Abstract
The major route of hepatitis C virus (HCV) transmission in the United States is injection drug use. We hypothesized that if a HCV vaccine was available, vaccination could impact HCV transmission among people who inject drugs by reducing HCV titers after viral exposure without necessarily achieving sterilizing immunity. To investigate this possibility, we developed a mathematical model to determine transmission probabilities relative to the HCV RNA titers of needle/syringe sharing donors. We simulated sharing of two types of syringes fitted with needles that retain either large or small amounts of fluid post-expulsion. Using previously published viral kinetics data from both naïve subjects infected with HCV and reinfected individuals who had previously cleared an HCV infection, we estimated transmission risk between pairs of serodiscordant injecting drug users, taking into account syringe type, rinsing, and sharing frequency. We calculated that the risk of HCV transmission through syringe sharing increased ~10-fold as viral titers (log10 IU/mL) increased ~25-fold. Cumulative analyses showed that, assuming sharing episodes every 7 days, the mean transmission risk over the first 6 months was >90% between two people sharing syringes when one had an HCV RNA titer >5 log10 IU/mL, regardless of infection outcome. For those with pre-existing immunity that rapidly controlled HCV, the cumulative risk decreased to 1–25% depending on HCV titer and syringe type. Our modeling approach demonstrates that even with transient viral replication following exposure during injection drug use, HCV transmission among people sharing syringes could be reduced through vaccination if a HCV vaccine were available.
One Sentence Summary:
A future HCV vaccine could reduce hepatitis C virus transmission among those sharing syringes, even in the presence of post-exposure viral replication in vaccinees.
Introduction
Injection drug use is the leading cause of hepatitis C virus (HCV) transmission in the United States (1) with an estimated 60% of all HCV infections attributed to sharing needles, syringes or other drug paraphernalia (2). As a consequence, people who inject drugs are an important target population for testing the efficacy of HCV vaccines under development and have been recruited to take part in a Phase I/II prophylactic HCV vaccine study (ClinicalTrials.gov Identifier: NCT01436357) designed to induce T cell responses to the HCV non-structural proteins. To date, the only efficacy data for prophylactic HCV vaccines have been obtained from chimpanzees infected with HCV [(reviewed in (3)]. These studies have shown that prophylactic vaccines have successfully induced HCV-specific immune responses and led to reductions in viral replication soon after challenge, indicating that the vaccines effectively primed antibody or T cell responses that could attenuate viral replication (4). However, none of these animal studies achieved sterilizing immunity by completely preventing infection upon viral challenge. Consistent with these findings, data on the spontaneous clearance of natural HCV infections show that pre-existing immune responses do not completely prevent infection and viral replication upon re-exposure in humans or chimpanzees (4, 5). A major problem following HCV infection is viral persistence, which can lead to chronic liver disease. Therefore, prevention of chronic liver disease rather than sterilizing immunity has come to be seen as an acceptable goal for HCV vaccines currently under development. As such, it has been proposed that vaccines would not need to achieve sterilizing immunity in injecting drug users in order to reduce disease spread. Transmission of low amounts of HCV (10 to 100 RNA copies) in naïve chimpanzees has been shown to result in initiation of HCV infection i.e., HCV RNA amplification in blood (6–9). In contrast, blood samples containing trace amounts of RNA (<10 RNA copies) have been shown to be non-infectious (6, 7, 10); this lack of infectivity may be dependent upon the RNA to infectious virus ratio in any given sample. Reverse titration studies in chimpanzees have shown that not all HCV RNA molecules are infectious and that more than one RNA copy is needed to initiate infection (11).
It is plausible to assume that the viral titer of transmitted blood is related to the probability of transmitting infection, in that small amounts of low-titer blood are less likely to contain infectious virus. We hypothesized that the HCV titer of an infected individual would directly impact the probability of transmitting infectious particles in the small volumes of blood contained within contaminated needles and syringes. Therefore, if titers are sufficiently reduced, vaccines would not need to achieve sterilizing immunity in people who inject drugs in order to reduce disease transmission. However, how low these viral titers would need to be to substantially reduce the chance of transmission from shared syringes with attached needles is unknown.
HCV transmission risk is challenging to study due to difficulties in culturing natural isolates and the limited availability of animal models to study viral replication (12). Nonetheless, several informative studies on HCV survival in injection equipment or transmission through syringe sharing have been completed (13–16). Simulations of injection practices and syringe contamination using HCV cell cultures have shown that although titers decline within the first few days (14, 16), the virus can maintain infectivity for prolonged periods of up to 6 weeks (13, 16) and therefore could potentially be transmitted to an uninfected individual despite extended periods between syringe sharing events. Additionally, the volume of residual blood in shared needles/syringes has been shown to directly impact the amount of virus that could be transferred (15). The amount of residual donor blood could be affected by the type of syringe used. High dead space syringes retain more fluid that cannot be expelled from the syringe (i.e. fluid remaining within the needle and between the syringe hub and the plunger following expulsion of liquid) than do low dead space syringes.
We anticipated that several factors could affect the probability of HCV transmission via syringe sharing (illustrated in fig. S1) including: (1) the proportion of blood transmitted from used syringes relative to syringe type (low or high dead space), (2) syringe rinsing, (3) the amount of HCV RNA in the blood of donors, (4) the relative volumes of blood and drug in the needle and syringe, and (5) the fraction of infectious HCV RNA in blood. How these factors could combine to affect HCV transmission remains unclear. To address this, we experimentally estimated HCV carry-over in two different combinations of syringes with needles, a low dead space syringe with a permanently attached small bore needle and a high dead space syringe with a detachable high bore needle. We developed an RNA titer-based mathematical analysis of HCV transmission between people sharing these two types of needle and syringe combinations. We applied this model to previously published viral kinetics data from HCV-infected subjects comprising naïve individuals (17, 18) and reinfected individuals who had previously cleared an HCV infection (19, 20). We then assessed the potential of T cell based HCV vaccines (i.e. vaccines that do not induce neutralizing antibodies) to subsequently reduce HCV transmission.
Results
HCV RNA carry-over in low and high dead space syringes
Figure 1 shows our study approach, equations used and sources of HCV data. We assessed HCV RNA carry-over from used needle and syringe combinations in vitro (Data file S1), as described in Materials and Methods. Throughout this study references to syringes or the term “syringe sharing” assumes the use of a syringe with an attached needle. We obtained mean RNA titers from HCV-positive human plasma, HCV-positive human plasma after syringe expulsion, and normal (uninfected) human plasma that had been drawn into contaminated syringes with attached needles with or without tap water rinse. These data are presented for low dead space syringes with a permanently attached needle (Fig. 2A) and high dead space syringes with a detachable needle (Fig. 2B). RNA extractions for normal plasma and water samples prior to syringe rinsing were always negative for RNA (Figs. 2A and 2B).
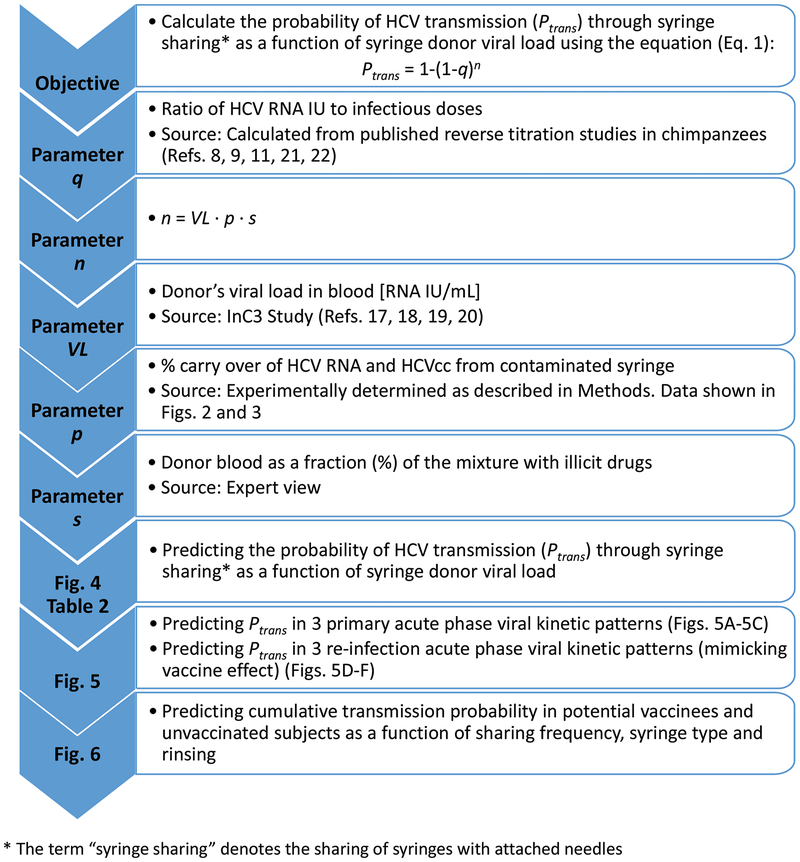
Fig. 1.
