Abstract
Although N-acetylaspartate (NAA) has long been recognized as the most abundant amino acid in neurons by far, its primary role has remained a mystery. Based on its unique tertiary structure, we explored the potential of NAA to modulate aggregation of amyloid-beta (Aβ) peptide 1–42 via multiple corroborating aggregation assays along with electron microscopy. Thioflavin-T fluorescence assay demonstrated that at physiological concentrations, NAA substantially inhibited the initiation of Aβ fibril formation. In addition, NAA added after 25 minutes of Aβ aggregation was shown to break up preformed fibrils. Electron microscopy analysis confirmed the absence of mature fibrils following NAA treatment. Furthermore, fluorescence correlation spectroscopy and dynamic light scattering measurements confirmed significant reductions in Aβ fibril hydrodynamic radius following treatment with NAA. These results suggest that physiological levels of NAA could play an important role in controlling Aβ aggregation in vivo where they are both found in the same neuronal compartments.
Keywords: N-acetylaspartate, amyloid beta, aggregation inhibitor, Thioflavin-T, fluorescence correlation spectroscopy, dynamic light scattering, transmission electron microscopy
1. Introduction
Amongst amino acids in the brain, NAA is second only to glutamate in total concentration (Tsai and Coyle, 1995). However, while high concentrations of glutamate are found in all compartments of the brain, NAA is predominantly located in axons and the soma, where it is by far the most abundant amino acid, at 12–15 mM (Benedetti et al., 2007; Glodzik et al., 2015). Although the high concentration of glutamate can easily be attributed to its major dual roles as a building block for protein synthesis and as the most abundant excitatory neurotransmitter (Watkins, 2000), surprisingly, the primary function of NAA has not been identified. Indeed, roles for NAA characterized thus far appear to represent general repurposing of the abundant amino acid rather than pointing to its essential cellular function. For example, once synthesized from acetate and aspartate in axons, NAA can be transferred to oligodendrocytes, where it is cleaved to release the acetate group to serve as a building block for myelin production (Chakraborty et al., 2001). However, it is unclear why such a complicated scheme would be specifically employed for building myelin since acetate is a common and easily produced end product in all cells. As such, this pathway may simply reflect a local recycling of NAA and its products to accommodate the high production and turnover in axons, which is essential to maintain a precise NAA concentration. In addition, while NAA has also been shown to act as a neuronal osmolyte helping with fluid balance (Taylor et al., 1994), similarly, it remains unclear why neurons specifically use NAA rather than many other more common osmolytes (Law, 1994).
Proton magnetic resonance spectroscopy (MRS) of the brain shows that the very large peak for NAA dominates the water-suppressed spectra, due primarily to its high concentration and unique structure. Since NAA is predominantly found in neuronal compartments and its relative concentration is highly stable under normal physiological conditions, its MRS signature has been established over decades as a reliable biomarker of neuronal integrity (Luyten and den Hollander, 1986; Rigotti et al., 2007). In neurodegenerative disorders and traumatic brain injury (TBI), regional decreases in NAA concentration are commonly found, particularly in the white matter (Brown et al., 2018; Cheng et al., 2002; Kantarci et al., 2007; Klunk et al., 1992; Vagnozzi et al., 2010; Wild et al., 1999). This is thought to represent either metabolic stress and dysfunction of mitochondria, particularly in axons where NAA is synthesized, or the decrease may signal a relative tissue loss of neuronal structures (Brooks et al., 2001; Cheng et al., 2002).
Based on its unique structure and high concentration in the brain, we hypothesize that NAA plays an important role in preventing or reversing aberrant aggregation of proteins and peptides, such as Aβ. If true, an imbalance of NAA might serve as a tipping point leading to unchecked aggregation of Aβ and the progressive formation of β-sheet rich oligomers, fibrils and plaques. TBI may be a prime example of this, where a decrease in NAA concentration in the white matter is accompanied by the massive genesis of Aβ peptides that accumulate in damaged axons (Cecil et al., 1998; Chen et al., 2004; Johnson et al., 2010; Johnson et al., 2012; Smith et al., 2003; Uryu et al., 2007). Release of this Aβ reservoir leads to the formation of diffuse plaques throughout the brain, remarkably within hours of injury, even in young individuals (Chen et al., 2004; Johnson et al., 2009; Roberts et al., 1991; Roberts et al., 1994). However, the capacity of normal or reduced levels of NAA to modulate this process has not been investigated.
Here we explored the potential role of NAA to inhibit or reverse Aβ aggregation using biochemical and electron microscopy analyses. Specifically, we examined the temporal effects of various biologically relevant concentrations of NAA alongside known inhibitors on Aβ aggregation, Morin and Phenol Red, in a Thioflavin-T (ThT) Aβ fibril formation assay. Results thus obtained were further verified by other complimentary methods, including electron microscopy, fluorescence correlation spectroscopy (FCS) and dynamic light scattering (DLS).
2. Materials and Methods
2.1. Thioflavin-T Amyloid-beta fibril formation assay
2.1.1. Amyloid Beta fibril formation
Thioflavin-T (ThT) (10 μL, 2 mM - Anaspec, Fremont CA, USA) was added to a well of a 96 well black non-binding plate (Corning Costar, Corning NY, USA). Either buffer (5 μL), or inhibitor (5 μL) was added to the ThT. One milliliter of buffer (50 mM Tris, 150 mM NaCl, pH = 7.2) was added to 0.25 mg of Amyloid beta 1–42 (Aβ42) (Anaspec, Fremont CA, USA). The solution was mixed by inversion, centrifuged at 10,000 rpm for 5 mins at 4°C to remove any precipitates. Aβ42 solution (85 μL) was then added to the well. The plate was placed in a Tecan Infinite M1000 plate reader (Tecan, Morrisville, NC, USA). Fluorescence was monitored at Ex/Em = 440/484 nm every 5 mins at 37°C with 15 secs shaking between readings.
