Abstract
Purpose:
This study evaluated the efficacy of retrobulbar ropivacaine plus dexmedetomidine compared with systemic fentanyl in pediatric vitreoretinal (VR) surgery.
Methods:
This prospective double-blind, randomized controlled study was performed in 60 children undergoing VR surgery, age from 2 to 7 years. After general anesthesia, the following procedure was administrated: (1) retrobulbar block with 0.5% ropivacaine plus dexmedetomidine 1 μg/kg (group RD, n = 20), (2) retrobulbar block with 0.5% ropivacaine (group RB, n = 20), and (3) control group with general anesthesia only (group F, n = 20). Hemodynamics, postoperative pain scores, anesthetics consumption (remifentanil, propofol, fentanyl), and emergence agitation were recorded.
Results:
Respiratory depression was observed in 7 of the 20 patients in group F after the laryngeal mask airway was removed in the operating room, compared with none in groups RD and RB. All patients in group F required intraoperative rescue fentanyl (average intraoperative fentanyl consumption, 26.6 ± 12.6 μg per patient). Some rescue fentanyl was required in group RB (three patients required one dose of rescue fentanyl). Patients in group RD required none. Groups RD and RB reported lower pain scores than group F at 4 h postoperatively (RD group: P < 0.001; RB group: P =0.002); pain scores in group RD were lower than that in group F at 6 h postoperatively (P < 0.001).
Conclusion:
Retrobulbar dexmedetomidine as an adjuvant to ropivacaine is a safe and effective alternative to systemic fentanyl. This regimen provides better pain management, hemodynamic stability, and stress response suppression in pediatric VR surgery.
Keywords: Pediatric, retrobulbar dexmedetomidine, ropivacaine, vitreoretinal surgery
Postoperative opioid-induced respiratory depression is the main cause of death and brain damage in the perioperativeperiod.[1,2] Regional anesthesia combined with general anesthesia decreased opioid-related adverse events in major pediatric surgeries.[3,4] Surgical stress associated with pediatric strabismus and vitreoretinal (VR) surgery has been reported.[5,6] Our hypothesis is retrobulbar dexmedetomidine, which is commonly used in China, could reduce the need of fentanyl and pain scores, meanwhile prolong the duration of analgesia. Thus, we administrated 1 μg/kg dexmedetomidine with retrobulbar block according to a previous study.[7]
Methods
The study was approved by the hospital's Ethics Committee. Written consents were obtained from the parents of the participating patients.
Patients of American Society of Anesthesiologists physical status I to II, age 2–7 years, who underwent elective VR surgery at our hospital, were selected for the study. All surgeries were performed vitrectomy without scleral buckling. Exclusion criteria include consent not obtained, upper airway infection, allergy to anesthetics, and eye tumors or infections.
Sixty patients were randomized to receive general anesthesia plus retrobulbar dexmedetomidine (group RD) or retrobulbar block (group RB), or general anesthesia alone (group F). The randomization was performed in a 1:1:1 ratio using SPSS 25.0 (IBM Inc., Chicago, IL, USA). A nurse, not involving in patient management, prepared all the anesthetics. The parent, investigator, and anesthesiologist were blinded to the group assignments.
After induction, 1 mL of venous blood was withdrawn to test the baseline stress response biomarkers. An intravenous infusion of 2–4 mL/kg/h Ringer's lactate was maintained during the surgery. The patients were monitored by electrocardiography, pulse oximetry (SPO2), heart rate (HR), noninvasive blood pressure (BP), capnography (ETCO2), and bispectral index (BIS). General anesthesia was induced with propofol 3 mg/kg, rocuronium 0.6 mg/kg, and remifentanil 3 μg/kg in all the three groups. A laryngeal mask airway (LMA) was inserted to secure the airway. Patients in group RD were administered retrobulbar block with 0.5% ropivacaine 0.1 mL/kg plus dexmedetomidine 1 μg/kg and group RB with 0.5% ropivacaine 0.1 mL/kg only. A blunt 5-cm needle was inserted at the inferolateral margin of the orbit along the orbital floor until its tip was posterior to the eye at the apex of the orbit, and the assigned anesthetics were injected. Anesthesia was maintained with propofol (guided by the BIS value, with dosages set to maintain a target BIS value of 40–60) and remifentanil (0.1 μg/kg/min). When there were signs of inadequate anesthesia (15% increase of baseline HR or BP), a bolus of fentanyl 1 μg/kg was given to all three groups.
