Abstract
An elevated circulating level of trimethylamine N-oxide (TMAO) has been identified as a risk factor for numerous diseases, including cardiovascular disease (CVD) and colon cancer. TMAO is formed from trimethylamine (TMA)-precursors such as choline via the combined action of the gut microbiota and liver. We conducted a Mediterranean diet intervention that increased intakes of fiber and changed intakes of many other foods containing fat to increase the relative amount of mono-unsaturated fats in the diet. The Mediterranean diet is associated with reduced risks of chronic diseases and might counteract the pro-inflammatory effects of increased TMAO formation. Therefore, the purpose of this study was to determine if the Mediterranean diet would reduce TMAO concentrations. Fasting TMAO concentrations were measured before and after six-months of dietary intervention in 115 healthy people at increased risk for colon cancer. No significant changes in plasma TMAO or in the ratios of TMAO to precursor compounds were found in either the Mediterranean group or the comparison group that followed a Healthy Eating diet. TMAO concentrations exhibited positive correlations with age and markers of metabolic health. TMAO concentrations were not associated with circulating cytokines, but the relative abundance of Akkermansia mucinophilia in colon biopsies was modestly and inversely correlated with baseline TMAO, choline, and betaine serum concentrations. These results suggest that broad dietary pattern intervention over six months may not be sufficient for reducing TMAO concentrations in an otherwise healthy population. Disruption of the conversion of dietary TMA to TMAO should be the focus of future studies.
Keywords: carnitine, choline, betaine, inflammation, Akkermansia mucinophilia
A Mediterranean diet does not reduce circulating TMAO, a metabolite that is associated with chronic disease risks.
INTRODUCTION
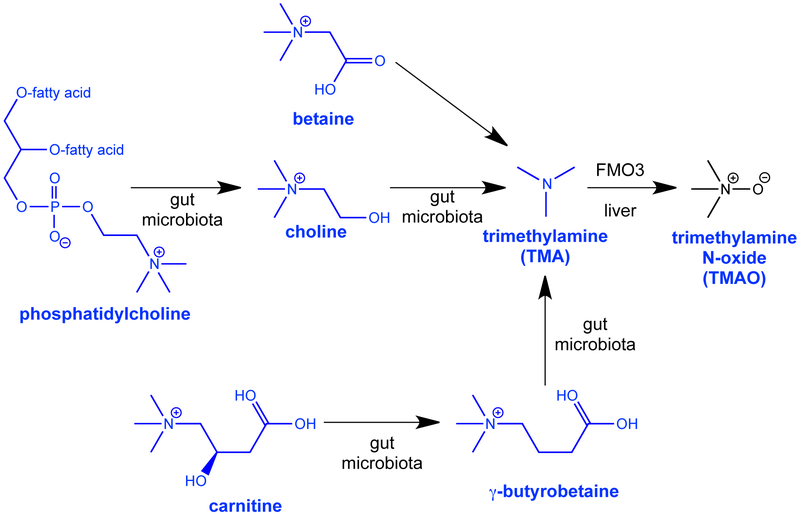
Trimethylamine N-oxide (TMAO) formation and circulating concentrations have recently been identified as risk factors for cardiovascular diseases (CVD) in clinical studies1–3. Much less is known about associations of TMAO with risks of cancer, but two studies have shown associations of serum TMAO with risk of colon cancer and a third study found associations with serum choline4–6. Formation of TMAO is dependent upon the release of trimethylamine (TMA) by the gut microbiota from choline, phosphatidylcholine, betaine and carnitine, from dietary sources such as eggs, dairy, and red meat. TMA is subsequently absorbed into circulation and transformed to TMAO by hepatic flavin-containing monooxygenase 3 (FMO3)7,8. Structures of these compounds and their biosynthesis are shown in Figure 1. The mechanism by which TMAO promotes atherosclerosis appears to involve induction of inflammatory pathways, inhibition of reverse cholesterol transport and hepatic bile synthesis, and increased foam cell formation7,9. Consumption of red meat and animal products is also risk factor for colorectal cancer10. At present, it is unclear as to the mechanisms by which TMAO could influence colon cancer risk, but this could be related to the pro-inflammatory pathways that increase risks of both CVD and cancer 11.
Figure 1.
Biosynthetic pathway of trimethylamine N-oxide (TMAO) from dietary substrates (choline, carnitine, betaine, and phosphatidylcholine).
Current research is underway to evaluate dietary strategies to reduce TMAO concentration by influencing TMA release by the gut microbiome7,12,13. However, previous studies from our group have shown that traditional approaches to modulating the composition and function of the microbiome (prebiotics and probiotics), as well as short-term flavonoid intervention14, failed to reduce circulating TMAO concentrations 15,16. A choline analog (3,3,-dimethylbutanol) has emerged as an effective inhibitor of choline metabolism and subsequent TMAO production in rodents8, but this compound is currently not approved for human use. Further research is needed into dietary strategies to blunt increases in TMAO given that a diet high in choline and carnitine is associated with increased TMAO production15,17 and intake of these nutrients alone is not associated with CVD risk, but rather conversion to TMAO appears to be the core problem18.
The Mediterranean diet is characterized by increased consumption of legumes, fruits, vegetables, cereals, and olive oil, and reduced intakes of red meat and fats found in Western diets19. Numerous reports indicate that following a Mediterranean diet may decrease CVD risk20. The impact of the Mediterranean diet on TMAO levels remains unclear, although several reports have indicated a TMAO lowering effect related to consumption of a Mediterranean diet. Filippis et al. reported a significant inverse correlation between adherence to a Mediterranean diet21 (based on an 11-unit dietary score) and urinary TMAO concentrations in a sample of vegetarians, vegans, and ominvores in Italy22. In the PREDIMED study (a randomized trial of Mediterranean vs low-fat diets in persons without diabetes), the Mediterranean diet was found to significantly decrease urinary TMAO compared to a low-fat diet in a Spanish sub-population 23. In a cross-sectional study within an Italian PREDIMED population, serum TMAO decreased with increased adherence to the Mediterranean Diet supplemented with extra-virgin olive oil [(rich in monounsaturated fatty acids (MUFA)]24. These previous studies were all conducted in Mediterranean populations. Little data exist on the impact of the Mediterranean diet on TMAO concentration in North American populations where rates of colon cancer are relatively high25.
