Abstract
IMPORTANCE
Biomarkers that predict suicidal behavior, especially highly lethal behavior, are urgently needed. In cross-sectional studies, individuals with depression who attempt suicide have lower midbrain serotonin transporter binding potential compared with those who do not attempt suicide, and higher serotonin1A binding potential in the raphe nuclei (RN) is associated with greater lethality of past suicide attempts and suicidal intent and ideation.
OBJECTIVES
To determine whether serotonin transporter binding potential in the lower midbrain predicts future suicide attempts and whether higher RN serotonin1A binding potential predicts future suicidal ideation and intent and lethality of future suicide attempts.
DESIGN, SETTING, AND PARTICIPANTS
In this prospective 2-year observational study, a well-characterized cohort of 100 patients presenting for treatment of a major depressive episode of at least moderate severity underwent positron emission tomography using carbon 11-labeled N-(2-(1-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl))-N-(2-pyridyl)-cyclohexanecarboxamide ([11C]WAY-100635), a serotonin1A antagonist; a subset of 50 patients also underwent imaging with carbon 11-labeled 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)- benzonitrile ([11C]DASB), a serotonin transporter radioligand. Imaging was performed at Columbia University Medical Center from May 3, 1999, to March 11, 2008. Follow-up was completed on May 28, 2010, and data were analyzed from August 1, 2013, to March 1, 2016.
EXPOSURES
Patients were treated naturalistically in the community and followed up for 2 years with documentation of suicidal behavior, its lethality, and suicidal ideation and intent.
MAIN OUTCOMES AND MEASURES
Suicide attempt or suicide.
RESULTS
Of the 100 patients undergoing follow-up for more than 2 years (39 men; 61 women; mean [SD] age, 40.2 [11.2] years), 15 made suicide attempts, including 2 who died by suicide. Higher RN serotonin1A binding potential predicted more suicidal ideation at 3 (b = 0.02; t = 3.45; P = .001) and 12 (b = 0.02; t = 3.63; P = .001) months and greater lethality of subsequent suicidal behavior (b = 0.08; t = 2.89; P = .01). Exploratory analyses suggest that the serotonin1A binding potential of the insula (t = 2.41; P = .04), anterior cingulate (t = 2.27; P = .04), and dorsolateral prefrontal cortex (t = 2.44; P = .03) were also predictive of lethality. Contrary to our hypotheses, suicidal intent was not predicted by serotonin1A binding potential in any brain region (F1,10 = 0.83; P = .38), and midbrain serotonin transporter binding potential did not predict future attempts (log-rank ; P = .54), possibly owing to low power.
CONCLUSIONS AND RELEVANCE
Greater RN serotonin1A binding potential predicted higher suicidal ideation and more lethal suicidal behavior during a 2-year period. This effect may be mediated through less serotonin neuron firing and release, which affects mood and suicidal ideation and thereby decision making.
Suicidal behavior occurs in the context of a diathesis characterized by impairments in mood and emotion regulation, decision making, problem solving, and social appraisal.1 Suicidal behavior is also associated with specific biomarkers,2 mostly involving the serotonergic system and hypothalamic pituitary adrenal axis. We found individuals with major depressive disorder (MDD) who attempt suicide (attempters) have lower in vivo midbrain serotonin transporter binding compared with those who do not attempt suicide (nonattempters or control individuals),3,4 suggesting that the differences in the proportions of suicide attempters studied may contribute to discrepant positron emission tomography (PET) findings in MDD.5,6 Similarly, attempters with MDD showed lower serotonin transporter binding in the midbrain and pons4 compared with controls. In contrast, a small study using iodine 123-labeled beta [3H]2-beta-carbomethoxy-3-beta-[4’-iodophenyl]tropane7single-photon emission computed tomography linked higher serotonin transporter binding in the globus pallidus to suicidal behavior. Using fluorodeoxyglucose F 18-labeled PET, a previous investigation8 found attempters with MDD had lower regional rates of cerebral glucose metabolism in the right dorsolateral prefrontal cortex (PFC) compared with nonattempters with MDD, a discrepancy that roughly doubled after fenfluramine administration, which suggested impaired serotonergic response.9 In a serotonin2A binding study using 123I-5-I-R91150 and single-photon emission computed tomography, deliberate self-harming patients who did not use medication had less frontal serotonin2A binding compared with controls. Thus, most studies implicate serotonergic dysfunction in suicidal behavior.
Moreover, high-lethality attempters with depression showed lower PFC rates of cerebral glucose metabolism than low-lethality attempters, a difference enhanced after fenfluramine administration.10 Lower ventromedial PFC activity was associated with lower impulsivity, higher suicidal intent, and more lethal suicide attempts. Likewise, Leyton et al11 found that, compared with controls, higher-lethality attempters had less carbon 11-labeled α-methyl L-tryptophan trapping in orbital PFC and less tracer uptake inversely correlated with suicidal intent. A recent study12 reported that raphe nuclei (RN) serotonin1A binding potential was greater in high-compared with low-lethality attempters and positively correlated with suicidal ideation, lethality, and intent of the most recent suicide attempt. Of note, PFC serotonin1A binding potential correlated with suicidal ideation, but not lethality.12 Hence, more pronounced serotonergic dysfunction may lead to more lethal suicidal behavior.