After the surgical procedures, propofol and remifentanil infusions were stopped. Residual neuromuscular block was reversed with neostigmine 50 μg/kg and atropine 0.01 mg/kg. LMA was removed after ensuring respiratory sufficiency. Postoperative neuroendocrine response biomarkers were tested with 1 mL of venous blood. All the patients were monitored for 2 h in postanesthesia care unit (PACU). Patients with Aldrete recovery score ≥9 were discharged to the ward. The time intervals between propofol/remifentanil discontinuation and LMA removal or eye opening in response to verbal stimuli were recorded.
The primary endpoint was the postoperative pain score (Faces, Legs, Activity, Cry and Consolability – FLACC), which was assessed by an observer blinded to the study immediately after LMA removal (FLACC scores ranging from 0 to 10, with 10 representing the most painful experience). Pain score was recorded at 0.5, 1, 2, 4, 6, 12, and 24 h after surgery. When the pain score was >5, rescue analgesic was administered using fentanyl 0.5 μg/kg intravenously in the PACU or oral acetaminophen 10 mg/kg in the ward. The secondary endpoints include remifentanil and propofol consumption, the incidence of emergence agitation, and sedation level. HR and mean arterial BP (MAP) were recorded at T0 (baseline), T1 (10 min after retrobulbar block), T2 (before incision), T3 (incision), T4 (intraocular exploration), T5 (immediately after LMA removal), and T6 (upon arriving PACU). The incidence of emergence agitation was defined as Pediatric Anesthesia Emergence Delirium (PAED) score ≥12. Sedation level was assessed using Richmond Agitation Sedation Scale (RASS) score ranging from −5 to 4. The PAED score was recorded as 0 when the RASS score was less than −2.
The blood that was withdrawn before anesthesia and after LMA removal was used to test the stress response biomarkers, including plasma interleukin-2 (IL-2), IL-6, IL-10, and tumor necrotic factor α (TNF-α). All samples were centrifuged at 4°C, and the plasma was collected and stored at −70°C. Analyses were performed with a commercially available radioimmune kit (Abcam UK Bio, Shanghai, China).
Respiratory depression is defined as a persistent respiratory rate <10 breaths/min or persistent oxygen desaturation <92% for longer than 1 min. Oculocardiac reflex (a decrease in HR >20%) was treated by cessation of the surgical stimulus, if ineffective atropine 0.01 mg/kg was administrated. Complications related to the retrobulbar block, such as retrobulbar hemorrhage or eyeball perforation, were documented.
Statistical analysis
Our pediatric VR surgery database revealed that pediatric VR surgery under general anesthesia with adjunctive fentanyl was associated with FLACC pain scores of 4.3 ± 1.3. The sample size was calculated based on an α of 0.05 and β of 0.1. Sample size of 17 subjects per group would be required for a 1.5 points decrease in pain scores compared with group F. Sixty subjects were enrolled in this study, which allowed about 10% incomplete follow-up or dropout. Statistics are presented as mean ± standard deviation or median (interquartile range) using SPSS version 25.0 (IBM Inc.). Categorical data are presented as numbers. Comparisons between groups were analyzed using independent t-test or Fisher's exact test as appropriate. For pain scores that were not normally distributed, comparisons were performed using Mann–Whitney U-test. Time-course measurements of hemodynamic variables were analyzed using repeated measures analysis of variance, and intergroup differences were compared using one-way analysis of variance. Significance was defined as *P < 0.05.
