Abstract
Background
Staging of liver fibrosis is critical in guiding the treatment of chronic hepatitis B (CHB) virus. Many efforts have been made toward the research of noninvasive techniques, mostly focusing on hepatitis B e-antigen (HBeAg)-positive [HBeAg(+)] CHB patients, whereas HBeAg(+) and HBeA-negative [HBeAg(−)] represent different stages of hepatitis B virus infection. Thus, in this study, we aimed to search for routinely available clinical noninvasive liver fibrosis markers and separately analysed the markers in HBeAg(+) and HBeAg(−) CHB patients.
Methods
Patients with CHB who were treatment naive and who underwent a liver biopsy at our hospital from 1 January 2016 to 31 April 2017 were enrolled. Liver histology was scored using the Scheuer classification system. The area under the receiver operator curve was used to determine the diagnostic accuracy.
Results
A total of 191 patients, including 104 HBeAg(+) and 87 HBeAg(−) treatment-naive CHB patients, were enrolled in this study. Serum alkaline phosphatase (ALP) levels increased gradually in all patients and separately in HBeAg(−) CHB patients, but not in HBeAg(+) CHB patients. ALP was an independent factors predicting significant fibrosis (S≥2) in all of the patients and separately in HBeAg(−) patients, with area under the receiver operator curves of 0.651 and 0.717, respectively. Further, the optimal cut-off value of ALP (>69.5 IU/l) for distinguishing HBeAg(−) CHB patients with significant fibrosis was determined (S≥2).
Conclusion
Serum ALP levels can identify significant fibrosis (S≥2) in treatment-naive HBeAg(−) CHB patients and could potentially reduce the need for liver biopsies and help to guide the clinical treatment of CHB.
Keywords: alkaline phosphatase, chronic hepatitis B, fibrosis, noninvasive techniques
Introduction
Chronic hepatitis B (CHB) virus infection is a public health problem worldwide. Repeated replication of the hepatitis B virus (HBV) and host immune response lead to hepatocyte wound healing, followed by abnormal hyperplasia of connective tissue, eventually leading to fibrosis and even cirrhosis, liver failure and hepatocellular carcinoma. Approximately one million individuals die each year of late-stage chronic HBV infection-related liver disease 1. Therefore, it is important to diagnose and stage liver fibrosis before cirrhosis develops and to perform potentially curative treatments in early-stage liver fibrosis.
Liver biopsy remains the gold standard for the evaluation of liver fibrosis stage. However, it has several disadvantages, such as its invasive nature and association with potential complications (range from mild abdominal pain to severe hemorrhage and injury to the biliary system), sampling error and its uselessness for dynamic surveillance of liver fibrosis 2,3. Consequently, considerable effort has been invested in the last decades in the search for noninvasive techniques that can replace liver biopsy in liver fibrosis assessment. Many noninvasive models, such as the aspartate transaminase-to-platelet ratio index (APRI) 4, fibrosis-4 (FIB-4) 5, Fibrotest 6 and Forn et al. 7, have been used to stage fibrosis. However, these models were developed for chronic hepatitis C and only distinguish cirrhosis from no or minimal fibrosis conditions. Further, the use of these models in the staging of the degree of liver fibrosis in patients with CHB is also controversial 8–10. In recent years, some other noninvasive indicators, such as CHI3L1 11 and Golgi protein 73 12, have been used specifically for CHB; however, they might not be available routinely and might be costly. Therefore, a robust noninvasive indicator specifically for CHB patients on the basis of routinely available clinical markers is urgently needed.
Hepatitis B e-antigen (HBeAg)-positive [HBeAg(+)] and HBeAg-negative [HBeAg(−)] patients have different stages of natural history of HBV infection, and they have different virus replication and biochemical conditions and may have different outcomes 13. Some noninvasive tests 14–16 are conventional and inexpensive, and are mainly for HBeAg(+) CHB patients, but not HBeAg(−) CHB patients. Those noninvasive parameters that are applicable for HBeAg(+) CHB might not be suitable for HBeAg(−) CHB patients. Therefore, it is important to differentiate HBeAg(+) and HBeAg(−) CHB when searching for and verifying noninvasive fibrosis markers. Thus, in the present study, we aimed to search for routinely available clinical noninvasive liver fibrosis markers and to analyse the markers in HBeAg(+) and HBeAg(−) patients separately.
