Abstract
Background:
Pathological laughing and crying (PLC) encompasses episodes of involuntary laughing, crying, or both that are contextually incongruous with the individual's subjective mood. Despite a 10% to 46% prevalence in people with multiple sclerosis (MS) and reduced quality of life, localization of neuroanatomical lesions associated with PLC remains poorly delineated.
Methods:
The relationship between posterior fossa lesions and PLC in people with MS was examined using a retrospective medical record review of people with MS (2012–2016) who had completed the Center for Neurologic Study–Liability Scale (CNS-LS) and had undergone 1.5-T magnetic resonance imaging within 6 months of each other.
Results:
Medical record review identified 80 potential cases, with 77 included. Brainstem and cerebellar lesions were counted, measured, and compared between people with MS who had positive results on the CNS-LS (scores ≥17, n = 22) with those who had negative results on the CNS-LS (scores ≤16, n = 55). Initial χ2 analysis showed no significant difference in lesion numbers in people with MS without (CNS-LS score ≤16) versus with (CNS-LS score ≥17) PLC. When analyzing only people with MS without evidence of depression, a significant inverse relationship was identified such that fewer posterior fossa lesions on automated magnetic resonance imaging was associated with the presence of PLC.
Conclusions:
Posterior fossa lesion load is not indicative of which individuals could develop PLC. Further investigations to delineate the primary source of PLC symptoms would aid in diagnosis and treatment of this condition.
Keywords: Affect, Mood, Magnetic resonance imaging (MRI), Multiple sclerosis (MS), Pathological laughing and crying, Pseudobulbar
Pathological laughing and crying (PLC), also known as pseudobulbar affect, is defined as episodes of involuntary laughing, crying, or both that are contextually inappropriate or incongruous with the individual's subjective mood state.1,2 It is a dysfunction in emotional expression and not directly associated with disorders of mood, which encompass problems of emotional experience. Pathological laughing and crying may affect patients with a wide array of neurologic disorders, including stroke, brain tumor, traumatic brain injury, Parkinson disease, and amyotrophic lateral sclerosis. Research has focused primarily on PLC due to stroke, tumor, and trauma. A growing body of literature has begun to examine the association between PLC and multiple sclerosis (MS). The mean prevalence in patients with MS is approximately 10%; however, prevalences as high as 46% have been reported in some MS cohorts.3,4
Multiple sclerosis–specific research into functional impact is sparse; however, subjective reports from people with MS and PLC support a common theme of embarrassment from outbursts,1,5,6 leading to impaired social interactions and isolation.7,8 Examination of the burden of PLC on patients with neurologic disorders (not specifically MS) found that patients with PLC scored lower on both quality of life and work quality and impairment questionnaires. Twenty-four percent of the patients reported PLC as the primary reason for becoming homebound.9 Despite its significant effect on quality of life, PLC continues to be underreported and undertreated. In 2015, Vidovic et al4 found that 42.4% of patients did not inform their neurologists of new-onset affect dysregulation. In a survey with 2318 respondents (not MS specific), only 41% of patients who discussed affect disinhibition with clinicians were appropriately diagnosed as having PLC, and of these, 50% received treatment.10 A better understanding of the underpinnings of PLC will aid in the development of targeted diagnostic and therapeutic tools to alleviate the functional impact of this condition.
The localization of neuroanatomical lesions causing PLC in people with MS continues to be poorly delineated. The multilesional nature of MS makes it especially difficult to assess; thus, the pathophysiology of PLC has largely been based on postmortem studies. The original theory by Wilson11 in 1924 postulated that motor cortex lesions and internal capsule disruption led to disinhibition of the brainstem nuclei that regulated emotional expression, manifesting as inappropriate laughing and crying.