No brain imaging study has examined these biomarkers prospectively to evaluate their capacity for predicting suicidal behavior. Given the findings in MDD of lower midbrain serotonin transporter binding in past suicide attempters and higher RN serotonin1A binding correlated with more severe suicidal ideation and greater lethality and intent of past attempts, our primary hypotheses were that, in patients with depression, PET scanning will demonstrate that (1) lower midbrain serotonin transporter binding potential predicts future suicide attempts and (2) greater RN serotonin1A binding potential predicts attempts of greater lethality and intent and greater suicidal ideation during a 2-year follow-up.
Methods
One hundred patients with MDD aged 18 to 65 years with Hamilton Depression Rating Scale13 (17-item) scores greater than 15 were included in the study. Exclusion criteria consisted of lifetime exposure to 3,4-methylenedioxymethamphetamine (Ecstasy) more than 2 times, alcohol or substance use disorders in the previous 6 months, unstable medical conditions, pregnancy, lactation, psychosis, and schizophrenia. Participants had been medication free for at least 3 weeks (6 weeks for fluoxetine hydrochloride and 4 weeks for antipsychotics); 62 patients required a medication washout period. Short-acting benzodiazepines for anxiety or insomnia were permitted as needed until 72 hours before scans. Data were collected from May 3, 1999, to March 11, 2008. This study was approved by the institutional review board of New York State Psychiatric Institute, and all patients provided written, informed consent.
Portions of this sample have been included in previous publications (eMethods in the Supplement).3,12,14–16 Assessments included a history and physical examination, routine blood tests, pregnancy test, urine toxicologic screen, the Structured Clinical Interview for DSM-IV,17 Hamilton Depression Rating Scale,13 Beck Depression Inventory,18 Global Assessment Scale,19 Beck Scale for Suicide Ideation (BSSI),20 Brown Goodwin Aggression History Scale,21 Buss-Durkee Hostility Inventory,22 and Barratt Impulsivity Scale.23 Suicidal behavior was documented with the Columbia Suicide History Form,24 using Columbia Classification Algorithm of Suicide Assessment.25 For attempters only, the Beck Suicide Intent Scale26 and Medical Lethality Scale26 rated each attempt’s intent and medical sequelae (range, 0 [no injury] to 8 [fatal]).
Radiochemistry, Input Function Measurement, and Image Acquisition and Analysis
Carbon 11-labeled N-(2-(1-(4-(2-methoxyphenyl)-1-piperazinyl) ethyl))-N-(2-pyridyl)-cyclohexanecarboxamide ([11C]WAY-100635), a serotonin antagonist, and carbon 11-labeled 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile ([11C]DASB), a serotonin transporter radioligand, were synthesized with assessment of arterial input function, metabolites, and plasma free-fraction (fP) measurements as previously described.27,28 Image acquisition and binding estimation were as previously reported for [11C]DASB3 and [11C]WAY-100635.12
Outcome Measures for Midbrain Serotonin Transporter and Serotonin1A Region of Interest Binding Potential
We derived [11C]DASB midbrain volume of distribution (VT) values using likelihood estimation in the graphical approach to reduce inherent noise-dependent bias.29,30 The [11C]DASB VT/fP was calculated as before,3 as were outcome measures for sensitivity analyses (BPF,BPP, and BPND31). For serotonin1A region-of-interest (ROI) binding potential (BPF), the ROI contours and BPF were generated as previously reported.12 The ROIs included the RN, amygdala, hippocampus, parahippocampal gyrus, anterior cingulate, medial and dorsolateral PFC, and insular, parietal, temporal, orbital, and occipital cortices. Imaging data acquisition and processing details are found in eMethods in the Supplement.
Prospective Assessments
Patients received treatment in the community. At 3, 12, and 24 months, suicidal ideation was assessed. For each follow-up suicide attempt, suicidal intent and lethality were assessed, as at baseline. Follow-up was completed on May 28, 2010, and data were analyzed from August 1, 2013, to March 1, 2016.
Statistical Analysis
Midbrain [11C]DASB Binding Potential and Future Suicide Attempts
Survival analysis using the log-rank test evaluated the time to the first suicide attempt in 2 groups defined by a median split of midbrain [11C]DASB VT/fP. To account for technical variability in [11C]DASB VT/fP, a sensitivity analysis used an analysis of variance model with log-transformed midbrain [11C]DASB VT/fP (to reduce skewness) as the dependent variable and with each observation weighted by the inverse squared errors of the respective [11C]DASB VT/fP. These SEs were estimated using a bootstrap algorithm that accounts for errors in metabolite, plasma, and brain data.32 Weighting based on the precision of binding potential estimates ensures that more reliable observations have more influence on results. Weights were also winsorized at the 90th percentile to avoid having outliers in the weight function, which also prevents a small number of patients from having an outsized influence on model parameters. Suicide attempt status during follow-up was the independent variable. Sensitivity analyses using the same analytic strategy assessed BPF, BPP, and BPND.
RN [11C]WAY-100635 BPF, Future Attempt Lethality and Intent, and Future Suicidal Ideation
Log-transformed RN serotonin1A BPF was modeled using a weighted linear regression model with maximal attempt lethality during follow-up and sex as independent variables. Weights were based on the BPF estimate’s precision, as above. Associations between lethality and log-transformed serotonin1A BPF in all 12 ROIs were explored using a single mixed-effects model with the following fixed effects: maximal attempt lethality during follow-up, ROI, lethality by ROI indicator interaction, and sex. A patient-specific random intercept term accounted for intrapatient correlation in BPF across ROIs. If the interaction term was not significant, it was removed from the model and only the main effect was reported. If the interaction term was significant, exploratory ROI-specific analyses similar to that for the RN were conducted for the remaining 11 ROIs. The ROI-specific exploratory models were adjusted using the Benjamini-Hochberg approach to control the false discovery rate.
