Abstract
Background:
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune disease characterized by unpredictable attacks of the optic nerves and spinal cord that cause neurologic deficits, including weakness, numbness, bowel/bladder dysfunction, and pain and reduced vision and can ultimately lead to blindness and paralysis. We assessed the effects of NMOSD on quality of life.
Methods:
Adult patients with NMOSD treated at a US academic neurology clinic completed the EQ-5D and several other measures of functional status and quality of life. The EQ-5D scores and correlations across measures were evaluated, and scores were compared with those of patients with multiple sclerosis and US norms.
Results:
Twenty-one patients (90% women; mean age, 42.8 years; mean disease duration, 8.2 years) were included. The mean EQ-5D score was 0.74. Most patients reported at least some problems with mobility, pain/discomfort, usual activities, and/or anxiety/depression. Greater proportions of patients reported moderate or severe problems with mobility and pain/discomfort than they did with self-care, usual activities, or anxiety/depression. In a multivariate model, only the Brief Pain Inventory was a significant independent predictor of overall EQ-5D score.
Conclusions:
Neuromyelitis optica spectrum disorder has a substantial effect on multiple domains of quality of life. Pain seems to be among the primary drivers of the EQ-5D scores in NMOSD.
Keywords: EQ-5D, Multiple sclerosis (MS), Neuromyelitis optica spectrum disorder, Pain, Quality of life
Neuromyelitis optica spectrum disorder (NMOSD) is a rare relapsing autoimmune disease that preferentially targets the optic nerves and spinal cord, leading to blindness and paralysis.1 The incidence of NMOSD in the United States is one to two per 100,000, with a total prevalence of 4000 to 8000, accounting for approximately 1.5% of all cases of demyelinating diseases. It accounts for a higher percentage of demyelinating diseases in countries with greater proportions of nonwhite individuals, including Mexico, Japan, West Indies, and Cuba,2–7 but is nonetheless rare in those countries as well. In the United States, the nonwhite population is overrepresented, as are women, by a ratio of 6.5:1.8 The average age at diagnosis is 41 years, with a broad range from 3 to 81 years. A clinical diagnosis of NMOSD can be made in a patient with relapsing autoimmune attacks of the spinal cord and optic nerves in which multiple sclerosis (MS) is considered less likely because of longitudinal inflammation in the spinal cord and absence of brain involvement.9 A biomarker for NMOSD, the NMO–immunoglobulin G, which binds to the aquaporin-4 water channel, is associated with NMOSD at increasing sensitivities between 70% and 87%, and specificity of more than 99%, implying a near-certain diagnosis of NMOSD in any seropositive patient with inflammation in the spinal cord or optic nerves.10
If left untreated, patients with NMOSD will have a relapse every 7 months, on average.11 Use of an immunosuppressive medication reduces the average frequency of relapse to every 15 months.11 Although the severity of each relapse varies, most leave residual neurologic disability, despite optimal acute intervention.12,13 Residual neurologic deficits resulting from NMOSD attacks of the spinal cord include weakness, numbness, incoordination, problems with mobility, bowel/bladder dysfunction, and pain; attacks of the optic nerve causing vision impairment affect the patient's ability to perform activities of daily living.14,15 Among the residual neurologic deficits from acute NMOSD relapses, pain is the most common, with more than 80% of patients reporting daily pain and/or pain that limits daily function.16 Furthermore, the unpredictability of future attacks and subsequent increasing disability contribute to anxiety, and experiencing new relapses or worsening of symptoms promotes low mood.
Previous studies on the quality of life of patients with NMOSD have focused on specific topics such as depression or pain.16–18 One, using semistructured interviews, took a broader view of overall health status and well-being.14 We used the five-level EQ-5D (EQ-5D-5L [EuroQol, Rotterdam, Netherlands]) instrument to assess the health status of an opportunistic sample of patients with NMOSD and compared their scores with those of patients with MS as reported in the literature. We also analyzed the individual contributions of each EQ-5D dimension in the study sample to understand the drivers of the overall score.
Methods
This study was approved by the Johns Hopkins institutional review board. All participants were recruited from the Johns Hopkins NMO Clinic and the Johns Hopkins Hospital at a Patient Day event hosted at the hospital on October 6, 2014. Eligibility was determined by screening patients who met the 2015 International Panel for Neuromyelitis Optica Diagnostic criteria.9 Patients had to be 18 years or older to participate and able to understand the consenting process.