Case studies have provided further support for the posterior fossa as a critical location for the development of PLC. In 2006, a 38-year-old man with MS exhibiting new onset of pathological laughter during a relapse with no other neurologic deficits showed a single new plaque in the pontine base on repeated magnetic resonance imaging (MRI).12 A postmortem case study in 2007 of an 80-year-old man with PLC revealed significant cerebellum atrophy with a flattened pons, whereas the cortex, basal ganglia, and midbrain showed only age-associated atrophy.12
In the first complete MRI study of people with MS and PLC, Ghaffar et al2 found that people with MS and PLC had an overall significantly greater hyperintense lesion volume localized to five distinct regions compared with controls: bilateral inferior parietal, bilateral medial inferior frontal, and the brainstem. Ghaffar et al's work suggested a major role for brainstem lesions after work by Poeck and Pilleri13 confirming that cortical lesions alone were insufficient to cause PLC. We hypothesized that posterior lesion load would be more strongly associated with PLC in MS. Our preliminary analysis assessed the relationship between lesions in the posterior fossa and PLC in patients with MS, focusing on lesion load, using quantitative automated MRI analyses.
Methods
This study was granted approval by the Western University Health Sciences Research Ethics Board (HSREB). Due to the retrospective nature of the study, as well as the lack of any involvement of the patient (chart review only), the HSREB granted a waiver of written informed consent.
Sample Selection
Medical records from the MS Clinic in London, Ontario, Canada, between January 1, 2012, and December 31, 2016, were reviewed for potential cases. The inclusion criteria were completion of the Center for Neurologic Study–Lability Scale (CNS-LS)14 and having had an MRI for clinical reasons within 6 months of completing the CNS-LS. The CNS-LS is a standard part of the cognitive battery in people with MS at our center. In addition to the two criteria noted previously herein, patients were included if they 1) were determined to have definite MS of any type15; 2) had no other neurologic diagnosis associated with PLC, such as stroke or dementia; 3) had not received corticosteroid treatment in the previous 6 weeks or relapsed in the previous 3 months; and 4) had completed the Hospital Anxiety and Depression Scale (HADS), a sensitive and specific measure of major depression and general anxiety validated in MS. The HADS was included because of the common comorbidity of depression and PLC.16 The HADS depression subscale (HADS-D) has been shown to have sensitivity and specificity of 90% and 87.3%, respectively, in the MS population.
The CNS-LS is a validated measure of PLC in people with MS. It is a seven-item self-report measure examining symptoms of PLC during the previous week. Sample items include “I find that even when I try to control my laughter, I am often unable to do so” and “I find that I am easily overcome by laughter.” Participants respond on a scale from 1 (applies never) to 5 (applies most of the time), for a maximum score of 35 and a minimum score of 7. A score of 17 or greater has shown sensitivity and specificity for the presence of PLC in people with MS.17
MRI Analysis
For MRI data, only brain scans obtained on the same 1.5-T clinical service MRI used for standard clinical care at the London MS Clinic were included. These clinical scans were completed as per full MS MRI protocol, with a slice thickness of 5 mm. T2-weighted axial images were examined and corroborated with fluid-attenuated inversion recovery images when in doubt about lesion presence. These data were collected by two authors (J.A.L. and S.M.) and reviewed/confirmed by a neuroradiologist with expertise in MS (M.S.) using picture archiving and communication system (Centricity; GE Healthcare, Milwaukee, WI) and free DICOM viewer (MicroDICOM) software. Both brainstem and cerebellar lesions were examined—specifically, the total number of posterior fossa lesions with an axial diameter of 2 mm or greater, as well as total lesions in the brainstem and cerebellum separately (Figure 1). The previously mentioned measures were selected based on previous MRI lesion analysis studies and the 2010 McDonald diagnostic criteria, which by convention uses 1.5-T scanner T2-weighted images to detect lesions in noncortical regions of the brain.2,18,19 These measures have been shown to have strong intrarater and interrater reliability.18
Figure 1.