Primary and exploratory analyses of serotonin1A BPF and the Beck Suicide Intent Scale score for the most lethal attempt during follow-up were identical to those for lethality. In analyses examining the relationship between serotonin1A BPF and future BSSI, we included attempters and nonattempters whose Hamilton Depression Rating Scale score was greater than 10, a score below which few have suicidal ideation.33 Three separate models tested the association at 3 (51 of 134 patients), 12 (47 or 134 patients), and 24 (39 of 134 patients) months, using the same strategy used for maximum lethality (eg, 1 primary analysis examining the association between future BSSI score and RN serotonin1A BPF and exploratory analyses of the association between future BSSI score and serotonin1A BPF in all 12 ROIs [all regions in 1 model]; and follow-up exploratory analyses of future BSSI and serotonin1A BPF in each ROI, if appropriate). Exploratory analyses used the Benjamini-Hochberg adjustment.
We conducted several sensitivity analyses. Given our cross-sectional findings,12 we calculated the correlation between base-line and future lethality scores and examined the association between RN serotonin1A BPF and future lethality while controlling for baseline lethality and sex. Sensitivity analyses excluding attempters whose attempt was of zero lethality assessed whether nondamaging attempts were biologically different. We also adjusted our model for clinical predictors of attempt lethality to explore whether binding provides additional information to that provided by these measures. Finally, we explored the effect of recent medication status on RN serotonin1A BPF’s association with follow-up attempt lethality by adjusting the weighted regression model described above using an indicator variable (medication washout vs no washout).
Results
One hundred patients (39 men; 61women; mean [SD] age, 40.2 [11.2] years) underwent [11C]WAY-100635 PET scans and 50 also underwent [11C]DASB scans; all had follow-up data (median follow-up, 748 days; range, 76–1294 days; time censored at 800 days). Fifty-one participants were past suicide attempters. During follow-up, 13 patients made a nonfatal attempt and 2 additional patients died by suicide. Clinical and demographic data are found in Table 1.
Table 1.
Baseline Characteristics of the 100 Participants
| Characteristic | Patients |
|---|---|
| Male sex, No. (%) | 39 (39) |
| Childhood, No. (%) | |
| Abuse | 51(51) |
| Separation younger than 15y (n = 99) | 38 (38) |
| Comorbid past substance abuse, No. (%) (n = 99) | 28 (28) |
| Cigarette smoking, No. (%) (n = 99) | 24 (24) |
| Borderline personality disorder, No. (%) (n = 95) | 17(18) |
| History of suicide attempt, No. (%) | 51 (51) |
| Age, mean (SD), y | 40.2 (11.2) |
| Hamilton Depression Rating Scale score, mean (SD)a | 19.5 (4.9) |
| Beck Depression Inventory score, mean (SD) (n = 99)b | 28.6 (10.1) |
| Beck Hopelessness Scale score, mean (SD)c | 12.0 (5.8) |
| Scale for Suicidal Ideationd | |
| 2 wk prior (n = 85) | 8.9 (9.3) |
| Current (n = 99) | 6.2 (7.3) |
| Brown-Goodwin Aggression History Scale score, mean (SD) (n = 98)e | 18.5 (5.6) |
| Buss-Durkee Hostility Inventory score, mean (SD) (n = 88)f | 38.7 (11.9) |
| Barratt Impulsivity Scale score, mean (SD) (n = 88)g | 57.7 (18.2) |
| No. of past suicide attempts, mean (SD) (n = 51) | 2.4 (1.9) |
| Maximal lethality of suicide attempts score, mean (SD) (n = 51)h | 2.6 (1.7) |
Scores range from 0 to 54, with higher scores indicating more severe depression.
Scores range from 0 to 63, with higher scores indicating more severe depression.
Scores range from 0 to 20, with higher scores indicating more severe hopelessness.
Scores range from 0 to 48, with higher scores indicating more severe ideation.
Scores range from 10 to 40, with higher scores indicating more severe aggression.
Scores range from 0 to 66, with higher scores indicating more severe hostility.
Scores range from 0 to 96, with higher scores indicating more severe impulsivity.
Scores range from 0 to 8, with higher scores indicating fatal injury.
Midbrain [11C]DASB BPF and Future Suicide Attempts
Only 5 patients with [11C]DASB data manifested suicidal behavior during follow-up, which limited statistical power. Future suicidal behavior was not associated with lower midbrain serotonin transporter VT/fP (log-rank ; P = .54). Similarly, a weighted analysis of variance model showed no difference in midbrain serotonin transporter VT/fP between future attempters and nonattempters (log scale difference: b = 0.006; SE = 0.08; t = 0.07; P = .94). Sensitivity analyses with 3 other [11C]DASB outcome measures (BPP, BPF, and BPND) were not different in future attempters.
VT/fP, Future Attempt Lethality and Intent, and Future Suicidal Ideation
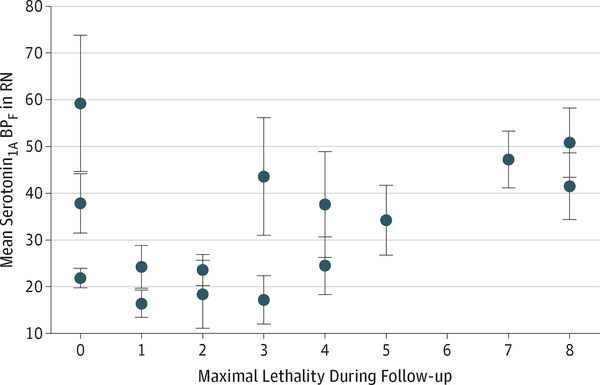
Higher lethality of future attempts was associated with higher RN serotonin1A BPF in the weighted regression model testing our primary hypothesis (b = 0.08; t = 2.89; P = .01) (Figure 1) and remained significant after adjusting for baseline lethality (P = .02). The mixed-effects exploratory model with BPF in 12 ROIs (including RN) as a response variable and ROI, lethality, and their interaction as independent variables showed an interaction between ROI BPF and lethality (F11,14 = 1.91; P = .04), indicating that the relationship between lethality and BPF was not uniform across ROIs. We found an association between future lethality and higher serotonin1A BPF in the insula, but not after adjustment for multiple testing (Table 2).