Study design, equation parameters and sources of data. The goals of each analysis are shown together with corresponding figures relating to data generated.
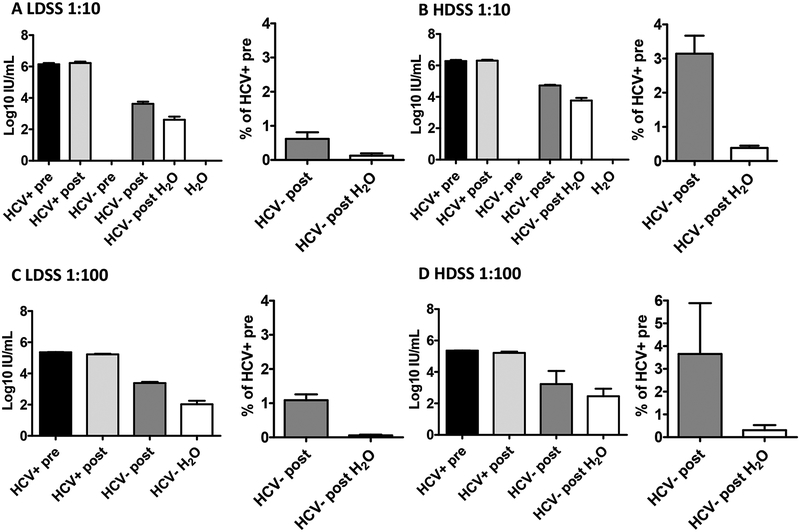
Fig. 2. RNA carry-over of HCV Positive Human Plasma in two different syringe types with attached needles.
Mean RNA titers and percentage carry-over for HCV-positive human plasma samples (diluted 1:10) after being carried over in (A) low dead space syringes (LDSS) and (B) high dead space syringes (HDSS) (mean of 3 independent experiments). Mean RNA titers and percentage carry-over for human plasma samples (diluted 1:100) after being carried over in (C) low dead space syringes and (D) high dead space syringes (mean of 3 independent experiments). Bars represent s.e.m. Means were calculated using log-transformed data. HCV+ pre= HCV-positive plasma before uptake into a syringe; HCV+ post= HCV-positive plasma after expulsion from the syringe; HCV- pre= HCV-negative plasma before uptake into a syringe; HCV- post= HCV-negative plasma after uptake and expulsion from a contaminated syringe; HCV- post H2O= HCV-negative plasma after uptake and expulsion from a contaminated syringe rinsed in tap water.
HCV-positive plasma before and after syringe expulsion showed similar titers for both syringe types (5.36 to 6.79 log10 IU/mL) (Figs. 2A and 2B left panels). We observed carry-over of HCV RNA in 100% of low (17/17) and high (15/15) dead space syringe samples when no rinsing was employed. Titers ranged from 2.27 to 4.32 log10 IU/mL and 4.54 to 4.97 log10 IU/mL for low and high dead space syringes, respectively (Figs. 2A and 2B). After rinsing, we observed HCV RNA carry-over in 83% (10/12) and 100% (12/12) of low and high dead space samples, respectively, with carry-over titers ranging from 1.57 to 3.81 log10 IU/mL and 2.51 to 4.39 log10 IU/mL (Figs. 2A and 2B). These data indicate that although water rinsing reduced the amount of HCV RNA carried over in syringe equipment, there was still a risk of HCV transmission.
We used the above values to estimate percentage carry-over of any HCV-positive sample in low (Fig. 2A) and high (Fig. 2B) dead space syringes for any single sharing event. The average percent carry-over without rinsing was 0.6% (ranging from 0.02 to 2.6%) for low dead space syringes and 3.1% (range 1.4 to 7.2%) for high dead space syringes (Figs. 2A and B). When rinsing was applied, these values were reduced to 0.12% (range 0.003 to 0.8%) for low dead space syringes and 0.38% (range 0.03 to 0.77%) for high dead space syringes. When these studies were repeated using HCV-positive plasma diluted 1:100, we obtained similar carry-over values (Figs. 2C to 2D), indicating that HCV RNA carry-over was independent of the starting RNA titer.
Cell culture HCV (HCVcc) carried over in low and high dead space syringes
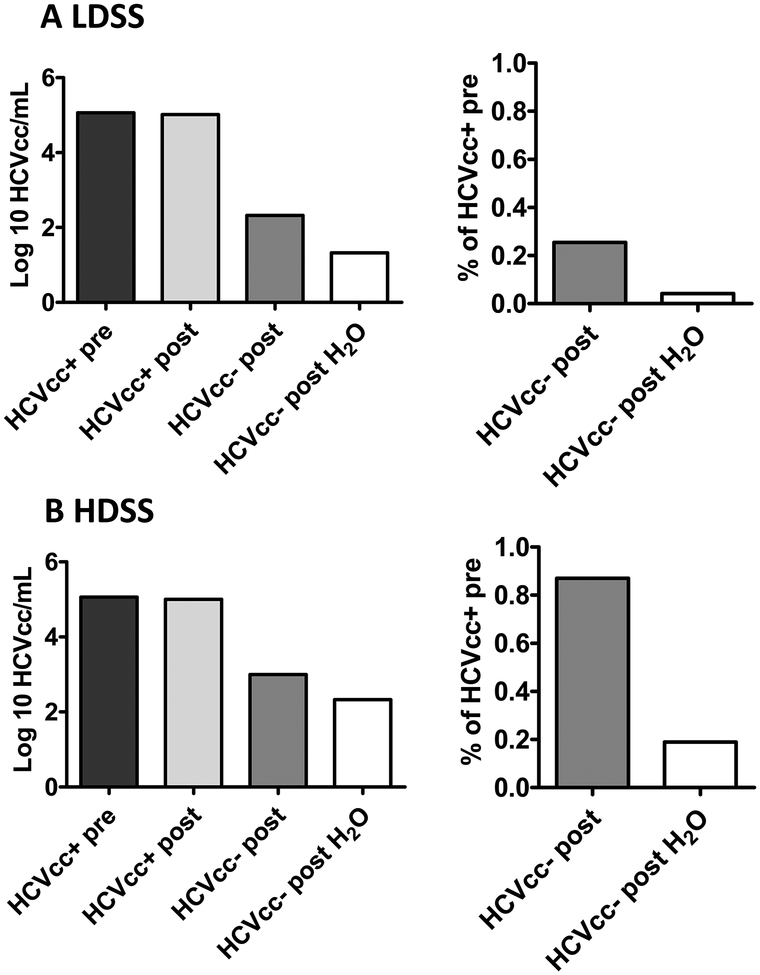
We wished to assess if the percentage transmission of infectious virus particles was consistent with the carry-over values obtained for HCV RNA. It was possible that infectious HCV may not have been carried over as easily as RNA or that manipulations with needles and syringes could have impacted the infectivity of the sample. In such a case, using RNA carry-over to estimate viral transmission could result in inaccurate predictions. We performed the same carry-over experiments using a cultured 1b/JFH1 chimeric virus (HCVcc) (Data file S1). The HCVcc virus titers calculated for syringe-treated samples are shown in Figs. 3A and 3B for low and high dead space syringes with attached needles, respectively. Consistent with the results seen for RNA titers in plasma, we saw similar amounts of infectious virus in the starting samples before and after expulsion for both syringe types. We obtained mean viral titers of 4.9 to 5.1 log10 focus forming units (ffu)/mL for the HCVcc pre- and post-expulsion samples, respectively (Figs. 3A and 3B, left panels). We also observed carry-over of infectious virus using both syringe types, with or without rinsing, although as seen for the plasma studies, more virus was carried over using high dead space syringes (ranges 4.9 to 5.1 log10 ffu/mL, unrinsed; 2.3 to 2.4 log10 ffu/mL, rinsed) (Fig. 3B) compared to low dead space syringes (ranges 2.4 to 2.5 log10 ffu/mL, unrinsed; 0 to 2 log10 ffu/mL, rinsed) (Fig. 3A). The percentage carry-over for unrinsed and rinsed low dead space syringes was 0.26% (range 0.23% to 0.28%) and 0.04% (range 0.003% to 0.08%), respectively (Fig. 3A). For high dead space syringes, the percentage carry-over was 0.87% with no rinsing and 0.19% with rinsing (range 0.16% to 0.22%) (Fig. 3B). Thus, using cell culture HCV, we found the percentage transmission of infectious virus was consistent with that obtained for RNA in human plasma.