2.1.2. Inhibitors
Known inhibitors Morin (Porat et al., 2006) and Phenol Red (Nerelius et al., 2009) (5 μL, 2 mM - Anaspec, Fremont CA, USA) were used as positive inhibitor controls and were added to Aβ42. Various concentrations of NAA (Sigma-Aldrich, St Louis MO USA) were tested (5 μL, final concentrations: 1 μM, 10 μM, 100 μM, 1 mM, 10 mM, 15 mM, 100 mM).
2.1.3. Thioflavin-T analysis
Background fluorescence readings from ThT controls (averaged from triplicate wells) were subtracted from the readings of all wells. Duplicate wells for each condition were completed for each separate experiment. Each fluorescence value was normalized to the maximum fluorescence obtained when only Aβ42 was present and expressed in percentage.
2.1.4. Transmission Electron Microscopy (TEM)
Undiluted samples (3 μL) were taken from the different conditions and time points, placed on carbon grids and negatively stained using a 2% (w/v) uranyl acetate solution. The grids were imaged using a Jeol-1010 transmission electron microscope (75,000 – 120,000 X).
TEM images were analyzed using ImageJ software (NIH, USA). Aβ42 monomers and oligomers were evenly distributed in a monolayer across the field of view, allowing for the length of Aβ42 monomers/oligomers to be measured from one end to the other, and the width to be measured perpendicular to the length. The length of fibrils were traced from one end to the other, and if the fibril went off the field of view, this was denoted as >90nm since the total length was unknown. Three to four images were taken per experiment with 450 to 700 measurements per condition.
2.2. Particle Size Distribution Analysis
2.2.1. Peptide Synthesis and Labeling
Human Aβ42 was produced for size distribution experiments via solid phase peptide synthesis on a Liberty Blue microwave-assisted synthesizer (CEM Corporation, Matthews, NC) using a standard Fmoc-protection strategy. Before cleavage from resin, a portion was treated with 5(6)-tetramethylrhodamine (TAMRA), leading to a fluorescently labeled Aβ42 peptide with TAMRA appended at the N-terminus (Bilgicer and Kumar, 2004). Labeled and unlabeled peptides were cleaved from the resin using trifluoroacetic acid and purified via reverse-phase high-pressure liquid chromatography (HPLC). Before fibril formation, peptides were monomerized by dissolving in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) at 1 mg/ml, drying under a nitrogen stream, and placing under vacuum for 1 hour. Peptide films were reconstituted in buffer at 50 μM to begin fibril formation.
2.2.2. Fluorescence Correlation Spectroscopy
Aβ42 fibrils were prepared at a 1:10,000 molar ratio of TAMRA-labeled to unlabeled peptide and incubated at 37°C for 18 hours in buffer (50 mM Tris, pH = 7.2) with periodic agitation.
The microscope used for the FCS measurements has been described in detail elsewhere (Chowdhury et al., 2007). Briefly, the excitation light source at 514 nm (~150 μW) was obtained from an argon ion laser (Spectra-Physics, Mountain View, CA) focused into a microscope objective (Nikon, 60×, NA 1.2, oil immersion), with the emission collected through the same objective and separated by a dichroic mirror. A 100 μm pinhole was used to select the confocal volume. Data collection was accomplished using avalanche photodiode detectors (Perkin-Elmer, Vaudreuil, Canada) and a fast correlator card (National Instruments, Austin, TX) which performed the autocorrelation of the intensity signal. The confocal volume was calibrated using R6G (Molecular Probes), a dye with a known diffusion constant. FCS traces were collected for 10 mins for each of the following conditions: Aβ42 before incubation, Aβ42 incubated for 18 hours, and Aβ42 incubated for 18 hours and then incubated with 15 mM NAA an additional 1 hour. The resultant autocorrelation curves were fit with a 3D diffusion model using a maximum entropy method (MEM) (Sengupta et al., 2003), using the computer program MEMFCS version 1.0f written by Osman Bilsel (UMass Medical School).
Diffusing particles of identical size produces a FCS curve that is characterized by a single diffusion time (τD) which is determined by the following equation:
where r0 is determined from the R6G calibration and is approximately 0.3 ± 0.02 μm for our setup. On the other hand, samples containing diffusing particles of different sizes will give rise to a FCS curve that requires multiple or a distribution of τD values to describe, which can be done by the MEM analysis.
If the diffusing species are assumed to be spherical, the hydrodynamic radius (Rh) can be calculated from the diffusion coefficient by the Stokes-Einstein relationship (Chowdhury et al., 2007):
where kB is the Boltzmann constant, T is the absolute temperature, and η is the viscosity of the solvent. It is important to note that the reported hydrodynamic radii are not the actual fibril and oligomer dimensions as the actual morphologies of the various species may not conform to a spherical model. However, the distributions are still representative of the relative amounts of fibrils and oligomers under the given conditions.