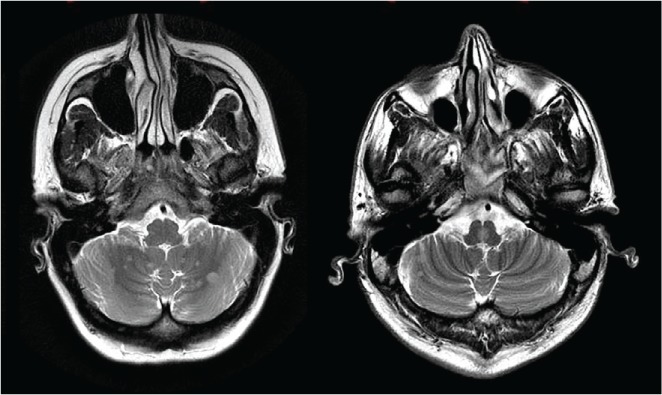
T2-weighted axial images demonstrate multiple hyperintense lesions in posterior fossa, seen in cerebellar hemispheres and in middle brainstem
Demographic Information
Other data collected included sex, age, time since MS diagnosis, education level, subtype of MS, history of depressive symptoms, medications at the time of CNS-LS administration, and Expanded Disability Status Scale score.
Statistical Analysis
Descriptive statistics were used to describe the characteristics of people with MS who were included in the study. Pearson correlations (r) and Student t tests were used, as appropriate, to identify potential covariates or confounders in relation to PLC scores. A P = .100 was used as the criterion for significant differences when examining confounding factors. A χ2 analysis was used to compare the number of posterior fossa lesions, as well as the brainstem and cerebellum separately, in people with MS who demonstrated PLC compared with those who did not on the CNS-LS. Next, the same analysis was performed when the sample was restricted to people with MS without depressive symptoms (HADS-D score <8) owing to the confounding effect of depression on CNS-LS scores. In the first analysis, depression was not controlled for. However, because depression can be comorbid with PLC, a planned secondary analysis was conducted controlling for depression. Due to the exploratory nature of this study, statistical significance was not adjusted for multiple comparisons, and a P ≤ .05 was used.
Because there is scant literature regarding the association of posterior fossa lesions and PLC, a sample size calculation could not be performed for this study. We elected to complete a retrospective medical record review of available patients between January 1, 2012, and December 31, 2016, and assess all potential cases that met the inclusion criteria.
Results
Of the medical records reviewed, 80 potential cases were identified of patients who had undergone MRI and completed a CNS-LS questionnaire within 6 months of each other. Three cases were excluded: two due to a diagnosis of clinically isolated syndrome and one due to lack of a HADS-D score. Demographic information is given in Table 1. The median HADS-D score was 4.0 (range, 0–17). There were 57 participants with a HADS-D score less than 8 (normal), 19 with a HADS-D score of 8 or greater (depressed), and 1 with an incomplete HADS-D questionnaire.
Table 1.
Demographic characteristics of study sample (N = 77)
| Characteristic | Value |
|---|---|
| Age, y | |
| Mean ± SD | 39.3 ± 11.0 |
| Range | 18.0–65.0 |
| Female sex | 51 (66.2) |
| Education, y | |
| Mean ± SD | 14.1 ± 2.3 |
| Range | 9.0–22.0 |
| MS course | |
| Relapsing remitting | 66 (85.7) |
| Secondary progressive | 7 (9.1) |
| Primary progressive | 4 (5.2) |
| EDSS scorea | |
| Median | 2.0 |
| Range | 0.0–6.5 |
| Mild (<3.5) | 58 (75.3) |
| Moderate (3.5–5.5) | 11 (14.3) |
| Severe (≥6.0) | 7 (9.1) |
| Disease duration, y | |
| Mean ± SD | 5.3 ± 7.3 |
| Range | 0.5–34.0 |
| Disease-modifying therapy | |
| Yes | 37 (48.1) |
Note: Unless otherwise indicated, values are given as number(percentage).