Fig. 3. Carry-over of HCV cell culture virus (HCVcc) positive samples in two different syringe types with attached needles.
Shown are mean titers and percentage carry-over of HCVcc before uptake into (A) a low dead space syringe (LDSS) (B) a high dead space syringe (HDSS). HCVcc+ pre= HCVcc-positive sample before uptake into a syringe; HCVcc+ post= HCVcc-positive sample after expulsion from the syringe; HCVcc- post= HCVcc-negative plasma after uptake and expulsion from a contaminated syringe; HCVcc- post H2O= HCVcc-negative plasma after uptake and expulsion from a contaminated syringe rinsed in tap water. Data represent the mean values of 2 independent experiments. Percentages were calculated as described for Figure 2 using HCVcc titers instead of HCV RNA titers. Means were calculated using log transformed data. Ranges taken from all experiments are reported in the text.
Determining Transmission Probability of HCV
To calculate transmission probabilities, we used the RNA carry-over data obtained from our studies with the human HCV plasma samples. The human plasma samples were the most representative of both the virus type and matrix (plasma is more similar to blood than cell culture media) that would be transferred during syringe sharing events. Additionally, the empirical data obtained with these plasma samples gave higher carry-over and greater variability in carry-over percentages compared to our studies with HCVcc and, therefore, represented the highest risk scenario in terms of virus transmission.
We predicted the probability of transmission (Ptrans) for individual syringe sharing events as a function of viral load for low and high dead space syringes with needles attached using an 11:1 RNA to infectivity ratio, considering both rinsed and unrinsed syringes (Eq. 1 and Fig. 4). Table 1 shows the source for each of the parameters used in Eq. 1 and the values, where applicable. The 11:1 infectivity ratio (value q) was determined from empirical data generated in chimpanzee reverse titration studies using acute phase plasma prior to seroconversion (table S2) (8, 9, 11, 22). These studies have provided direct comparisons between RNA titers and infectious titers of HCV RNA-positive samples in the absence of neutralizing antibodies. This type of sample would be applicable to individuals immunized with a T cell based vaccine as they would not have circulating antibodies to HCV envelope proteins that might influence viral infectivity.
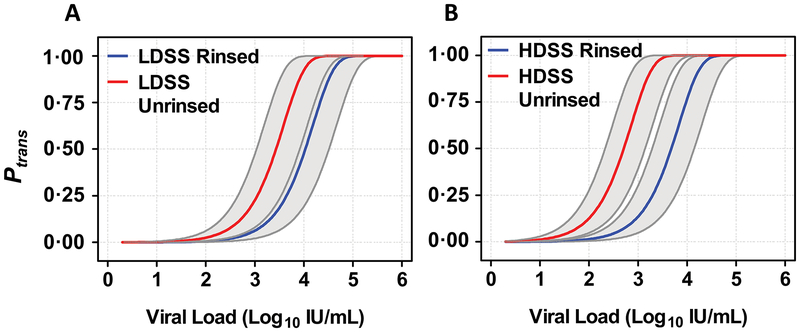
Fig. 4. Probability of HCV Transmission Based on HCV RNA titer.
Probability of transmission (Ptrans) was calculated using Eq.1, described in Materials and Methods, for increasing viral load as a function of syringe type, with and without rinsing. Set viral loads were taken and combined with randomly chosen ranges and frequencies for RNA carry-over and relative volume of donor blood in the drug mixture (ranges for these parameters are shown in Table 1). (A) Calculated values for Ptrans as a function of viral load using rinsed and unrinsed low dead space syringes (LDSS). (B) Calculated values for Ptrans as a function of viral load using rinsed and unrinsed high dead space syringes (HDSS). Grey bands show the interquartile range based on Monte Carlo sensitivity analyses.
Table 1. Equation 1 parameters, values and sources.
Transmission probability was calculated based on Equation 1 (Eq. 1). The parameters VL, s, ρ, and q used in Eq. 1 are described together with values and their ranges. Sources are identified; either calculated in this study, taken from previously published data or estimated from experts familiar with practices employed by injecting drug users. IU, international units; HCV, hepatitis C virus; HDSS, high dead space syringe; LDSS, low dead space syringe; IQR, interquartile range (Q1-Q3).
| Parameter | Description [units] | Value [Range] | Scenario | Source |
|---|---|---|---|---|
| n | Number [#] of transferred HCV RNA IUs by contaminated syringe | VL·ρ·s | Calculated as described in text and in Fig. 1 | |
| VL | Donor’s viral load in blood [RNA IU / mL] | Variable [0 to 6 log10 IU/mL] |
Experimental | Values input for testing (Fig. 4 and Table 1) |
| Time-dependent: 3 primary acute-phase viral kinetic patterns | Self-limiting, transient, chronic outcome (Figs. 5A, B and C) | InC3 Study (17–20) | ||
| Time-dependent: 3 reinfection viral kinetic patterns | Self-limiting (Low and high titer) and chronic outcome (Figs. 5D, E and F) | |||
| s | Donor blood as a fraction of the mixture with illicit drugs [%] | 25% [5%−50%] | Expert view | |
| ρ | HCV RNA carry-over | 0.6% [0.02 to 2.6%] |
LDSS | Estimated in the current study (Fig. 2) |
| 3.1% [1.4 to 7.2%] |
HDSS | |||
| 0.12% [0.003 to 0.8%] |
LDSS + rinse | |||
| 0.38% [0.03 to 0.77%] |
HDSS + rinse | |||
| q | Ratio of infectious doses to HCV RNA IU | 17% [IQR 7–33%] |
Table S2 based on published studies (8, 9, 11, 21, 22) |
We calculated that Ptrans was ≥90% if an individual had a titer >5 log10 IU/mL, regardless of syringe type or use of a rinse step (Table 2). With low dead space syringes Ptrans increased 10-fold as titers increased from ~3.2 to ~4.7 log10 IU/mL (rinsed) and ~2.6 to ~4.1 log10 IU/mL (unrinsed) (Fig. 4A). When using high dead space syringes the risk was greater and increased 10-fold as titers increased from ~2.8 to ~4.3 log10 IU/mL (rinsed) and ~1.9 to ~3.4 log10 IU/mL (unrinsed) (Fig. 4B). If an individual had an HCV titer below ~2.3 log10 IU/mL, then Ptrans for sharing low dead space syringes was predicted to be <5% (Table 2, table S1). In contrast, if high dead space syringes were to be shared, the viral titer would need to be <1.6 log10 IU/mL in order to reduce Ptrans to <5%.
Table 2: Probability (mean ± standard deviation) of HCV transmission as a function of viral load.