Results
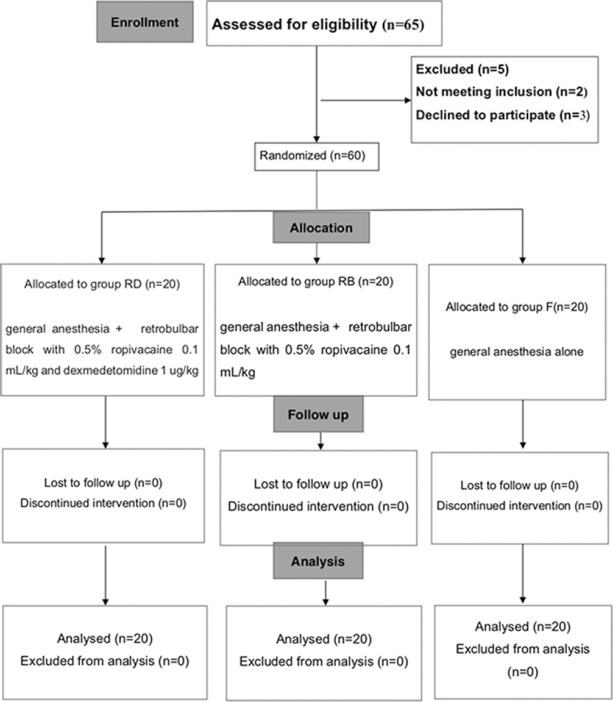
We initially assessed 65 patients for eligibility for participation in this study [Fig. 1]. Two patients did not meet the inclusion criteria, and three patients declined to participate. A total of 60 patients were randomized to treatment allocation and their data were included in the analysis. Patient's age, body mass index, gender, and duration of surgery did not differ among the groups [Table 1].
Figure 1.
Consort 2010 flow diagram
Table 1.
Clinical profile of patients enrolled in each treatment group
| Variable | Group RD (n=20) | Group RB (n=20) | Group F (n=20) |
|---|---|---|---|
| Gender, M:F | 16:24 | 21:19 | 23:17 |
| Age (years) | 4.5±1.2 | 4.4±1.6 | 4.2±2.0 |
| Weight (kg) | 16.4±3.9 | 17.1±4.2 | 16.2±3.8 |
| BMI (kg/m2) | 21.68±2.81 | 20.68±3.10 | 21.10±2.76 |
| Duration of surgery (min) | 85.63±28.42 | 81.83±25.89 | 77.38±23.45 |
BMI=Body mass index; SD=Standard deviation. Values are mean±SD or number. No statistically significant differences were noted between the groups
A significant difference in propofol consumption was observed [Table 2]. Propofol consumption was decreased significantly in group RD compared with group RB (206.2 ± 61.4 vs 261.7 ± 62.9, P = 0.009), while groups RB and group F did not show significant difference (P = 0.237). Remifentanil consumption was comparable among the three groups [Table 2]. All patients in group F were administered fentanyl as rescue analgesic during the surgery (i.e., 7 patients required one dose of fentanyl rescue 1 μg/kg; 11 patients required two doses, and 2 patients required three doses). The average intraoperative fentanyl consumption was 26.6 ± 12.6 μg per patient. Fewer patients in group RB required fentanyl rescue (only three patients required one dose). Patients in group RD did not require rescue analgesia [Table 2].
Table 2.
Surgical data
| Variable | Group RD (n=20) | Group RB (n=20) | Group F (n=20) | P* | P# |
|---|---|---|---|---|---|
| Propofol consumptions (mg) | 206.2±61.4 | 261.7±62.9 | 286.2.2±69.4 | 0.009 | 0.237 |
| Remifentanil consumptions (µg) | 261.3±68.7 | 264.4±76.8 | 278.5±81.2 | 0.747 | 0.425 |
| Intraoperativefentanyl rescue (µg per patient) | 0 | 3.1±7.8 | 26.6±12.6 | 0.000 | 0.000 |
SD=Standard deviation. Values are expressed as mean±SD or number. *P-value compares group RD and group RB. #P-value compares between group RB and group F. P<0.05 was considered statistically significant
We did not observe any obvious difference in the preoperative plasma TNF-α, IL-2, IL-6, and IL-10 levels among three groups. The postoperative plasma TNF-α and IL-6 levels were greater in group F than in group RB (P = 0.000). The IL-2 levels were also higher in group RB than group RD (P = 0.031). There was no difference in IL-10 levels among the groups postoperatively (P = 0.059) [Table 3].
Table 3.
