Abstract
The potential applications of cationic poly(ionic liquids) range from medicine to energy storage, and the development of efficient synthetic strategies to target innovative cationic building blocks is an important goal. A post-polymerization click reaction is reported that provides facile access to trisaminocyclopropenium (TAC) ion-functionalized macromolecules of various architectures, which are the first class of polyelectrolytes that bear a formal charge on carbon. Quantitative conversions of polymers comprising pendant or main-chain secondary amines were observed for an array of TAC derivatives in three hours using near equimolar quantities of cyclopropenium chlorides. The resulting TAC polymers are biocompatible and efficient transfection agents. This robust, efficient, and orthogonal click reaction of an ionic liquid, which we term ClickabIL, allows straightforward screening of polymeric TAC derivatives. This platform provides a modular route to synthesize and study various properties of novel TAC-based polymers.
Keywords: click chemistry, gene delivery, ionic liquids, polyelectrolytes, polymers
Graphical Abstract
They just clicked: A stable bis(dialkylamino) cyclopropenium chloride salt can efficiently react with polymers containing secondary amines to yield cationic polyelectrolytes. The dialkylamino groups in this click transformation can be modulated to yield various polyelectrolytes bearing soft cationic moieties. Some of these cyclopropenium-based polymers give high transfection efficiencies and are less cytotoxic than linear polyethyleneimine (PEI).
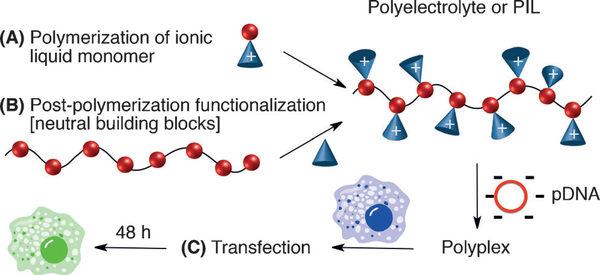
The ability to control the macromolecular architecture and synthetic tunability[1] of cationic building blocks has contributed to the widespread use of poly(ionic liquids) (PILs, Figure 1A), or polyelectrolytes, in various applications[2] ranging from gene delivery vectors[3] to alkaline fuel cells.[4] As the understanding of structure–property relationships with respect to charge density, repeat-unit composition, and macromolecular structure in such polymeric systems has developed,[5] so too has the need for synthetic strategies to target new classes of these materials (Figure 1B).[6] However, manipulating the functionality, processability, and Coulombic interactions of PILs presents a significant challenge,[7] and the development of detailed structure–property relationships for cellular transfection applications has been limited. Chemical transformations that overcome such obstacles have the potential to broaden our fundamental understanding of polyelectrolytes in modern technologies, particularly gene- based therapies.[8]
Figure 1.
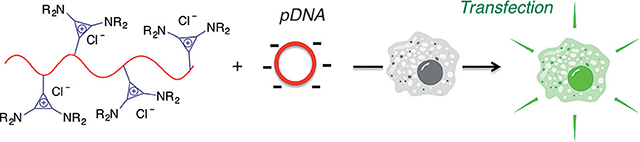
Common synthetic strategies to access polyelectrolytes through a) polymerization of ionic liquids that contain a polymerizable unit; and b) modification of the polymer backbone with a neutral group that yields charged moieties or by directly using a charged functional group to couple to the backbone. c) PIL/pDNA polyplexes transfect cells and induce luciferase expression, resulting in cell luminescence.
We recently reported the synthesis of trisaminocyclopropenium (TAC)-based polymers, where the formal charge is on carbon but is highly delocalized within the monomer—a soft cation.[9] Initial structure–property studies of functional TAC PILs with regard to ionic conductivity and processability have underscored the necessity for an alternative synthetic strategy, since performing many polymerizations is cumbersome, and polymers comprising different TAC derivatives show batch-to-batch variation. Thus, a new method for synthesizing TAC-based polyelectrolytes is needed to simultaneously control the macromolecular architecture and molecular composition of the TAC repeat units. Akin to what Coates and co-workers have demonstrated with alkaline-stable imidazolium ionic liquids (ILs), the ability to elaborate cationic building blocks towards complex structures that are not commercially available is crucial to optimize performance for a given application.[10] Therefore, straightforward access to a variety of amino substituents on the TAC scaffold could facilitate optimization, inform design principles, and elucidate chemical structure–property relationships within a single family of materials to improve performance in applications such as nonviral gene delivery.