Abbreviations: EDSS, Expanded Disability Status Scale; MS, multiple sclerosis.
aNo EDSS score for one patient.
The mean (± SD) CNS-LS score of the entire cohort was 13.9 ± 5.0, with 22 patients (28.6%) meeting the criteria for confirmed PLC (CNS-LS score ≥17). A significant difference in CNS-LS scores was found for men versus women (12.0 vs 14.9, P = .014), relapsing versus progressive courses of MS (14.4 vs 11.2, P = .046), and level of education (Pearson r = −0.207, n = 77, P = .071). These significant differences indicated possible confounders.
On MRI, 42 patients (54.5%) had lesions in the posterior fossa, ranging from 0 to 28 (brainstem, 0–16; cerebellum, 0–12). Of the 42 participants with lesions, 13 met the defined criteria for PLC (CNS-LS score ≥17) (Table 2). The presence, or absence, of posterior fossa lesions was not predictive of PLC (χ211,77 = 0.25, P = .612). An analysis comparing the number of lesions in people with MS without PLC (CNS-LS score ≤16, n = 55) and those with PLC (CNS-LS score ≥17, n = 22) was conducted both uncontrolled and controlling for the possible confounders listed previously herein (Table 3). The number of lesions was not significantly different in people with MS without versus with PLC (χ211,77 = 12.903, P = .300). This finding did not change when controlling for potential confounders.
Table 2.
Presence of posterior fossa lesions by PLC score
| Posterior fossa lesionsa | Participants, No. | ||
|---|---|---|---|
| No PLC (CNS-LS score ≤16) | PLC (CNS-LS score ≥17) | Total | |
| No | 26 | 9 | 35 |
| Yes | 29 | 13 | 42 |
| Total | 55 | 22 | 77 |
Abbreviations: CNS-LS, Center for Neurologic Study–Liability Scale; PLC, pathological laughing and crying.
aPosterior fossa refers to both brainstem and cerebellum.
Table 3.
Distribution of posterior fossa lesions with PLC scores without removal of HADS-D–positive participants
| Posterior fossa lesions,a No. | Participants, No. | ||
|---|---|---|---|
| No PLC (CNS-LS score ≤16) | PLC (CNS-LS score ≥17) | Total | |
| 0 | 26 | 9 | 35 |
| 1 | 8 | 4 | 12 |
| 2 | 1 | 4 | 5 |
| 3 | 3 | 0 | 3 |
| 4 | 5 | 1 | 6 |
| 5 | 5 | 2 | 7 |
| 6 | 2 | 0 | 2 |
| 7 | 2 | 1 | 3 |
| 8 | 1 | 0 | 1 |
| 13 | 1 | 0 | 1 |
| 16 | 1 | 0 | 1 |
| 28 | 0 | 1 | 1 |
| Total | 55 | 22 | 77 |
Abbreviations: CNS-LS, Center for Neurologic Study–Liability Scale; HADS-D, Hospital Anxiety and Depression Scale depression subscale; PLC, pathological laughing and crying.
aPosterior fossa refers to both brainstem and cerebellum.
Assessment also individually compared brainstem lesions only and cerebellum lesions only with CNS-LS scores. There were 37 patients (48.1%) with brainstem lesions and 24 (31.2%) with cerebellum lesions. These were not mutually exclusive counts, and some members of the cohort overlapped between the two groups (ie, some of those with brainstem lesions also had cerebellum lesions, for a total of 42 patients with lesions). No significance was detected when comparing only brainstem lesions (χ29,77 = 4.078, P = .906) or only cerebellar lesions (χ25,77 = 6.989, P = .221) with CNS-LS scores. Owing to the possible effect of depressive symptoms on the CNS-LS score, we removed patients with depressive symptoms on the HADS-D score. As noted previously herein, 19 participants had a HADS-D score of 8 or greater, indicating depression. The one individual with an incomplete HADS-D questionnaire was also removed from further analyses due to the possibility of a positive depression score. Of the 57 remaining participants, 12 (21.1%) met the criteria for PLC (Table 4). First, we examined whether the presence or absence of lesions in the posterior fossa was predictive of PLC (χ21,57 = 0.684, P = .408), and it was not. When the analysis was restricted to people with MS without evidence of depression (HADS-D score <8), there was a significant difference (χ29,57 = 17.882, P = .037) such that participants with PLC had fewer lesions in the posterior fossa than those without PLC. Expressed conversely, those without PLC as per the CNS-LS score showed significantly more lesions in the posterior fossa.