Preoperative and postoperative stress response biomarkers
| Variable | Group RD | Group RB | Group F | P* | P# |
|---|---|---|---|---|---|
| Preoperative value (pg/mL) | |||||
| IL-2 | 28.5±4.6 | 32.3±7.6 | 26.2±7.8 | 0.274 | 0.347 |
| IL-6 | 20.4±5.6 | 19.1±6.1 | 21.6±6.7 | 0.223 | 0.641 |
| IL-10 | 14.6±6.4 | 16.3±5.1 | 15.5±4.8 | 0.520 | 0.633 |
| TNF-α | 45.9±7.9 | 42.2±8.5 | 43.1±9.4 | 0.363 | 0.377 |
| Postoperative value (pg/mL) | |||||
| IL-2 | 33.8±5.4 | 38.1±10.2 | 41.9±9.4 | 0.031 | 0.186 |
| IL-6 | 22.5±7.2 | 25.4±8.2 | 38.6±10.3 | 0.272 | 0.000 |
| IL-10 | 15.9±2.9 | 18.6±4.7 | 17.8±3.1 | 0.632 | 0.104 |
| TNF-α | 47.7±7.7 | 45.4±7.7 | 55.2±9.0 | 0.377 | 0.000 |
TNF=Tumor necrotic factor; SD=Standard deviation. Values are expressed as mean±SD or number. *P-value compares group RD and group RB. #P-value compares between group RB and group F. P<0.05 was considered statistically significant
LMA removal time was comparable among the three groups. Time to eye opening was significantly prolonged in group RD compared with group RB (P = 0.000). Fewer patients in groups RD and F experienced emergence agitation in the PACU compared with group RB (n = 4, 17, and 7, respectively, P = 0.001). We did not observe any retrobulbar-related complication, such as retrobulbar hemorrhage or globe perforation. None of the patients experienced postoperative nausea or vomiting in either group [Table 4].
Table 4.
Surgical data
| Variable | Group RD (n=20) | Group RB (n=20) | Group F (n=20) | P* | P# |
|---|---|---|---|---|---|
| LMA removal time (min) | 7.4±2.4 | 6.9±1.9 | 6.5±2.9 | 0.427 | 0.523 |
| Eye opening time (min) | 34.9±8.9 | 11.4±8.6 | 14.5±3.8 | 0.000 | 0.126 |
| The number of emergence agitation | 4 | 7 | 17 | 0.000 | 0.000 |
| Post vomit and nausea (%) | 0 | 0 | 0 | >0.05 | >0.05 |
| Retrobulbar-related complication | 0 | 0 | 0 | >0.05 | >0.05 |
LMA=Laryngeal mask airway; SD=Standard deviation. Values are expressed as mean±SD or number. *P-value compares group RD and group RB. #P-value compares between group RB and group F. P<0.05 was considered statistically significant
Patients in both groups RD and RB reported lower pain scores than those in group F 4 h postoperatively (RD group: P < 0.001; RB group: P =0.002) [Fig. 2]. Groups RD and RB did not show significance in pain scores (P = 0.182). Six-hour postoperatively, only group RD showed lower pain score compared with group F (P < 0.001). The FLACC scores were similar among the three groups at all other measured time points [Fig. 2].
Figure 2.
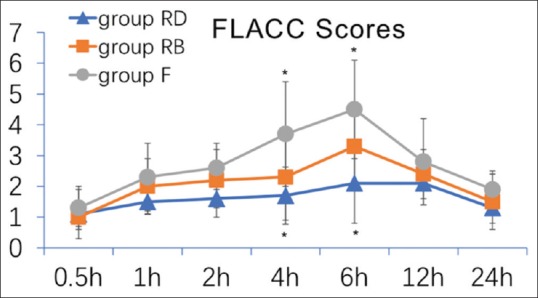
Children Postoperative Pain Scale score in the three groups. Data are presented as mean ± SD; significance was compared with group F (*P < 0.05).