To estimate the probability of HCV transmission (Eq. 1) means were calculated using a Monte Carlo procedure. For parameter ρ (Eq. 1), samples were generated using the truncated normal distribution with the standard deviation equal to ¼ of the experimentally determined range. For parameter s, values were chosen from a uniform distribution between 0.05 and 0.5, and q values were chosen from a gamma distribution with calculated median and Q1-Q3 as 17% and 7%−33%, respectively, (table S2). HDSS, high dead space syringe; LDSS, low dead space syringe.
| Mean HCV transmission probability (± SD) | ||||
|---|---|---|---|---|
| LDSS | HDSS | |||
| HCV titer [log10 IU/mL] |
Unrinsed | Rinsed | Unrinsed | Rinsed |
| 1.0 | 0.57(±1.25) | 0.16(±0.30) | 2.58(±4.16) | 0.30(±0.55) |
| 1.5 | 1.78(±3.53) | 0.51(±0.96) | 7.58(±10.36) | 0.97(±1.72) |
| 2.0 | 5.43(±8.60) | 1.67(±3.00) | 19.97(±20.84) | 3.09(±5.10) |
| 2.5 | 14.12(±17.34) | 4.89(±7.85) | 40.81(±30.08) | 8.66(±12.00) |
| 3.0 | 31.00(±28.03) | 12.95(±16.67) | 65.67(±31.68) | 21.18(±22.10) |
| 3.5 | 54.04(±33.41) | 28.73(±27.39) | 84.54(±25.09) | 42.25(±30.94) |
| 4.0 | 75.50(±30.68) | 50.95(±33.81) | 94.26(±16.41) | 66.45(±32.01) |
| 4.5 | 88.90(±23.29) | 72.52(±31.79) | 98.04(±9.78) | 84.66(±25.26) |
| 5.0 | 95.31(±15.79) | 87.22(±24.31) | 99.37(±5.73) | 94.12(±16.89) |
| 5.5 | 98.13(±10.09) | 94.79(±16.41) | 99.78(±3.29) | 97.89(±10.19) |
| 6.0 | 99.30(±6.03) | 97.91(±11.36) | 99.94(±1.25) | 99.31(±6.11) |
Impact of HCV RNA Kinetics on the Probability of Transmission
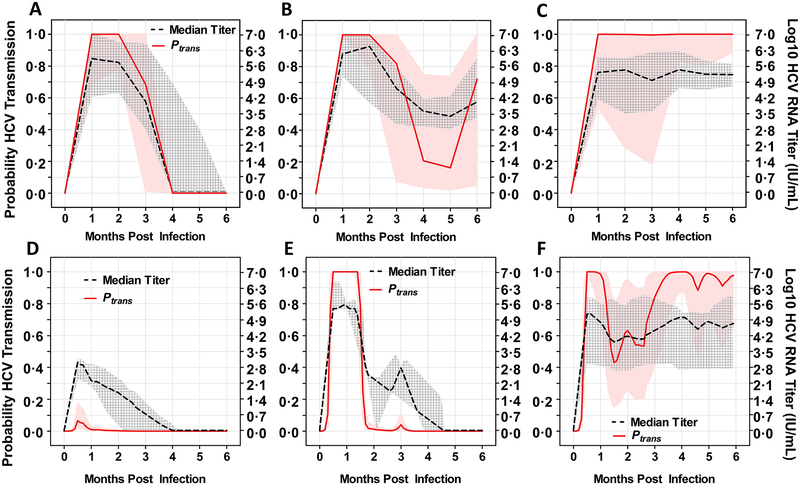
Our calculations of Ptrans were based on viral titers in a syringe donor at a single time point. However, titers during acute HCV infection fluctuate until they eventually result in spontaneous clearance or long-term persistence (also called chronicity) (17). We previously reported (17) three a priori-defined RNA kinetic patterns during primary acute infection in human subjects: full control (spontaneous clearance of HCV RNA within 6 months post infection; n=52) (Fig. 5A), incomplete control (≥1 log10 IU/mL decline in HCV RNA following the peak titer followed by persistence; n=44) (Fig. 5B) and persistence (increase or < 1 log10 IU/mL decline in HCV RNA following the peak titer; n=66) (Fig. 5C). Using median RNA titers calculated from subjects classified into the three different kinetic patterns (Figs. 5A to 5C), we estimated Ptrans for any single syringe-sharing event using a rinsed low dead space syringe with an attached needle. The full control group showed a reduction in Ptrans to 40% by month three and elimination by month four (Fig. 5A). The incomplete control group showed a reduction in Ptrans in months 4 to 5 following transient control of HCV replication; however, this was followed by a later increase in Ptrans upon loss of control and a return of viral titers to higher levels at 6 months (Fig. 5B). In contrast, the persistent infection group maintained transmissibility risk at >90% throughout the first 6 months of the acute phase of infection (Fig. 5C).
Fig. 5. Probability of HCV transmission via a rinsed LDSS during acute phase infection and reinfection.
Probability of transmission (Ptrans) (solid red) and syringe donors’ median viral titers (dotted black) following different HCV infection outcomes in naïve patients (A, B and C) and patients who had cleared primary infections and experienced reinfection (D, E and F). Data are presented as the median HCV RNA titer for patients grouped by outcome: (A) naïve acute phase, self-clearing (B) naïve acute phase, incomplete control (C) naïve acute phase, persistence (D) reinfection acute phase, low titer with rapid clearance (E) reinfection acute phase, high titer with rapid clearance (F) reinfection acute phase, chronic infection. Gray cross-hatched bands represent the interquartile range of measured data points of all individuals in the group at the given time points. Pink bands show the interquartile range of transmission probability based on Monte Carlo sensitivity analyses.
Successfully vaccinated individuals would ideally have immune responses that would control HCV replication, resulting in viral titers that were lower and of shorter duration than those seen in naïve subjects, as has been reported for HCV reinfection in people who had previously cleared an infection (5, 19). Upon analysis of serial HCV RNA data obtained within 1 to 90 days after confirmed reinfection in subjects who had previously successfully cleared a known primary infection (19), we observed three different outcomes: rapid clearance (within 4 months)/low titer (Fig. 5D), rapid clearance/high titer (Fig. 5E), and chronic infection (Fig. 5F).
To simulate the potential impact of a T cell based vaccine on HCV transmission between people who inject drugs and share syringes, we analyzed RNA profiles from subjects who were reinfected following spontaneous clearance of HCV. We applied median RNA profiles from each of the three identified groups to our transmission equation to estimate Ptrans for any single syringe sharing event using rinsed low dead space syringes with an attached needle over a 6-month period. During the first month following exposure, Ptrans was reduced by more than 90% in the rapid clearance/low titer group (Fig. 5D), compared to the first month of acute infection in naïve individuals (Figs. 5A to 5C), and transmission was eliminated by month 2. Transmission probability was >99% during the first 6 weeks in the rapid clearance/high titer group (Fig. 5E), but was almost zero from 6 weeks onwards (Fig. 5E). In contrast, the chronic infection group maintained transmissibility risk at > 40% throughout the 6 months and showed a risk of transmission at >90% for the majority of the time period analyzed (Fig. 5F).
Cumulative Transmission Probabilities in Potential Vaccinees
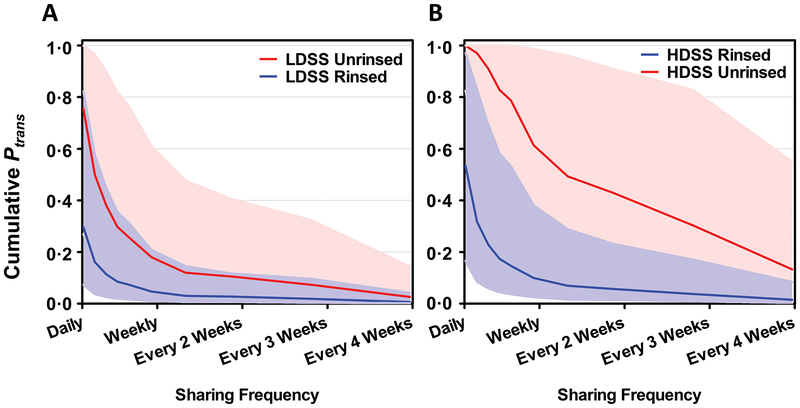
The calculations above apply to single syringe sharing events between an infected person and an uninfected syringe recipient. To understand transmission risk based on syringe sharing over time, we calculated the cumulative probability of transmission between an infected and uninfected syringe sharer over 6 months for high (daily) to low (monthly) sharing frequencies using either rinsed or unrinsed, low or high dead space syringes with attached needles for all of the infection profiles shown in Fig. 5. For high titer individuals (>5 log10 IU/mL), HCV transmission probabilities were >99.9% throughout the 6-month time period studied regardless of sharing frequency, syringe type, and irrespective of viral kinetics. This included all acute phase profiles in naïve subjects (Figs. 5A to 5C) and reinfected subjects other than those with a rapidly clearing, low titer profile (Figs. 5E and 5F). When we applied kinetics from the rapidly clearing, low titer reinfected group (Fig. 5D), we found that the frequency of syringe sharing had a significant impact on cumulative transmission, although reduction also depended upon syringe type and rinsing. For example, if rinsed syringe sharing occurred daily, the cumulative probability of transmission was <50% (Figs. 6A and B). Moreover, if sharing frequency decreased to weekly for rinsed syringes, the cumulative transmission probability declined to <~10%, and further still with less frequent sharing (Figs. 6A and B).