Cationic polymers are among the most common nonviral gene delivery vectors because of their ability to complex with the negatively charged phosphate backbone of DNA, and the formation of these polyplexes can prevent the degradation of genetic material and encourage cellular uptake (Figure 1C).[8a,11] However, if the electrostatic cohesion between the polymer and DNA is too strong for adequate release of DNA into the cell, transfection efficiency can be dramatically suppressed)[12] In fact, Schmuck and co-workers have shown that the specific nature of the association between the cationic building block and the DNA, and the ability to manipulate these Coulombic interactions, is instrumental for the optimization of transfection efficiency.[13] It is therefore important to study how various types of building blocks affect transfection.[3b,8d,14] Considering that trisaminocyclopropenium ions are remarkably stable cations that have been observed to only weakly associate with their counterions,[15] we sought to investigate how these moieties would behave as transfection agents. Furthermore, because the cyclopropenium cation is stable across a broad pH range,[16] we postulated that the resulting polyplexes would be particularly robust. For these reasons, along with the acute control of macromolecular architecture and molecular structure this system permits, we anticipated that the development of a post-polymerization strategy towards TAC polymers would serve as an effective approach to synthesizing transfection agents.
The modification of polymer backbones with functional groups by using modular and efficient chemistries, especially “click” reactions, is particularly desirable for materials commercialization.[17] The limited tolerance of myriad functional groups in controlled polymerization techniques (Figure 1A) renders post-polymerization functionalizations (PPF, Figure 1B) an attractive route to complex macromolecular structures of polyelectrolytes.[18] PPF is especially attractive for PILs, since charged groups are incompatible with most size-exclusion chromatography (SEC) columns. As a result, many studies of PILs ignore effects of molecular mass and dispersity (Ð), correlating physical properties solely to the structure of the repeat units.[19] A more complete understanding of macromolecular systems can be achieved in materials with well-defined and narrow molecular weight distributions.[20] Herein, we report a new type of click reaction between bis(dialkylamino)cyclopropenium chloride (BACCl) ILs[21] and polymers containing secondary amines, along with a transfection study. While many reported procedures to obtain other cationic PILs through PPF involve reactions with large excesses of the quaternizing agent (3–10 equiv) and long reaction times (up to three days),[22] we found this conjugation reaction to proceed in approximately 3 h under mild conditions, with stoichiometric amounts of reactants. Therefore, we designate these reactions as “click” in the context of polymer chemistry,[23] thus establishing the BACCl ion as a clickable ionic liquid, or ClickabIL.
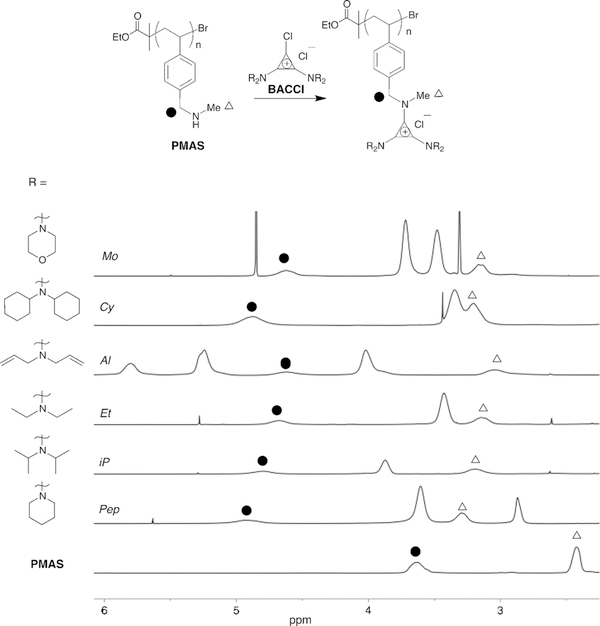
To obtain a well-defined neutral polymer precursor, we synthesized poly(methylaminostyrene) (PMAS, Figure 2), a polymer containing a secondary amine as a pendant group. Because monomeric PMAS does not polymerize by reversible addition–fragmentation chain-transfer polymerization or under atom-transfer radical-polymerization (ATRP) conditions, we elected to protect the secondary amine with a tert-butyloxycarbonyl (Boc) protecting group.[24] The Boc-protected monomer readily polymerizes by ATRP to yield polymers of controllable molecular mass and narrow dispersity (Ð), which can be characterized by size-exclusion chromatography at this step. Further details of the synthetic methods are available in the Supporting Information.