Patients and methods
Patients
Patients with CHB who were treatment naive and who underwent liver biopsy at the First Affiliated Hospital, College of Medicine, Zhejiang University, from 1 January 2016 to 31 April 2017 were enrolled. Also, 337 healthy controls were enrolled in this study. All of the patients had been hepatitis B virus surface antigen (HBsAg)-positive for at least 6 months before study entry. The exclusion criteria were as follows: (a) causes of liver disease other than HBV; (b) antiviral therapy with nucleoside (acid) or interferon; (c) coinfection with hepatitis A, C or E or HIV; (d) co-existence of alcoholic liver disease, nonalcoholic fatty liver disease or autoimmune liver diseases; (e) compensated or decompensated liver cirrhosis or hepatocellular carcinoma; (f) immunosuppressive treatment; and (g) relevant patient laboratory and clinical data that were incomplete. The demographic, clinical and laboratory data were reviewed by a trained team of physicians and were entered in duplicate into a computerized system. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, which conformed to the ethical guidelines of the Helsinki Declaration. Informed consent was obtained from the enrolled patients.
Liver biopsies and the staging of fibrosis
The staging of liver fibrosis was determined by liver biopsies. The process of percutaneous liver biopsies is as follows: ultrasound was performed to select the best puncture point (usually located in the right liver; the reason may as follows: the right liver is relatively large and close to the right abdominal wall and there is no organ around the right liver. It is least likely to damage other organs), which was marked, and local anaesthesia of the skin, disinfection and liver biopsy were performed at the previously marked site using an 18 G biopsy needle. The liver biopsy specimens were fixed in 4% buffered formalin and embedded in paraffin and were then stained with haematoxylin and eosin. Liver tissue obtained by biopsy containing at least six portal tracts was used in analyses. Liver fibrosis stages (S≥2) were scored using the Scheuer classification 17: no fibrosis (S0); portal fibrosis (S1); septum formation (S2); architectural distortion (S3); and cirrhosis, probable or definite (S4). “Significant fibrosis” was defined as a Scheuer score equal to or greater than 2 (S≥2). “Insignificant fibrosis” was defined as a Scheuer fibrosis score less than or equal to 1 (S≤1).
Statistical analysis
Statistical analyses were carried out using SPSS software (version 19.0; SPSS Inc., Chicago, Illinois, USA). The results were expressed as the mean±SD, medians with interquartile ranges (p25–p75) and numbers (percentages). The means for continuous variables were compared using Student’s t-test or one-way analysis of variance for normally distributed data and the Mann–Whitney test for non-normally distributed data. The categorical variables were analysed by performing the χ2-test or Fisher’s exact test. Independently predicting indicators of fibrosis stage of CHB patients were analysed using univariate analysis and multivariate (binary) logistic regression analysis. Receiver operating characteristic (ROC) curves and areas under the receiver operating characteristic curves (AUROC) were calculated to evaluate alkaline phosphatase (ALP) for liver fibrosis stages. All P-values were based on a two-tailed test of significance (P<0.05).
Results
Patients characteristics
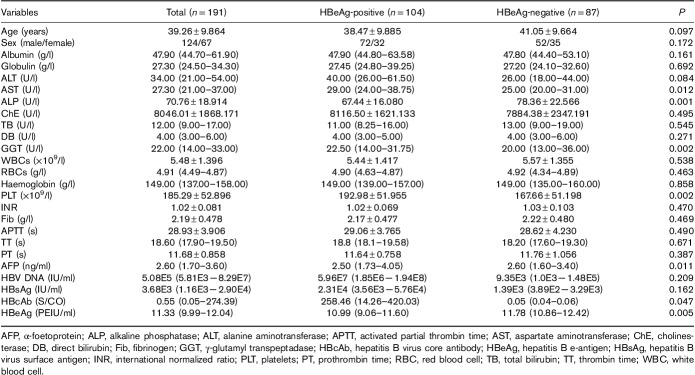
A total of 191 patients were enrolled in this study: 104 patients were HBeAg(+) and 87 were HBeAg(−). The characteristics of the patients are presented in Table 1. There were some differences between HBeAg(+) and HBeAg(−) patients. The levels of aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), platelets (PLTs), HBsAg and HBeAg were higher in HBeAg(+) patients than in HBeAg(−) patients (P<0.05). ALP, α-foetoprotein (AFP) and hepatitis B virus core antibody (HBcAb) were higher in HBeAg(−) patients (P<0.05).