Accordingly, the objective of this study was to perform a secondary analysis of samples from a previous clinical study of healthy subjects in the U.S. for fasting serum TMAO concentrations. The subjects were at elevated risk for colon cancer and were randomized to follow a Mediterranean or a Healthy Eating diet (based on the Healthy People 2010 dietary recommendations) for six months. Our hypothesis was that consumption of a Mediterranean diet, which is thought to have anti-inflammatory effects, would be associated with reduced circulating concentrations of TMAO. The results showed that this was not the case, although at baseline TMAO was significantly associated with cardiovascular risk factors.
METHODS
Study participants and samples.
Serum samples were obtained from a previously completed clinical trial, the Healthy Eating Study for Colon Cancer Prevention 25. The study was approved by the University of Michigan Institutional Review Board (HUM00007622) and registered at clinicaltrials.org (NCT00475722). All experiments were performed in accordance with the guidelines in the US Public Health Service Act and its amendments (the “Common Rule”) and in Guidelines for Good Clinical Practice (GCP) of the International Conference of Harmonization (ICH). Informed consents were obtained from human participants of this study. The secondary analysis of de-identified samples was approved by the Virginia Tech IRB (approval #17–173) and performed at Virginia Tech. Briefly, 120 healthy people at increased risk of colon cancer, BMI 18.5–35 kg/m2, were recruited and enrolled from the Ann Arbor, MI area by community advertising during 2007–2010, and 93 subjects finished six months of study participation. Full details of this trial have been published previously26.
Study participants were randomized to follow either a Healthy Eating diet based on the Healthy People 2010 dietary recommendations or a Mediterranean style diet for 6 months. As previously described, the Healthy Eating diet goals consisted of consumption of less than 35% of total caloric intake from fat with less than 10% caloric intake from saturated fat, and 2 servings/day of fruit, 3 servings/day of vegetables, and 3 servings/day of whole grains. The Mediterranean diet program consisted of consuming fats in a targeted ratio of 1:2:5 for PUFA:saturated fatty acid (SFA):MUFA, consuming 7–9 servings/day of fruits and vegetables, consumption of foods rich in omega-3 fatty acids at least twice a week, and at least 3 servings/day of whole grains25. The dietary fat goal on the Mediterranean arm was achieved by asking subjects to avoid high fat foods (prime cuts and untrimmed cuts of red meat, cheese, fried meats, high fat bread products such as biscuits and muffins) and to include lower fat foods (poultry breast, lean red meat, fish, low fat bread products) in order to accommodate increased intakes of foods that are high in MUFA and low in PUFA (namely olive oil, olives, avocados, hazelnuts, macadamia nuts). The goal was to maintain baseline fat intake as a percentage of energy. The number of food group exchanges per day for grains was adjusted recognizing that some of the carbohydrates will be provided by the fruit and vegetable goals (7–9 servings/day depending on individual energy needs). The resulting diet was therefore a low-fat, high fruit-vegetable diet with high MUFA foods added back to maintain total fat intake at baseline levels.
The primary goal of the original study was to evaluate changes in colonic eicosanoid concentrations. Dietary intakes of carotenoids, as determined from analysis of food records, roughly doubled in both study arms and this was confirmed by blood carotenoids 25,27. Blood and unprepped colon biopsies collected by flexible sigmoidoscopy were obtained before and after the 6-month intervention.
Dietary intake data for each subject at each time point was an average of four days at baseline, four days at 6 months (two recalls and two written records at each time point), and three days at 3 months (one recall and two written records). The un-announced recalls were performed using the 5-pass method28 to increase accuracy, and the written food records were reviewed with the subjects to probe for details. In addition to probing review of self-reported intakes, accuracy was likely increased by training subjects with regard to serving size estimation, and provision of a pictorial guide of serving sizes. Blood carotenoids and fatty acids changed in the expected directions27. The food records and recalls were analyzed using the Nutrition Data System for Research software (version 2010, Nutrition Coordinating Center, University of Minnesota).
Measures of inflammatory stress.
Blood concentrations of cholesterol, triglycerides, C-reactive protein (CRP), and cytokines were made as previously described29. Briefly, plasma cytokines (IL1β, IL6, IL8, interferon γ, TNFα, IL4, IL10, and IL13) were measured using ELISA DuoSets from R&D Systems (Minneapolis, MN). Serum lipopolysaccharide binding protein (LBP) was analyzed by enzyme-linked immunosorbent assay (ELISA) from Cell Sciences (Canton, MA). Measures of serum total cholesterol, high-density lipoprotein cholesterol (HDL), and triglycerides were performed using a Cobas Mira Chemistry analyzer (Roche Diagnostics Corporation, Indianapolis). CRP in serum was measured using a latex immunoturbimeteric assay. Plasma glucose was measured using a hexokinase colorimetric assay, and C-peptide was measured using an Immulite chemiluminescent assay (Diagnostics Products Corporation, Los Angeles, CA). The homeostasis model of assessment for insulin resistance (HOMA2-IR) was calculated from fasting C-peptide and glucose using an online calculator from the University of Oxford (The HOMA Calculator version 2.2)30.
Colonic microbiota analyses.
Bacteria adhering to the biopsies were identified by isolating DNA and sequencing the V4 region of the bacterial-specific 16S rRNA gene as described previously 31–33. Sequencing was done on the Illumina MiSeq platform (San Diego, CA). Subsampling was done to 1047 sequences per sample. Genus level taxonomic classifications were made using a modified version of the Ribosomal Database Project (RDP) training set 9 within mothur34,35.
The Shannon diversity index (H), which accounts for richness and evenness of species present, and the inverse Simpson index, another diversity metric, were calculated as previously described36. The community distance index (θYC) was calculated using the method of Yue and Clayton that accounts for proportions of both shared and non-shared species, producing a “Nonparametric Maximum Likelihood Estimator” index, with values between 0 and 1 indicating increasing community diversity37. The mean inter-individual θYC was calculated for samples both at baseline and 6 months after averaging θYC for all pairs of values at each time point for each sample33.
UPLC-MS/MS analyses of TMAO and precursor molecules.
TMAO, L-carnitine, choline, betaine and γ-butyrobetaine were measured as described previously14–16,38 with minor modifications. The serum aliquots used for the present analyses of TMAO and related compounds were prepared from fasting blood samples obtained in EDTA tubes and stored at −80˚C. The samples had been thawed and refrozen once. Of the 213 samples collected from 120 study participants, 205 samples from 115 subjects were available: n=115 at baseline and n=90 post-intervention. Immediately prior to sample preparation, 1 mL of an internal standard (IS) stock solution comprised of 25 μM choline chloride-d9, 25 μM betaine HCl-d9, 25 μM TMAO-d9, and 120 μM L-carnitine-d9 in water (TMAO-d9 and L-carnitine-d9 were from Cambridge Isotope Laboratories, Tewksbury, MA, all other internal standards were from Sigma, St. Louis, MO) was diluted 100-fold with acetonitrile (ACN). Samples were thawed at room temperature, and 25 μL serum was combined with 300 μL diluted IS solution. Samples were vortexed, centrifuged (17,000 x g, 3 min, room temperature), and the supernatant was filtered using a PTFE (4 mm, 0.2 μm) filter into a certified Waters HPLC vial (Milford, MA) with a 150 μL deactivated glass insert.