HR was significantly slower in group RD after retrobulbar dexmedetomidine (P = 0.000) when compared to the baseline [Fig. 3]. HR was significantly slower in group RD after retrobulbar dexmedetomidine compared with group F (P = 0.000), except 10 min after retrobulbar dexmedetomidine, at incision, and during intraocular exploration (P = 0.002, 0.001, and 0.001, respectively). In group RB, HR was significantly slower before incision, at incision, and during intraocular exploration (P = 0.019, 0.014, and 0.001, respectively) compared with group F. In group RD, MAP was significantly reduced at 10 min after retrobulbar dexmedetomidine when compared to the baseline, and compared with groups RB and F. In group F, MAP was significantly elevated at incision and during intraocular exploration relative to groups RD and RB (P < 0.001).
Figure 3.
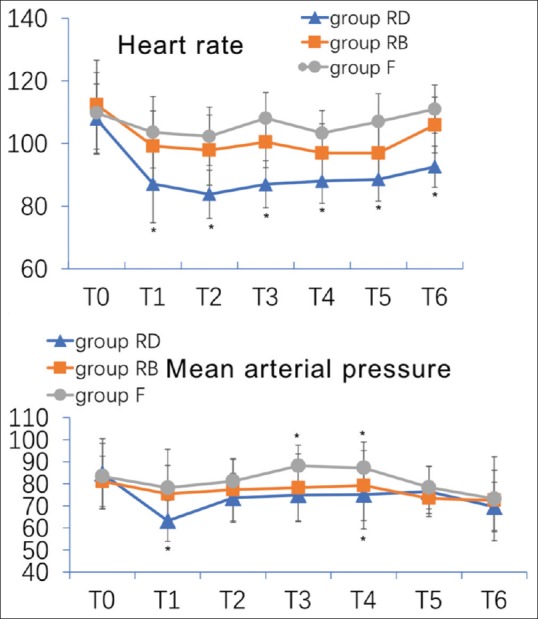
Intraoperative hemodynamic recordings. Heart rate and mean arterial blood pressure. T0 = baseline; T1 = 10 min after retrobulbar block; T2 = before incision; T3 = incision; T4 = intraocular exploration; T5 = immediately after LMA removal; T6 = upon arriving PACU. Data are presented as mean ± SD; significance was compared with group F (*P < 0.05). PACU: postanesthesia care unit
Seven patients in group F developed respiratory depression after LMA removal in the operating room, which was resolved by administration of oxygen. Respiratory depression did not occur in any of the patients in groups RD and RB. Oculocardiac reflex was observed in one patient in group F, but was resolved by stopping the surgical stimulus; oculocardiac reflex did not occur in any patients in groups RD and RB. Retrobulbar block–related complications did not occur in any patient.
Discussion
VR surgery, which is the most painful ophthalmologic procedure, involves extensive intraocular and extraocular manipulation.[8] Research has demonstrated that regional anesthesia in children is comparable to that in adults in terms of safety.[9] Dexmedetomidine is a highly selective α2 adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesic-sparing effects and has more stable respiratory and hemodynamic profiles compared with remifentanil.[10] This single-center randomized double-blind clinical trial in children showed that retrobulbar ropivacaine with dexmedetomidine eliminated the need for intraoperative fentanyl in pediatric VR surgery, and consequently, eliminated the respiratory depression associated with opioid consumption.
Retrobulbar block with ropivacaine is a common, safe, and effective form of regional anesthesia; however, this technique may not entirely eliminate the need for intraoperative fentanyl. The retrobulbar dexmedetomidine used in this study provided better relief of postoperative pain and hemodynamic and neuroendocrine response than retrobulbar ropivacaine or general anesthesia itself. However, these beneficial effects of dexmedetomidine were accompanied by prolonged time to emergence from anesthesia and increased length of stay in the PACU.