Table 1.
Clinical characteristics of the study population
Associations between histological fibrosis stage and clinical and laboratory data
In all 191 patients, patients with significant fibrosis had higher levels of AST, ALP, GGT, AFP and HBcAb (P<0.05) and lower levels of PLT and HBeAg than in patients with insignificant fibrosis. However, the above significant predicting indicators were then analysed by multivariate (binary) logistic regression. We found that only ALP was an independent factor for significant fibrosis in CHB patients (P=0.035) (Table 2).
Table 2.
Multiple logistic regression analysis of factors associated with significant fibrosis in chronic hepatitis B patients
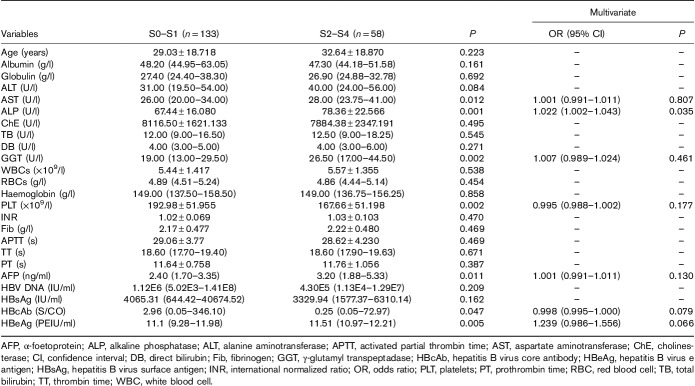
In HBeAg(−) patients, univariate analysis showed that alanine aminotransferase, ALP, GGT, activated partial thrombin time and HBsAg levels were associated with significant fibrosis (P<0.05). However, subsequent multiple logistic regression analysis indicated that only ALP was associated with significant fibrosis (P=0.007) (Table 3).
Table 3.
Multiple logistic regression analysis of factors associated with significant fibrosis in hepatitis B virus e-antigen negative chronic hepatitis B patients
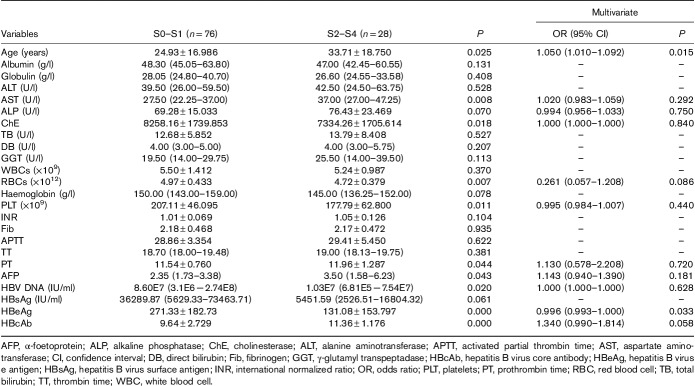
In HBeAg(+) patients, univariate analysis showed that age, AST, ALP, cholinesterase, red blood cells, PLT, prothrombin time, AFP, HBV DNA, HBeAg and HBcAb levels were associated with significant fibrosis (P<0.05). However, subsequent multiple logistic regression analysis indicated that age and HBeAg were associated with significant fibrosis (P=0.015 and 0.033) (Table 4).
Table 4.
Multiple logistic regression analysis of factors associated with significant fibrosis in hepatitis B virus e antigen positive chronic hepatitis B patients
Correlations between serum alkaline phosphatase levels and liver fibrosis stages in chronic hepatitis B patients
In healthy controls, the mean level of serum ALP was 59.97±14.533 U/l. In all of the CHB patients, the mean levels of serum ALP in different fibrosis stages were as follows: S0–S1, 67.44±16.080 U/l; S2, 78.34±21.907 U/l; and S3–S4: 78.39±24.0349 U/l. The mean levels of ALP in healthy controls were significantly lower than those for stages S0–S1, S2 and S3–S4 of the CHB patients. (P=0.000, 0.000, and 0.000). In CHB patients, with the aggravation of fibrosis stages, the level of ALP increased gradually, and the difference among the three fibrosis stages was significant (P=0.000). The mean levels of ALP in stages S0–S1 were significantly lower than those for stages S2 and S3–S4 (P=0.000 and 0.003), whereas there was no significant difference between stages S2 and S3–S4 (0.991) (Table 5 and Fig. 1).