Fig. 6. Cumulative probability of transmission (Ptrans) via shared syringes over 6 months for the reinfected low titer group based on frequency of sharing.
This patient group corresponds to that shown in Fig. 5A. (A) LDSS, low dead space syringe; (B) HDSS, high dead space syringe. Data are shown for rinsed (blue line) or unrinsed (red line) syringes. Bands show the interquartile range of cumulative transmission probability based on Monte Carlo sensitivity analyses. Of note, all viral kinetic profiles for other groups, either following primary infection or re-infection, resulted in a cumulative transmission probability of >99.9% over a 6-month period.
Transmission Risk as a Function of Viral Load over Time
Our cumulative analyses demonstrated that transmission risk should be estimated as a function of viral load over time, not just a single event with a single titer. We calculated the area under the curve (AUC) using our plots of viral titer against time (Fig. 5). Applying this to our cumulative analyses revealed that individuals with AUCs of over ~100,000 Titers*Months had a >99% chance of viral transmission to their sharing partner regardless of syringe type or sharing frequency. This value corresponds, for example, to a titer (log10 IU/mL) of >5 for 1 month, 4.5 for over 3 months, or 4.2 for over 6 months. In cases where the individual’s viral load and time of infection resulted in AUCs <100,000 Titers*Months (e.g. the individual shown in Fig. 5D), reducing syringe sharing frequency could considerably decrease the likelihood of transmission (Fig. 6).
A Potential Non-Linear Relationship between Ptrans and Viral Load
In order to consider non-linear changes in Ptrans as a function of viral load, we also tested a Hill function as an alternative to Eq. 1. The Hill function (Eq. 2) can be formulated as , where n and q are the same as in Eq. 1, p50 represents the value of nq for which Ptrans = 50% (fig. S2A), and β controls the steepness of the change in Ptrans (fig. S2B). Eq. 2 qualitatively matches Eq. 1 for β = 1.4 and p50 = 0.46 for low dead space syringes (fig. S3). Reconsidering our studies of transmission probability over time (Figs. 5 and 6), we found that a difference in β did not lead to qualitative changes in transmission probability over time (fig. S4) or the extent to which lower sharing frequency reduced transmission probability (fig. S5). For example, for rinsed low dead space syringes and rapid clearance with low viral load, we found that in our analysis using Eq. 1 the overall transmission probability was ~30% for daily sharing and <1% for monthly sharing, whereas for an extreme example of a Hill function with β = 0.5, the transmission probability was 95% for daily sharing and 10% for monthly sharing (fig. S5). These results show that even under very different Ptrans steepness conditions, low sharing frequency using rinsed low dead space syringes could greatly reduce transmission probability, consistent with Eq. 1.
Discussion
In order to reduce the spread of HCV in people who inject drugs, effective interventions are needed that will reduce the chances of virus transmission (23). Vaccine development or antiviral treatment may be able to augment other efforts that can impact infection incidence, such as harm reduction initiatives (e.g. needle and syringe programs, opioid substitution therapy, and behavioral counseling (24, 25)). However, low HCV titers may still be transiently present in individuals receiving vaccines (4). To assess how low viral titers need to be in order for the spread of the virus to be impacted, we performed simulated syringe sharing experiments using low and high dead space syringes with needles attached and developed an equation for HCV transmission based on viral titers when either of these syringe types is used in combination with the needles tested in our studies. We also examined the effect of water rinsing and sharing frequency on the probability of transmission using these two syringe types.
Our approach used empirical data generated in a set of experiments designed to assess RNA carry-over from an infected syringe donor to an uninfected individual (Figs 2 and 3). Our RNA carry-over data were consistent with prior studies on syringe dead space (15, 26). The average difference in dead space between low and high dead space syringes has been estimated as 10-fold (27). We found a 3 to 6-fold difference in the mean carry-over of viral RNA between these two types of syringes. There is conflicting data in the literature as to the benefit of low vs high dead space syringes in preventing virus transmission despite the lower amount of contaminated blood that can be transmitted from the former [reviewed in (27)]. Our data can partially explain these discrepancies. Using an RNA to infectivity ratio of 11:1, we estimated that syringe sharing could result in >90% transmission probability for any single event if HCV titers were >5 log10 IU/mL (28). This high transmission risk could explain reports showing that needle sharing programs have been less successful at preventing the spread of HCV compared to HIV in people who inject drugs (29), while still acknowledging the general positive impact of needle sharing programs on reducing HCV transmission (28). The inclusion of additional prevention strategies, including opioid substitution therapy along with high coverage of needle and syringe exchange programs, will be essential for universally reducing HCV transmission rates (30). Conversely, we show that if viral titers were to be reduced below 2.3 log10 IU/mL, the transmission probability could almost completely be eliminated (<5%) for low dead space syringes. For high dead space syringes, the titer would need to be reduced below 1.6 log10 IU/ (63 IU/mL). Thus, if infected individuals test negative for HCV RNA using highly sensitive assays (detection limits <20 IU/mL), transmission risk would be close to zero.
One limitation of our work assessing transmission probability is the use of an RNA titer to infectivity ratio established from reverse titration studies in chimpanzees (table S2), as such a ratio has not been established for human transmission. The RNA to infectivity ratio was also established using plasma obtained during the acute phase of infection prior to seroconversion (8, 9, 11, 21). Thus, the presence of neutralizing antibodies to HCV in a plasma sample may have an impact on the proportion of transmitted virus that can result in an infection. Such antibodies are known to be present in sera from chronically infected patients (31, 32) and in patients that spontaneously clear infections (5). The presence and effectiveness of neutralizing antibodies is difficult to analyze mathematically, but such a scenario would not apply to vaccinees receiving T cell based vaccines targeting only the non-structural proteins of HCV. If future vaccine clinical trials or infectivity studies establish new infectivity to RNA ratios, such as 1:100 or 1:1000, our approach (Eq. 1) could be adapted using a recalculated parameter q. We also assumed a linear relationship between the viral titer and HCV transmission probability using Eq. 1. If transmission is found to change in a non-linear fashion as a function of viral load, e.g. when neutralizing antibodies are present, our approach from Eq. 1 could be adapted to Eq. 2. However, our predicted data, using Eq. 1, are consistent with studies on occupational exposure that demonstrated a relationship between virus titer and infection in exposed health care workers (33). It was shown that the risk of transmission increased 11-fold when health care workers were exposed to sera with a high viral load (>6 log10 copies/mL) compared with exposure to sera with a lower viral load (≤4 log10 copies/mL) (33).
