Abstract
Life starts with a zygote, which is formed by the fusion of a haploid sperm and egg. The formation of a blastomere by cleavage division (nuclear division without an increase in cell size) is the first step in embryogenesis, after the formation of the zygote. Blastomeres are responsible for reprogramming the parental genome as a new embryonic genome for generation of the pluripotent stem cells which then differentiate by Waddington’s epigenetic landscape to create a new life. Multiple authors over the past 150 years have proposed that tumors arises from development gone awry at a point within Waddington’s landscape. Recent discoveries showing that differentiated somatic cells can be reprogrammed into induced pluripotent stem cells, and that somatic cell nuclear transfer can be used to successfully clone animals, have fundamentally reshaped our understanding of tumor development and origin. Differentiated somatic cells are plastic and can be induced to dedifferentiate into pluripotent stem cells. Here, I review the evidence that suggests somatic cells may have a previously overlooked endogenous embryonic program that can be activated to dedifferentiate somatic cells into stem cells of various potencies for tumor initiation. Polyploid giant cancer cells (PGCCs) have long been observed in cancer and were thought originally to be nondividing. Contrary to this belief, recent findings show that stress-induced PGCCs divide by endoreplication, which may recapitulate the pattern of cleavage-like division in blastomeres and lead to dedifferentiation of somatic cells by a programmed process known as “the giant cell cycle”, which comprise four distinct but overlapping phases: initiation, self-renewal, termination and stability. Depending on the intensity and type of stress, different levels of dedifferentiation result in the formation of tumors of different grades of malignancy. Based on these results, I propose a unified dualistic model to demonstrate the origin of human tumors. The tenet of this model includes four points, as follows. 1. Tumors originate from a stem cell at a specific developmental hierarchy, which can be achieved by dualistic origin: dedifferentiation of the zygote formed by two haploid gametes (sexual reproduction) via the blastomere during normal development, or transformation from damaged or aged mature somatic cells via a blastomere-like embryonic program (asexual reproduction). 2. Initiation of the tumor begins with a stem cell that has uncoupled the differentiation from the proliferation program which results in stem cell maturation arrest. 3. The developmental hierarchy at which stem cells arrest determines the degree of malignancy: the more primitive the level at which stem cells arrest, the greater the likelihood of the tumor being malignant. 4. Environmental factors and intrinsic genetic or epigenetic alterations represent the risk factors or stressors that facilitate stem cell arrest and somatic cell dedifferentiation. However, they, per se, are not the driving force of tumorigenesis. Thus, the birth of a tumor can be viewed as a triad that originates from a stem cell via dedifferentiation through a blastomere or blastomere-like program, which then differentiates along Waddington’s landscape, and arrests at a developmental hierarchy. Blocking the PGCC-mediated dedifferentiation process and inducing their differentiation may represent a novel alternative approach to eliminate the tumor occurrence and therapeutic resistance.
Keywords: Tumor origin, Polyploid giant cancer cells, The giant cell cycle, Blastomere, Blastomere-like, Dedifferentiation, Differentiation, Maturation arrest
1. Introduction
“Half a century of cancer research had generated an enormous body of observations about the behavior of the disease, but there were essentially no insights into how the disease begins and progresses to its life-threatening conclusions” [1]
Dr. Robert A. Weinberg
Understanding how cancer arises and progresses to become a life-threatening disease has been a focus of intensive study by generations of investigators. However, there is no generally accepted theory of how cancer originates and progresses. In the past 50 years, cancer biologists have focused on a model in which cancer is viewed as arising from genetic and molecular alterations in somatic cells and tumors are interpreted as clusters of fast-replicating mutant cells that survive or die according to principles of via Darwinian evolution [2–4]. This simplified working model satisfies the desire, common in this era of molecular and genetic revolution, to find simple causes for complicated diseases. However, reducing the origins of cancer to a single or a few mutations or a specific cell type is insufficient to explain the tumor represents a benign or malignant organs or pluripotent stem cell-derived teratomas that have recapitulated all three germ layers of human development. The cancer research community would benefit from a new conceptual framework that can rationally interpret the huge amount of existing molecular data generated in the past 50 years as well as the different tumor types observed by pathologists over the past 150 years. It is time to rethink “cancer biology.”