Table 5.
Correlation between serum alkaline phosphatase levels and liver fibrosis stages in chronic hepatitis B patients
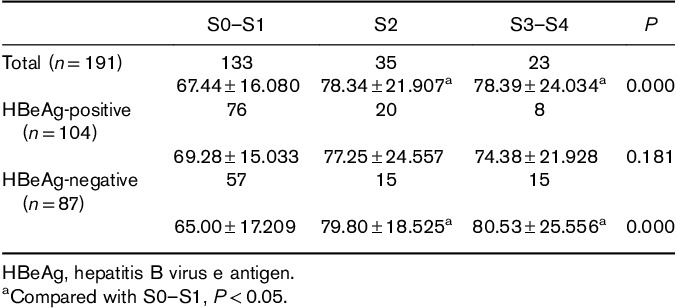
Fig. 1.
Correlation between serum alkaline phosphatase levels and liver fibrosis stages in healthy controls and chronic hepatitis B patients.
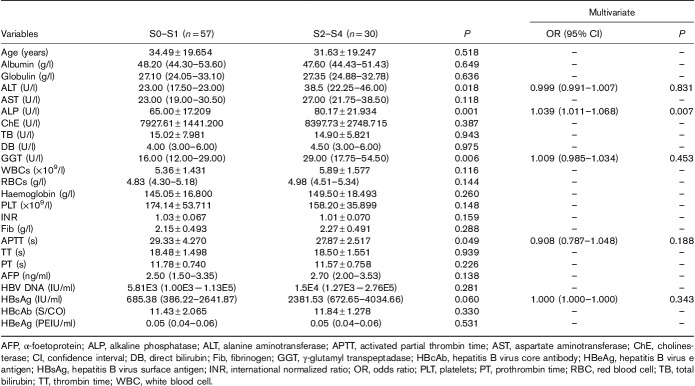
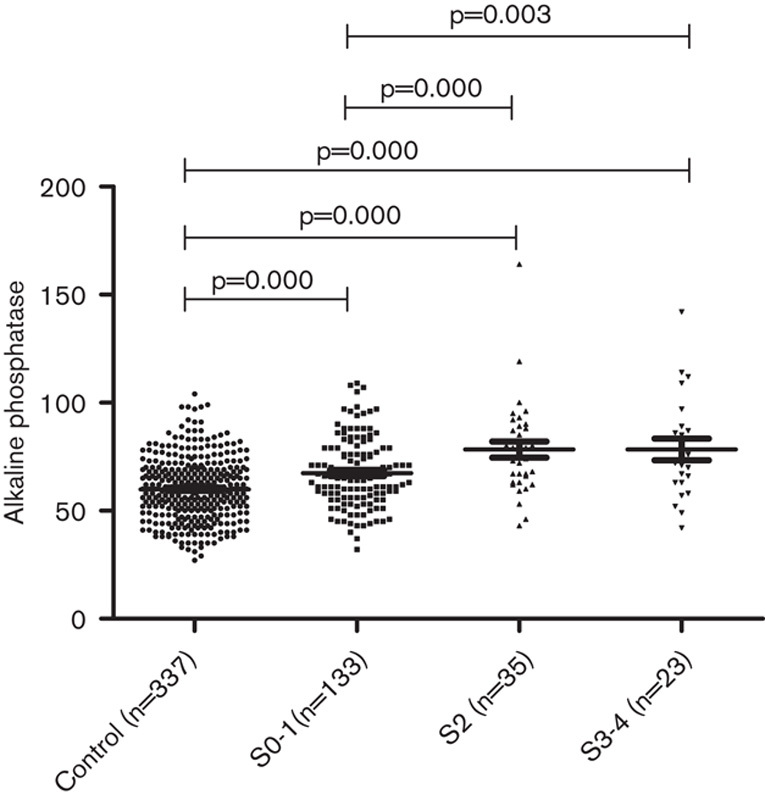
In the HBeAg(−) patients, the mean levels of serum ALP in different fibrosis stages were as follows: S0–S1, 65.00±17.209 U/l; S2, 79.80±18.525 U/l; and S3–S4, 80.53±25.556 U/l. The mean levels of ALP in healthy controls were significantly lower than those for the three stages of the HBeAg(−) CHB patients (P=0.024, 0.000, and 0.000). In the HBeAg(−) patients, with the aggravation of fibrosis stages, the level of ALP increased gradually and the difference among the three fibrosis stages was significant (P=0.000). The mean levels of ALP in stages S0-1 were significantly lower than those in stages S2 and S3–S4 (P=0.001 and 0.001), whereas there was no significant difference between stages S2 and S3–S4 (P=0.897) (Table 5 and Fig. 2).