Samples (5 μL) were immediately analyzed by UPLC-MS/MS on a Waters Acquity H-class UPLC with a triple quadrupole (TCD) detector. UPLC separations were performed with a Waters BEH HILIC column (2.1 × 100 mm; 1.7 μm particle size) with a BEH HILIC VanGuard pre-column (2.1 × 5 mm; 1.7 μm). Column and sample temperatures were 30 and 10°C, respectively. The mobile phases were 15 mM ammonium formate, pH 3.5 (A) and ACN (B). The flow rate was 0.65 mL/min, and isocratic elution was achieved (20% A/80% B) over 3 min. Following separation, analytes were quantified using electrospray ionization (ESI) in (+)-mode. Source and capillary temperatures were 150 and 400°C, respectively. Capillary voltage was +0.60 kV, and desolvation and cone gas (both N2) flow rates were 800 and 20 L/h, respectively. Compounds were quantified using optimized multi-reaction monitoring (MRM) functions (Table S1). MRMs were optimized to achieve 12 points/10 s peak, and the detection span was ± 0.2 amu. Quantification was performed using ratios of the target analyte and respective IS peak areas, based on authentic external standard curves (1/X weighting) prepared using a wide range of target analyte concentrations (500 μM-0.1 nM; TMAO, betaine, L-carnitine, choline, and γ-butyrobetaine HCl from Sigma) and the same IS concentrations used to prepare the serum samples.
Data analysis and statistics.
Data were analyzed using SPSS version 24 (IBM, Amourk, NY, USA). For comparisons of two groups, two-sample, two-sided, t-tests were used for continuous variables and Chi-square tests for categorical variables. For analyses of changes over time, mixed models ANOVA was used, with outcome variables transformed as needed to achieve normality. Subject age and smoking status were covariates. Correlations of TMAO and related analytes with dietary intakes, biomarkers of inflammatory status and anthropometric variables were completed using Spearman correlations. Figures were generated using GraphPad Prism v.7 (LaJolla, CA, USA). Adjustment for false discovery rates was done using the method of Benjamini and Hochberg39.
Metabolic health was determined by published criteria that predict metabolic syndrome30,40. One point was awarded for each of the following criteria, with a lower score indicating lower risk of metabolic syndrome: 1) high waist circumference (>34.6 inches women, >40.1 inches for males), 2) low HDL (<40 mg/dl in men or <50 mg/dl in women or taking cholesterol medications), 3) high triglycerides (>150 mg/dl), 4) high glucose (>100 mg/dl), 5) high blood pressure (>130 mm systolic or >85 mm diastolic or taking blood pressure medications), and 6) insulin resistant by the Homeostatic Model Assessment of Insulin Resistance (HOMA2IR >1.8).
RESULTS
Effects of intervention on dietary intakes and concentrations of TMAO and TMA-precursors.
Of the 213 samples originally collected from 120 study participants, 205 samples from 115 subjects were available for this secondary analysis: 115 at baseline and 90 post-intervention. The demographic characteristics of the 115 subjects analyzed in the present analysis did not differ appreciably from the 120 in the full study set as published41. The 115 subjects for the present analysis were 72% female, 89% Caucasian, 11% current smoker, mean age 52 ± 12 years, and mean BMI 27.0 ± 3.7 kg/m2 (Table S2). No significant differences in demographic or dietary characteristics were observed between subjects in the two diet treatment arms at baseline (Table S2).
As described previously41, significant dietary changes were observed after six months of intervention in both dietary treatment arms, and at six months subjects reported meeting 82% of goals for the Mediterranean arm and 88% of the goals for the Healthy Eating arm. Both groups reported significantly increased consumption of fruits, vegetables, and whole grains and reduced consumption of red meat as a result of the dietary interventions. The primary difference in diet was related to fat intake. The Healthy Eating group exhibited a decrease in total fat consumption and saturated fat consumption, while the Mediterranean diet group reduced intake of n-6 PUFAs and increased intake of MUFAs27. A significant increase in fish intake was reported in the Mediterranean diet study participants after the 6-month intervention. The Healthy Eating group also reported significantly reduced dairy intake after the intervention (Table 1).
Table 1.
Dietary intakes in each diet arm before and after intervention.
| Measurea | Healthy Eating Diet |
Mediterranean Diet |
||
|---|---|---|---|---|
| Baseline (n=57) |
6
Months (n=45) |
Baseline (n=58) |
6
Months (n=45) |
|
| Dietary Intakesb | ||||
| Fish, Servings/day | 0.53 (0.99) | 0.93 (1.12) | 0.41 (0.69) | 1.47 (2.09)c |
| Red meat, servings/day | 2.79 (1.90) | 1.58 (1.54)c | 2.35 (1.83) | 1.20 (1.22)c |
| Eggs, servings/day | 0.44 (0.50) | 0.47 (0.54) | 0.36 (0.40) | 0.44 (0.64) |
| Dairy, servings/day | 3.33 (1.92) | 2.45 (1.47)c | 3.08 (1.96) | 2.49 (1.48) |
Data shown are mean and SD at each time point
Each participant was asked to collect two days of food records and to provide two 24-hour recalls at each time point. Fish servings include fish and shellfish. Red meat servings include all forms of beef, lamb, pork, wild game, cold cuts and red meat snacks such as jerky. Dairy servings include cheese, milk, foods made with milk, and butter.
Significantly different from baseline within the same food category as determined from mixed models ANOVA with diet group and time as factors, adjusted for baseline age and smoking status. For both fish and red meat intakes, there was a significant fixed effect of time. The data were normalized using natural log transformation for dietary intakes of fish and dairy. Square root transformation was used for dietary intakes of eggs and red meat.