In this article, I revisit the theories of cancer arising in the context of normal development, including the century-old embryonic theory of cancer and its variants that have been proposed in recent years.I then review recent discoveries regarding induced pluripotency and the formation of stress-inducd polyploidy or polyploid giant cancer cells (PGCCs) as a potential endogenous mechanism for somatic cell de-differentiation (reprogramming), which give rise to tumors. On the basis of this new knowledge, I propose a new, unified theory of human tumors, named the dualistic origin for human tumors. This theory accounts for all tumors observed in oncopathology including embryonic/germ cell tumors, organ-derived tumors, and benign and malignant tumors.
I use terms found in Kumar et al.’s Robbins and Cotran Pathologic Basis of Disease, a textbook for first- or second-year medical students [5]. As described herein, a tumor is equivalent to a neoplasia or neoplasm and is defined as “an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of the normal tissues, and persists in the same excessive manner after cessation of the stimuli which evoked the change” as stated by eminent pathologist R. A. Willis [6]. Tumors can be divided into embryonic or germ cell origin and an adult-organ origin. On the basis of histopathologic appearance and clinical behavior, tumors can be further divided into malignant and benign. Malignant tumors are equivalent to cancer and display a poor level of tissue differentiation, resembling the primitive tissue from which they are derived. Benign tumors display good differentiation. These terms will be used as described here to avoid any confusion that can arise from the use of tumor as a synonym for cancer, a practice observed in many articles in the oncology literature.
2. Normal development and induced dedifferentiation
The human life cycle, from zygote to adult organism, is characterized by phases of de-differentiation (or reprogramming) and differentiation [7,8]. During the first three to four days after fertilization, the zygote divides without growth in cell size to generate two blastomeres, further rounds of cell division result in four, eight, and then 16-cell blastomeres. The eight-cell blastomeres start to compact into a cellular mass with indistinguishable cell borders, a process termed compaction. The further division of this cell mass resembles a mulberry and is known as morula (the Latin word for mulberry), before forming a fluid-filled cyst, known as the blastocyst. During this period, the cells alternate between the S and M phases without the G1 and G2 phases, initially dividing synchronously but gradually moving to the asynchronous division associated with shortened telomeres, which leads to genomic chaos [9,10]. The cell mass then differentiates into a trophoectoderm and inner cell mass in this cyst, which is the first step toward an ultimate cell fate determination [11].
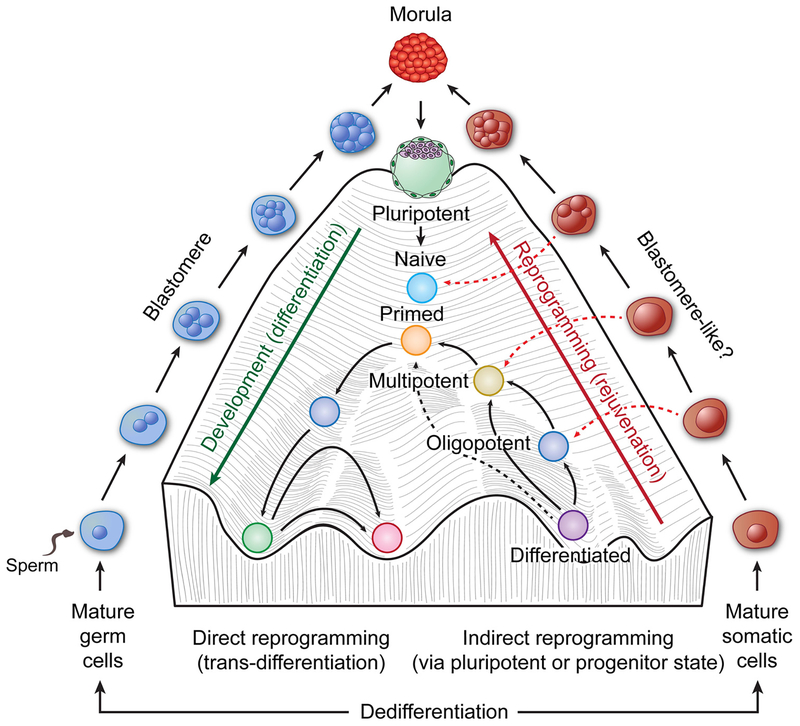