Table 4.
Distribution of posterior fossa lesions with PLC score after removal of HADS-D–positive participants
| Posterior fossa lesions,a No. | Participants, No. | ||
|---|---|---|---|
| No PLC (CNS-LS score ≤16) | PLC (CNS-LS score ≥17) | Total | |
| 0 | 21 | 4 | 25 |
| 1 | 7 | 1 | 8 |
| 2 | 0 | 4 | 4 |
| 3 | 3 | 0 | 3 |
| 4 | 4 | 1 | 5 |
| 5 | 4 | 1 | 5 |
| 6 | 2 | 0 | 2 |
| 7 | 2 | 1 | 3 |
| 8 | 1 | 0 | 1 |
| 13 | 1 | 0 | 1 |
| Total | 45 | 12 | 57 |
Abbreviations: CNS-LS, Center for Neurologic Study–Liability Scale; HADS-D, Hospital Anxiety and Depression Scale depression subscale; PLC, pathological laughing and crying.
aPosterior fossa refers to both brainstem and cerebellum.
Discussion
This analysis showed that compared with those without PLC, people with MS with PLC had fewer lesions in the posterior fossa when controlling for depressive symptoms. Compared with previous work implicating brainstem and cerebellum lesions in PLC,2,12,13,20,21 the present results are not in line with the predicted hypothesis; however, they provide insight and further our understanding of the underpinnings of PLC. The present results suggest that lesion localization alone, specifically, in the posterior fossa, is insufficient to predict the development of PLC in people with MS. It is possible that lesions in the pons, or another brainstem localization, would more specifically be related to PLC; however, the present sample size did not allow for this more specific analysis. Future directions could consider shifting the focus from anatomical localization to encompass a more global lens involving facets such as functional connectivity and neurotransmission.
Pioneering work by Wilson on the anatomical localization of the “laughing and crying center” of the brain pointed toward the posterior fossa as a target for lesions inducing dysregulated affect.11 Further analysis throughout the 1950s corroborated Wilson's original hypothesis with postmortem findings of internal capsule, pontine, and brainstem lesions in patients exhibiting PLC.22
The present study was undertaken as a follow-up to the initial MRI study by Ghaffar et al2 and as a preliminary analysis focusing on posterior fossa lesions as a whole. We focused primarily on lesion presence and number of lesions, versus the exact location in the posterior fossa and the size of lesions. These results were counterintuitive to what was anticipated based on previous results, but they remain valuable by indicating that lesion load in the posterior fossa does not seem to be the determining, or at least not the dominant, factor in whether an individual does or does not develop PLC.
Wilson's original hypothesis posited that the pons itself may contain the laughing and crying center.11 It is possible, however, that pontine lesions are only one localization associated with the development of PLC. An alternative consideration is that atrophy, either as a primary process or due to demyelination in the cortex with connections in the pons, may affect the expression of PLC. The literature is scant on distinguishing effects of atrophy versus lesions themselves. Most studies focus on the appearance of new lesions. Therefore, it may be of value moving forward to assess for atrophy in the pons compared with the presence of lesions. Lesion size is another factor that has not been extensively investigated in previous studies and may warrant further investigation.