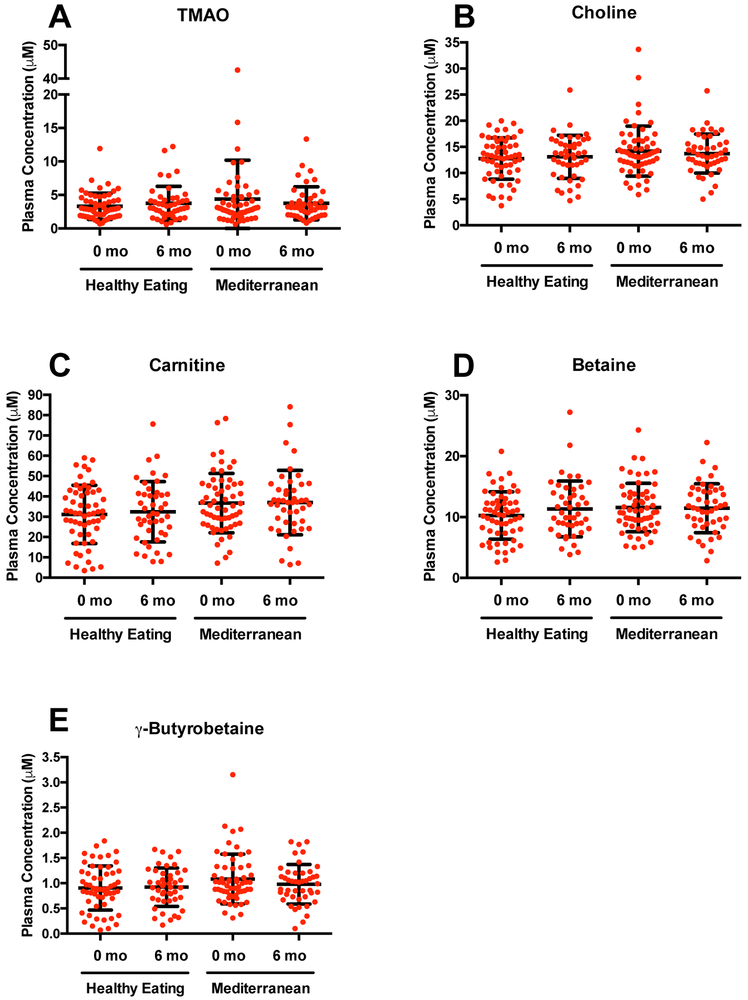
The fasting serum concentrations of TMAO, choline, carnitine, betaine and γ-butyrobetaine at baseline and after 6 months of the intervention are shown in Figure 2 and Table 2. No changes were observed in serum concentrations of TMA-precursors or TMAO after intervention in either treatment arm. Additionally, the ratios of TMAO to TMA-precursors (representative of the conversion of TMA to TMAO)42 remained unchanged in both treatment arms (Table 2). However, the ratios of TMAO to betaine and γ-butyrobetaine were slightly, but not significantly, reduced after the Mediterranean diet intervention, whereas only the ratio of γ-butyrobetaine to TMAO was slightly reduced after the Healthy Diet intervention. When looking specifically at the characteristics of subjects who demonstrated increases or decreases in TMAO concentration post-intervention (Table S3), no differences in reported dietary changes were observed.
Figure 2.
Distributions of fasting serum concentration of TMAO (A), choline (B), carnitine (C), betaine (D) and γ-butyrobetaine (E) at baseline (0 months) and after 6 months of dietary intervention with a Healthy Eating or Mediterranean diet. Mixed models ANOVA indicated no significant changes in concentrations of any analyte (or TMAO/precursor ratios, not shown) after intervention (see Results).
Table 2.
Concentrations of TMAO, relevant serum measures and dietary intakes in each diet arm before and after intervention. Data shown is mean and SD at each time pointa.
| Measurea | Healthy Eating Diet |
Mediterranean Diet |
||
|---|---|---|---|---|
| Baseline, n=57 | 6 Months, n=45 | Baseline, n=58 | 6 Months, n=45 | |
| Serum Concentrations or Concentration Ratios | ||||
| TMAO, µM | 3.3 (2.0) | 3.8 (2.5) | 4.4 (5.8) | 3.8 (2.5) |
| Betaine, µM | 10.2 (3.9) | 11.3 (4.6) | 11.6 (4.0) | 11.5 (4.0) |
| Choline, µM | 12.8 (4.0) | 13.1 (4.1) | 14.2 (4.8) | 13.7 (3.7) |
| Carnitine, µM | 31.3 (14.3) | 32.4 (14.9) | 36.7 (14.6) | 37.0 (15.9) |
| γ-Butyrobetaine, µM | 0.9 (0.4) | 0.9 (0.4) | 1.1 (0.5) | 1.0 (0.4) |
| TMAO:Betaine | 0.35 (0.25) | 0.34 (0.20) | 0.43 (0.83) | 0.34 (0.19) |
| TMAO:Choline | 0.26 (0.14) | 0.28 (0.15) | 0.31 (0.40) | 0.28 (0.18) |
| TMAO:Carnitine | 0.12 (0.07) | 0.12 (0.06) | 0.13 (0.19) | 0.11 (0.06) |
| TMAO:γ-Butyrobetaine | 4.47 (2.79) | 4.24 (2.28) | 4.82 (9.06) | 4.12 (2.22) |
Data shown is mean and SD at each time point. None of the measures were significantly changed by dietary intervention as determined from mixed models ANOVA using diet group and time as factors and adjustment for baseline age and smoking status. TMAO concentration and its ratios with precursor molecules were natural log transformed before analysis; the other variables did not require transformation.
We also calculated changes in TMAO, choline, carnitine, betaine, and γ-butyrobetaine concentrations during the interventions are shown in Figure S1 (absolute changes) and Figure S2 (fold changes) to demonstrate the variability in responses. In terms of fold (relative) changes, the mean fold changes for TMAO were 0.201 ± 0.718 (mean ± SD) and 0.298 ± 0.910 in the Healthy Eating and Mediterranean arms, respectively (Figure S2A). The mean fold changes for all other compounds were essentially near zero for both study arms (Figure S2B-E). It should be noted that the greatest variation in fold changes was observed for the microbial metabolites (TMAO and γ-butyrobetaine at baseline) compared to the dietary substrates.
Demographic and dietary characteristics of subjects with low and high TMAO concentrations at baseline.
Since there was little effect of diet intervention on TMAO variables, we then evaluated whether any demographic or dietary characteristics of subjects with relatively low and high baseline TMAO concentrations differed. The median TMAO concentration in the 115 subjects was 2.92 µmol/L at baseline (Table 3). A total of 57 study participants had TMAO readings above the median and 58 participants had TMAO readings below the median. BMI (p = 0.029), age (p = 0.016), waist circumference (p = 0.006), and blood pressure (p = 0.018) were significantly lower in the subjects with TMAO concentrations below the median compared to the above median. Additionally, there were significantly more females than males (p = 0.007) with TMAO concentrations below the median TMAO concentrations at baseline.