After obtaining written informed consent, the EQ-5D-5L questionnaire was given to each patient to complete while seated at a table in a quiet place. The EQ-5D is a standardized validated measure of health status developed by the EuroQol Group to provide a simple, generic measure of health for clinical and economic appraisals.19 It consists of a questionnaire that assesses five dimensions of health thought to affect quality of life: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The new 5L version of the EQ-5D offers five possible responses to each of the questions (no problems, slight problems, moderate problems, severe problems, extreme problems/unable to do). The set of responses to each of the five questions represents a person's health state, or profile. This profile can be mapped into a single score, or utility value, using an equation developed by presenting possible profiles to a sample of the general population and asking them to address how much life expectancy they would give up to relieve fully the problems presented by the profile. The score is typically on a continuum between −1 (worst possible health) and +1 (best possible health).20 The EQ-5D also includes a visual analogue scale (EQ-VAS) whereby respondents are asked to provide an assessment of their overall health in a range from 0 (worst imaginable health state) to 100 (best imaginable health state). The EQ-VAS provides complementary information to the profile by reflecting the respondent's overall perception of current health. Both the profile-based utility score and the EQ-VAS can be used to assess changes over time or to compare health status across diseases and conditions.
Other metrics were collected, including Beck Depression Inventory (BDI), Fatigue Severity Scale (FSS), Brief Pain Inventory (BPI), and patient-administered Expanded Disability Status Scale (EDSS) scores.
All data were deidentified before analysis. The ratings on the individual dimensions of the EQ-5D (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) were compiled for each patient. Because the 5L version of the instrument is quite new, value sets for the US population are available only for the older three-level (3L) version (three possible responses for each question: no problems, some problems, severe problems/unable to do). A crosswalk created by EuroQol researchers uses a mapping approach to estimate a value set for the EQ-5D-5L based on the available value set for the EQ-5D-3L.21 Statistical analysis was performed using JMP version 11.2 (SAS Institute Inc, Cary, NC). The distributions of each score and its components were derived, including means, medians, and standard deviations where appropriate. Linear correlations among instruments and subscales were explored. Multivariate regression of the overall EQ-5D score against the other scores was performed to identify the best predictors.
Results
Twenty-four patients met the inclusion criteria and were enrolled in this study. Three patients did not have sufficient clinical data available and were subsequently excluded from the analysis. The demographic and clinical characteristics of the remaining 21 patients are detailed in Table 1. The mean ± SD age at the time of completing the EQ-5D-5L was 42.8 ±10.6 (median, 43.0) years, with a mean ± SD disease duration of 8.2 ± 5.0 (median, 9.0) years. The female-to-male ratio was 19:2, and there were 8 African American and 13 white participants. The mean ± SD EDSS score was 5.0 ± 1.8 (median, 5.5), which corresponds to disability severe enough to impair full daily activities and ability to work a full day without special provisions; at an EDSS score of 5.0, patients are still able to walk 200 m without aid or rest. The demographic and clinical profile of the study population is consistent with that of the broader NMOSD population in the United States.
Table 1.
Characteristics of the 21 study patients with neuromyelitis optica spectrum disorder
| Characteristic | Value |
|---|---|
| Female sex | 19 (90.5) |
| Age at survey, mean, y | 42.8 |
| Disease duration, mean, y | 8.2 |
| Race | |
| Black | 8 (38.1) |
| White | 13 (61.9) |
| Anti–aquaporin-4 seropositive | 17 (81.0) |
| Expanded Disability Status Scale score | 5.0 ±1.8 |
| Beck Depression Inventory score | 14.6 ±7.6 |
| Fatigue Severity Scale score | 46.5 ±14.2 |
| Brief Pain Inventory score | 4.5 ±2.7 |
Note: Unless otherwise indicated, values are given as number (percentage) or mean ± SD.
Scores on the other metrics are also included in Table 1. The mean ± SD BDI score was 14.6 ± 7.6 (median, 16), which falls on the lower (ie, less depressed) end of the spectrum of mild depression (range, 14–19). Minimal depression was reported by 42.9% of patients, mild depression by 28.6%, and moderate depression by 28.6%. The mean ± SD FSS score was 46.5 ± 14.2 (median, 53.0), where a score greater than 36 indicates severe fatigue, and the mean ± SD BPI score was 4.5 ± 2.7 (median, 4.75). Just more than half of the patients (52.4%) reported mild pain (score of <5); 19.0%, moderate pain (score of 5–7); and 28.6%, severe pain (score of >7–10).