Figure 1. Correlation Between Maximal Lethality of Suicide Attempts During Follow-up and Serotonin1A Binding Potential (BPF) in the Raphe Nuclei (RN).
Each data point represents the mean RN BPF for a single suicide attempter; error bars indicate SEs. Lethality is scored from 0 to 8, with 0 indicating no injury and 8 indicating fatal injury, using the Beck Suicide Intent Scale20 and Medical Lethality Scale.26
Table 2.
Association Between Serotonin1A BPF in 12 ROIs and Lethality of Follow-up Suicide Attemptsa
| Log-Transformed PBF per ROI | With Zero-Lethality Attempters |
Without Zero-Lethality Attempters |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | t Value | P Value | Adjustedb | Estimate | t Value | P Value | Adjustedb | |
| RN | 0.08 | 2.88 | .01 | NA | 0.12 | 4.43 | .002 | NA |
| Amygdala | 0.01 | 0.48 | .64 | 0.64 | 0.02 | 0.68 | .51 | 0.51 |
| Hippocampus | 0.05 | 1.61 | .13 | 0.15 | 0.07 | 1.82 | .10 | 0.11 |
| Parahippocampal gyrus | 0.05 | 1.65 | .12 | 0.15 | 0.07 | 2.13 | .06 | 0.08 |
| Temporal lobe | 0.05 | 1.64 | .12 | 0.15 | 0.08 | 2.13 | .06 | 0.08 |
| Anterior cingulate gyrus | 0.06 | 1.79 | .09 | 0.15 | 0.09 | 2.27 | .04 | 0.08 |
| Dorsal PFC | 0.07 | 1.84 | .09 | 0.15 | 0.10 | 2.44 | .03 | 0.08 |
| Medial PFC | 0.06 | 1.65 | .12 | 0.15 | 0.09 | 2.05 | .07 | 0.08 |
| Orbital PFC | 0.05 | 1.56 | .14 | 0.15 | 0.09 | 2.13 | .06 | 0.08 |
| Insula | 0.05 | 2.31 | .04 | 0.15 | 0.07 | 2.41 | .04 | 0.08 |
| Occipital lobe | 0.05 | 1.71 | .11 | 0.15 | 0.09 | 2.17 | .05 | 0.08 |
| Parietal lobe | 0.06 | 1.71 | .11 | 0.15 | 0.09 | 2.24 | .05 | 0.08 |
Abbreviations: BPF, binding potential; NA, not applicable in primary analysis; PFC, prefrontal cortex; RN, raphe nuclei; ROI, region of interest.
Patients underwent imaging with carbon 11-labeled 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile ([11C]DASB).
Significance levels for exploratory analyses are adjusted using the Benjamini-Hochberg method.
After removing 3 patients with zero-lethality follow-up attempts in an exploratory analysis, we found a positive correlation between serotonin1A BPF and future lethality (b = 0.09; t9 = 2.38; P = .04) across all ROIs (interaction with ROI: F11,11 = 0.99; P = .45). The ROI-specific post hoc analyses without these 3 patients showed significant associations between future lethality and serotonin1A BPF in the RN, anterior cingulate, dorsolateral PFC, and insula. After Benjamini-Hochberg correction, these regions showed a strengthened but nonsignificant association (Table 2).
Adjusting for the presence of a comorbid personality disorder did not affect results, and we found no evidence of a differential correlation between future lethality and BPF based on comorbid diagnoses. Adjusting for clinical or demographic variables at the time of the baseline scan (Table 1), such as age, severity of depression, aggression, impulsivity, and suicidal ideation, did not alter the significance of lethality’s association with RN serotonin1A BPF. Indeed, RN serotonin1a BPF, adjusted for sex, explained more variability than independent variables such as the Beck Depression Inventory score (adjusted R2 = 0.33 [P = .03] vs adjusted R2 = 0.16 [P = .19], respectively).
Suicidal intent associated with the most lethal suicide attempt during follow-up was not associated with RN serotonin1A BPF (b = −0.006; t = −0.20; P = .84). An exploratory model with all 12 ROIs was not significant (F1,10 = 0.83; P = .38). Removing zero-lethality attempters did not change results.