Table 3.
Baseline characteristics of study subjects who had TMAO serum concentrations above or below the mediana (2.92 µM).
| Parameterb | Above Median, n=57 |
Below Median, n=58 |
P-Valuea |
|---|---|---|---|
| BMI (kg/m2) | 27.9 (3.5) | 26.3 (4.1) | 0.029 |
| Age (years) | 55 (11) | 49 (13) | 0.016 |
| Caucasian (number, %) | 54 (93%) | 48 (84%) | 0.152 |
| Female (number, %) | 36 (62%) | 48 (84%) | 0.007 |
| Current smoker (number, %) | 6 (11%) | 5 (9%) | 1.000 |
| College Graduate (number, %) | 45 (78%) | 45 (79%) | 0.860 |
| Physical Activity, metabolic equivalents/day | 17 (12) | 21 (15) | 0.165 |
| High waist circumferenceb (number, %) | 31 (53%) | 16 (28%) | 0.006 |
| High blood pressurec
(number, %) |
31 (53%) | 18 (32%) | 0.018 |
| Taking NSAIDs regularlyd (number, %) | 15 (26%) | 9 (16%) | 0.184 |
Mean TMAO was 5.8 µmol/L (SD 5.6) for subjects above the median and 2.0 µmol/L (SD 0.6) for subjects below the median.
Data shown is mean and SD or number and percent. P-values are from independent samples t-tests (two-sided), from Chi square tests for categorical variables, or from Fisher’s Exact test for current smoking.
In terms of baseline dietary characteristics, several significant differences between subjects with high or low baseline TMAO concentrations were observed (Table S4). Red meat intake (p = 0.025) and glycemic load (p = 0.042) were significantly different dietary attributes, with red meat being consumed in greater quantities in the subjects with TMAO concentrations above the median.
Relationships of diet with TMA-precursors at baseline.
A correlation matrix of TMAO metabolites and dietary intakes of TMA-precursors at baseline is shown in Table 4. TMAO was positively correlated with red meat intake (ρ = 0.261) and negatively correlated with fiber intake (ρ = −0.197). The ratios of TMAO to betaine, choline, and carnitine, which are at least partially representative of the conversion of TMA-precursors to TMAO, were also positively correlated with red meat intake (ρ = 0.188, 0.281, and 0.211, respectively), implying that red meat consumption stimulates the conversion of TMA substrates to TMAO. In contrast, the ratios of TMAO to betaine, choline, and carnitine had a modest negative correlation with fish consumption, suggesting reduced conversion rates, but this was not statistically significant. The ratio of TMAO to betaine was negatively correlated with fiber consumption (ρ = −0.178), while choline and carnitine concentration was negatively correlated with legume intake (ρ = −0.197 and −0.188 respectively).
Table 4.
Spearman correlations of serum TMAO concentrations and related measuresa with dietary factors at baseline in 115 study participants.
| Dietary Intake |
TMAO µM |
Betainea µM |
Cholinea µM |
Carnitinea µM |
γ- Butyro-betaine µM |
TMAO: Betaine |
TMAO: Choline |
TMAO: Carnitine |
TMAO: γ- Butyro-betaine |
|---|---|---|---|---|---|---|---|---|---|
| Fish, servings/day | −0.027 | −0.076 | −0.086 | 0.036 | −0.172 | −0.027 | −0.076 | −0.086 | 0.036 |
| Red meat, servings/day | 0.261 | 0.115 | 0.062 | 0.033 | 0.139 | 0.198 | 0.281 | 0.211 | 0.149 |
| Eggs, servings/day | −0.179 | −0.029 | −0.065 | −0.058 | 0.011 | −0.101 | −0.130 | −0.118 | −0.121 |
| Dairy, servings/day | 0.021 | 0.024 | 0.056 | −0.010 | 0.014 | 0.033 | −0.038 | −0.048 | −0.044 |
| Percent fat, of energy | 0.026 | −0.098 | 0.022 | −0.047 | −0.091 | 0.069 | 0.035 | 0.023 | 0.086 |
| Fiber, g/1000 kcal | −0.194 | −0.026 | −0.060 | −0.076 | −0.169 | −0.178 | −0.146 | −0.040 | −0.009 |
| Legumes, servings/day | −0.181 | −0.077 | −0.197 | −0.188 | −0.110 | −0.108 | −0.088 | 0.034 | 0.001 |
TMA-precursors that are converted to TMAO.
Correlation coefficients with an absolute values of 0.178 and greater had nominal p-values <0.05, but after corrections for false discovery rates, none of the correlations were significant.
TMAO status and metabolic health risks at baseline.