2.2.3. Dynamic Light Scattering
The analysis of size distribution in polydisperse systems such as Aβ aggregates has been accomplished using DLS experiments (Bitan et al., 2003). Samples of synthetic Aβ42 (WT, without TAMRA label) were prepared at 15 mM in cold buffer (50mM Tris, 150mM NaCl, pH = 7.2), sonicated for 5 min and centrifuged at 10,000 rpm for 5 mins at 4°C to remove any precipitates. 50 μL were removed from the top of the centrifuge tube and placed in a quartz cuvette and measurements were taken in a DyanaPro Nanostar (Wyatt Technology) with the cuvette heater set at 37°C. The cuvette was gently agitated every 10 mins. After 100 mins, 15 mM NAA was added. For all measurements, the collection software reports a fitting size distribution of the underlying diffusing particles according to their percentage of the mass composition. This fit accounts for the larger scattering signal of the larger aggregates. Specifically, the diffusion coefficient (D) is related to scattering decay rate (Γ) and the magnitude of the scattering vector (q) by the following equation (Chu, 1991):
Furthermore, the scattering vector magnitude is dependent on the scattering angle (θ) by the following equation:
where n0 is the solvent index of refraction (1.33) and λ0 is the wavelength of the incident laser (658 nm). Larger particles will have a larger scattering vector magnitude. Upon finding a diffusion coefficient for the components via a least squares fitting, the hydrodynamic radius (Rh) is calculated via the Stokes-Einstein relationship in the same manner as FCS.
3. Results
3.1. Fluorescence & morphology studies:
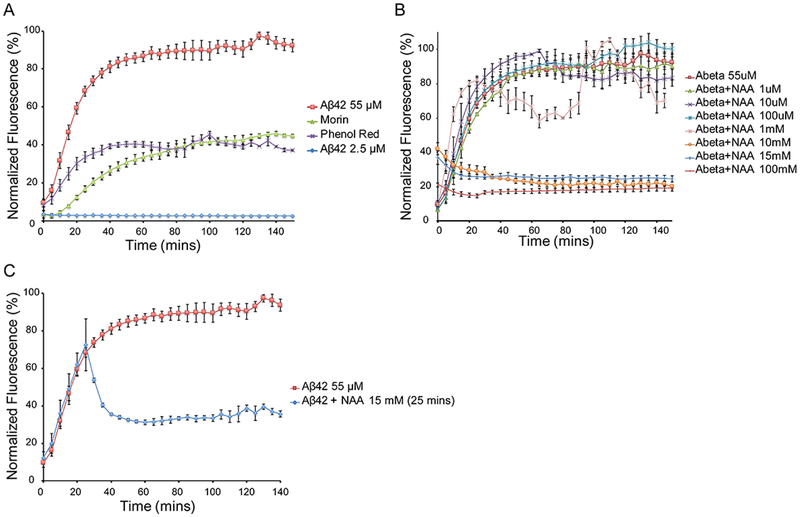
In order to assess the ability of NAA to inhibit Aβ42 aggregation, we used ThT to detect β-sheet formation (Naiki et al., 1989). When ThT binds to β-sheets, a strong fluorescence signal is emitted allowing for the kinetics of β-sheet formation to be monitored over time (LeVine, 1999). Human Aβ42 was solubilized in buffer, incubated at 37°C, agitated every 5 mins and the ThT fluorescence monitored. Untreated Aβ42 showed a characteristic increase in β-sheet formation with a very short lag phase followed by a growth phase which plateaus around 60 mins (Figure 1A). This short lag phase was likely due to the presence of preformed aggregates that were not sufficiently removed during centrifugation. Known β-sheet inhibitors Phenol Red and Morin showed characteristic inhibition of the growth phase reaching maximums of 37% and 45% respectively.
Figure 1. Monitoring Aβ42 aggregation using ThT at 37°C with agitation every 5 mins.
A) Aggregation of Aβ42 either untreated or treated with known inhibitors Morin and Phenol Red. B) Aggregation of Aβ42 following addition of NAA at concentrations from 1 μM to 100 mM added at time 0. C) Aggregation of Aβ42 with 15 mM NAA added at 25 mins.
NAA samples at various concentrations were added to Aβ42 at time 0 in order to assess the inhibitory effects of aggregation / β-sheet formation (Figure 1B, Supporting Figure 1 and 5). NAA concentrations below 10 mM had no effect on β-sheet formation, however concentrations of 10mM and above showed significant inhibition. Final ThT fluorescence levels were 20%, 25% and 19% for 10 mM, 15 mM and 100 mM respectively. NAA alone had no effect on ThT fluorescence (Supporting Figure 1). A NAA concentration of 15mM was chosen for all subsequent experiments based on numerous studies showing NAA concentrations above 10mM (Baslow, 2003; Glodzik et al., 2015; Grams et al., 2011; Inglese et al., 2008).
In order to assess if NAA has any effect on preformed β-sheets, 15mM NAA was added in at 25 mins (Figure 1C). This time point reflected approximately 75% of maximum ThT fluorescence. ThT fluorescence intensity significantly decreased following addition of NAA, reaching a final ThT fluorescence of 35%.
Samples of Aβ42 were analyzed using TEM in order to assess morphology of fibril formation with and without NAA addition (Figure 2). TEM images of untreated Aβ42 samples at time 0 showed small oligomeric sized aggregates (Figure 2A). Samples taken from untreated Aβ42 following 150 mins of incubation and agitation showed two distinct populations of aggregates, with one consisting of long mature fibrils that tend to stick together and another with shorter individual fibrils. The longer mature fibrils appear to have some helical twist as shown in Figure 2B (red arrows). Samples with NAA added initially and sampled at 150 mins showed predominantly small aggregates with some short protofibrils (Figure 2C). Samples with NAA added at time 25 mins showed predominantly small aggregates (Figure 2D).