The overall EQ-5D scores for patients with NMOSD ranged from 0.412 to 1.000, with a mean ± SD of 0.738 ± 0.163, which is lower than the US norm of 0.867 and the world norm of 0.902.22 The mean EQ-5D score for the present NMOSD population is within the range but higher than most of the values reported for patients with MS, where average scores of 0.41 to 0.77 have been reported.23–28 One study that also used the EQ-5D-5L found a mean score of 0.59 in their MS population.26 The health profiles compiled from the EQ-5D in the NMOSD population show that no patients reported extreme problems on any of the five domains, but most patients reported at least some problems with mobility (66.7%), usual activities (61.9%), pain/discomfort (76.2%), or anxiety/depression (71.4%). One-third of the patients (33.3%) reported problems with self-care. Pain/discomfort and mobility were the two domains with the highest proportion of patients reporting moderate or severe problems (61.9% and 52.4%, respectively) (Figure 1). One-third of the patients (33.3%) reported moderate or severe problems with usual activities, and almost one-fourth (23.8%) reported moderate or severe problems with anxiety/depression. Less than 5% of participants reported moderate or severe problems with self-care.
Figure 1.
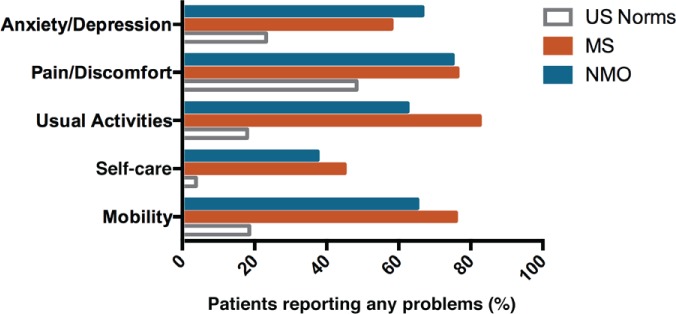
EQ-5D domain scores by severity
The health profiles compiled from the EQ-5D in the neuromyelitis optica spectrum disorder population show that no patients reported extreme problems on any of the five domains, but most patients reported at least some problems with mobility (66.7%), usual activities (61.9%), pain/discomfort (76.2%), or anxiety/depression (71.4%). Pain/discomfort and mobility were the two domains with the highest proportion of patients reporting moderate or severe problems (61.9% and 52.4%, respectively).
Figure 2 compares the present findings with US norms and scores reported for select MS populations. Patients with NMOSD and patients with MS are substantially more likely to report problems in each of the EQ-5D domains than the normative US population. The proportion of patients with NMOSD who reported problems was generally within the ranges found in MS, although patients with NMOSD were less likely to report problems with usual activities but more likely to report problems with anxiety/depression.
Figure 2.
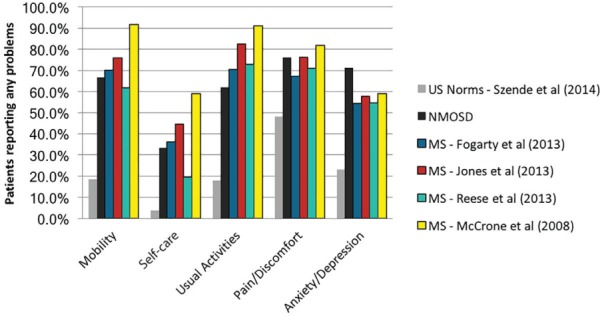
Proportions of patients reporting problems across EQ-5D domains: comparison of present findings with US norms and scores reported for select multiple sclerosis (MS) populations
Patients with neuromyelitis optica spectrum disorder (NMOSD) and those with MS are substantially more likely to report problems in each of the EQ-5D domains than the normative US population. The proportions of patients with NMOSD who reported problems were generally within the ranges found in patients with MS, although patients with NMOSD were less likely to report problems with usual activities but more likely to report problems with anxiety/depression.
Almost two-thirds of patients reported moderate or severe problems with pain/discomfort on the EQ-5D. In a comparison of the EQ-5D with the other four instruments, the EQ-5D was most closely correlated with the BPI (r2 = 0.67) (Table 2). The EQ-5D correlations were much weaker with the BDI (r2 = 0.32), the EDSS (r2 = 0.28), and the FSS (r2 = 0.24). Consistent with the univariate correlations, the BPI was the only measure significantly associated with the EQ-5D in the multivariate analysis.
Table 2.