Figure 2.
1H NMR spectra of TAC polymers show complete functionalization of PMAS with BACCl ClickablLs bearing various alkyl substituents. Full NMR spectra are available in the Supporting Information.
The deprotected PMAS undergoes smooth addition to BACCl salts as shown in Figure 2. The choice of BACCl can tailor the physical properties of the resulting polymers through control of solubility properties or through the introduction of functional groups via the amino substituents (e.g., diallylamino). We subjected PMAS to functionalization with six different BACCl derivatives, containing isopropyl (iP), ethyl (Et), allyl (Al), cyclohexyl (Cy), morpholine (Mo), or piperidine (Pep) substituents (Figure 2). Proton nuclear magnetic resonance (1H NMR) spectra of these polymers reveal that the starting material is fully converted into the corresponding TAC-containing polymer, as evidenced by the peak shifts noted in Figure 2. Notably, the positive charge is not generated by a reaction between neutral reactants in this new approach, in contrast to quaternization PPF reactions.[25] Instead, BACCl salts are directly coupled to neutral homopolymers containing secondary amines. While our original report notes differences in the solubility profiles and thermal properties of TAC polymers comprising different amino substituents,[9a] this chemistry provides a direct approach to study the effect that various functional groups exert on macromolecular properties, while keeping the effects of dispersity and degree of polymerization constant.
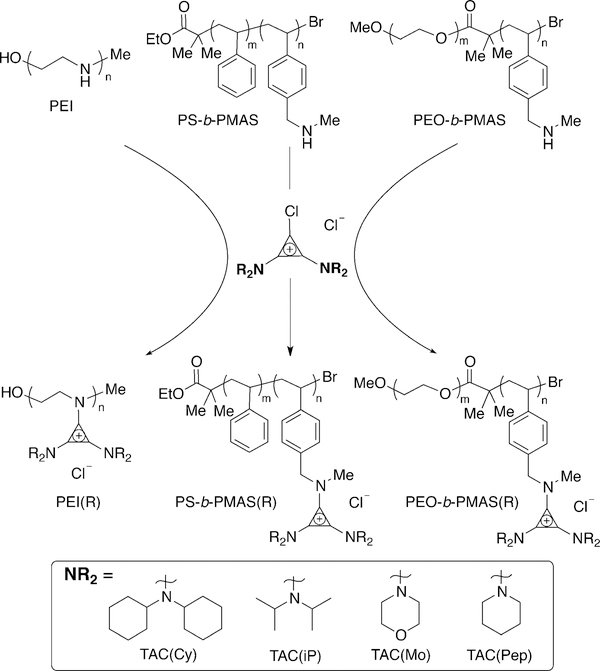
The modularity of this method is highlighted by the diverse set of functional groups obtained using the same parent polymer; only minor changes in procedure (e.g., use of co-solvent) are needed to accommodate structural diversity. In this vein, diblock copolymers were synthesized through polymerization of the Boc-protected monomer onto both polyethylene oxide and polystyrene macro-initiators by ATRP (Figure 3). SEC traces show that a narrow dispersity is maintained after copolymerization in both cases (Figures S1 and S2 in the Supporting Information). Following their successful deprotection, the PMAS blocks in the PEO and PS diblock copolymers were fully functionalized with both hydrophobic and hydrophilic BACCls (Cy, iP, and Mo, from most to least hydrophobic) without the need to modify the procedures used to prepare the corresponding homopolymers. The adaptable nature of this chemistry will encourage systems development over individual polymer design.
Figure 3.
Post-polymerization functionalization of polymers containing secondary amines through the addition of BACCl ClickabILs.