Figure 2. Representative TEM images of Aβ42 untreated or treated with NAA.
A) Untreated Aβ42 at time 0, B) untreated Aβ42 at 150 mins, C) Aβ42 treated with NAA added at time 0, and D) Aβ42 treated with NAA added at 25 mins. Scale bars: 100 nm (Ai, Bi, Bii, Ci, Di), 50nm (Aii, Biii, Cii, Dii).
The TEM images were analyzed for aggregate/protofibril/fibril width and length (Figure 3). Untreated Aβ42 at 150 mins show an increase in width with a significant proportion of fibrils longer than 95 nm (lengths of >1000 nm was observed) (Figure 3B). When NAA was added initially and sampled at 150mins, aggregate width is reduced but the length of the reduced protofibrils is longer when comparing to that obtained at 0 mins (Figure 3C).
Figure 3. Analysis of Aβ42 aggregate / fibril width and length measurements from TEM images with or without 15 mM NAA.
Aβ42 widths (A, C, E) and lengths (B, D, F) at time 0 (A, B), 150 mins (C, D) and 150 mins following addition of NAA (E, F).
Since NAA is a relatively strong acid, we examined if the effects observed may be due to pH. We therefore either adjusted an untreated Aβ42 solution down to the pH of Aβ42 + NAA or adjusted the pH of Aβ42 + NAA up to 7.4 (Supporting Figure 2). Adjusting the pH of Aβ42 + NAA up to 7.4 still results in inhibition of ThT fluorescence. Adjusting the pH of Aβ42 down still resulted in β-sheet formation but the process was delayed compared to that of the unadjusted pH. The effects of two different concentrations of glutamate chosen based on published glutamate concentrations, had no effect on ThT fluorescence (Pouwels and Frahm, 1998; Srinivasan et al., 2005).
3.2. FCS & DLS studies
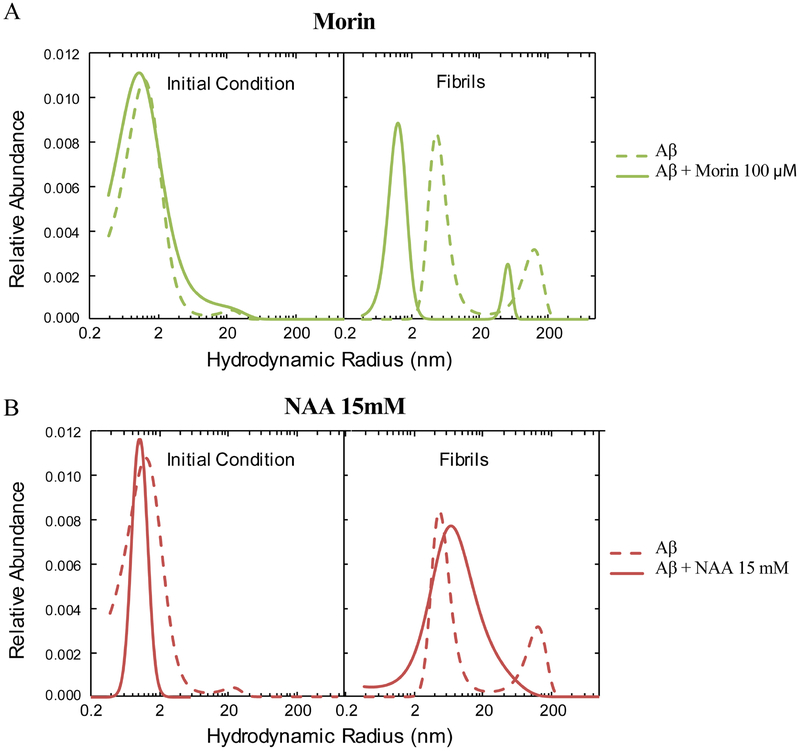
A fluorescence probe, 5(6)-tetramethylrhodamine (TAMRA), was introduced at the N-terminus of Aβ42 (hereafter referred to as Aβ42-TAMRA) for FCS studies in order to further investigate and confirm NAA’s disruption effect toward amyloid β-sheet formation. This methodology allows for the analysis of particle size distribution based on the time each labeled particle takes to diffuse through a small confocal volume. ThT fluorescence measurements confirmed that Aβ42-TAMRA peptides form β-sheets (increases in ThT fluorescence - Supporting Figure 3) and fibrils (TEM imaging - Supporting Figure 4). FCS curves were analyzed by the MEM and the results are shown in Figure 4. The dashed lines represent the distributions of hydrodynamic radii (Rh) of the Aβ42-TAMRA samples at the outset of the experiment (Initial Condition) and after 18 hours of incubation at 37°C with periodic agitation. The initial condition trace exhibits a single peak centered at 1.2 nm, while the fibril sample contains two peaks centered at 5.0 nm and 124 nm. In separate experiments, both the initial-condition and fibril samples were treated with Morin (100 μM) (Figure 4A) or NAA (15 mM) (Figure 4B), resulting in markedly different size distributions. Treatment of the initial-condition samples with Morin or NAA resulted in single peaks centered at 1.2 nm and 1.1 nm, respectively. Furthermore, the distribution upon addition of Morin to fibrils after 18 hours incubation exhibits two peaks centered at 1.2 nm and 53 nm. Most notable is the addition of NAA to pre-aggregated samples resulting in a single, broad peak with maximum abundance at an Rh value (7.2 nm) between those of the two distinct populations in the untreated fibril sample.