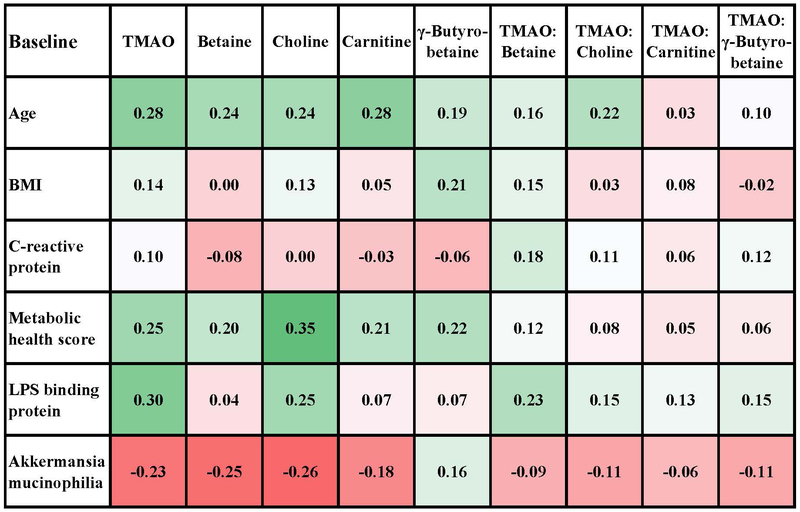
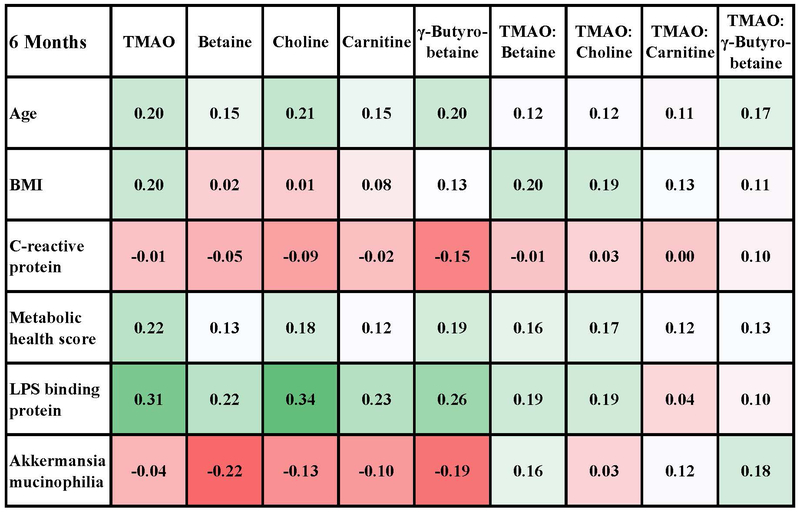
Relationships between markers of metabolic health risks and TMAO metabolites were also evaluated at baseline and post intervention (Figure 3A-B) using a heat map for exploration of the direction and strength of associations. Overall, correlations were modest but generally in the same direction at baseline and at 6 months. At baseline and post intervention, all TMAO metabolites were positively correlated with age and the 6-factor metabolic health score. For the individual components of the metabolic health score (not shown), all metabolites except TMAO were positively correlated with waist:hip ratio, and all metabolites except betaine were positively correlated with HOMA-2IR score. Serum CRP was not correlated with TMAO or any of the precursor molecules at baseline or post intervention. Relationship with plasma cytokine concentrations were also evaluated (Table S5) and none were statistically significant. LBP was, however, positively associated with TMAO concentrations at baseline, and this correlation remained statistically significant after correction for false discovery rates.
Figure 3A.
Spearman correlations of TMAO and precursor molecules with measures of health risks and microbial variables at baseline (n=86). Metabolic score was calculated as the number of factors that were abnormal: 1) high waist circumference (>34.6 inches for women, >40.1 inches for men), 2) low HDL (<40 mg/dl for men or <50 mg/dl for women or taking cholesterol medications), 3) high triglycerides (>150 mg/dl), 4) high glucose (>100 mg/dl), 5) high blood pressure (>130 mm systolic or >85 mm diastolic, or taking blood pressure medications), and 6) insulin resistance by the Homeostatic Model Assessment of Insulin Resistance (HOMA2IR>1.8). The number of samples for which microbiota analyses were available at baseline was 86. The supplementary data shows correlations with the relative abundance of other microbiome variables, but the correlation with Akkermansia mucinophilia was the strongest.
Figure 3B.
Spearman correlations of TMAO and precursor molecules with measures of health risks and microbial variables after 6 month intervention (n=90). Metabolic score was calculated as the number of factors that were abnormal: 1) high waist circumference (>34.6 inches for women, >40.1 inches for men), 2) low HDL (<40 mg/dl for men or <50 mg/dl for women or taking cholesterol medications), 3) high triglycerides (>150 mg/dl), 4) high glucose (>100 mg/dl), 5) high blood pressure (>130 mm systolic or >85 mm diastolic, or taking blood pressure medications), and 6) insulin resistance by the Homeostatic Model Assessment of Insulin Resistance (HOMA2IR>1.8). The number of samples for which microbiota analyses were available at baseline was 90. The supplementary data shows correlations with the relative abundance of other microbiome variables, but the correlation with Akkermansia mucinophilia was the strongest.
Bacterial populations and diversity at baseline.
Baseline relationships between TMAO metabolites and bacterial populations were examined and as shown in Table S6. TMAO concentration was negatively and modestly correlated with the relative abundance of the Akkermansia mucinophilia genus adhering to colonic biopsies at baseline and post intervention (Figure 3A-B) and with the Verrucomicrobia family of which Akkermansia mucinophilia is a major OTU we identified. There were no significant correlations with the relative abundance of major phyla, the Firmicutes:Bacteroidetes or Prevotella:Bacteroides ratios, or diversity indices (Shannon, inverse Simpson and the Yue and Clayton theta-YC distance metric). Given that no significant changes to TMAO or TMA-substrate concentrations were observed, relationships with microbial profiles were not assessed post intervention.
DISCUSSION
Previous studies have linked circulating TMAO concentration with heightened concentration of inflammatory processes implicated in the etiology of CVD and cancer2,3,5,43. The goal of the present study was to determine whether dietary intervention with a Mediterranean or Healthy Eating diet would reduce concentrations of circulating TMAO and alter the ratios of circulating TMAO to TMA-precursors. There was no significant reduction in TMA-precursors or TMAO was observed after either intervention (Table 2). The ratios of TMAO and TMA-precursors, representative of the conversion of substrates to TMAO, were also unchanged after the intervention. Interestingly, subjects with TMAO concentrations higher than the median value at baseline were significantly older and had significantly higher BMIs, waist circumferences, and elevated blood pressure compared to subjects with TMAO concentrations below the median value (Table 3).
The present study population was unique compared to other studies that examined Mediterranean dietary patterns and TMAO concentration in that the population was based in North America. Additionally, the subjects were at increased risk for colon cancer but were otherwise healthy16, 22, 38. The dietary intervention was not associated with reductions in serum TMAO concentration despite elevated fasting concentrations at baseline compared with another study from our group looking at younger adults with lower baseline TMAO concentration38. At baseline, mean TMAO concentration (median 2.92 µM) in this study population were higher than that reported in young, healthy adults (~1 μM) by us15, 38. TMAO and TMA substrate concentrations were, however, modestly positively correlated with the 6-factor metabolic health score (Figure 3A-B). Both dietary interventions caused a shift in dietary trends away from red meat that contains TMA substrates27. Despite this, circulating TMAO and TMA-substrate concentrations remained unchanged in both treatment groups (Figures 2, S1-2). This suggests that neither of these diets appears to significantly disrupt the net production of TMAO nor the conversion of TMA substrates to TMAO by way of gut microbes and liver enzymes, (Figure 1)44. This was somewhat surprising. A recent study showed that reduced red meat intake, as a part of a controlled feeding program, reduced circulating TMAO in just four weeks17. In our study, subjects reported consuming more fish after intervention, and some forms of fish are high in TMAO. It is also possible that part of the reason for the lack of effect on TMAO concentration could be that diet was assessed by survey and recall methods rather than controlled feeding. This could have resulted in error due to self-report. Unlike the baseline diet that presumably had been followed for some time, the intervention was instituted over only 6 months and time was required to meet the dietary goals after randomization.