The functions of blastomeres include erasing the epigenetic memory from the parental genome in the zygote, activating the embryonic genome, and beginning the formation of a blastocyst, which will give rise to embryoblasts and trophoblast stem cells [11]. The key function of blastomeres is to dedifferentiate the zygote formed by the mature gametes to forget the parents and to generate embryoblasts for an entire new organism together with the trophoblasts that give rise to placenta that supports its growth [11]. Differentiation from embryoblasts to adult is a highly deterministic process of tissue development, in which pluripotent cells become successively more differentiated as they roll down the “canal” to their eventual fate, a process that embryologist Conrad Waddington first termed the “epigenetic landscape” in 1950 [8,12] (Fig. 1, center). A co-culture of embryonic stem cells with trophoblast stem cells is sufficient to form a blastocyst, which is at the very top of this epigenetic landscape, capable of initiating embryogenesis in mice [13].
Fig. 1.
Waddington’s landscape of development (center) in the context of blastomere-mediated dedifferentiation of gametes (left), and a hypothesized blastomere-like process of dedifferentiation of somatic cells for tumor initiation (right). Following fertilization, the zygote is dedifferentiated all the way to the top of Waddington’s hill, where the compaction/morula generates a blastocyst made of trophectoderm and inner cell mass. The inner cell mass then differentiates into naïve and then primed pluripotent, multipotent, and oligopo-tent cells, and development is finally arrested at the bottom of the “canal” for a mature organism. The mature stable cells can be reprogramed via various inducing methods. The previously unknown blastomere-like pathway for dedifferentiation to generate various stem cells for tumor initiation is shown on the right side. The diagram of Waddington’s landscape and rejuvenation is adapted from these two references [12, 207].
In 2006, it was discovered that mature somatic cells can be reprogrammed into pluripotent stem cells by four ectopically introduced transcription factors [14]. This revolutionary finding, together with the other revolutionary finding that somatic cell nuclear transfer can be used to successfully clone animals [15–17], fundamentally changed our understanding of developmental biology, regenerative medicine, and tumors. While the reprogramming in these studies was induced artificially, the data demonstrates that: (i) mature somatic cells are very plastic and can be induced into pluripotent stem cells using transcription factors or other stressors; and (ii) development can potentially be reversed (rejuvenated) in the appropriate context. These induced pluripotent stem cells can then give rise to a teratoma in vitro and in vivo, which is a tumor composed of mature somatic tissue (benign) or immature embryonic tissue (malignant) from one or all three germ layers, as well as other somatic cell-derived tumors including Wilms’ tumor, uroepithelial carcinoma, skin papilloma, and intestinal polyp [18]. Premature termination of reprogramming in vivo also leads to the development of Wilms’ tumor by the altered epigenetic landscape without genetic mutations [19]. Furthermore, it has been shown that somatic cells can be reprogrammed into pluripotent stem cells in vivo as a result of tissue damage and senescence [20]. These studies demonstrate that somatic-derived pluripotent stem cells can serve as the origin of teratoma, as well as somatic cell-derived tumors. This provides a novel source of origin not only for teratoma, as these tumors are generally believed to be derived from germ cells [21], but also a new source of origin for somatic cell-derived tumors. However, it remains unclear whether there is an intrinsic mechanism in somatic cells that can be induced in vivo to give rise to a tumor without exogenously introduced reprogramming factors.