For single syringe sharing events, we observed a significant impact on transmission risk from individuals who had pre-existing immune responses to HCV (i.e. those who had previously cleared infections) and rapidly controlled the virus (Fig. 5D) compared to naïve, exposed subjects with no pre-existing immunity (Figs. 5A to 5C) and those with little or no control of their secondary infection (Fig 5F). In all naïve, acute phase subjects the risk of transmission was predicted to be ≥50% within the first 3 months regardless of outcome (clearance or persistence), whereas transmission was eliminated or reduced to <5% in the reinfected subjects that rapidly cleared the virus within 1 (Fig. 5D) to 2 (Fig. 5E) months following re-exposure. Our mathematical modeling analyses suggest that immunity-inducing HCV vaccination could have a marked long-term effect on HCV spread through a population of injecting drug users and emphasizes the need to reduce titers in HCV-infected people who inject drugs in order to reduce HCV transmission. When analyzing cumulative risk, we found that the frequency of syringe sharing remained a pivotal factor in potential virus spread between syringe sharing partners, even if their HCV titers were reduced. This has implications for the maintenance of harm reduction strategies to address potential risk compensation (i.e., re-engaging in high risk practices) by vaccinated drug users who perceive no risk of reinfection or transmission to others. We considered a defined 6-month period following infection but did not assess the longer-term impact of rapid clearance in potential vaccinees. An individual with high titers (>5 log10 IU/mL) for a short period would be infectious for the first week or month but non-infectious for the remaining period; in contrast, a chronically infected individual would remain infectious for the full 6 months and beyond. Both the long-term vaccination effects of reduced/transient viral load and the transmission probabilities developed here could serve as important concepts in a comprehensive and complex modeling approach (e.g., agent-based) that considers the events of sharing syringes in space and time along with the dynamic and complex interplay of factors at the individual level (e.g., risk behavior), the structural level (e.g., access to clean needles and syringes), and the social level (e.g., injection networks) (34). Such comprehensive models have the potential to fully investigate the impact of reduced viral titers, reduced periods of infection, and multiple syringe sharing partners on the spread and long-term prevalence of HCV.
Applying our models of HCV transmission via syringe sharing to RNA kinetics data, we developed a basis for understanding transmission relative to viral titers (Eq. 1) and predicted the titers necessary to substantially reduce transmission probability among serodiscordant people who inject drugs. Of note, we show that for accurate consideration of transmission risk due to syringe sharing, probability should be estimated as a function of viral load over time, not just a single event with a single titer. Overall, our data strongly support previous reports that viral load is an important factor in HCV transmission (33), similar to the relative increase in HIV (35) and herpes simplex virus transmission (36) risk via sexual intercourse with increased viral load. Notably, our analyses indicate that vaccination of people who inject drugs would not need to achieve sterilizing immunity to reduce HCV transmission provided that harm reduction strategies also were maintained.
Materials and Methods
Study Design
The objective of this study was to assess whether reduced HCV RNA titers in vaccinees would reduce the spread of the virus within drug user populations where contaminated syringes were shared. Data on transmission of HCV RNA from contaminated low and high dead space syringes with attached needles was generated through in vitro studies (3 independent experiments). Each experiment consisted of drawing HCV-positive human plasma or cell culture virus into a clean syringe via the attached needles, expelling the sample, drawing normal (HCV-negative) human plasma into the same syringe and testing the RNA titer in the expelled, contaminated plasma. These data were used to calculate the percentage of RNA transmitted from syringes and used in a model-based mathematical analysis to calculate the probability of RNA being transmitted to an HCV-naïve injecting drug user from an HCV-infected injecting drug user based upon the RNA titer in the infected individual. Previously published HCV RNA kinetic data from the InC3 study were obtained for naive (n=162) and reinfected (n=21) subjects (17–20) and a mathematical analysis was applied to calculate the transmission probability for a single sharing event at each timepoint over a 6-month period based on the median titers observed. We extended these studies to consider the cumulative probability of HCV transmission if the same two serodiscordant injecting drug users shared syringes on a regular basis over a 6-month period (i.e. daily, weekly or monthly) based on the previously published viral kinetics data. Each sharing event was considered to use a newly contaminated syringe with attached needle, not the same syringe, and we assumed no loss of infectivity due to delay between contamination and use by the naïve individual. For the human HCV RNA data, all participants provided written informed consent and protocols were approved by local human subject research review committees.
HCV Patient Data
Data for well-characterized HCV RNA kinetics from naïve and reinfected subjects were obtained from the InC3 Study (17–20). All participants provided written informed consent and protocols were approved by local human subject research review committees. Among 643 participants, we studied two subgroups in detail: those with well-characterized acute infection (17) and those with reinfection (19).
Among 162 participants with well-characterized primary acute infection, i.e. HCV RNA detected within three months of the date of infection and a subsequent HCV RNA test within 120 days of the peak RNA, we previously identified three a priori-defined patterns of HCV RNA kinetics: i) spontaneous clearance, ii) partial viral control with persistence (≥ 1 log10IU/mL decline in HCV RNA levels following peak) and iii) viral plateau with persistence (increase or <1 log10IU/mL decline in HCV RNA levels following peak). The estimated date of HCV infection was calculated based on a hierarchy using all serological (anti-HCV antibodies), virological (HCV RNA), and clinical data (symptoms and liver function tests) as previously described (17). Briefly, for individuals testing HCV RNA positive and anti-HCV negative, date of infection was estimated as four weeks prior to HCV RNA detection. For individuals with symptomatic acute HCV, date of infection was estimated as six weeks prior to onset of symptoms (jaundice or ALT >400 IU/L). For individuals with a negative anti-HCV test followed by either a positive anti-HCV or positive HCV RNA test, seroconversion was assumed to occur at the mid-point between the last negative and the first positive test. Date of infection in this group was deemed to be six weeks prior to the estimated seroconversion date if the first positive test was an anti-HCV test and four weeks prior to the estimated seroconversion date if the first positive test was only an HCV RNA test. Overall, 32% of participants had spontaneous clearance, 27% had partial viral control with persistence and 41% had viral plateau with persistence (17).
To simulate viral kinetics in vaccinees, i.e. those with pre-existing immunity, we first identified 28 individuals with re-infection based on evidence of spontaneous clearance as previously described (17) (two consecutive undetectable HCV RNA tests at least one month apart) or viral suppression (one undetectable HCV RNA test) after primary infection (HCV seroconversion), followed by reappearance of HCV viremia and a change in HCV viral sequence. We excluded 7 individuals who had no quantifiable viral RNA titer, only a qualitative result, (n=3) or only one data point (n=4). Among the remaining 21 individuals, 16 had one episode, three had two episodes and two had three episodes (total of 28 episodes). Among 27 episodes, 10 episodes (37%) were considered chronic and 17 episodes (63%) were considered self-clearing. The time of re-infection was calculated based on the mid-point between the final undetectable HCV RNA test and the first detectable HCV RNA test indicating HCV reinfection (19).
Viral titer imputation for reinfected subjects
In order to analyze viral kinetics in reinfected subjects, we estimated the viral load before the virus was first detected to in turn estimate the date at which the individual was first re-infected (19). We assumed that in the first two weeks following reinfection, the viral load increased in an exponential manner to that of the first measured data point. If the first measured data point was before 0.5 months we assumed an exponential increase up to that point. From the time t=0.5 months to the first measured time, we assumed that the viral load was constant. We then used linear interpolation, using a log scale, to obtain a continuous graph of the viral load at any given time during the infection.
We next determined population-level statistics by classifying individuals as belonging to one of three classes: low-titer (<4log10 IU/mL) with rapid clearance (within 4 months) (n=12), high-titer (>4.0 log10 IU/mL) with rapid clearance (n=5), or chronic infection (persistence of virus for more than 6 months) (n=10). Within each group, we used the previously defined linear interpolation function to estimate the viral load of each individual at each time point. We then estimated the viral load of the group as the median of the values. Since the data on different individuals extends to different time points, data for later time points may not have been available for all individuals. In these cases we calculated the median of the viral load values of the remaining individuals.
HCV Samples
RNA studies used HCV genotype 1b human plasma (kindly provided by Mei-Ying Yu, CBER/FDA), which had been used to generate part of a CBER HCV RNA panel (37). Infectious virus studies used 1b/JFH1 chimeric HCVcc (38).