To expand upon the macromolecular architectures accessible with ClickabIL chemistry, we functionalized commercially available linear polyethyleneimine (PEI) with BACCls. When protonated, PEI is a cationic polyelectrolyte that is frequently used as a cellular transfection agent.[26] However, its cytotoxicity, especially at high doses, limits its widespread use in transfection applications.[27] We postulated that functionalizing PEI with cyclopropenium units might make it less toxic and potentially a better transfection agent. PEI was subjected to the ClickabIL reaction conditions described above (Figure 3), and quantitative functionalization was observed for all BACCls.
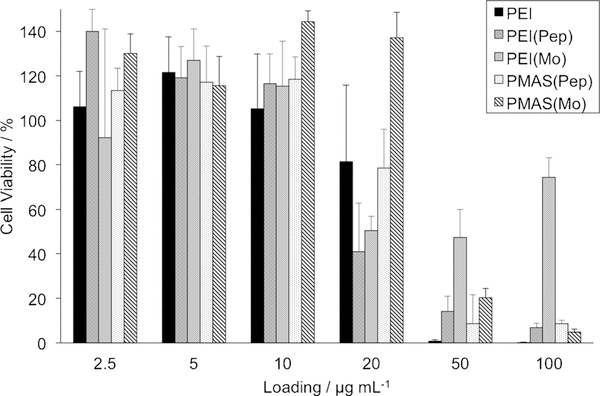
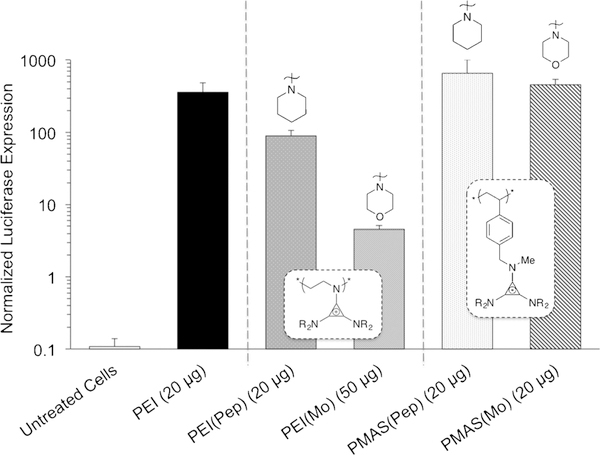
We next sought to determine whether this transformation is relevant to biomedical applications, such as gene delivery. While we synthesized many polymers, we focused on homopolymers that are highly water-soluble. Furthermore, to simplify comparisons between materials, we elected to change only subtle elements of the TAC structure on two different polymer backbones. Cytotoxicity assays and luciferase transfection experiments in HEK-293T cells revealed a significant dependence on the chemical structure of the pendant TAC ion, namely its amino substituents, and on the identity of the polymer backbones (PEI and PMAS). All four TAC polymers showed a similar toxicity profile to linear PEI (25 kgmol−1) at low dosages. However, at high loadings, polyTACs were found to be more biocompatible, especially those bearing a styrene backbone (Figure 4). Functionalizing PEI with TAC(Mo) endowed the polymer with low toxicity, giving a cell viability of approximately 50% at both 50 and 100 μgmL−1 loading.
Figure 4.
Viability of HEK-293T cells following 48 h incubation with TAC-functionalized polymers at various doses, as measured by trypan blue dye exclusion. Error bars show the standard deviation of triplicate measurement.