Figure 4. FCS distribution plots of hydrodynamic radii of Aβ42 from MEM fitting.
Aβ42 was treated with 100 μM Morin (A) or 15 mM NAA (B).
Particle size distributions obtained from DLS measurements are presented in Figure 5. Unlike the FCS experiments, these measurements were obtained with unlabeled Aβ42 samples, and the resultant signal comes from the intensity of scattered light from particles of various sizes. Due to the larger relative intensity of signals arising from oligomer and fibril components, the results show a portion of large species even in the initial condition, with peaks centered at 0.42 nm and 39.3 nm (Figure 5A). After incubation and periodic agitation, two distributions are observed (14.3 nm and 178 nm), representing larger aggregates and fibrils (Figure 5B). Finally, after addition of 15 mM NAA, the distribution closely resembles that observed at the initial condition; two peaks are centered at 0.70 nm and 83.8 nm, with the majority of the contribution from small monomeric and oligomeric Aβ42 species (Figure 5C).
Figure 5. Aβ42 particle size distributions measured using DLS.
Aβ42 particle size was measured initially (A), after 100 mins incubation (B), and after treatment with 15 mM NAA (C).
4. Discussion
We show a new and potentially significant biological function of neuronal NAA, as a surprisingly effective agent for inhibiting and even reversing Aβ42 aggregation. Using several different but complementary techniques, we consistently found that at physiological concentrations, NAA impairs Aβ42 aggregation and reduces fibril formation. These findings may have important implications for TBI and neurodegenerative disorders, where persistent decreases in NAA concentrations are commonly found to coincide with progressive Aβ pathogenesis.
The function of amyloid precursor protein (APP) has also been somewhat of a mystery, but is believed to play a role in synapse formation and neural plasticity. Found in many brain cells, it is most concentrated in synapses and axons (Findeis, 2007). APP undergoes numerous post-translational modifications, including what is considered pathological cleavage by γ- and β-secretases resulting in release of Aβ fragments 1–40 and 1–42, found in both acute and long-term TBI and in Alzheimer’s disease (Gentleman et al., 1997; Murphy and LeVine, 2010; Roberts et al., 1991; Victor et al., 2014). These peptide fragments readily self-assemble into aggregates, with Aβ42 showing more rapid aggregation behavior (Jarrett et al., 1993) and greater toxicity (Pike et al., 1991). Aβ aggregation progresses from monomeric to oligomeric species onto protofibril and mature fibrils (Verma et al., 2015). These Aβ species may go on to form extracellular plaques (Dickson, 1997), or interfere with synaptic transmission and memory function (Takahashi et al., 2002). Toxicity may include changes in ion homeostasis (Lin et al., 2001) and neuron death (Paradis et al., 1996)
A number of strategies to reduce aggregation burden have been attempted over the years, none of which have proved to be clinically successful. These include immunotherapy through immunization against Aβ42 (Nicoll et al., 2006), reducing Aβ production through beta secretase inhibitors (Kennedy et al., 2016), and small molecules (Cohen et al., 2006; Necula et al., 2007a; Podlisny et al., 1998; Salomon et al., 1996; Tomiyama et al., 1996; Yang et al., 2005), which have been shown to either inhibit oligomerization or fibrillation or both, implying that they follow distinct pathways (Necula et al., 2007b). Despite these failures, there remains great interest stopping or reversing progression of Aβ pathologies, which are thought to precede and potentially promote additional neurodegenerative changes, such as tau pathologies (Stancu et al., 2014).
Here, we examined the potential of physiological levels of NAA to modify Aβ aggregation kinetics and progression to fibril formation using several well-established techniques in the study of amyloid formation, i.e. ThT fluorescence, EM, DLS and FCS. The benzothiol dye, ThT has been used for decades to visualize and clinically diagnose amyloid fibrils, with PiB-PET based on its derivative (Klunk et al., 2001). ThT fluorescence increases significantly following binding to β-sheets, which NMR has shown to start from the protofibril stage (Zhang et al., 2009), and has been used to both stain tissue and monitor real time self-assembly in vitro (LeVine, 1993; LeVine, 1997). While ThT renders a qualitative assessment of β-sheet formation, both FCS and DLS allow for a more quantitative determination of the size distributions of various aggregated species in solution (Bitan et al., 2003; Mittag et al., 2014). EM analysis allows for direct observation of the physical morphology and characteristics of protofibrils and fibrils.
During in vitro self-assembly kinetic experiments, ThT fluorescence increase typically follows a sigmoidal shape with an initial lag phase followed by a period of rapid growth and finally reaching a plateau. We observed a very short if any, lag phase in our results likely due to the presence of preformed aggregates (that we were unable to sufficiently remove using centrifugation), which rapidly elongate. This fibril formation was confirmed by long fibrils observed in EM images and the appearance of slowly diffusing species in the FCS and DLS measurements. The FCS results correspond qualitatively to those observed using DLS.
We show that adding NAA with concentrations 10mM and above at the onset of the experiment significantly inhibits β-sheet formation as measured using ThT fluorescence. This inhibition of aggregation was not complete since the final ThT fluorescence was above the baseline starting value. Evidence of this reduced β-sheet formation can also be observed in EM images where the majority of Aβ1–42 are either shorter protofibrils or in small oligomeric forms.