3. Theories suggesting that cancer arises from development gone awry
3.1. Embryonic theories of cancer
Successful generation of induced pluripotent stem cells from somatic cells and the observation that these can lead to the formation of teratoma forces us to revisit the concept of cancer being embryonic origin, one of the oldest theories in pathology and cancer biology. The first two articles on the embryonic properties of cancer were published in the 19th century. Recamier (1829) [22] and Remak (1854) [23] proposed the so-called embryonic rest hypothesis of cancer origin, which was further elaborated by Durante (1874) [24] and Cohnheim (1875) [25]. Hansemann (1875) showed that cancer budding is similar to polar body extrusion in oocytes and proposed a gametogenic origin of cancer, using the word anaplasia (Latin for “grow backward”) to describe this process [26,27]. In addition, in 1855, Rudolf Virchow, the father of pathology and medicine, considered that connective tissues including fibroblasts and adipose tissue were full of germ cells that could give rise to cancer [28]. In 1902, embryologist John Beard developed a “trophoblast theory of cancers”, a particular interpretation of germ cells, which considered that as a result of germ cell derived trophoblasts, cancers become irresponsible [29]. These theories suggested that embryonic remnants in adult tissues or gamete-like cells could be activated to give rise to tumors.
In 1895, Wilms gave the first histological description of teratoma (called “embryoma” due to its resemblance to an embryo) and adopted the germ cell origin described first by Marchand in 1890. In this theory, the teratoma is considered to be the eggs that escape oogenesis [30]. Together with Bonnet, Marchand soon developed the blastomere theory for teratoma [30]. According this theory, the blastomere, i.e. the normal embryonic cells produced during cleavage, can escape from their normal developmental trajectory to form the teratoma, which provides an explanation as to why tissues from all three germ layers as well as these cell clusters resemble blastocyst. This was first description of the origin of teratoma. Askanazy [30] provided the first experimental proof of the embryonic origin of the teratoma. However, these concepts fell out of favor due to the lack of additional experimental support and also the shift in research efforts toward infectious diseases, influenced by the First and Second World Wars [31].
Not until almost half a century later, did Steven provide a series of experiments to generate teratoma by transplanting mouse embryo [32,33]. During the same period of time, Kleinsmith and Pierce showed that a single embryonic carcinoma cell was capable of multilineage differentiation [34]. Based on this work, Pierce proposed that tumors as caricatures of the process of tissue renewal [35]. Pierce et al. also proved that solid tumors, including squamous cell carcinoma, could differentiate into benign squamous cells [35–37]. On the basis of these data, Pierce and Sell [38,39] proposed that cancer is a developmental problem caused maturation arrest. Supporting this view, the malignant cells can be differentiated into normal tissue in mice if they are placed into a normal blastocyst [40,41].
One controversial issue is whether the targets are embryonic somatic cells or germinal cells formed via parthenogenesis, a natural form of asexual reproduction without fertilization that occurs in plants and some invertebrate animals. An unfertilized egg that can replicate itself to start embryogenesis can be a potential origin of a tumor, a view that was first proposed by Beutner in 1926 [42]. By grafting embryos with genetically impaired germ cells, Mintz et al. showed that malignant teratocarcinoma can arise from embryonal somatic cells [43]. These works provided the first experimental proof of the somatic origin of these totipotent cell-derived tumors, supporting the blastomere theory proposed by Bonnet and Marchand more than seven decades ago.
Another theory positing that cancer represents development gone wrong is the “tissue organization field theory,” or TOFT, which describes carcinogenesis as being comparable to organogenesis [44,45]. Similarities between gametogenesis and cancer development have recently been recognized, owing to the activation and expression in tumors of cancer/testis antigen and other genes normally expressed only in the germline and in embryonic cells [46]. Cancer has been proposed to be a “somatic cell pregnancy” [46,47].
The embryonic theory of cancer has also been put forward under other names, including oncogermination [48], parthenogenesis [49], and being very similar to small embryonic/epiblast-like stem cells [50]. At the experimental level, the premature termination of Yamanaka factor-induced reprogramming leads to tumor development in vivo [19]. Activation of a pluripotent stem cell program has been reported in bone marrow-derived cells [51,52], and germline traits have been reported in hepatoma-derived cells associated with metastasis [53]. These data demonstrate the somatic embryonic origin of human tumors, and the activation of germline traits could be as a potential result of the differentiation of embryonic somatic cells into tumors.