It is also important to emphasize the inconsistencies between the prevalence of posterior fossa lesions with that of PLC. The prevalence of PLC in people with MS has been shown to range from 10% to 46%.3,4 Current literature reports the detection of posterior fossa lesions between 63% and 92% in people with MS.23–25 Therefore, a substantial percentage of individuals with posterior fossa lesions will not develop PLC. Rocca et al,25 whose work examined the specific distribution of lesions in the posterior fossa, showed that 90% of participants had lesions in the pons on MRI. The discrepancy in prevalence between PLC and posterior fossa lesions is in line with the results of the present study. Furthermore, it suggests that lesions in these areas alone are unlikely to be sensitive or specific for PLC. Difficulties in pinpointing a definitive anatomical localization indicate that future investigations should focus on alternative origins for dysregulated affect. One such basis may instead involve functional networks, which have not yet been examined with respect to PLC in people with MS. Another area that has had minimal work to date is neurotransmitter dysregulation.
The present study has several strengths to acknowledge. This study is the first MRI analysis using the CNS-LS questionnaire as opposed to Poeck's four criteria for PLC.26 The CNS-LS is a measure of PLC validated specifically for people with MS versus Poeck's criteria, which have been used more broadly and are more subjective in nature. The more stringent criteria and cutoff for PLC in this cohort are likely responsible for the lower number of patients being identified as having PLC despite a large percentage expressing certain symptoms of the condition.
Depression presents a complex problem in the realm of PLC. Although previous literature indicates depression as a possible confounder for the CNS-LS questionnaire and PLC results,27 it has also been found to be significantly associated with crying-predominant PLC.28 The criteria for PLC diagnosis define an expression of excessive emotion devoid of experienced emotion.3 The CNS-LS score can be affected by depressive symptoms, making the CNS-LS result falsely positive. However, those with depressive symptoms, by definition, experience the emotions related to decreased mood and, therefore, breach the accepted standards for classification of PLC. Previous work has used the Patient Health Questionnaire-9 (PHQ-9) to distinguish those with depression from PLC, or mixed presentations. Because we did not have PHQ-9 results, we did not initially control for depression due to the possibility of losing truly positive PLC participants in the analysis. After finding no significant results in the primary analysis, the secondary analysis was restricted to people with MS without evidence of depression (HADS-D score <8) to control for the confounding effects of preexisting or concurrent mood disorders. Doing so revealed an inverse relationship between lesion number and incidence of PLC.
Several limitations exist in the present study that must be acknowledged. It was a retrospective analysis and, therefore, we were unable to assess firsthand the PLC symptoms the cohort reported to gather qualitative data. Self-reported measures through questionnaires were used for both PLC and depression instead of direct interviewing; therefore, no comparison can be drawn between questionnaire results and the clinical picture. We did not have participants who completed the Patient Health Questionnaire-9 (PHQ-9), which has previously been used to distinguish depression from PLC. As a result, it was possible that even patients with a positive HADS-D score were simultaneously PLC positive; this possibility was controlled for in the secondary analysis. Other possible confounders identified on analysis included differences in CNS-LS scores between men and women, relapsing and progressive courses of MS, and level of education. Previous work has indicated that people with MS who exhibit PLC symptoms are more severely physically disabled than those who do not and are usually in the chronic progressive phase of the disease.3 Cognitive impairment, specifically in verbal fluency and verbal learning, has also been shown in people with MS with PLC.4 Both findings are associated with a poorer prognosis. In comparison, the present cohort was composed primarily of patients with a relapsing form of MS and lower Expanded Disability Status Scale scores, which may have been a confounder for the results. Only 28.6% of the cohort met the requirements for PLC using the CNS-LS questionnaire. Despite this limitation, this cohort is the largest group of patients with MS to be analyzed in an MRI study to date regarding PLC and the only study to use the CNS-LS questionnaire to determine cohorts with and without PLC. Finally, it is possible that a higher-Tesla MRI would better delineate the role of brainstem lesions in PLC in people with MS.