In order to investigate whether NAA could either inhibit further fibril formation or breakdown already preformed protofibrils / fibrils, we allowed the aggregation to proceed for 25 mins before adding NAA. We observed that the intensity of ThT fluorescence exhibits a significant decrease soon after NAA addition and subsequently reaches a plateau that is slightly above the level observed when NAA was added in the beginning of the aggregation process. This suggests that NAA may be either breaking up already preformed fibrils, stabilizing protofibrils thereby inhibiting elongation, or both. This is evident from the EM images where we observe mainly short protofibrils, i.e. no mature fibrils. Similarly, there is a substantial shift of the FCS and DLS peaks towards faster timescales following addition of NAA, showing significant decreases in aggregate size. In particular, FCS shows complete elimination of the second large diameter peak following NAA addition in comparison to Morin inhibition which shifts both peaks to smaller Rh values.
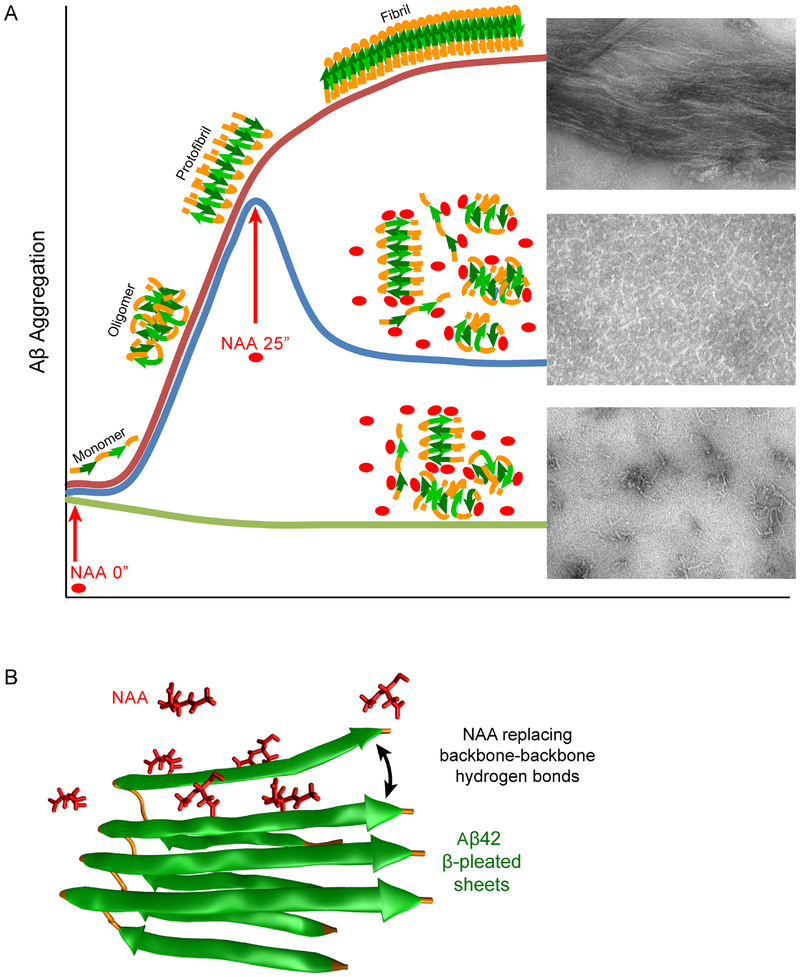
While the current experiments cannot determine a molecular mechanism for the observed inhibition of Aβ aggregation by NAA, we propose a potential interaction scheme (Figure 6). NAA may stabilize small oligomeric and fibril species and preventing further organization into elongated fibrils. This is supported by elevated initial ThT signal and residual fluorescence at the end of experiments involving NAA; this hypothesis is also supported by the EM, DLS, and FCS results. Because NAA has three carbonyl groups, it is reasonable to consider that it could form several hydrogen bonds with peptide backbones, replacing backbone-backbone hydrogen bonds normally favoring the formation of β-sheets and leading to the elongation phase of fibrillation.
Figure 6. Schematic of proposed NAA action on Aβ42 aggregation.
A) Graphical representation of Aβ42 aggregation / fibril formation either untreated or treated with NAA at time 0 or 25 mins. B) Proposed mechanism of NAA interaction with Aβ42, with several NAA replacing backbone-backbone hydrogen bonds. Green ribbons represent Aβ42 β-pleated sheets; several NAA molecule are shown in red ball-and-stick models. Adapted from PDB: 2BEG.
These results may have implications for Aβ plaque formation in vivo. In pathological circumstances such as severe TBI, a 20% reduction in NAA is consistently observed in the white matter, measured with proton magnetic resonance spectroscopy (Cecil et al., 1998; Marino et al., 2007; Signoretti et al., 2008; Smith et al., 1998). Notably, in the same population of patients, there is a rapid development of Aβ plaques, identified post-mortem and in surgically excised tissue (Abu Hamdeh et al., 2017; Ikonomovic et al., 2004; Roberts et al., 1994; Smith et al., 2003). NAA has also been shown to decline as Alzheimer’s disease progresses (Adalsteinsson et al., 2000), with NAA levels correlating with both the severity of disease as assessed post-mortem (Kantarci et al., 2008) and higher Aβ burden, demonstrated with positron emission tomography studies (Nedelska et al., 2017).