Fig. 2.
Correlation between serum alkaline phosphatase levels and liver fibrosis stages in healthy controls and hepatitis B e-antigen negative chronic hepatitis B patients.
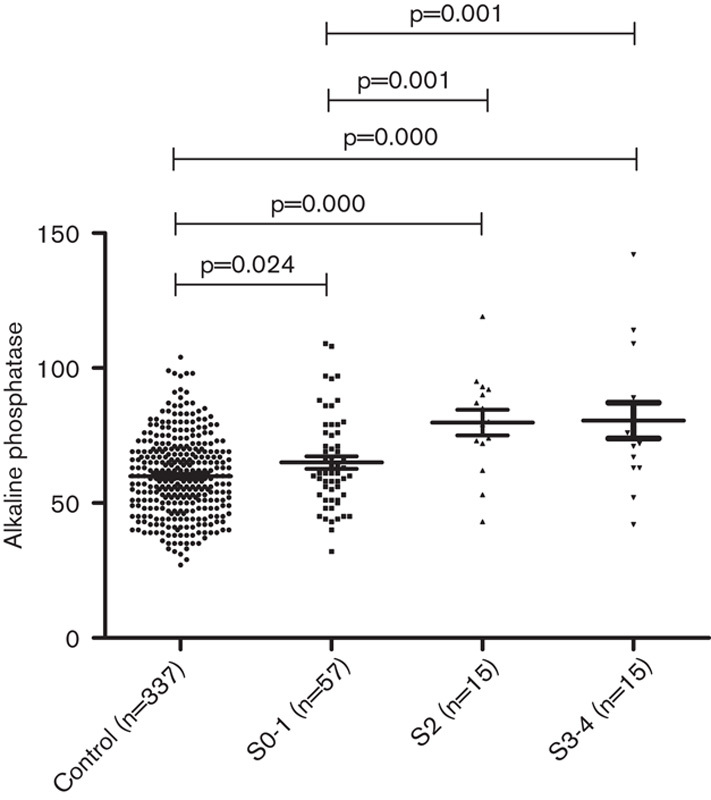
In the HBeAg(+) CHB patients, the mean levels of serum ALP in different fibrosis stages were as follows: S0–S1, 69.28±15.033 U/l; S2, 77.25±24.557 U/l; S3–S4, and 74.38±21.928 U/l. However, in the HBeAg(+) CHB patients, the difference among the three stages was not significant, and there were no differences between stages S0–S1 and S2 or S3–S4 (Table 5). In contrast, the mean levels of ALP in healthy controls were significantly lower than those for the three stages of the HBeAg(+) CHB patients (P=0.000, 0.000, and 0.009).
Area under the receiver operator curves of serum alkaline phosphatase levels associated with significant fibrosis
The ROC curves of serum ALP levels for predicting significant fibrosis in all of the CHB patients are shown in Fig. 3. The AUROC was 0.651, and the 95% confidence interval ranged from 0.566 to 0.736. The ROC curves of serum ALP levels for predicting significant fibrosis in HBeAg(−) CHB patients are shown in Fig. 4. The AUROC was 0.717 and the 95% confidence interval ranged from 0.601 to 0.833. Further, the optimal cut-off was 69.5I U/l, with a sensitivity of 73.3% and a specificity of 66.7%. The AUROCs of APRI and FIB-4 were 0.580 and 0.500 in HBeAg(−) CHB patients. The AUROCs of APRI and FIB-4 were 0.601 and 0.658 in all of the CHB patients.