Simulated Syringe Sharing
Two syringe types (insulin and tuberculin) were chosen to represent low and high dead space syringes, commonly used for injecting various types of drugs (39). For low dead space syringes we used 0.5 mL U-100 insulin syringes with fixed 27-gauge 0.5 inch needles (Terumo Medical, MD). For high dead space syringes we used 1 mL tuberculin syringes fitted with Precision Glide 23-gauge 1 inch needles (Becton Dickenson, NJ). To simulate real-life situations where syringes are rinsed in an attempt to reduce transmission prior to sharing, we used tap (chlorinated) water for rinsing between simulated contamination and uptake of negative plasma.
HCV Positive Plasma
HCV positive plasma was diluted 1:10 or 1:100 in normal human plasma (Innovation Research, Novi, MI); 0.5 mL was drawn into a syringe via the needle and expelled. Subsequently, 0.5 mL of normal plasma was drawn into the same syringe and expelled into a clean tube. In rinsing studies, 0.5 mL of tap water was drawn into the syringe and expelled to waste prior to drawing up normal plasma (0.5 mL). Samples were extracted as 100 μL aliquots and assessed for RNA titers by real-time RT-PCR, limit of detection 200 RNA copies/mL (40). Using this real-time RT-PCR assay we previously obtained ratios of 2.7 RNA copies per HCV IU using WHO international standards (40), therefore all titers were divided by 2.7 for expression as IU/mL. Syringe-treated samples negative in the real-time RT-PCR assay were assigned a value of half of the limit of detection (i.e. 100 RNA copies/mL or 37 IU/mL). Percentage carry-over was calculated by dividing the non-log transformed HCV RNA titer in the contaminated, expelled plasma in each individual experiment by the non-log transformed titer of HCV RNA in the positive plasma in the same experiment and multiplying by 100. Each experiment was repeated 2 to 4 times generating 4 to 5 extracted aliquots per experiment to give a total of 8 to 17 data points per result.
1b/2a Chimeric cell culture HCV
Chimeric cell culture HCV samples were diluted 1:5 in normal plasma and subjected to the same treatment described above except that tap water was passed through a 0.2 μm filter. Titers were assessed using Huh7.5 cells as previously described (38). Briefly, diluted virus samples were inoculated onto Huh7.5 cells, 100μl per well in duplicate. Following incubation at 37°C for three hours inocula were removed and replaced with fresh cell growth medium. Cells were incubated for 3 days at 37°C then fixed and stained as previously described (38). Experiments were performed twice. Pre-carry-over samples were diluted 10-fold and tested in triplicate to assess titers, and carry-over samples were diluted 2-fold and tested in duplicate. Percentage carry-over was calculated as described above using FFU/mL titers in place of RNA titers.
Simulations, Sensitivity, and Statistical Analyses
Assuming infectivity by one infectious unit is independent of infectivity by other infectious units, we used a binomial function (Equation [Eq.] 1 below), reminiscent of a binomial function used by Rottingen et al. (41), to estimate the probability of a syringe sharing event leading to transmission of an infectious dose between an HCV-infected and serodiscordant individual (Ptrans), where n = the number of transferred HCV RNA units and q = the ratio of infectious dose to HCV RNA units (Tables 1 and S2).
We used published data from 12 chimpanzee reverse titration studies employing multiple HCV genotypes (8, 9, 11, 22) to calculate that the median number of RNA molecules required to result in an infection is ~9 with a range of 3 to 13 (table S2). The transformed infectivity to RNA ratios (q) were best described as a gamma distribution with calculated median and Q1-Q3 as 17% and 7%−33%, respectively (Table s2).
| (Eq.1) |
We simulated Ptrans as a function of syringe type (low or high dead space) and syringe rinsing (with or without a rinse step). We determined that n was based upon viral load (IU/mL) in the syringe donor’s blood (VL), percent carry-over from the syringe-needle combination based on empirical RNA data generated in this study (ρ), and the relative volume of donor blood in the mixture (s) (outlined in Table 1). Parameter n was determined by multiplying each of these three parameters. We took set viral loads and chose randomly from ranges and frequencies for RNA carry-over (ρ) and relative volume of donor blood in the mixture (s). Parameter s accounts for situations where blood is drawn into a syringe prior to injection to ensure that the dissolved drugs are injected into a vein (42). We assumed that VL does not diminish between contamination and syringe sharing.
Sensitivity analyses used 1000 Monte Carlo samples (RStudio version 3.2.0, Boston, MA). Statistical comparisons were performed using Mann Whitney test and Graphpad Prism software 5.0. P-values <0.05 were considered statistically significant. Area Under the Curve (AUC) was calculated using the Trapezoidal Rule and implemented in Python (version 2.7).
Cumulative transmission probability
We calculated Ptrans for each group using the pre-established values for ρ, s and q from the in vitro experiments and literature as described above. For the variable representing the viral load, VL, we used the median viral load of all individuals in the given group at the specific time of the injection.
We assumed that the first injection took place at t = 0 (which was also the time of infection). The next injection took place at time t = freqinjection, where freqinjection represents the frequency with which the individual injected with shared syringes. We assumed that the individual continued to inject (via shared syringes) with a frequency equal to freqinjection and used the viral load corresponding to that time to calculate the probability of transmission. The overall probability of no transmission occurring is and thus the overall probability of transmission is . This approach calculates the likelihood of transmission from one specific viremic individual to another specific non-infected individual with whom they share syringes.
Considering a non-linear relationship between Ptrans and viral load
A Hill function as an alternative to Eq. 1 can also be used, reminiscent of (43). The Hill function could be formulated as Eq. 2 where p50 represents the value of n‧q (n and q as described in Table 1) for which Ptrans = 50%, (fig. S2A) and β controls the steepness of the change in Ptrans (fig. S2B):
| (Eq.2) |
Supplementary Material
Acknowledgements
We thank M-Y. Yu (CBER/FDA) for the kind gift of human HCV genotype 1b human plasma and M. E. Mackesy-Amiti and L. Ouellet for guidance on syringes and syringe-sharing practices.
Funding
Financial support for this work was provided by the Food and Drug Administration intramural funds [Program Number Z01 BK 04010–11 LHV to M.E.M], the National Institutes of Health [grant numbers R01-AI078881 to H.D. and 1R01-GM121600–01A1 to H.D. and B.B.] and the Victorian Operational Infrastructure Support Program to the Burnet Institute. Q.C. was a recipient of a CBER/FDA sponsored Oak Ridge Institute for Science and Education (ORISE) fellowship. B.H. and R.S.D. were recipients of Australian National Health and Medical Research Council (NHMRC) Early Career Fellowships.
Footnotes
Supplementary Materials
Figure S1. Illustration of HCV transmission via a shared syringe.
Figure S2. Sensitivity analysis of Eq. 2 (Hill function) as a function of viral load.
Figure S3. Ptrans generated by Eq. 1 and Eq. 2.
Figure S4. Probability of HCV transmission via a rinsed low dead space syringe during reinfection using Eq. 2.
Figure S5. Cumulative probability of transmission via shared needles over 6 months for the reinfected low titer group based on frequency of sharing using Eq. 2.
Table S1. Critical probabilities of HCV transmission and corresponding viral log10 IU/mL
Table S2. Estimated q distribution calculated from chimpanzee reverse-titration experiments.
Data file S1. Titers obtained for in vitro syringe studies
Competing interests
The authors declare that they have no competing interests.
Data and materials availability
All mathematical equations used to generate the data and all data supporting the findings of this study are presented in the manuscript. HCVcc used in this study can be obtained by contacting Marian Major at the Center for Biologics Evaluation and Research, US Food and Drug Administration, followed by completion of a Material Transfer Agreement .