In our study, seven patients in group F developed respiratory depression after LMA removal in the operating room. This can be mainly attributed to two factors: (1) intraoperative rescue fentanyl was not required with preemptive retrobulbar dexmedetomidine, whereas all children in group F required fentanyl. Since all opioids carry the risk of respiratory depression,[1] this might have been a possible reason. (2) Dexmedetomidine has the unique characteristic of providing analgesia without causing respiratory depression[11] and provides a stable respiratory profile even in children with severe preoperative airway impairment.[12]
In this study, preemptive retrobulbar dexmedetomidine was seen to provide effective analgesia, as shown by the fact that there was no need for intraoperative or postoperative rescue fentanyl. The pain scores immediately after surgery were significantly lower in group RD/RB than group F. At 6-h postoperatively, pain scores only in group RD were lower than that in group F, which may be due to the following reasons. The α2 adrenergic receptor agonists have been shown to prolong the duration of action of ropivacaine and improve the quality of analgesia by causing local vasoconstriction and increasing the potassium conductance in Aδ and C fibers.[13,14] This is beneficial in longer duration surgery. These drugs may also potentiate the action of local anesthetics by entering the central nervous system either through systemic absorption or by diffusion into the cerebrospinal fluid through the sheath of the optic nerve. Dexmedetomidine has been used as an adjunct to spinal anesthesia without apparent toxicity.[15,16] Previous studies indicate that dexmedetomidine protects the spinal cord from lidocaine-induced spinal neurotoxicity by regulating protein kinase expression and glutamate release.[17] Future studies are warranted to support or dispel concerns regarding neurotoxicity of retrobulbar dexmedetomidine.
The incidence of emergence agitation after ophthalmologic surgery is particularly high owing to visual disturbances. In this study, retrobulbar dexmedetomidine decreased the incidence of emergence agitation, which agrees with previous studies.[18,19] Our data indicated that dexmedetomidine prolonged the time to eyeopening, but did not prolong the LMA removal time. This is due to dexmedetomidine's sedation effect.
The results of our study indicate that dexmedetomidine can be used as an adjunct to general anesthesia to suppress stress responses. VR surgery is categorized as a minor surgery, but it is one of the most painful ophthalmologic procedures. There was a steep increase in the postoperative IL-2, TNF-α, and IL-6 in group F, and these levels differ significantly among the groups. We demonstrated that retrobulbar block and dexmedetomidine could alleviate IL-2, TNF-α, and IL-6 release, which was also reported in previous studies.[20]
In this study, hemodynamic variables were blunted during the surgical procedures in groups RD and RB. HR was significantly slower in group RD relative to group F during most of the intraoperative period. Single-injection retrobulbar block with dexmedetomidine was effective in preventing the surgical trauma-related hemodynamic response and did not cause sudden decreases in HR. Here, we report that this regimen was clinically well-tolerated, although it resulted in a transient increase in systemic and pulmonary pressures and a decrease in HR.[21] One possible reason for this difference is that VR surgeries are minor, short, and highly localized, with few associated systemic diseases.
Conclusion
In summary, we demonstrated that retrobulbar dexmedetomidine as an adjuvant to ropivacaine is a safe and effective alternative to systemic fentanyl, providing better pain management, hemodynamic stability, and stress response suppression in pediatric VR surgery. Future studies could include (1) determining optimal dose range for dexmedetomidine and (2) conducting studies with larger patient population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the assistance provided by Ms. Zexin Chen, Department of Anesthesiology, Zhejiang University, with the statistical analysis. They also gratefully acknowledge the assistance of Dr. Haifeng Hou, Department of Nuclear Medicine, Zhejiang University, for providing advice regarding this study.