Fig. 3.
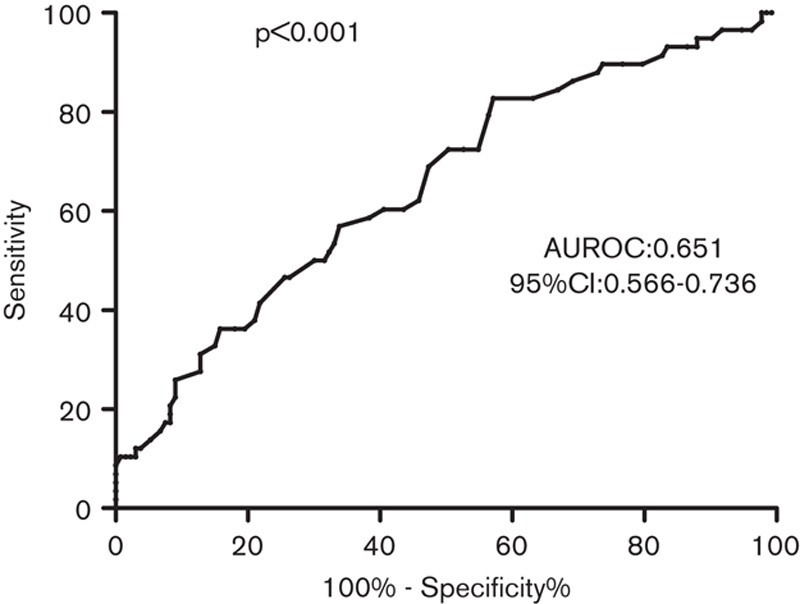
Receiver operating characteristic curves of alkaline phosphatase levels used to distinguish significant fibrosis in chronic hepatitis B patients. AUROC, areas under the receiver operating characteristic curves; CI, confidence interval.
Fig. 4.
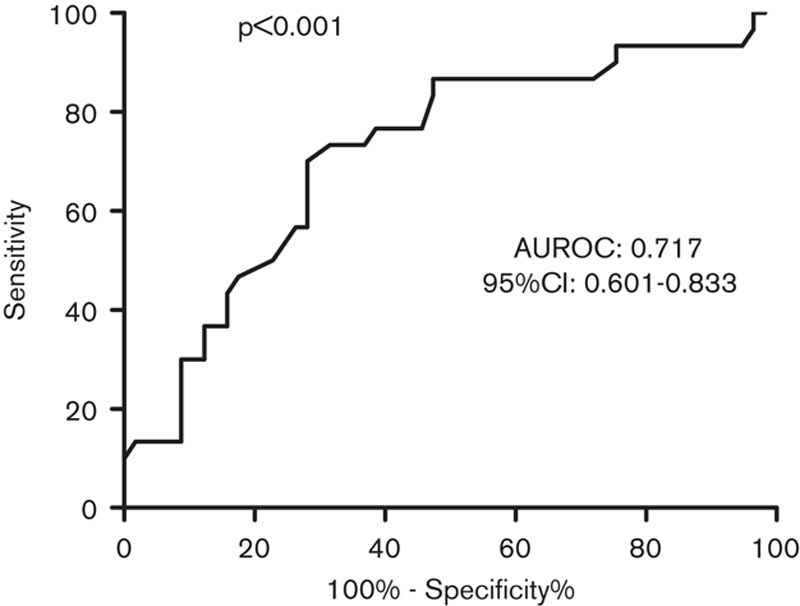
Receiver operating characteristic curves of alkaline phosphatase levels used to distinguish significant fibrosis in hepatitis B e-antigen negative chronic hepatitis B patients. AUROC, areas under the receiver operating characteristic curves; CI, confidence interval.
Discussion
According to CHB guidelines 13,18,19, patients should be considered for antivirus therapy when they have moderate to severe fibrosis (S≥2). Therefore, it is important to stage significant (S≥2) and insignificant (S≤1) fibrosis in CHB patients. Liver biopsy is the gold standard for staging fibrosis. However, its limitations prevent its wide application. Thus, there is an urgent need for research on noninvasive procedures or tests to stage liver fibrosis.