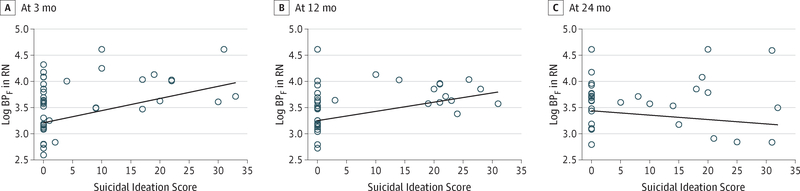
The BPF of RN serotonin1A correlated positively with suicidal ideation severity (BSSI score) at 3 (b = 0.02; t = 3.45; P = .001) and 12 (b = 0.02; t = 3.63; P = .001) months of follow-up, but not at 24 months (Figure 2). In mixed-effects exploratory models encompassing all 12 ROIs, higher BSSI scores at 3 and 12 months were associated with higher serotonin1A BPF (F11,40 = 9.64 [P = .03] and F11,34 = 5.11 [P = .03], respectively), but not 24 months, with differences in the strength of the association across ROIs at both time points (interactions with ROI: P = .003 at 3 months and P < .001 at 1 year) (Table 3). This association was present for every ROI at 3 months and all ROIs except the amygdala at 12 months. Sensitivity analyses showed that recent medication status (required washout vs no washout) did not affect serotonin1A BPF in any ROI (F1,97 = 0.008; P = .93) and did not predict future attempts (log-rank test ; P = .68) or lethality (t13 = 0.98; P = .34).
Figure 2. Serotonin1A Binding Potential (BPF) in Raphe Nuclei (RN) by Suicide Ideation Severity.
Serotonin1A BPF was measured at baseline; suicide ideation severity was measured at 3, 12, and 24 months using the Beck Scale for Suicide Ideation.20 Scores ranged from 0 to 30, with higher scores indicating more severe ideation. Standard errors of the BP estimates were adjusted for in the calculation of the regression estimates.
Table 3.
Serotonin1A ROI Analysis Results for Suicidal Ideation During Follow-upa
| Log-Transformed PBF per ROI | 3 mo |
12 mo |
24 mo |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | P Value | Adjustedb | Estimate | P Value | Adjustedb | Estimate | P Value | Adjustedb | |
| RN | 0.02 | .001 | NA | 0.01 | <.001 | NA | −0.008 | .21 | NA |
| Amygdala | 0.02 | <.001 | 0.002 | 0.004 | .26 | 0.26 | −0.01 | .03 | 0.29 |
| Hippocampus | 0.02 | <.001 | 0.002 | 0.009 | .03 | 0.03 | −0.007 | .18 | 0.29 |
| Parahippocampal gyrus | 0.01 | .01 | 0.02 | 0.01 | .004 | 0.02 | −0.009 | .10 | 0.29 |
| Temporal lobe | 0.01 | .005 | 0.01 | 0.007 | .02 | 0.03 | −0.008 | .11 | 0.29 |
| Anterior cingulate gyrus | 0.01 | .02 | 0.02 | 0.009 | .004 | 0.02 | −0.008 | .12 | 0.29 |
| Dorsal PFC | 0.01 | .02 | 0.02 | 0.009 | .01 | 0.02 | −0.006 | .24 | 0.29 |
| Medial PFC | 0.01 | .01 | 0.02 | 0.008 | .01 | 0.02 | −0.005 | .29 | 0.29 |
| Orbital PFC | 0.01 | .02 | 0.02 | 0.007 | .03 | 0.03 | −0.006 | .24 | 0.29 |
| Insula | 0.01 | .006 | 0.01 | 0.009 | .006 | 0.02 | −0.005 | .29 | 0.29 |
| Occipital lobe | 0.01 | .01 | 0.02 | 0.008 | .01 | 0.02 | −0.006 | .22 | 0.29 |
| Parietal lobe | 0.01 | .02 | 0.02 | 0.008 | .01 | 0.02 | −0.007 | .16 | 0.29 |
Abbreviations: BPF, binding potential; NA, not applicable in the primary analysis; PFC, prefrontal cortex; RN, raphe nuclei; ROI, region of interest.
Patients underwent imaging with carbon 11-labeled 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile ([11C]DASB).
Significance levels for exploratory analyses are adjusted using the Benjamini-Hochberg method.
Discussion
This study is the first prospective examination of whether serotonin transporter and serotonin1A binding in vivo predict suicidal behavior and its lethality. Consistent with postmortem studies of suicides with depression suicide 34,35 and with cross-sectional imaging findings,12 higher RN serotonin1A BPF predicted greater lethality of future suicidal behavior during 2 years. These results suggest that RN serotonin1A BPF represents a predictive biomarker of more lethal suicidal behavior, a serious clinical outcome.
Higher RN serotonin1A BPF may lead to more lethal suicidal behavior via more severe suicidal ideation, and, indeed, higher RN serotonin1A BPF predicted greater suicidal ideation at 3 and 12 months. However, the association between RN serotonin1A BPF and suicidal intent for future suicide attempts was not detected, possibly owing to insufficient statistical power, because only 15 subsequent suicidal acts were reported.
Vt/fp and Likelihood of Future Suicide Attempts
Lower serotonin transporter binding in ventromedial PFC and other brain regions is found in suicides in postmortem studies.36–40 These and cross-sectional findings3 led us to hypothesize that lower midbrain serotonin transporter Vt/fp would predict suicide attempts during a 2-year period. This prediction was not verified in our subsample, perhaps because only 5 attempts occurred during follow-up.
Although 2 meta-analyses examining the serotonin transporter LPR polymorphism report an association of suicidal behavior and the serotonin transporter LPR low-expressing S allele,41,42 we and others3,43–49 have reported no association of in vivo binding with lower-expressing alleles in the gene, although not all studies agree.50–52 A more robustly powered study with a larger sample of future suicide attempters may determine whether serotonin transporter binding predicts suicide risk.