Multivariate regression of EQ-5D-5L scores against other measures
| Term | Estimate | Standard error | t Ratio | Probability>|t| |
|---|---|---|---|---|
| Intercept | 0.9766 | 0.092651 | 10.54 | <0.0001 |
| Expanded Disability Status Scale | −0.001066 | 0.016787 | −0.06 | 0.9501 |
| Beck Depression Inventory | 0.000097 | 0.005126 | 0.02 | 0.9851 |
| Fatigue Severity Scale | −0.000804 | 0.002354 | −0.34 | 0.7372 |
| Brief Pain Inventory | −0.044765 | 0.014415 | −3.11 | 0.0068 |
Discussion
To our knowledge, this is the first study to use a standardized, commonly used assessment tool, the EQ-5D, to evaluate the overall quality of life of patients with NMOSD. We found that these patients rate their quality of life lower than does the general US population but somewhat higher than do patients with MS. The EQ-5D scores were most closely correlated with pain scores, which is different from MS, where scores are largely driven by anxiety/depression and mobility. We found that the pain scale score was the only independent predictor of the EQ-5D score. These findings suggest that the EDSS, BDI, FSS, and BPI each provide unique and valuable information about health status in NMOSD and that the EQ-5D should be used in addition to, rather than as a replacement for, these other instruments.
The present findings are generally consistent with those of published studies that used the EQ-5D in MS. Interestingly, EQ-5D scores were better in the NMOSD cohort compared with national MS scores, although NMOSD relapses tend to be more severe and damaging. On average, relapses in NMOSD tend to be more severe than in MS, and resulting neurologic disability is higher because of involvement of spinal cord function on mobility and pain.29 The higher rates of anxiety/depression seen in NMOSD may be attributable to the unpredictable nature and potential severity of NMOSD relapses. Patients with NMOSD tend to have less severe brain involvement than those with MS, which may explain the more benign scores in usual activities and self-care.
There are three significant limitations to this study. First is the small sample size due to the low prevalence of NMOSD. The study sample was limited to 21 patients treated at a single NMOSD clinic. Nevertheless, the demographic and clinical characteristics of the population were representative of the broader NMOSD population. The second significant limitation is that the newer 5L version of the EQ-5D was used but the valuation was derived from a crosswalk from older 3L-based values. It is unknown what, if any, distortion this introduces. The third major limitation is that the EQ-5D instrument was administered at a different time than the EDSS, BDI, FSS, and BPI measures. Furthermore, we were limited by the data collected. For example, although patients with NMOSD commonly experience both neuropathic and spastic pain,16 the BPI fails to differentiate among types of pain. The type of pain may influence quality of life in different ways. Although the time between administrations of the different questionnaires was only a few days, and no patients experienced any relapses in the time between questionnaires, it is nonetheless possible that the patients' health status had changed over those few days and the questionnaires were not addressing the same state.
In conclusion, further studies in larger cohorts are needed to clarify the relationships among co-occurring symptoms including depression, anxiety, fatigue, and cognitive function on quality of life. Nonetheless, NMOSD has a substantial effect on multiple domains of quality of life, with pain emerging as a primary driver of the EQ-5D scores in this population.
PRACTICE POINTS
Patients with neuromyelitis optica spectrum disorder (NMOSD) were more likely to report problems with all aspects of quality of life covered by the EQ-5D.
Almost two-thirds of patients with NMOSD reported moderate or severe problems with pain/discomfort on the EQ-5D.
The Brief Pain Inventory was a significant independent predictor of overall EQ-5D score.
Acknowledgments
Ms. Mealy is a PhD candidate at the Johns Hopkins University School of Nursing.
Financial Disclosures
Dr. Boscoe works for Alexion Pharmaceuticals. Dr. Levy currently receives research support from the National Institutes of Health, Maryland Technology Development Corporation, Sanofi, Genzyme, Alexion, Alnylam, Shire, Acorda, and Apopharma; has received personal compensation for consultation from Alexion, Acorda, and Genzyme; and serves on the scientific advisory boards for Alexion, Acorda, and Quest Diagnostics. The other authors declare no conflicts of interest.
Funding/Support
This work was supported by Alexion Pharmaceuticals. No one from Alexion was involved in data collection.