Additional analyses were conducted at baseline since there was little change in TMAO after intervention. There were negative associations between TMAO and total fiber. Choline and carnitine intake were negatively associated with legume intake at baseline (Table 4). The Mediterranean and Healthy Eating dietary interventions both largely focused on dietary fat and fruit/vegetables and did not appreciably affect the microbiota33. Examination of how fiber and legumes influence the TMAO biosynthetic pathway could be topic of future studies. However, it is possible that the negative correlation between TMAO and fiber could simply be due to decreased consumption of red meat and fiber could actually have no direct effect on TMA production. Moreover, a previous study from our group indicated that an inulin intervention did not reduce serum TMAO concentrations16.
Despite previous reports of an association between TMAO and elevated cytokine concentration7, 9, in this study subjects with higher TMAO concentration did not exhibit significantly higher cytokine concentration, perhaps since the subjects in the present study were all reasonably healthy (Table S5). It is worth noting, however, that subjects who demonstrated increased concentration of TMAO post-intervention exhibited slightly but not significantly higher concentrations of lipopolysaccharide binding protein, a marker of increased intestinal permeability (Table S3).
Collectively, these findings are consistent with prior intervention studies targeting TMAO. Both acute15,38 and long term45 studies examining the effect of a diet recommended for prevention of cardiovascular risks on TMAO concentration have concluded that fasting TMAO concentration may not be easily altered by modifying the diet. Our findings were, however, different than other studies specifically examining the relationship between TMAO and a Mediterranean diet in southern Europe 21,22. Our study was unique in that we examined serum concentration of TMAO rather than urine concentration, and our study population was an older North American population. Overall, it may be the case that long-term habitual diet across the majority of the lifespan is more important than relatively short-term changes later in life. For the effects of diet on the intestinal microbiome, this has been shown to be the case46,47.
While the present study adds to the body of literature regarding dietary modification and TMAO concentration, several limitations must be acknowledged. Firstly, this study did not specifically examine vascular health and the subjects were not recruited based on CVD risk or existing disease. Secondly, this was a relatively short dietary intervention of six months and 1–2 months was required for subjects to meet the intervention goals48. It is possible that longer-term adherence to a Mediterranean diet, or a high MUFA Mediterranean diet, would generate different results. Thirdly, it must be mentioned that this was a behavioral intervention and adherence was based on self-report of dietary intake. Finally, the Mediterranean diet group significantly increased fish intake after intervention. It is possible that this elevated fish consumption could have contributed to the lack of effect on TMAO concentration because salt-water fish produce TMAO for use in osmotic regulation49. This elevated fish consumption could have caused an increase in pre-formed TMAO consumption in this group, assuming that the types of fish predominantly consumed were salt-water varieties.
Future efforts should be directed towards more targeted manipulation of gut microbes directly involved in TMA release and conversion of TMA to TMAO via FMO3 in the liver. Of note in this study were the significant and modest negative correlations between TMAO, betaine, and choline with the relative abundance of the phylum Verrucomicrobia and the major genus identified within that phylum, Akkermansia mucinophilia, observed at baseline (Table S3). Elevated abundance of Akkermansia mucinophilia has been linked in mice and human studies with reduced risk factors comprising the metabolic syndrome including obesity and insulin sensitivity13,50, as well as atherosclerosis51. Moreover, the FMO3 enzyme family, which is directly involved in the conversion of TMA to TMAO is typically upregulated in an obese or insulin resistant state.45 It would be interesting to determine if increasing Akkermansia mucinophilia population in the gut, perhaps by way of polyphenol supplementation52,53, would have an impact on the conversion of TMA to TMAO in future studies. Another area worthy of exploration is the impact of fish consumption as part of a Mediterranean diet on TMAO levels. It was not possible to study the effects of salt-water fish consumption in this study, but given that this is a potential source of pre-formed TMAO49, it would be beneficial to investigate the effects of Mediterranean diets with and without fish on TMAO concentrations.
CONCLUSION
This study supports previous findings that serum TMAO concentrations are increased by age, metabolic syndrome risk factors and systemic exposure to lipopolysaccharide. Six months of dietary intervention with either Healthy Eating or Mediterranean goals, however, did not alter fasting TMAO concentration in a population of subjects at elevated risk for colon cancer. Approaches that are targeted to alter the TMAO biosynthetic pathway via larger alterations in the gut microbiota may be needed to reduce TMAO production.