RN [11C]WAY-100635 BPF, Future Attempt Lethality and Intent, and Future Suicidal Ideation
Serotonin1A somatodendritic autoreceptors inhibit RN serotonin neuron firing.53 Thus, more autoreceptors can lead to less firing and serotonin release from nerve terminals.53,54 That higher RN serotonin1A BPF predicts more lethal suicidal behavior agrees with cross-sectional studies showing that higher-lethality attempters have low cerebrospinal fluid levels of 5-hydroxyindoleacetic acid, a marker of low serotonin turnover,55 and with reports of blunted prolactin response to serotonergic challenge in high-lethality suicide attempters.10 The effect of higher RN serotonin1A BPF on lethality of future suicidal behavior is also consistent with the observation that low cerebrospinal fluid 5-hydroxyindoleacetic acid levels predict suicide death among discharged inpatients.56,57
How might elevated RN serotonin1A BPF lead to a complex outcome such as a suicide attempt? We observed that RN serotonin1A BPF predicted more pronounced suicidal ideation at 3 and 12 months, which could lead to more lethal attempts. Moreover, other parameters associated with low serotonergic tone, as would be expected in individuals with greater RN serotonin1A BPF, are predictors of more lethal suicidal behavior. For example, we found that a lower response to fenfluramine, an index of serotonergic hypofunction, was associated with higher-lethality behavior and low impulsivity.10 Low impulsivity may increase lethality owing to careful planning,10,58 although impulsive individuals may make high-lethality attempts if they choose very lethal methods (eg, firearms, jumping). On the other hand, we have previously reported that those with low serotonin1A BPF—the opposite of what we found here—have greater aggression,59 found in some studies to be linked to more lethal suicidal behavior.60,61 Our sample had relatively low aggression (mean score, 18.5), perhaps indicating that aggression was not a key driver of suicidal behavior for this study population.
Exploratory post hoc analyses suggested that greater serotonin1A BPF in insula, in addition to RN, predicted more lethal attempts. When those whose future suicide attempts had zero lethality were excluded, serotonin1A BPF in anterior cingulate cortex and dorsolateral PFC also predicted greater lethality, although these findings did not survive the Benjamini-Hochberg adjustment. The anterior insula has close neuroanatomic connections to the anterior cingulate cortex. Together, they are pivotal to decision making involving risk62 (weighing risks and benefits of a decision) and in emotional and social cognition, putatively mediated by von Economo neurons,63 and implicated in suicidal behavior previously.1 Thus, lethality may be a consequence of the individual’s underestimate of the risk of a negative effect on loved ones during decision making about whether to attempt suicide.64 Similarly, disturbances in emotional and social cognition could lead to underestimation of the social and emotional consequences of the suicidal behavior for others. This insula-anterior cingulate cortex system is also implicated in switching from default mode network to executive mode, which enlists the dorsolateral PFC,62,65 critical for reappraisal and contextual processing to assess the salience of negative stimuli.66 Moreover, inhibitory effects of serotonin1A heteroreceptors on glutamatergic and γ-aminobutyric acid-transmitting cells that drive hemodynamic responses to functional demands are hypothesized to regulate activation of the default mode network.67 Thus, attempt lethality may result from excessive default network activity leading to negative ruminative states,66 perhaps potentiating suicidal preoccupation.
We did not find an association of serotonin1A BPF with the suicidal intent associated with future suicide attempts, in contrast to findings in past suicide attempters. However, higher RN serotonin1A BPF predicted more suicidal ideation (BSSI score) at 3 and 12 months, and exploratory analyses showed that greater serotonin1A BPF in 12 ROIs also predicted a greater BSSI score at 3 and 12 months, but with differences in the strength of that relationship across ROIs. Although more severe suicidal ideation at 3 and 12 months of follow-up may lead to well-planned, more lethal suicide attempts, we were unable to detect such an association in this study. In addition, whether elevated BSSI scores at 3 and 12 months reflect more persistent or chronic suicidal ideation, thereby increasing risk, is unknown. We are currently investigating this question.
Systems other than serotonergic neurotransmission are linked to suicidal behavior, for example, γ-aminobutyric acid, glutamatergic, hypothalamic-pituitary-adrenal, and noradrenergic systems.2 Many of these systems interact with the serotonin system. Too few future suicide attempts limit statistical power, but this study is one of the largest prospective PET brain imaging studies in mood disorders ever published. We did not have data regarding medication exposure during follow-up. Finally, [11C]WAY-100635, a serotonin1A antagonist, binds to high- and low-affinity states, limiting its utility for examining high-affinity agonist receptors.
Conclusions
We found that greater RN serotonin1A BPF, measured with [11C]WAY-100635 PET, predicted more lethal suicidal behavior during a 2-year period. Our results suggest that this association is at least partly mediated through more severe suicidal ideation. Identifying neurobiological characteristics of high-lethality suicide attempters has intrinsic scientific importance, and discovery of molecular-level markers of high-lethality behavior may eventually improve clinical screening to detect those at risk for suicide.
Supplementary Material
Key Points.
Question
Are in vivo brain serotonergic indices predictive of more lethal suicidal behavior?
Findings
The binding potential of the serotonin1A receptor in the raphe nuclei predicts more lethal suicidal behavior and greater suicidal ideation during a 2-year follow-up.
Meaning
Serotonin system hypofunction is a key risk factor for suicidal behavior with greater morbidity and mortality; treatments that target this system pharmacologically or that impact the effects of low serotonergic tone on suicidal ideation, such as cognitive therapy for suicidal behavior, may aid in stemming the epidemic of suicides.
Acknowledgments
Funding/Support: This study was supported by grants MH40695, MH62185, MH074813, MH090964, and MH48514 from the US Public Health Service.