3.2. Cancer stem cell theory of cancer
In the past quarter century, a modified version of the embryonic theory of cancer has been intensively pursued that reduces the embryo to a specific stem cell type, termed “cancer stem cells”. Cancer stem cells have been proposed to be the major cause of tumor growth and drug resistance. The experiments that support this theory can be traced back to as early as 1960 based on the successful demonstration of the clonal nature of spleen colonies in transplanted bone marrow in mice [54]. This theory has been revitalized based on a study that showed that a subset of leukemia cells enriched by a combination of cell surface markers could successfully generate an entire tumor population in nude mice after a serial transplantation assay [55]. Similar experiments were conducted on a variety of solid tumors [56]. However, despite initial excitement, these subsequent studies on cancer stem cells showed that their tumor-initiating ability was not strictly dependent on the marker expression, and the tumor growth was also dependent on the mouse strain used [57–61]. In solid tumors, the cancer stem cells and the differentiated cells all grow together. In order to define the properties of cancer stem cell using serial transplantation assay, the cells in the tissue must be dissociated from their native microenvironment, resulting in changes in cell behavior and marker expression, which largely reflect their ability to adapt the mouse environment rather than the tumorigenic and differentiating properties of stem cells.
Genetic lineage tracing allows the identification of stem cells in solid tissue in situ without mechanical dissociation and has overcome some of the technical challenges associated with transplantation [62–64]. However, while lineage tracing is useful for identifying cells wired along defined developmental hierarchies, such as intestines and hair follicles, this technique has little value in high-grade tumors, such as melanoma or high-grade carcinomas from various organs, as the tumor cells in these tumors are very plastic and are poorly wired along a developmental hierarchy. Therefore, the cancer stem cell concept, although it generated much excitement and promise when it was first proposed, has been challenged in recent years [65–67].
3.3. Unanswered questions
Despite the long history of the embryonic theory of cancer and its variants, including the cancer stem cell theory, and the excitement about induced pluripotent stem cells and cancer stem cells, the relationship between embryogenesis and cancer stem cells remains elusive. In addition, cancer stem cells have not been conclusively linked to any specific stages of embryonic development. Given the fact that a tumor represents a disease of abnormal development, it is intuitive to assume that tumor may have simply hijacked the earliest developmental pathway for its origin. As a blastomere is at the very beginning of embryogenesis to dedifferentiate the imprinted parental genome in the zygote to create an embryonic one, it seems logical to assume that the birth of a tumor may involve a blasomere-like developmental mechanism in order to dedifferentiate somatic cells along the reverse slope of Waddington’s epigenetic landscape for tumor development (Fig. 1). However, despite the blastomere theory for teratoma being proposed by Marchard and Bonnet more than a century ago [30], it has barely been mentioned in the cancer research literature during last a half century. It was only recently, that the link between somatic-derived PGCCs and blastomeres has been made [68,109], demonstrating that blastomere-like structures and functions can also be induced from somatic cells.
4. Polyploidy in normal embryogenesis and polyploidy in tumors
Polyploidy, the presence of more than two sets of homologous chromosomes, can occur in both mononucleated and multinucleated cells. Polyploidy can be as a result of an endoreplication cell cycle without entering mitosis (endocycle), or entering mitosis with or without cytokinesis (endomitosis) failed at any stages of mitosis [69]. Polyploidy may play a key role in nonmalignant physiological and pathological processes. There are several excellent reviews on normal and pathologic functions of polyploidy, and readers are encouraged to refer to these for further information, particularly on the role of polyploidy in other normal developmental processes [70–73].