Supplementary Material
ACKNOWLEDGEMENT
Funding for the present work was provided, in part, by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture (APN, KPD). Funding for the clinical study (ZD) was provided by NIH grant RO1 CA120381, Cancer Center Support Grant P30 CA046592 (Immunology Core Laboratory). The clinical study used core resources supported by the University of Michigan Medical School Host Microbiome Initiative, a Clinical Translational Science Award, NIH grant UL1RR024986 (the Michigan Clinical Research Unit), by the Michigan Diabetes Research Center, NIH grant 5P60 DK20572 (Chemistry Laboratory), and the Michigan Nutrition and Obesity Research Center, NIH grant P30 DK089503. Funding for LEG and MEB was provided by the Virginia Tech Translational Obesity Research Interdisciplinary Graduate Education program (TOR IGEP).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Brown JM and Hazen SL, Annual review of medicine, 2015, 66, 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL and Tang WW, Journal of the American College of Cardiology, 2016, 67, 2620–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ufnal M, Zadlo A and Ostaszewski R, Nutrition, 2015, 31, 1317–1323. [DOI] [PubMed] [Google Scholar]
- 4.Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y and Cheng T-YD, Cancer research, 2014, 74, 7442–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu R, Wang Q and Li L, BMC genomics, 2015, 16, S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guertin KA, Li XS, Graubard BI, Albanes D, Weinstein SJ, Goedert JJ, Wang Z, Hazen SL and Sinha R, Cancer Epidemiology and Prevention Biomarkers, 2017, cebp–0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu J, Zhang Q and Mi M, MBio, 2016, 7, e02210–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ and Hazen SL, Cell, 2015, 163, 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson AL and Bäckhed F, Nature Reviews Cardiology, 2017, 14, 79. [DOI] [PubMed] [Google Scholar]
- 10.Chan DSM, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E and Norat T, PLOS ONE, 2011, 6, e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klampfer L, Curr Cancer Drug Targets, 2011, 11, 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostertag LM, Philo M, Colquhoun IJ, Tapp HS, Saha S, Duthie GG, Kemsley EK, de Roos B, Kroon PA and Le Gall G, Journal of proteome research, 2017, 16, 2516–2526. [DOI] [PubMed] [Google Scholar]
- 13.Aron-Wisnewsky J and Clément K, Nature Reviews Nephrology, 2016, 12, 169. [DOI] [PubMed] [Google Scholar]
- 14.Angiletta CJ, Griffin LE, Steele CN, Baer DJ, Novotny JA, Davy KP and Neilson AP, Food & Function, 2018, 9, 5350–5361. [DOI] [PubMed] [Google Scholar]
- 15.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW and Davy KP, Obesity, 2015, 23, 2357–2363. [DOI] [PubMed] [Google Scholar]
- 16.Baugh ME, Steele CN, Angiletta CJ, Mitchell CM, Neilson AP, Davy BM, Hulver MW and Davy KP, Nutrients, 2018, 10, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM and Hazen SL, Eur Heart J, , DOI: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer KA and Shea JW, Nutrients, , DOI: 10.3390/nu9070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M and Lapetra J, New England Journal of Medicine, 2013, 368, 1279–1290. [DOI] [PubMed] [Google Scholar]
- 20.Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bulló M and Barrubés L, Progress in Cardiovascular Diseases, 2018, 61, 62–67. [DOI] [PubMed] [Google Scholar]
- 21.Agnoli C, Krogh V, Grioni S, Sieri S, Palli D, Masala G, Sacerdote C, Vineis P, Tumino R, Frasca G, Pala V, Berrino F, Chiodini P, Mattiello A and Panico S, J Nutr, 2011, 141, 1552–1558. [DOI] [PubMed] [Google Scholar]
- 22.Filippis FD, Pellegrini N, Vannini L, Jeffery IB, Storia AL, Laghi L, Serrazanetti DI, Cagno RD, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW and Ercolini D, Gut, 2016, 65, 1812–1821. [DOI] [PubMed] [Google Scholar]
- 23.Vázquez-Fresno R, Llorach R, Urpi-Sarda M, Lupianez-Barbero A, Estruch R, Corella D, Fitó M, Arós F, Ruiz-Canela M, Salas-Salvadó J and Andres-Lacueva C, Journal of Proteome Research, 2015, 14, 531–540. [DOI] [PubMed] [Google Scholar]
- 24.Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Somma CD, Maisto M, Tenore GC, Colao A and Savastano S, Nutrition, 2018, 62, 7–17, DOI: 10.1016/j.nut.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD and Jemal A, CA: A Cancer Journal for Clinicians, 2017, 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 26.Sidahmed E, Cornellier ML, Ren J, Askew LM, Li Y, Talaat N, Rapai MS, Ruffin MT, Turgeon DK, Brenner D, Sen A and Djuric Z, J. Hum. Nutr. Diet, 2014, 27, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen A, Ren J, Ruffin MT, Turgeon DK, Brenner DE, Sidahmed E, Rapai ME, Cornellier ML and Djuric Z, Cancer Prevention Research, 2013, 6, 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conway JM, Ingwersen LA and Moshfegh AJ, Journal of the American Dietetic Association, 2004, 104, 595–603. [DOI] [PubMed] [Google Scholar]
- 29.Umoh FI, Kato I, Ren J, Wachowiak PL, Ruffin MT, Turgeon DK, Sen A, Brenner DE and Djuric Z, European journal of nutrition, 2016, 55, 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menendez JA, Lupu R and Colomer R, European journal of cancer prevention, 2005, 14, 263–270. [DOI] [PubMed] [Google Scholar]
- 31.Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD and Young VB, Infection and immunity, 2015, 83, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozich JJ, Westcott SL, Baxter NT, Highlander SK and Schloss PD, Applied and environmental microbiology, 2013, AEM–01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djuric Z, Bassis CM, Plegue MA, Ren J, Chan R, Sidahmed E, Turgeon DK, Ruffin IV MT, Kato I and Sen A, Journal of the Academy of Nutrition and Dietetics, 2018, 118, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Garrity GM, Tiedje JM and Cole JR, Applied and environmental microbiology, 2007, 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR and Tiedje JM, Nucleic acids research, 2013, 42, D633–D642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begon M, Harper J and Townsend C, Ecology: individuals, populations, and communities. 3rd ed Blackwell Scientific, Oxford, UK, 1996. [Google Scholar]
- 37.Yue JC and Clayton MK, Communications in Statistics-theory and Methods, 2005, 34, 2123–2131. [Google Scholar]
- 38.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW and Davy KP, Nutrition Research, 2015, 35, 858–864. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y and Hochberg Y, Journal of the royal statistical society. Series B (Methodological), 1995, 289–300. [Google Scholar]
- 40.Huang PL, Disease models & mechanisms, 2009, 2, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djuric Z, Ruffin IV MT, Rapai ME, Cornellier ML, Ren J, Ferreri TG, Askew LM, Sen A, Brenner DE and Turgeon DK, Contemporary clinical trials, 2012, 33, 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang WHW, Wang Z, Wu Y, Fan Y, Koeth RA and Hazen S, Journal of the American College of Cardiology, 2013, 61, E1398. [Google Scholar]
- 43.Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA and Solas M, Nutrients, , DOI: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ and Hazen SL, Cell, 2016, 165, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer B, Machann J, Schick F, Fritsche A, Häring H-U, Xu G, Lehmann R and Stefan N, Scientific Reports, 2016, 6, 26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA and Knight R, Science, 2011, 334, 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z and Knight R, British Journal of Nutrition, 2015, 113, S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidahmed E, Cornellier ML, Ren J, Askew LM, Li Y, Talaat N, Rapai MS, Ruffin MT, Turgeon DK, Brenner D, Sen A and Djuric Z, J Hum Nutr Diet, 2014, 27, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammerschlag N, Marine and Freshwater Behaviour and Physiology, 2006, 39, 209–228. [Google Scholar]
- 50.Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, Fournier M, Lecours M-A, Desjardins Y, Roy D, Levy E and Marette A, Mol Metab, 2017, 6, 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Lin S, Vanhoutte PM, Woo CW and Xu A, Circulation, 2016, CIRCULATIONAHA; –115. [DOI] [PubMed] [Google Scholar]
- 52.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ and Raskin I, Diabetes, 2015, db141916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anhê FF, Pilon G, Roy D, Desjardins Y, Levy E and Marette A, Gut microbes, 2016, 7, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.