References
- 1.Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel-Lewis T, et al. Postoperative opioid-induced respiratory depression: A closed claims analysis. Anesthesiology. 2015;122:659–65. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 2.Mamie C, Habre W, Delhumeau C, Argiroffo CB, Morabia A. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Paediatr Anaesth. 2004;14:218–24. doi: 10.1111/j.1460-9592.2004.01169.x. [DOI] [PubMed] [Google Scholar]
- 3.Dumans-Nizard V, Le Guen M, Sage E, Chazot T, Fischler M, Liu N. Thoracic epidural analgesia with levobupivacaine reduces remifentanil and propofol consumption evaluated by closed-loop titration guided by the bispectral index: A double-blind placebo-controlled study. Anesth Analg. 2017;125:635–42. doi: 10.1213/ANE.0000000000001996. [DOI] [PubMed] [Google Scholar]
- 4.Shah RD, Suresh S. Applications of regional anaesthesia in paediatrics. Br J Anaesth. 2013;111:i114–24. doi: 10.1093/bja/aet379. [DOI] [PubMed] [Google Scholar]
- 5.Yao L, Zhao H, Jiang B, Feng Y. Retrobulbar block in pediatric vitreoretinal surgery eliminates the need for intraoperative fentanyl and postoperative analgesia: A randomized controlled study. Reg Anesth Pain Med. 2017;42:521–6. doi: 10.1097/AAP.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 6.Kallio H, Lindberg LI, Majander AS, Uutela KH, Niskanen ML, Paloheimo MP. Measurement of surgical stress in anaesthetized children. Br J Anaesth. 2008;101:383–9. doi: 10.1093/bja/aen204. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Q, Huang DY, Zhao YL, Wang GH, Liu YX, Zhong L, et al. Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth. 2013;110:420–4. doi: 10.1093/bja/aes385. [DOI] [PubMed] [Google Scholar]
- 8.Subramaniam R, Subbarayudu S, Rewari V, Singh RP, Madan R. Usefulness of pre-emptive peribulbar block in pediatric vitreoretinal surgery: A prospective study. Reg Anesth Pain Med. 2003;28:43–7. doi: 10.1053/rapm.2003.50032. [DOI] [PubMed] [Google Scholar]
- 9.Walker BJ, Long JB, Sathyamoorthy M, Birstler J, Wolf C, Bosenberg AT, et al. Complications in pediatric regional anesthesia: An analysis of more than 100,000 blocks from the Pediatric Regional Anesthesia Network. Anesthesiology. 2018;129:721–32. doi: 10.1097/ALN.0000000000002372. [DOI] [PubMed] [Google Scholar]
- 10.Chen KZ, Ye M, Hu CB, Shen X. Dexmedetomidine vs remifentanil intravenous anaesthesia and spontaneous ventilation for airway foreign body removal in children. Br J Anaesth. 2014;112:892–7. doi: 10.1093/bja/aet490. [DOI] [PubMed] [Google Scholar]
- 11.Hammer GB, Philip BM, Schroeder AR, Rosen FS, Koltai PJ. Prolonged infusion of dexmedetomidine for sedation following tracheal resection. Paediatr Anaesth. 2005;15:616–20. doi: 10.1111/j.1460-9592.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen KZ, Ye M, Hu CB, Shen X. Dexmedetomidine vs remifentanil intravenous anaesthesia and spontaneous ventilation for airway foreign body removal in children. Br J Anaesth. 2014;112:892–7. doi: 10.1093/bja/aet490. [DOI] [PubMed] [Google Scholar]
- 13.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang Q, Huang DY, Zhao YL, Wang GH, Liu YX, Zhong L, et al. Caudal dexmedetomidine combined with bupivacaine inhibit the response to hernial sac traction in children undergoing inguinal hernia repair. Br J Anaesth. 2013;110:420–4. doi: 10.1093/bja/aes385. [DOI] [PubMed] [Google Scholar]
- 15.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Zhao B, She Y, Song X. Dexmedetomidine ameliorates lidocaine-induced spinal neurotoxicity via T inhibiting glutamate release and the PKC pathway. NeuroToxicology. 2018;69:77–83. doi: 10.1016/j.neuro.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Tsiotou AG, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: A double-blind, randomized study. Paediatr Anaesth. 2018;28:632–8. doi: 10.1111/pan.13397. [DOI] [PubMed] [Google Scholar]
- 19.Song IA, Seo KS, Oh AY, Baik JS, Kim JH, Hwang JW, et al. Dexmedetomidine injection during strabismus surgery reduces emergence agitation without increasing the oculocardiac reflex in children: A randomized controlled trial. PLoS One. 2016;12:11. doi: 10.1371/journal.pone.0162785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wang B, Zhang LL, He SF, Hu XW, Wong GT, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122:1202–10. doi: 10.1213/ANE.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 21.Jooste EH, Muhly WT, Ibinson JW, Suresh T, Damian D, Phadke A, et al. Acute hemodynamic changes after rapid intravenous bolus dosing of dexmedetomidine in pediatric heart transplant patients undergoing routine cardiac catheterization. Anesth Analg. 2010;111:1490–6. doi: 10.1213/ANE.0b013e3181f7e2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]