References
- 1.Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. 2012;2012 doi: 10.1155/2012/460825. 460825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuroiwa Y, Igata A, Itahara K et al. Nationwide survey of multiple sclerosis in Japan: clinical analysis of 1,084 cases. Neurology. 1975;25:845–851. doi: 10.1212/wnl.25.9.845. [DOI] [PubMed] [Google Scholar]
- 3.Cabre P, Heinzlef O, Merle H et al. MS and neuromyelitis optica in Martinique (French West Indies) Neurology. 2001;56:507–514. doi: 10.1212/wnl.56.4.507. [DOI] [PubMed] [Google Scholar]
- 4.Rivera JF, Kurtzke JF, Booth VJ, Corona VT. Characteristics of Devic's disease (neuromyelitis optica) in Mexico. J Neurol. 2008;255:710–715. doi: 10.1007/s00415-008-0781-2. [DOI] [PubMed] [Google Scholar]
- 5.Cabre P. Environmental changes and epidemiology of multiple sclerosis in the French West Indies. J Neurol Sci. 2009;286:58–61. doi: 10.1016/j.jns.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera-Gomez JA, Bonnan M, Gonzalez-Quevedo A et al. Neuromyelitis optica positive antibodies confer a worse course in relapsing-neuromyelitis optica in Cuba and French West Indies. Mult Scler. 2009;15:828–833. doi: 10.1177/1352458509104585. [DOI] [PubMed] [Google Scholar]
- 7.Asgari N. Epidemiological, clinical and immunological aspects of neuromyelitis optica (NMO) Dan Med J. 2013;60 B4730. [PubMed] [Google Scholar]
- 8.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–1180. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 9.Wingerchuk DM, Banwell B, Bennett JL. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeon A, Fryer JP, Apiwattanakul M et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009;66:1134–1138. doi: 10.1001/archneurol.2009.178. [DOI] [PubMed] [Google Scholar]
- 11.Kimbrough DJ, Mealy MA, Simpson A, Levy M. Predictors of recurrence following an initial episode of transverse myelitis. Neurol Neuroimmunol Neuroinflamm. 2014;1 doi: 10.1212/NXI.0000000000000004. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud H, Petrak A, Mealy M et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22:185–192. doi: 10.1177/1352458515581438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiter I, Gahlen A, Borisow N et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79:206–216. doi: 10.1002/ana.24554. [DOI] [PubMed] [Google Scholar]
- 14.Mutch K, Methley A, Moore P, Jacob A. Life on hold: the experience of living with neuromyelitis optica. Disabil Rehabil. 2014;36:1100–1107. doi: 10.3109/09638288.2013.833301. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, Mutch K, Elsone L et al. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. 2014;20:1658–1661. doi: 10.1177/1352458514522103. [DOI] [PubMed] [Google Scholar]
- 16.Qian P, Lancia S, Alvarez En et al. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol. 2012;69:1482–1487. doi: 10.1001/archneurol.2012.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanamori Y, Nakashima I, Takai Y et al. Pain in neuromyelitis optica and its effect on quality of life: a cross-sectional study. Neurology. 2011;77:652–658. doi: 10.1212/WNL.0b013e318229e694. [DOI] [PubMed] [Google Scholar]
- 18.Chanson JB, Zephir H, Collongues N et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol. 2011;18:836–841. doi: 10.1111/j.1468-1331.2010.03252.x. [DOI] [PubMed] [Google Scholar]
- 19.Devlin NJ, Krabbe PFM. The development of new research methods for the valuaiton of EQ-5D-5L. Eur J Health Econ. 2013;14(suppl 1):S1–S3. doi: 10.1007/s10198-013-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 21.van Hout B, Janssen MF, Feng YS et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Szende A, Janssen B, Cabases J, editors. Self-reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer Netherlands; 2014. [PubMed] [Google Scholar]
- 23.Orme M, Kerrigan J, Tyas D et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10:54–60. doi: 10.1111/j.1524-4733.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- 24.Moore F, Wolfson C, Alexandrov L, Lapierre Y. Do general and multiple-sclerosis-specific quality of life instruments differ? Can J Neurol Sci. 2004;31:64–71. doi: 10.1017/s0317167100002857. [DOI] [PubMed] [Google Scholar]
- 25.Jones KH, Ford DV, Jones PA et al. How people with multiple sclerosis rate their quality of life: an EQ-5D survey via the UK MS register. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065640. e65640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogarty E, Walsh C, Adams R et al. Relating health-related quality of life to disability progression in multiple sclerosis, using the 5-level EQ-5D. Mult Scler. 2013;19:1190–1196. doi: 10.1177/1352458512474860. [DOI] [PubMed] [Google Scholar]
- 27.Kuspinar A, Mayo NE. A review of the psychometric properties of generic utility measures in multiple sclerosis. Pharmacoeconomics. 2014;32:759–773. doi: 10.1007/s40273-014-0167-5. [DOI] [PubMed] [Google Scholar]
- 28.Reese JP, Wienemann G, John A et al. Preference-based Health status in a German outpatient cohort with multiple sclerosis. Health Qual Life Outcomes. 2013;11:162. doi: 10.1186/1477-7525-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrone P, Heslin M, Knapp M et al. Multiple sclerosis in the UK: service use, costs, quality of life and disability. Pharmacoeconomics. 2008;26:847–860. doi: 10.2165/00019053-200826100-00005. [DOI] [PubMed] [Google Scholar]