Alternatively, NAA may facilitate proteolysis of Aβ. Endogenous Aβ degrading enzymes, such as neprilysin (Saido and Leissring, 2012), reduce Aβ peptide concentrations in neuronal compartments and can also be used by microglia to digest extracellular Aβ plaques (Lee and Landreth, 2010). However, neprilysin is most effective in degrading the least aggregated Aβ species, where the cleavage sites are most readily accessed (Kanemitsu et al., 2003; Morelli et al., 2003). Therefore, by inhibiting or reversing Aβ aggregation, NAA may work synergistically with neprilysin towards more rapid Aβ proteolysis.
The current results support initiation of a new line of investigation to elucidate potential mechanisms of NAA interactions with Aβ in the in vivo environment, where these two molecules are naturally found in the same neuronal compartments. In addition to the potential implications of endogenous NAA modulating amyloid pathogenesis and disease progression, identification of these processes in vivo could reveal novel therapeutic opportunities.
Supplementary Material
Highlights:
Physiological levels of NAA prevent and reverse Aβ42 β-sheet formation in vitrio
Electron microscopy confirmed the absence of mature fibrils with NAA treatment
Assays confirmed reductions in Aβ42 fibril radius following NAA treatment
These findings may reveal a new role for NAA in vivo in controlling Aβ aggregation
Acknowledgements
This work was supported by National Institutes of Health grants NS092398 (D.H.S), NS038104 (D.H.S), P41-GM104605 (F.G) and the PA Consortium on Traumatic Brain Injury 4100077083 (D.H.S). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- Aβ
Amyloid-beta
- NAA
N-acetylaspartate
- MRS
magnetic resonance spectroscopy
- ThT
Thioflavin-T
- FCS
fluorescence correlation spectroscopy
- DLS
dynamic light scattering
- MEM
maximum entropy model
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no competing financial interests.
References
- Abu Hamdeh S, et al. , 2017. Rapid amyloid-beta oligomer and protofibril accumulation in traumatic brain injury. Brain Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adalsteinsson E, et al. , 2000. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet. 355, 1696–7. [DOI] [PubMed] [Google Scholar]
- Baslow MH, 2003. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 28, 941–53. [DOI] [PubMed] [Google Scholar]
- Benedetti B, et al. , 2007. Reproducibility of the whole-brain N-acetylaspartate level across institutions, MR scanners, and field strengths. AJNR Am J Neuroradiol. 28, 72–5. [PMC free article] [PubMed] [Google Scholar]
- Bilgicer B, Kumar K, 2004. De novo design of defined helical bundles in membrane environments. Proc Natl Acad Sci U S A. 101, 15324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G, et al. , 2003. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 100, 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks WM, et al. , 2001. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 16, 149–64. [DOI] [PubMed] [Google Scholar]
- Brown M, et al. , 2018. Magnetic resonance spectroscopy abnormalities in traumatic brain injury: A meta-analysis. J Neuroradiol. 45, 123–129. [DOI] [PubMed] [Google Scholar]
- Cecil KM, et al. , 1998. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg. 88, 795–801. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, et al. , 2001. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 78, 736–45. [DOI] [PubMed] [Google Scholar]
- Chen XH, et al. , 2004. Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol. 165, 357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LL, et al. , 2002. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging. 20, 527–33. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, et al. , 2007. Fluorescence correlation spectroscopic study of serpin depolymerization by computationally designed peptides. J Mol Biol. 369, 462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, 1991. Laser Light Scattering Basic Principles and Practice. Academic Press. [Google Scholar]
- Cohen T, et al. , 2006. Inhibition of amyloid fibril formation and cytotoxicity by hydroxyindole derivatives. Biochemistry. 45, 4727–35. [DOI] [PubMed] [Google Scholar]
- Dickson DW, 1997. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 56, 321–39. [DOI] [PubMed] [Google Scholar]
- Findeis MA, 2007. The role of amyloid beta peptide 42 in Alzheimer’s disease. Pharmacol Ther. 116, 266–86. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, et al. , 1997. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport. 8, 1519–22. [DOI] [PubMed] [Google Scholar]
- Glodzik L, et al. , 2015. Global N-acetylaspartate in normal subjects, mild cognitive impairment and Alzheimer’s disease patients. J Alzheimers Dis. 43, 939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams AE, et al. , 2011. Cerebral Magnetic Resonance Spectroscopy at 7 Tesla: Standard Values and Regional Differences. Academic Radiology. 18, 584–587. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, et al. , 2004. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 190, 192–203. [DOI] [PubMed] [Google Scholar]
- Inglese M, et al. , 2008. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage. 41, 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett JT, et al. , 1993. The C-terminus of the beta protein is critical in amyloidogenesis. Ann N Y Acad Sci. 695, 144–8. [DOI] [PubMed] [Google Scholar]
- Johnson VE, et al. , 2009. A neprilysin polymorphism and amyloid-beta plaques after traumatic brain injury. J Neurotrauma. 26, 1197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, et al. , 2010. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 11, 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, et al. , 2012. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu H, et al. , 2003. Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci Lett. 350, 113–6. [DOI] [PubMed] [Google Scholar]
- Kantarci K, et al. , 2008. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 248, 210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, et al. , 2007. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 28, 1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy ME, et al. , 2016. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS beta-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 8, 363ra150. [DOI] [PubMed] [Google Scholar]
- Klunk WE, et al. , 1992. N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 42, 1578–85. [DOI] [PubMed] [Google Scholar]
- Klunk WE, et al. , 2001. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 69, 1471–84. [DOI] [PubMed] [Google Scholar]