Role of Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Oquendo reports receiving royalties from the Research Foundation for Mental Hygiene (RFMH) for commercial use of the Columbia Suicide Severity Rating Scale (C-SSRS) and family ownership of stock in Bristol-Myers Squibb. Dr Miller reports receiving financial compensation for psychiatric evaluations of patients enrolled in medication studies sponsored by Pfizer and Orexigen Therapeutics and family ownership of stock in Johnson & Johnson. Dr Burke reports receiving royalties from the RFMH for commercial use of the C-SSRS. Dr Mann reports receiving royalties from the RFMH for commercial use of the C-SSRS. No other disclosures were reported.
Contributor Information
Maria A. Oquendo, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Hanga Galfalvy, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Gregory M. Sullivan, Tonix Pharmaceuticals, LLC, New York, New York.
Jeffrey M. Miller, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Matthew M. Milak, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
M. Elizabeth Sublette, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Sebastian Cisneros-Trujillo, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Ainsley K. Burke, New York State Psychiatric Institute, New York; Department of Psychiatry, Columbia University, New York, New York.
Ramin V. Parsey, Department of Psychiatry and Behavioral Science, Stony Brook University School of Medicine, Stony Brook, New York.
J. John Mann, New York State Psychiatric Institute, New York; Department of Radiology, Columbia University, New York, New York.
REFERENCES
- 1.van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. 2014;1(1):63–72. [DOI] [PubMed] [Google Scholar]
- 2.Oquendo MA, Sullivan GM, Sudol K, et al. Toward a biosignature for suicide. Am J Psychiatry. 2014;171 (12):1259–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JM, Hesselgrave N, Ogden RT, et al. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74 (4):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nye JA, Purselle D, Plisson C, et al. Decreased brainstem and putamen SERT binding potential in depressed suicide attempters using [11C]-zient PET imaging. Depress Anxiety. 2013;30(10):902–90. [DOI] [PubMed] [Google Scholar]
- 5.Selvaraj S, Murthy NV, Bhagwagar Z, et al. Diminished brain 5-HT transporter binding in major depression: a positron emission tomography study with [11C]DASB. Psychopharmacology (Berl). 2011; 213(2–3):555–562. [DOI] [PubMed] [Google Scholar]
- 6.Cannon DM, Ichise M, Rollis D, et al. Elevated serotonintransporterbindinginmajordepressive disorder assessed using positron emission tomography and [11C]DASB: comparison with bipolar disorder. Biol Psychiatry. 2007;62(8):870–87. [DOI] [PubMed] [Google Scholar]
- 7.Vang FJ, Ryding E,Träskman-Bendz L, van Westen D, Lindström MB. Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res. 2010;183(2):177–179. [DOI] [PubMed] [Google Scholar]
- 8.Sublette ME, Milak MS, Galfalvy HC, Oquendo MA, Malone KM, Mann JJ. Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Arch Suicide Res. 2013;17(4):434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153(2): 174–182. [DOI] [PubMed] [Google Scholar]
- 10.Oquendo MA, Placidi GP, Malone KM, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60(1):14–22. [DOI] [PubMed] [Google Scholar]
- 11.Leyton M, Paquette V, Gravel P, et al. alpha-[11C]Methyl-L-tryptophan trapping in the orbital and ventral medial prefrontal cortex of suicide attempters. Eur Neuropsychopharmacol. 2006;16(3):220–223. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan GM, Oquendo MA, Milak M, et al. Positron emission tomography quantification of serotonin(1A) receptor binding in suicide attempters with major depressive disorder. JAMA Psychiatry. 2015;72(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller JM, Hesselgrave N, Ogden RT, et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol Psychiatry. 2013;74(10):760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsey RV, Ogden RT, Miller JM, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsey RV, Oquendo MA, Ogden RT, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59(2): 106–113. [DOI] [PubMed] [Google Scholar]
- 17.First MB Sr, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P), Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Kovacs M, Weissman A Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47(2):343–352. [DOI] [PubMed] [Google Scholar]
- 21.Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1(2):131–139. [DOI] [PubMed] [Google Scholar]
- 22.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957; 21(4):343–349. [DOI] [PubMed] [Google Scholar]
- 23.Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychol Rep. 1965;16:547–554. [DOI] [PubMed] [Google Scholar]
- 24.Oquendo MAHB, Mann JJ. Risk factors for suicidal behavior: the utility and limitations of research instruments In: First MB, ed. Standardized Evaluation in Clinical Practice. Vol 22 Washington, DC: American Psychiatric Publishing; 2003:103–130. [Google Scholar]
- 25.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007; 164(7):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck AT, Beck R, Kovacs M Classification of suicidal behaviors, I: quantifying intent and medical lethality. Am J Psychiatry. 1975;132(3):285–287. [DOI] [PubMed] [Google Scholar]
- 27.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25 (7):785–793. [DOI] [PubMed] [Google Scholar]
- 28.Parsey RV, Slifstein M, Hwang DR, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison ofarterial and reference tisssue input functions. J Cereb Blood Flow Metab. 2000;20(7):1111–1133. [DOI] [PubMed] [Google Scholar]
- 29.Parsey RV, Ogden RT, Mann JJ. Determination of volume of distribution using likelihood estimation in graphical analysis: elimination of estimation bias. J Cereb Blood Flow Metab. 2003;23 (12):1471–1478. [DOI] [PubMed] [Google Scholar]