HBeAg(+) and HBeAg(−) stages represent different stages of HBV infection, with different virological, serological and biochemical levels 13. In the HBeAg(+) phase, an inactive stage presents mild liver injury with no or mild fibrosis, whereas the active stage presents significant liver injury with progressive fibrosis. In the HBeAg(−) stage, the inactive stage shows repaired liver injury with regressive fibrosis, whereas the active stage involves significant liver re-injury with progressive re-fibrosis 20. In this study, we found that HBeAg(+) patients had higher HBsAg, HBeAg, AST and GGT levels, whereas HBeAg(−) patients had higher HBcAb, ALP and AFP levels. Therefore, it is important to differentiate HBeAg(+) and HBeAg(−) CHB when searching for and verifying noninvasive fibrosis markers.
Here, we analysed the relationship between clinical and laboratory data and histological fibrosis in CHB patients. We found that AST, ALP, GGT, AFP, HBcAb, PLT and HBeAg were related to significant fibrosis. Surprisingly, only ALP was an independent factor for significant fibrosis by multiple logistic regression analysis in CHB patients. Then, we differentiated the HBeAg(+) and HBeAg(−) patients. ALP was also the only independent factor for significant fibrosis in HBeAg(−) patients. In HBeAg(+) patients, ALP was one of the factors for significant fibrosis in univariate analysis; however, subsequent multiple logistic regression analysis indicated that age and HBeAg were associated with significant fibrosis, similar to previous studies 5,21,22. Age was an important predictor because the progression of fibrosis is time dependent in CHB. In HBeAg(+) patients, significant fibrosis patients had lower HBeAg levels. The specific mechanism is unknown. However, this might occur because, from high levels of HBeAg to low levels of HBeAg, immune clearance is improved, injury and healing are more obvious, and the fibrosis is more aggravated.
ALP is a group of enzymes that catalyse the hydrolysis of monoesters of phosphoric acid, mainly distributed in the microvilli of the sinusoidal side of the liver cells and the capillary bile duct side. ALP is released into the intestine through bile. A large amount of ALP can be induced when the bile is released obstructively and the pressure of the bile capillaries is high. Then, ALP enters the blood through the lymphatic and hepatic sinuses, resulting in an increase in serum ALP.
In this study, we found that, the levels of ALP in healthy controls were significantly lower than those for the CHB patients. Also, with the aggravation of liver fibrosis, ALP increased gradually in all of the patients and separately in HBeAg(−) CHB patients, but not in HBeAg(+) CHB patients. Compared with insignificant fibrosis patients, significant (S2–S4) and advanced (S3, S4) fibrosis patients had higher ALP levels. Also, compared with APRI or FIB-4, serum ALP levels was better to indicating significant fibrosis. ALP was an independent factor for significant fibrosis in all of the patients and HBeAg(−) CHB patients. The AUROC value of serum ALP levels for significant fibrosis in all of the patients was 0.651, but it was more suitable for HBeAg(−) patients, with an AUROC value of 0.717. ALP is generally used for the diagnosis of cholestasis. It is generally believed that ALP is not sensitive to hepatocyte damage and fibrosis. However, in this study, we found that ALP can also predict fibrosis, which was also reported in schistosomiasis liver disease by Chen ZP 23 and in chronic hepatitis C by Ahmad et al. 24.
The present study showed that serum ALP levels could play an important role in predicting significant fibrosis in CHB patients, especially in HBeAg(−) patients. The interpretation could be as follows: hepatic fibrosis is a wound repair response characterized by the accumulation of extracellular matrix after liver injury. If the damage is acute or self-limiting, the change is transient and the liver structure returns to its normal composition. However, if the damage persists, chronic inflammation and accumulation of extracellular matrix will persist, leading to progressive replacement of the liver parenchyma by scar tissue – a process that can lead to significant or advanced fibrosis, even cirrhosis, and result in adverse consequences and high mortality 1. Liver injury because of CHB is usually the result of the host’s anti-HBV immune activation, which is chronic and persistent, resulting in hepatocyte injury, and accumulation of extracellular matrix, and proliferation of fibrous connective tissue. With the aggravation of fibrosis, hepatocyte destruction will worsen gradually, the pressure of the bile capillaries will also increase further and entry of ALP into the blood will increase further, resulting in an increase in serum ALP.