4.1. Polyploidy in normal embryogenesis
One critical topic that is not covered in previous reviews is the role of polyploidy in embryonic development. Although the blastomere stage of development is well-studied, most knowledge comes from inbreed genetically homogenous murine models [11,74]. In non-inbred genetically heterogeneous humans, the blastomere stage of development is relatively poorly understood [75], and the relevant studies are largely drawn from the field of assisted reproductive technology. Blastomere-stage embryos appear to be in a state of chaos and show marked genomic instability. In studies of fertilized eggs in vitro, approximately 60% of blastomeres grew in giant mononuclear or multi-nucleated forms [76,77]. Numerous chromosomal abnormalities (chromothripsis) were detected in normal developing human embryos [78]. The blastomerestage embryo shows increased aneuploidy, microcells, failed mitosis and cytokinesis, and endoreplication. Genetically, the interblastomere chromosome exchange may be mediated via microcells and horizontal genetic transfer [10,79]. These observations appear to be an enigma to reproductive biology on how any of us has made it this far with such a chaotic beginning [10]. Very recent findings demonstrate that there is dual-spindle formation in a zygote rather than previously believed single spindle in early mammalian embryos. These two spindles align their poles before anaphase, but the parental genomes are kept apart during the first cleavage. Multinucleated blastomeres are likely to be the result of erroneous divisions of the two spindle pole, starting from the first cleavage division [80]. The detailed process of blastomere development has been reviewed recently [74,81].
4.2. Polyploidy in tumors
Polyploidy in cancer has long been recognized by pathologists [26,27]. Endomitosis in tumors, a mechanism that generates PGCCs, was reported as early as 1953 [82]. Polyploidy has been reported conclusively in nearly 37% of all human tumors [83]. Mononucleated and multinucleated PGCCs are commonly observed in Barret’s esophagus, high-grade ovarian cancers and post-chemotherapy specimens [84–87] and are enriched at the invasion fronts and metastatic foci [88]. The number of PGCCs increases dramatically with the grade of malignancy in patients with breast cancer and patients with glioma [89,90]. Many recent publications report the detection of mononucleated and multinucleated PGCCs in blood specimens from cancer patients with the PGCCs appearing as macrophage-like circulating tumor cells [91,92]. It has been reported that PGCCs were visualized by fluorescence in situ hybridization, in a large majority of peripheral blood samples from patients with pancreatic adenocarcinoma [93]. In a study on mechanisms of polyploid phenotypes in breast cancer, GINS2 was identified as the highest-ranking endoreplication-inducing gene, suggesting this molecule could be a potential biomarker for aberrant cell proliferation, and therefore a potential therapeutic target [94].
PGCCs were generally considered to be terminally differentiated senescent cells because of their inability to execute mitosis [95]. Polyploidy is commonly found in senescent fibroblasts [96]. Traditionally, progression of polyploid animal cells was thought to be limited by several intrinsic mechanisms [97–99] and a number of published findings support this view. PGCCs have been shown to inhibit tumor growth [97–99] and induce senescence [100,101], and polyploidy can suppress tumor formation in normal liver [102]. Polyploidy induced by cytokinesis failure can trigger activation of the Hippo tumor suppressor pathway [103]. These studies support the inhibitory role of polyploidy in cell proliferation and disease progression.
On the other hand, the concept that PGCCs are non-dividing has been challenged by numerous other reviews. PGCCs were shown to be capable of continuously budding and generating mononucleated cells via non-mitotic mechanisms [104]. At the beginning of this century, several investigators have independently substantiated this observation in genotoxically induced cancer cells, senescent epithelial cells or fibroblasts. Illidge et al. and Erenpreisa et al. observed the budding of PGCCs from irradiated lymphoma cell lines [86,105]; while Sundaram et al. observed a similar budding process which they termed “neosis” [106]. At approximately same time, Walen observed genomically altered daughter cells from senescent epithelial cells [107,108], demonstrating that a similar process is involved in generating transformed clones from senescent polyploidy cells. This amitotic budding has been subsequently observed by multiple other investigators including my own lab [109–115].