Overall, this study was a preliminary analysis examining the relationship between lesion load in the posterior fossa and a validated measure of PLC in people with MS. When controlling for patients with depressive symptoms, analyses showed an inverse relationship between posterior fossa lesion number and PLC symptoms, suggesting that lesion load in the posterior fossa specifically does not determine which individuals may develop PLC. Future directions could involve expanding investigations outside the realm of pure anatomical localization to include functional networks and neurotransmitter efficacy in individuals with PLC. Until anatomical lesions or functional network identification are able to better predict PLC, clinical presentation remains the gold standard for diagnosis. Developing a better understanding of PLC will help improve the effectiveness of diagnosis and treatment of patients currently experiencing this underreported condition.
PRACTICE POINTS
Pathological laughing and crying (PLC) is common in people with MS, with a prevalence of 10% to 46%. Symptoms are routinely underreported and undertreated.
For people with MS, PLC has a significant effect on quality of life. Because the anatomical correlation of PLC remains uncertain, clinicians cannot rely on the presence or quantity of posterior fossa lesions to determine who to screen for PLC or as a basis to establish an accurate diagnosis. Clinical presentation remains the gold standard, with special consideration for depression as a confounder.
Future clinical research aiming to further understand the localization of lesions associated with PLC may aid in the development of more targeted diagnostic and treatment approaches. Consideration may be expanded to include functional brain networks, which to date remain unexamined in people with MS and PLC.
Financial Disclosures
Dr. Menon has disclosed relationships with Roche, EMD Serono, Sanofi (consulting fee); and Roche (contracted research). Dr. Morrow has disclosed relationships with Biogen, EMD Serono, Novartis, Roche, and Sanofi Genzyme (consulting fee, speakers' bureau, contracted research). The other authors declare no conflicts of interest.
Funding/Support
This study was funded through a grant from the MS Workforce of the Future program of the Foundation of the Consortium of Multiple Sclerosis Centers (CMSC). The funding organization had no role in design or conduct of the study, collection or analysis of data, or preparation of the manuscript.
Prior Presentation
Aspects of this study have been presented in abstract form at the CMSC Annual Meeting; May 24–27, 2017; New Orleans, Louisiana.
References
- 1.Parvizi J, Coburn KL, Shillcutt SD, Coffey CE, Lauterbach EC, Mendez MF. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci. 2009;21:75–87. doi: 10.1176/jnp.2009.21.1.75. [DOI] [PubMed] [Google Scholar]
- 2.Ghaffar O, Chamelian L, Feinstein A. Neuroanatomy of pseudobulbar affect: a quantitative MRI study in multiple sclerosis. J Neurol. 2008;255:406–412. doi: 10.1007/s00415-008-0685-1. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein A, Feinstein K, Gray T, O'Connor P. Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Arch Neurol. 1997;54:1116–1121. doi: 10.1001/archneur.1997.00550210050012. [DOI] [PubMed] [Google Scholar]
- 4.Vidovic C, Rovazdi MC, Kraml O, Kes VB. Pseudobulbar affect in multiple sclerosis patients. Acta Clin Croat. 2015;54:159–163. [PubMed] [Google Scholar]
- 5.Parvizi J, Anderson SW, Coleman OM et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 6.Elyas AE, Bulters DO, Sparrow OC. Pathological laughter and crying in patients with pontine lesions. J Neurosurg Pediatr. 2011;8:544–547. doi: 10.3171/2011.8.PEDS11265. [DOI] [PubMed] [Google Scholar]
- 7.Arciniegas DB, Topkoff J. The neuropsychiatry of pathologic affect: an approach to evaluation and treatment. Semin Clin Neuropsychiatry. 2000;5:290–306. doi: 10.1053/scnp.2000.9554. [DOI] [PubMed] [Google Scholar]