References
- 1.Williams IT, Bell BP, Kuhnert W, Alter MJ, Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch Intern Med 171, 242–248 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ, Epidemiology of hepatitis C virus infection. World. J Gastroenterol 13, 2436–2441 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Major ME, Prophylactic and Therapeutic Vaccination against Hepatitis C virus (HCV): Developments and Future Perspectives. Viruses 1, 144–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahari H, Feinstone SM, Major ME, Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology 139, 965–974 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL, Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138, 315–324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch MP, Murthy KK, Kleinman SH, Hirschkorn DF, Herring BL, Delwart EL, Racanelli V, Yoon JC, Rehermann B, Alter HJ, Infectivity in chimpanzees (Pan troglodytes) of plasma collected before HCV RNA detectability by FDA-licensed assays: implications for transfusion safety and HCV infection outcomes. Blood 119, 6326–6334 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shata MT, Tricoche N, Perkus M, Tom D, Brotman B, McCormack P, Pfahler W, Lee DH, Tobler LH, Busch M, Prince AM, Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology 314, 601–616 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama K, Kumagai J, Komiya Y, Mizui M, Yugi H, Kishimoto S, Yamanaka R, Tamatsukuri S, Tomoguri T, Miyakawa Y, Tanaka J, Yoshizawa H, Titration of hepatitis C virus in chimpanzees for determining the copy number required for transmission. Intervirology 47, 57–64 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM, Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol 76, 6586–6595 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veerapu NS, Park SH, Tully DC, Allen TM, Rehermann B, Trace amounts of sporadically reappearing HCV RNA can cause infection. J Clin Invest 124, 3469–3478 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engle RE, Russell RS, Purcell RH, Bukh J, Development of a TaqMan assay for the six major genotypes of hepatitis C virus: comparison with commercial assays. J Med Virol 80, 72–79 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukh J, Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology 142, 1279–1287 e1273 (2012) [DOI] [PubMed] [Google Scholar]
- 13.Doerrbecker J, Behrendt P, Mateu-Gelabert P, Ciesek S, Riebesehl N, Wilhelm C, Steinmann J, Pietschmann T, Steinmann E, Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis 207, 281–287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paintsil E, He H, Peters C, Lindenbach BD, Heimer R, Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis 202, 984–990 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binka M, Paintsil E, Patel A, Lindenbach BD, Heimer R, Survival of Hepatitis C Virus in Syringes Is Dependent on the Design of the Syringe-Needle and Dead Space Volume. PLoS One 10, e0139737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paintsil E, Binka M, Patel A, Lindenbach BD, Heimer R, Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis 209, 1205–1211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajarizadeh B, Grady B, Page K, Kim AY, McGovern BH, Cox AL, Rice TM, Sacks-Davis R, Bruneau J, Morris M, Amin J, Schinkel J, Applegate T, Maher L, Hellard M, Lloyd AR, Prins M, Dore GJ, Grebely J, InC3 Study Group, Patterns of hepatitis C virus RNA levels during acute infection: the InC3 study. PLoS One 10, e0122232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grebely J, Page K, Sacks-Davis R, van der Loeff MS, Rice TM, Bruneau J, Morris MD, Hajarizadeh B, Amin J, Cox AL, Kim AY, McGovern BH, Schinkel J, George J, Shoukry NH, Lauer GM, Maher L, Lloyd AR, Hellard M, Dore GJ, Prins M, InC3 Study Group, The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 59, 109–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacks-Davis R, Grebely J, Dore GJ, Osburn W, Cox AL, Rice TM, Spelman T, Bruneau J, Prins M, Kim AY, McGovern BH, Shoukry NH, Schinkel J, Allen TM, Morris M, Hajarizadeh B, Maher L, Lloyd AR, Page K, Hellard M, InC3 Study Group, Hepatitis C Virus Reinfection and Spontaneous Clearance of Reinfection--the InC3 Study. J Infect Dis 212, 1407–1419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grebely J, Morris MD, Rice TM, Bruneau J, Cox AL, Kim AY, McGovern BH, Shoukry NH, Lauer G, Maher L, Lloyd AR, Hellard M, Prins M, Dore GJ, Page K, InC3 Study Group, Cohort profile: the international collaboration of incident HIV and hepatitis C in injecting cohorts (InC3) study. Int J Epidemiol 42, 1649–1659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukh J, Meuleman P, Tellier R, Engle RE, Feinstone SM, Eder G, Satterfield WC, Govindarajan S, Krawczynski K, Miller RH, Leroux-Roels G, Purcell RH, Challenge pools of hepatitis C virus genotypes 1–6 prototype strains: replication fitness and pathogenicity in chimpanzees and human liver-chimeric mouse models. J Infect Dis 201, 1381–1389 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinstone SM, Alter HJ, Dienes HP, Shimizu Y, Popper H, Blackmore D, Sly D, London WT, Purcell RH, Non-A, non-B hepatitis in chimpanzees and marmosets. J. Infect. Dis 144, 588–598 (1981) [DOI] [PubMed] [Google Scholar]
- 23.Page K, Morris MD, Hahn JA, Maher L, Prins M, Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis 57 Suppl 2, S32–38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hope VD, Hickman M, Ngui SL, Jones S, Telfer M, Bizzarri M, Ncube F, Parry JV, Measuring the incidence, prevalence and genetic relatedness of hepatitis C infections among a community recruited sample of injecting drug users, using dried blood spots. J Viral Hepat 18, 262–270 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K, Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 174, 1974–1981 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oramasionwu CU, Bailey SC, Moore HN, Oramasionwu CO, Russell AL, Zule WA, Dead space in over-the-counter syringes: The implications for HIV and HCV transmission. Int J Drug Policy 26, 1282–1284 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Vickerman P, Martin NK, Hickman M, Could low dead-space syringes really reduce HIV transmission to low levels? Int J Drug Policy 24, 8–14 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, May M, Taylor A, De Angelis D, Cameron S, Parry J, Lyons M, Goldberg D, Allen E, Hickman M, The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 106, 1978–1988 (2011). [DOI] [PubMed] [Google Scholar]
- 29.MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, Taylor A, Roy K, Aspinall E, Goldberg D, Rhodes T, Hedrich D, Salminen M, Hickman M, Hutchinson SJ, Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy 25, 34–52 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Platt L, Reed J, Minozzi S, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Hickman M, Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA, Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA 101, 10149–10154 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinmann D, Barth H, Gissler B, Schurmann P, Adah MI, Gerlach JT, Pape GR, Depla E, Jacobs D, Maertens G, Patel AH, Inchauspe G, Liang TJ, Blum HE, Baumert TF, Inhibition of hepatitis C virus-like particle binding to target cells by antiviral antibodies in acute and chronic hepatitis C. J Virol 78, 9030–9040 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazdanpanah Y, De CG, Migueres B, Lot F, Campins M, Colombo C, Thomas T, Deuffic-Burban S, Prevot MH, Domart M, Tarantola A, Abiteboul D, Deny P, Pol S, Desenclos JC, Puro V, Bouvet E, Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 41, 1423–1430 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Dahari H, Boodram B, How to eliminate HCV in people who inject drugs in the USA. Lancet Infect Dis 18, 134–135 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH, Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342, 921–929 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A, Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface 11, 20140160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saldanha J, Heath A, Lelie N, Pisani G, Nubling M, Yu M, Calibration of HCV working reagents for NAT assays against the HCV international standard. The Collaborative Study Group. Vox. Sang 78, 217–224 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Kachko A, Kochneva G, Sivolobova G, Grazhdantseva A, Lupan T, Zubkova I, Wells F, Merchlinsky M, Williams O, Watanabe H, Ivanova A, Shvalov A, Loktev V, Netesov S, Major ME, New neutralizing antibody epitopes in hepatitis C virus envelope glycoproteins are revealed by dissecting peptide recognition profiles. Vaccine 30, 69–77 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Ciccarone D, Harris M, Fire in the vein: Heroin acidity and its proximal effect on users’ health. Int J Drug Policy 26, 1103–1110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puig M, Mihalik K, Yu MY, Feinstone SM, Major ME, Sensitivity and reproducibility of HCV quantitation in chimpanzee sera using TaqMan real-time PCR assay. J. Virol. Methods 105, 253–263 (2002) [DOI] [PubMed] [Google Scholar]
- 41.Rottingen JA, Garnett GP, The epidemiological and control implications of HIV transmission probabilities within partnerships. Sex Transm Dis 29, 818–827 (2002) [DOI] [PubMed] [Google Scholar]
- 42.Harm Reduction Coalition. Getting Off Right: A Safety Manual For Injection Drug Users. http://harmreduction.org/wp-content/uploads/2011/12/getting-off-right.pdf, Last Accessed June 2018 (2009).
- 43.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP, Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A 104, 17441–17446 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.