While structural modification of PEI with the TAC ions led to a lower transfection efficacy compared to the parent polymer (Figure 5), comparing the two modified PEI materials bearing TAC(Pep) and TAC(Mo) showed notable differences in transfection efficiency. These two materials differ in the chemistry at the 4-position of the six-membered ring : a simple variation between a methylene group and an ether oxygen. The PEI(Pep) polyplexes transfected cells almost as well as the PEI parent polymer, but polyplexes of PEI(Mo) exhibited poor transfection efficiency (Figure 5). Potentially, this difference may be attributed to the increased hydrophobicity of PEI(Pep) over PEI(Mo), a property that has been shown to play a key role in nonviral transfection agents.[28] Polyplexes of PEI and TAC polymers with plasmid DNA (pDNA), at the loadings noted in Figure 5, were further characterized by dynamic light scattering (DLS) for hydrodynamic diameter (DH) and zeta potential (ζ; Table 1). Since the Dh and surface charge values are similar for PEI(Pep), PEI(Mo), and unfunctionalized PEI polyplexes (Table 1), the observed discrepancy in transfection efficacy may be due to fine structural variations between each agent. However, it is challenging to draw conclusions at this stage, and further investigation of the complex relationships between transfection efficiency, TAC structure, and polymer molecular mass is ongoing.[29] Changing the backbone from PEI to PMAS resulted in smaller polyplexes that were also viable transfection agents [Figure 5, PMAS(Pep) and PMAS(Mo)]. All of our most effective formulations (Table 1 and Figure 5) are within the size regime that Zhou and co-workers outlined for high-efficiency transfection reagents, that is, less than 500 nm.[29] Furthermore, the PMAS-based materials exhibited optimal pDNA transfection at lower charge ratios than PEI and PEI(R) polymers (Table 1). Within the range of the error bars, the more hydrophobic PMAS(Pep) derivative and the PMAS(Mo) derivative show similar efficacy, unlike in the PEI systems. These preliminary results suggest that ClickabIL chemistry has great potential as a platform to tune the chemical composition of TAC-based polyelectrolytes, build detailed structure-property relationships, and inform design principles for the optimization of transfection agents.
Figure 5.
Luciferase expression in HEK-293T cells transfected with pDNA containing the firefly luciferase reporter gene using TAC polymers and 25 K linear PEI. Polymer backbones are noted in white boxes and amino substituents pictured above the respective bars. Luciferase expression is measured after 48 h incubation with specified polymer loadings (all with pDNA loading of 3 μgmL−1) and normalized by cell count. Error bars show the standard deviation of triplicate measurement.
Table 1:
Characterization of transfection agents and polyplexes corresponding to optimal transfection efficacy.
| Transfection Agent[a] | MM[b] [kDa] | Charge ratio[c] | DH [nm] | ζ potentia [mV] |
|---|---|---|---|---|
| PEI | 25 | 50:1 | 490±60 | 40±10 |
| PEI(Pep) | 166 | 8:1 | 425±100 | 65±5 |
| PEI(Mo) | 164 | 20:1 | 400±110 | 60±6 |
| PMAS(Pep) | 53 | 5.5:1 | 140±60 | 27±8 |
| PMAS(Mo) | 53 | 5.5:1 | 215±25 | 43±6 |
Polyplexes of polymers tested at the loadings noted in Figure 5.
Molecular mass of transfection agent, calculated based on commercial linear PEI; for PMAS(R) materials, PMAS was measured by SEC using PS standards of narrow dispersity, then calculated for the corresponding TAC group.
Ratio of either N to phosphate anions (PEI) or TAC to phosphate anions.
In this Communication, we have demonstrated that a variety of BACCl building blocks readily react with polymers containing pendant and main-chain secondary amines in a novel example of macromolecular click chemistry. Linking neutral polyamines that are either commercially available or rapidly assembled with charged BACCl Click- abILs furnishes diverse classes of well-defined TAC polymers under mild conditions. Biocompatibility and transfection capacity varied with BACCl identity and polymer backbone, and some materials showed similar transfection efficiency to PEI but with lower cytotoxicity. These data demonstrate that precise tuning of macromolecular properties through molecular structure is vital for the design of advanced materials. This chemistry is currently being used to explore a divergent approach to materials discovery and optimization of cyclopropenium macromolecules for a variety of applications.
Supplementary Material
Acknowledgements
This work was funded by the National Science Foundation (NSF CAREER DMR-1351293), ACS Petroleum Research Fund, and 3M Non-Tenured Faculty Award. J.S.B. is grateful for NSF and NDSEG fellowships. S.D.B is grateful for NSF fellowship. Y.J. thanks the Columbia Amgen Scholars Program and the Columbia Science Research Fellows.
Footnotes
Supporting information for this article can be found under: http://dx.doi.org/10.1002/anie.201605214.