- Law RO, 1994. Regulation of mammalian brain cell volume. J Exp Zool. 268, 90–6. [DOI] [PubMed] [Google Scholar]
- Lee CY, Landreth GE, 2010. The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna). 117, 949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H 3rd, 1993. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2, 404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine H 3rd, 1997. Stopped-flow kinetics reveal multiple phases of thioflavin T binding to Alzheimer beta (1–40) amyloid fibrils. Arch Biochem Biophys. 342, 306–16. [DOI] [PubMed] [Google Scholar]
- LeVine H 3rd, 1999. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 309, 274–84. [DOI] [PubMed] [Google Scholar]
- Lin H, et al. , 2001. Amyloid beta protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB J. 15, 2433–44. [DOI] [PubMed] [Google Scholar]
- Luyten PR, den Hollander JA, 1986. Observation of metabolites in the human brain by MR spectroscopy. Radiology. 161, 795–8. [DOI] [PubMed] [Google Scholar]
- Marino S, et al. , 2007. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 78, 501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag JJ, et al. , 2014. Simultaneous measurement of a range of particle sizes during Abeta1–42 fibrillogenesis quantified using fluorescence correlation spectroscopy. Biochem Biophys Res Commun. 448, 195–9. [DOI] [PubMed] [Google Scholar]
- Morelli L, et al. , 2003. Differential degradation of amyloid beta genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J Biol Chem. 278, 23221–6. [DOI] [PubMed] [Google Scholar]
- Murphy MP, LeVine H 3rd, 2010. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 19, 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H, et al. , 1989. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 177, 244–9. [DOI] [PubMed] [Google Scholar]
- Necula M, et al. , 2007a. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry. 46, 8850–60. [DOI] [PubMed] [Google Scholar]
- Necula M, et al. , 2007b. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 282, 10311–24. [DOI] [PubMed] [Google Scholar]
- Nedelska Z, et al. , 2017. 1H-MRS metabolites and rate of beta-amyloid accumulation on serial PET in clinically normal adults. Neurology. 89, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerelius C, et al. , 2009. Alpha-helix targeting reduces amyloid-beta peptide toxicity. Proc Natl Acad Sci U S A. 106, 9191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, et al. , 2006. Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol. 65, 1040–8. [DOI] [PubMed] [Google Scholar]
- Paradis E, et al. , 1996. Amyloid beta peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons. J Neurosci. 16, 7533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, et al. , 1991. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563, 311–4. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, et al. , 1998. Oligomerization of endogenous and synthetic amyloid beta-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 37, 3602–11. [DOI] [PubMed] [Google Scholar]
- Porat Y, et al. , 2006. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 67, 27–37. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J, 1998. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 39, 53–60. [DOI] [PubMed] [Google Scholar]
- Rigotti DJ, et al. , 2007. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR Am J Neuroradiol. 28, 1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts GW, et al. , 1991. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 338, 1422–3. [DOI] [PubMed] [Google Scholar]
- Roberts GW, et al. , 1994. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 57, 419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saido T, Leissring MA, 2012. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2, a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon AR, et al. , 1996. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry. 35, 13568–78. [DOI] [PubMed] [Google Scholar]
- Sengupta P, et al. , 2003. Measuring size distribution in highly heterogeneous systems with fluorescence correlation spectroscopy. Biophys J. 84, 1977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, et al. , 2008. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J Neurosurg. 108, 42–52. [DOI] [PubMed] [Google Scholar]
- Smith DH, et al. , 1998. Magnetic resonance spectroscopy of diffuse brain trauma in the pig. J Neurotrauma. 15, 665–74. [DOI] [PubMed] [Google Scholar]
- Smith DH, et al. , 2003. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 98, 1072–7. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, et al. , 2005. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 128, 1016–25. [DOI] [PubMed] [Google Scholar]
- Stancu IC, et al. , 2014. Models of beta-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener. 9, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, et al. , 2002. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 161, 1869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, et al. , 1994. Extracellular N-acetylaspartate in the rat brain: in vivo determination of basal levels and changes evoked by high K+. J Neurochem. 62, 2349–55. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, et al. , 1996. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem. 271, 6839–44. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT, 1995. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 46, 531–40. [DOI] [PubMed] [Google Scholar]
- Uryu K, et al. , 2007. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 208, 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, et al. , 2010. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 133, 3232–42. [DOI] [PubMed] [Google Scholar]
- Verma M, et al. , 2015. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann Indian Acad Neurol. 18, 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor MR, et al. , 2014. Adams and Victor’s Principles of Neurology. McGraw-Hill. [Google Scholar]
- Watkins JC, 2000. l-glutamate as a central neurotransmitter: looking back. Biochem Soc Trans. 28, 297–309. [PubMed] [Google Scholar]
- Wild JM, et al. , 1999. 1H spectroscopic imaging of acute head injury--evidence of diffuse axonal injury. MAGMA. 8, 109–15. [DOI] [PubMed] [Google Scholar]
- Yang F, et al. , 2005. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 280, 5892–901. [DOI] [PubMed] [Google Scholar]
- Zhang A, et al. , 2009. Structural differences between Abeta(1–40) intermediate oligomers and fibrils elucidated by proteolytic fragmentation and hydrogen/deuterium exchange. Biophys J. 96, 1091–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.