This work has a number of limitations. First, the study included only 191 cases and 87 HBeAg(−) cases. Second, this was a retrospective study. Third, the study lacked known serum fibrosis markers, including hyaluronic acid, type III procollagen, laminin and type IV collagen, and because of incomplete data, we could not compare these data with ALP. We will address these drawbacks in our future studies.
Conclusion
This study suggests that serum ALP levels were higher in treatment-naive CHB patients with significant fibrosis (S≥2) than in patients with no or mild fibrosis (S<2), especially in HBeAg(−) patients. Further, the optimal cut-off value of ALP (>69.5 IU/l) for distinguishing HBeAg(−) CHB patients with significant fibrosis was determined (S≥2). The use of this predictive score for serum ALP could potentially reduce the need for liver biopsies and help to guide the clinical management of treatment-naive HBeAg(−) CHB patients.
Acknowledgements
This study was supported by the national S&T major project (2017ZX10202202-003-004).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Jianhua Hu, Xiaoli Zhang, and Jueqing Gu contributed equally to the writing of this article.
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med 1997; 337:1733–1745. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest 2013; 123:1887–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. and American Association for the Study of Liver D. Liver biopsy. Hepatology 2009; 49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 4.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 5.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 6.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357:1069–1075. [DOI] [PubMed] [Google Scholar]
- 7.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002; 36:986–992. [DOI] [PubMed] [Google Scholar]
- 8.Wong GL, Wong VW, Choi PC, Chan AW, Chan HL. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2010; 31:1095–1103. [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010; 30:546–553. [DOI] [PubMed] [Google Scholar]
- 10.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012; 142:1293–1302.e4. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, Wu T, Mao J, Fang Y, Zhang J, Wu L, et al. CHI3L1 is a liver-enriched, noninvasive biomarker that can be used to stage and diagnose substantial hepatic fibrosis. OMICS 2015; 19:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Z, Li Z, Wang H, Liu Y, Xu Y, Mo R, et al. Algorithm of Golgi protein 73 and liver stiffness accurately diagnoses significant fibrosis in chronic HBV infection. Liver Int 2017; 37:1612–1621. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver. Electronic address eee and European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:370–398. [DOI] [PubMed] [Google Scholar]
- 14.Cheng PN, Tsai HW, Chiu YC, Ho CH, Wu IC, Chang TT. Clinical significance of serum HBsAg levels and association with liver histology in HBeAg positive chronic hepatitis B. J Clin Virol 2013; 57:323–330. [DOI] [PubMed] [Google Scholar]
- 15.Marcellin P, Martinot-Peignoux M, Asselah T, Batrla R, Messinger D, Rothe V, et al. Serum Levels of Hepatitis B Surface Antigen Predict Severity of Fibrosis in Patients With E Antigen-Positive Chronic Hepatitis B. Clin Gastroenterol Hepatol 2015; 13:1532–1539.e1. [DOI] [PubMed] [Google Scholar]
- 16.Hou FQ, Song LW, Yuan Q, Fang LL, Ge SX, Zhang J, et al. Quantitative hepatitis B core antibody level is a new predictor for treatment response in HBeAg-positive chronic hepatitis B patients receiving peginterferon. Theranostics 2015; 5:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clin Liver Dis 2002; 6:335–347. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018; 67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZQ, Lu W, Wang YB, Weng QC, Zhang ZY, Yang ZQ, et al. Measurement of the hepatitis B core-related antigen is valuable for predicting the pathological status of liver tissues in chronic hepatitis B patients. J Virol Methods 2016; 235:92–98. [DOI] [PubMed] [Google Scholar]
- 21.Zeng DW, Liu YR, Dong J, Zhu YY, Li YB, Chen J, et al. Serum HBsAg and HBeAg levels are associated with liver pathological stages in the immune clearance phase of hepatitis B virus chronic infection. Mol Med Rep 2015; 11:3465–3472. [DOI] [PubMed] [Google Scholar]
- 22.Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, et al. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int 2007; 27:969–976. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZP. Relationship among serum ALP marks of Child–Pugh in patients with cirrhosis. J Clin Med Pract 2005; 14:344–345. [Google Scholar]
- 24.Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, et al. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI). BMC Gastroenterol 2011; 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]