References
- [1].a) Hawker CJ, Wooley KL, Science 2005, 309, 1200–1205 [DOI] [PubMed] [Google Scholar]; b) Matyjaszewski K, Tsarevsky NV, Nat. Chem 2009,1, 276–288. [DOI] [PubMed] [Google Scholar]
- [2].a) Lodge TP, Science 2008, 321, 50–51 [DOI] [PubMed] [Google Scholar]; b) Bandar JS, Barthelme A, Mazori AY, Lambert TH, Chem. Sci 2015, 6, 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Ramos J, Forcada J, Hidalgo-Alvarez R, Chem. Rev 2014, 114, 367–428 [DOI] [PubMed] [Google Scholar]; b) Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Nat. Rev. Genet 2014,15, 541–555. [DOI] [PubMed] [Google Scholar]
- [4].a) Pan J, Chen C, Zhuang L, Lu J, Acc. Chem. Res 2012, 45, 473–481 [DOI] [PubMed] [Google Scholar]; b) Qiu B, Lin B, Qiu L, Yan F J. Mater. Chem 2012, 22, 1040–1045. [Google Scholar]
- [5].Wang Y, Kimura K, Dubin PL, Jaeger W, Macromolecules 2000, 33, 3324–3331. [Google Scholar]
- [6].a) Hunt JN, Feldman KE, Lynd NA, Deek J, Campos LM, Spruell JM, Hernandez BM, Kramer EJ, Hawker CJ, Adv. Mater 2011, 23, 2327–2331 [DOI] [PubMed] [Google Scholar]; b) Fang J, Wallikewitz BH, Gao F, Tu G, Mueller C, Pace G, Friend RH, Huck WTS, J. Am. Chem. Soc 2011,133, 683–685 [DOI] [PubMed] [Google Scholar]; c) Pan J, Chen C, Zhuang L, Lu J, Acc. Chem. Res 2012, 45, 473–481. [DOI] [PubMed] [Google Scholar]
- [7].Sing CE, Zwanikken JW, Olvera de la Cruz M, Nat. Mater 2014,13, 694–698. [DOI] [PubMed] [Google Scholar]
- [8].a) Zintchenko A, Philipp A, Dehshahri A, Wagner E, Bioconjugate Chem 2008, 19, 1448–1455 [DOI] [PubMed] [Google Scholar]; b) Jiang HL, Kwon JT, Kim YK, Kim EM, Arote R, Jeong HJ, Nah JW, Choi YJ, Akaike T, Cho MH, Cho CS, Gene Ther 2007,14, 1389–1398 [DOI] [PubMed] [Google Scholar]; c) Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang M, Adv. Funct. Mater 2009, 19, 2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, Moroni L, Dubruel P, Chem. Soc. Rev 2012, 41, 7147–7194. [DOI] [PubMed] [Google Scholar]
- [9].a) Jiang Y, Freyer JL, Cotanda P, Brucks SD, Killops KL, Bandar JS, Torsitano C, Balsara NP, Lambert TH, Campos LM, Nat. Commun 2015, 6, 5950. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bandar JS, Lambert TH, Synthesis 2013, 2485–2498. [Google Scholar]
- [10].Hugar KM, Kostalik HA, Coates GW, J. Am. Chem. Soc 2015,137, 8730–8737. [DOI] [PubMed] [Google Scholar]
- [11].a) Tseng Y-C, Mozumdar S, Huang L, Adv. Drug Delivery Rev 2009, 61, 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG, J. Intern. Med 2010, 267, 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yoon H, Dell EJ, Freyer JL, Campos LM, Jang W-D, Polymer 2014, 55, 453–464. [Google Scholar]
- [12].a) Yudovin-Farber I, Yanay C, Azzam T, Linial M, Domb AJ, Bioconjugate Chem 2005, 16, 1196–1203 [DOI] [PubMed] [Google Scholar]; b) Kawano T, Okuda T, Aoyagi H, Niidome T, J. Controlled Release 2004, 99, 329–337. [DOI] [PubMed] [Google Scholar]
- [13].Li M, Schlesiger S, Knauer SK, Schmuck C, Angew. Chem. Int. Ed 2015, 54, 2941–2944; Angew. Chem. 2015, 127, 2984–2987. [DOI] [PubMed] [Google Scholar]
- [14].De Smedt S, Demeester J, Hennink W, Pharm. Res 2000, 17, 113–126. [DOI] [PubMed] [Google Scholar]
