. Curr Opin Ophthalmol. 2016;27:387–92. doi: 10.1097/ICU.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 2.Mintz-Hittner HA, Kennedy KA, Chuang AZ BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: A 5-year retrospective analysis. Ophthalmology. 2015;122:1008–15. doi: 10.1016/j.ophtha.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arámbulo O, Dib G, Iturralde J, Duran F, Brito M, Fortes Filho JB. Intravitreal ranibizumab as a primary or a combined treatment for severe retinopathy of prematurity. Clin Ophthalmol. 2015;9:2027–32. doi: 10.2147/OPTH.S90979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salman AG, Said AM. Structural, visual and refractive outcomes of intravitreal aflibercept injection in high-risk prethreshold type 1 retinopathy of prematurity. Ophthalmic Res. 2015;53:15–20. doi: 10.1159/000364809. [DOI] [PubMed] [Google Scholar]
- 6.Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E, Costagliola C. Aflibercept in wet AMD: Specific role and optimal use. Drug Des Devel Ther. 2013;7:711–22. doi: 10.2147/DDDT.S40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart MW. What are the half-lives of ranibizumab and aflibercept (VEGF Trap-eye) in human eyes? Calculations with a mathematical model. Eye Reports. 2011;1:e5. [Google Scholar]
- 8.Tarrytown, NY, USA: Regeneron Pharmaceuticals, Inc; [Last accessed on 2012 Apr 02]. EYLEA™ (aflibercept) injection: US prescribing information. Available from: http://www.regeneron.com/Eylea/eylea-fpi.pdf . [Google Scholar]
- 9.Huang CY, Lien R, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefe's Arch Clin Exp Ophthalmol. 2018;256:479–87. doi: 10.1007/s00417-017-3878-4. [DOI] [PubMed] [Google Scholar]