- 8.Spencer RJ. Pathological Laughter and Crying in Multiple Sclerosis: Quality of Social Interactions [dissertation] Baltimore: University of Maryland; 2008. [Google Scholar]
- 9.Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29:775–798. doi: 10.1007/s12325-012-0043-7. [DOI] [PubMed] [Google Scholar]
- 10.Work SS, Colamonico JA, Bradley WG, Kaye RE. Pseudobulbar affect: an under-recognized and under-treated neurological disorder. Adv Ther. 2011;28:586–601. doi: 10.1007/s12325-011-0031-3. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SAK. Some problems in neurology. J Neurol Psychopathol. 1924;4:299–333. doi: 10.1136/jnnp.s1-4.16.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swamy MN, Johri S, Gorthi SP et al. Pathological laughter, multiple sclerosis, behavioural abnormality. Med J Armed Forces India. 2006;62:383–384. doi: 10.1016/S0377-1237(06)80117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poeck K, Pilleri G. Pathologisches lachen und weinen. Arch Neurol Psychiatry. 1964:92323–92370. [PubMed] [Google Scholar]
- 14.Moore SR, Gresham LS, Bromberg MB, Kasarkis EJ, Smith RA. A self report measure of affective lability. J Neurol Neurosurg Psychiatry. 1997;63:89–93. doi: 10.1136/jnnp.63.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2001;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler. 2009;15:1518–1524. doi: 10.1177/1352458509347150. [DOI] [PubMed] [Google Scholar]
- 17.Smith RA, Berg JE, Pope LE, Callahan JD, Wynn D, Thisted RA. Validation of the CNS emotional lability scale for pseudobulbar affect (pathological laughing and crying) in multiple sclerosis patients. Mult Scler. 2004;10:679–685. doi: 10.1191/1352458504ms1106oa. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein A, Roy P, Lobaugh N et al. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62:586–590. doi: 10.1212/01.wnl.0000110316.12086.0c. [DOI] [PubMed] [Google Scholar]
- 19.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocer B, Oner Y, Batur H et al. Pathological laughing as a manifestation in a clinically isolated brainstem syndrome: a case report. J Neuroimaging. 2009;19:291–294. doi: 10.1111/j.1552-6569.2008.00281.x. [DOI] [PubMed] [Google Scholar]
- 21.Takado Y, Igarashi S, Akaiwa Y, Terajima K, Tanaka K, Tsuji S. A case of multiple sclerosis with pathological laughing caused by pontine base lesions [in Japanese] Rinsho Shinkeigaku. 2002;42:519–522. [PubMed] [Google Scholar]
- 22.Ironside R. Disorders of laughter due to brain lesions. Brain. 1956;79:589–609. doi: 10.1093/brain/79.4.589. [DOI] [PubMed] [Google Scholar]
- 23.Skoric MK, Adamec I, Madaric VN, Habek M. Evaluation of brainstem involvement in multiple sclerosis. Can J Neurol Sci. 2014;41:346–349. doi: 10.1017/s0317167100017285. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima N, Fujihara K, Okita N et al. Clinical and MRI study of brainstem and cerebellar involvement in Japanese patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1999;67:153–157. doi: 10.1136/jnnp.67.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocca MA, Yousry TA, Yousry I, Iammcci G, Then Bergh F, Filippi M. Anatomic distribution of MS lesions in the posterior fossa. Proc Int Soc Magn Reson Med. 2000;8:1195. [Google Scholar]
- 26.Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clin Risk Manag. 2013;9:483–489. doi: 10.2147/TCRM.S53906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna J, Feinstein A, Morrow SA. The association of pathological laughing and crying and cognitive impairment in multiple sclerosis. J Neurol Sci. 2016;361:200–203. doi: 10.1016/j.jns.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Thakore NJ, Pioro EP. Laughter, crying, and sadness in ALS. J Neurol Neurosurg Psychiatry. 2017;88:825–831. doi: 10.1136/jnnp-2017-315622. [DOI] [PMC free article] [PubMed] [Google Scholar]