- [15].a) Weiss R, Brenner T, Hampel F, Wolski A, Angew. Chem. Int. Ed. Engl 1995, 34, 439–441; Angew. Chem. 1995, 107, 481–483; [Google Scholar]; b) Weiss R, Rechinger M, Hampel F, Wolski A, Angew. Chem. Int. Ed. Engl 1995, 34, 441–443; Angew. Chem. 1995, 107, 483–485. [Google Scholar]
- [16].a) Moss RA, Shen S, Krogh-Jespersen K, Potenza JA, Schugar HJ, Munjal RC, J. Am. Chem. Soc 1986, 108, 134–140 [Google Scholar]; b) Killops KL, Brucks SD, Rutkowski KL, Freyer JL, Jiang Y, Valdes ER, Campos LM, Macromolecules 2015, 48, 2519–2525. [Google Scholar]
- [17].Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ, Chem. Rev 2009,109, 5620–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].a) Laschewsky A, Curr. Opin. Colloid Interface Sci 2012, 17, 56–63 [Google Scholar]; b) Espeel P, Du Prez FE, Macromolecules 2015, 48, 2–14. [Google Scholar]
- [19].Choi UH, Lee M, Wang S, Liu W, Winey KI, Gibson HW, Colby RH, Macromolecules 2012, 45, 3974–3985. [Google Scholar]
- [20].a) Singh M, Odusanya O, Wilmes GM, Eitouni HB, Gomez ED, Patel AJ, Chen VL, Park MJ, Fragouli P, Iatrou H, Hadjichristidis, Cookson , Balsara NP, Macromolecules 2007, 40, 4578–4585 [Google Scholar]; b) Weber RL, Ye Y, Schmitt AL, Banik SM, Elabd YA, Mahanthappa MK, Macromolecules 2011, 44, 5727–5735. [Google Scholar]
- [21].a) Curnow OJ, Holmes MT, Ratten LC, Walst KJ, Yunis R, RSC Adv 2012, 2, 10794–10797 [Google Scholar]; b) Curnow OJ, MacFarlane DR, Walst KJ, Chem. Commun 2011, 47, 10248–10250. [DOI] [PubMed] [Google Scholar]
- [22].a) Choi J-H, Ye Y, Elabd YA, Winey KI, Macromolecules 2013, 46, 5290–5300 [Google Scholar]; b) Sudre G, Inceoglu S, Cotanda P, Balsara NP, Macromolecules 2013, 46, 1519–1527. [Google Scholar]
- [23].Barner-Kowollik C, Du Prez FE, Espeel P, Hawker CJ, Junkers T, Schlaad H, Van Camp W, Angew. Chem. Int. Ed 2011, 50, 60–62; Angew. Chem. 2011,123, 61–64. [DOI] [PubMed] [Google Scholar]
- [24].Janoschka T, Teichler A, Krieg A, Hager MD, Schubert US, J. Polym. Sci. Part A 2012, 50, 1394–1407. [Google Scholar]
- [25].a) Yuan J, Mecerreyes D, Antonietti M, Prog. Polym. Sci 2013, 38, 1009–1036 [Google Scholar]; b) Saha A, De S, Stuparu MC, Khan A, J. Am. Chem. Soc 2012,134, 17291–17297. [DOI] [PubMed] [Google Scholar]
- [26].a) Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP, Proc. Natl. Acad. Sci. USA 1995,92,7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Florea BI, Meaney C, Junginger HE, Borchard G, AAPS PharmSci 2002, 4, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, Mol. Ther 2005,11, 990–995. [DOI] [PubMed] [Google Scholar]
- [28].a) Kuchelmeister HY, Karczewski S, Gutschmidt A, Knauer S, Schmuck C, Angew. Chem. Int. Ed 2013, 52, 14016–14020; Angew. Chem. 2013,125,14266–14270 [DOI] [PubMed] [Google Scholar]; b) Yu T, Liu X, Bolcato-Bellemin A-L, Wang Y, Liu C, Erbacher P, Qu F, Rocchi P, Behr J-P, Peng L, Angew. Chem. Int. Ed 2012, 51, 8478–8484; Angew. Chem. 2012,124, 8606 – 8612 [DOI] [PubMed] [Google Scholar]; c) Buerkli C, Lee SH, Moroz E, Stuparu MC, Leroux J-C, Khan A, Biomacromolecules 2014,15, 1707–1715. [DOI] [PubMed] [Google Scholar]
- [29].Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM, Nat. Mater 2012,11, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.