- 30.Ogden RT. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat Med. 2003;22(22):3557–3568. [DOI] [PubMed] [Google Scholar]
- 31.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. [DOI] [PubMed] [Google Scholar]
- 32.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7(1):115–129. [DOI] [PubMed] [Google Scholar]
- 33.Riihimäki K, Vuorilehto M, Melartin T, Haukka J, Isometsa E. Incidenceand predictors of suicide attempts among primary-care patients with depressive disorders: a 5-year prospective study. Psychol Med. 2014;44(2):291–302. [DOI] [PubMed] [Google Scholar]
- 34.Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42(6):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression: postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18 (18):7394–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2012;15(4): 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114(3):807–815. [DOI] [PubMed] [Google Scholar]
- 38.Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. [DOI] [PubMed] [Google Scholar]
- 39.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995; 688(1–2):121–133. [DOI] [PubMed] [Google Scholar]
- 40.Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia: a postmortem study. Arch Gen Psychiatry. 1993;50(10):810–818. [DOI] [PubMed] [Google Scholar]
- 41.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter, II: suicidal behavior. Mol Psychiatry. 2003;8(7):646–653. [DOI] [PubMed] [Google Scholar]
- 42.Lopez de Lara C, Dumais A, Rouleau G, et al. STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry. 2006;59(2):114–120. [DOI] [PubMed] [Google Scholar]
- 43.Güzey C, Allard P, Brannstrom T, Spigset O. Radioligand binding to brain dopamine and serotonin receptors and transporters in Parkinson’s disease: relation to gene polymorphisms. Int J Neurosci. 2012;122(3):124–132. [DOI] [PubMed] [Google Scholar]
- 44.Kobiella A, Reimold M, Ulshofer DE, et al. How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry. 2011;1:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murthy NV, Selvaraj S, Cowen PJ, et al. Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage. 2010;52(1):50–54. [DOI] [PubMed] [Google Scholar]
- 46.Rhodes RA, Murthy NV, Dresner MA, et al. Human 5-HTtransporteravailabilitypredicts amygdala reactivity in vivo. J Neurosci. 2007;27 (34):9233–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oquendo MA, Hastings RS, Huang YY, et al. Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry. 2007; 64(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parsey RV, Hastings RS, Oquendo MA, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163(1):48–51. [DOI] [PubMed] [Google Scholar]
- 49.Shioe K, Ichimiya T, Suhara T, et al. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48(4):184–188. [DOI] [PubMed] [Google Scholar]
- 50.Kalbitzer J, Frokjaer VG, Erritzoe D, et al. The personality trait openness is related to cerebral 5-HTT levels. Neuroimage. 2009;45(2):280–285. [DOI] [PubMed] [Google Scholar]
- 51.Reimold M, Smolka MN, Schumann G, et al. Midbrain serotonin transporter binding potential measured with [11C]DASB is affected by serotonin transporter genotype. J Neural Transm (Vienna). 2007;114(5):635–639. [DOI] [PubMed] [Google Scholar]
- 52.Praschak-Rieder N, Kennedy J, Wilson AA, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol Psychiatry. 2007;62(4):327–331. [DOI] [PubMed] [Google Scholar]
- 53.Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. LifeSci. 1991;48(18):1779–1786. [DOI] [PubMed] [Google Scholar]
- 54.Kelly JS, Larkman P, Penington NJ, Rainnie DG, McAllister-Williams H, Hodgkiss J. Serotonin receptor heterogeneity and the role of potassium channels in neuronal excitability. Adv Exp Med Biol. 1991;287:177–191. [DOI] [PubMed] [Google Scholar]
- 55.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41(2): 162–171. [DOI] [PubMed] [Google Scholar]
- 56.Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? Int J Neuropsychopharmacol. 2006;9(4):465–474. [DOI] [PubMed] [Google Scholar]
- 57.Nordstrom P, Samuelsson M, Asberg M, et al. CSF 5-HIAA predicts suicide risk after attempted suicide. Suicide Life Threat Behav. 1994;24(1):1–9. [PubMed] [Google Scholar]
- 58.Baca-García E, Diaz-Sastre C, Basurte E, et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. J Clin Psychiatry. 2001;62(7):560–564. [DOI] [PubMed] [Google Scholar]
- 59.Parsey RV, Oquendo MA, Simpson NR, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954(2):173–182. [DOI] [PubMed] [Google Scholar]
- 60.Doihara C, Kawanishi C, Yamada T, et al. Trait aggression in suicide attempters: a pilot study. Psychiatry Clin Neurosci. 2008;62(3):352–354. [DOI] [PubMed] [Google Scholar]
- 61.Placidi GP, Oquendo MA, Malone KM, Huang YY, Ellis SP, Mann JJ. Aggressivity, suicide attempts, and depression: relationship to cerebrospinal fluid monoamine metabolite levels. Biol Psychiatry. 2001;50(10):783–791. [DOI] [PubMed] [Google Scholar]
- 62.Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci. 2014;1316:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evrard HC, Forro T, Logothetis NK Von Economo neurons in the anterior insula of the macaque monkey. Neuron. 2012;74(3):482–489. [DOI] [PubMed] [Google Scholar]
- 64.Joiner TE, Pettit JW, Walker RL, et al. Perceived burdensomeness and suicidality: two studies on the suicide notes of those attempting and those completing suicide. J Soc Clin Psychol. 2002;21(5): 531–545. [Google Scholar]
- 65.Klein TA, Ullsperger M, Danielmeier C Error awareness and the insula: links to neurological and psychiatric diseases. Front Hum Neurosci. 2013;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. [DOI] [PubMed] [Google Scholar]
- 67.Hahn A, Wadsak W, Windischberger C, et al. Differential modulation of the default mode network via serotonin-1A receptors. Proc Natl Acad Sci USA. 2012;109(7):2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.