Polyploidy was found to facilitate the bypassing of a senescence-induced replication blockade, which effectively stimulates tumor progression [116,117]. Tetraploid cells facilitate both cancer cell survival and neoplastic transformation [95,118–120]. Gamma-irradiated tumor cells are capable of both self-renewal via PGCC formation, and PGCC de-polyploidization into paradiploid progeny [121]. Treatment of human colon cancer cells with ionizing radiation resulted in the development of viable and radiation-resistant PGCCs [114]. Docetaxel-induced PGCCs were resistant to treatment and underwent a proliferative “mitotic slippage” phase in prostate cancer [122]. Evolution of the colorectal cancer genome was accelerated via whole-genome doubling and polyploidy [123]. PGCCs with supernumerary, unstable chromosomes are likely to drive chemotherapy resistance and disease relapse across multiple types of cancers, including colon cancer, gastric cancer, and prostate cancers [124–126]. Metastatic tumors can be generated from single highly chemotherapy-resistant giant fibrosarcoma cells [110]. In addition, PGCCs have been identified in blood samples from patients with metastatic breast cancer, and higher numbers of PGCCs in blood were shown to be an independent predictor for worse progression-free survival and overall survival in these patients [91].
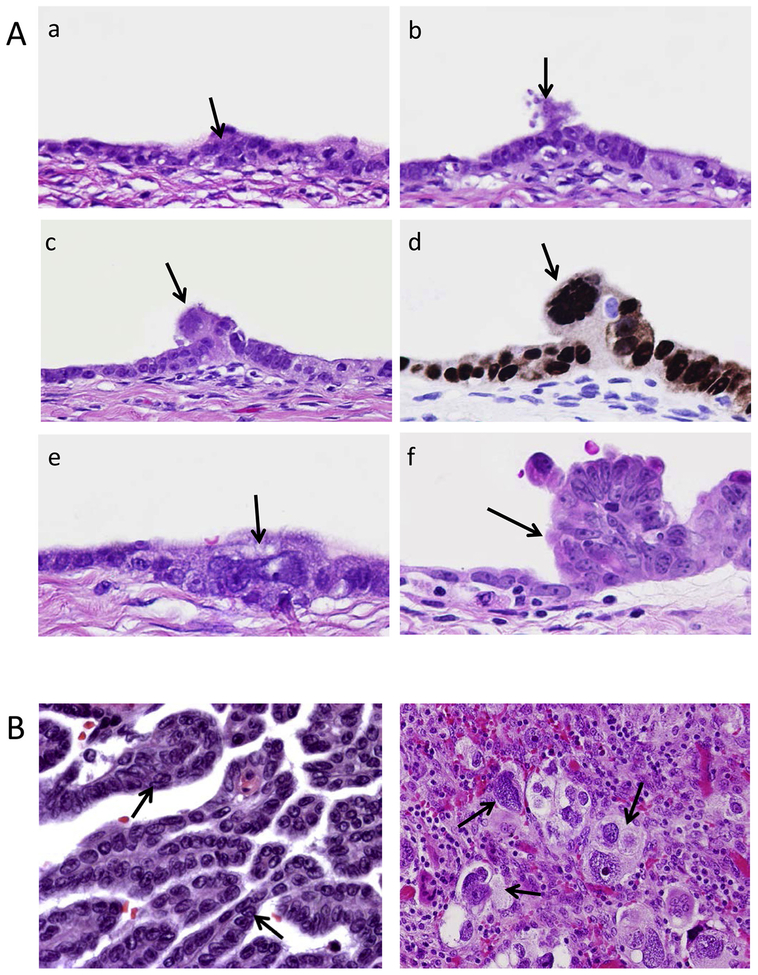
Multinucleated PGCCs are observed at the initiation site where noninvasive carcinoma transitions into invasive high-grade serous carcinoma (Fig. 2A). Multinucleated or mononucleated giant cells are observed in high-grade serous carcinoma but rarely in low-grade serous carcinoma (Fig. 2B). We demonstrated that CoCl2-treated ovarian cancer cells could be dedifferentiated into PGCCs with an acquired capacity to generate embryonic-like stemness and produce diploid progeny cells via budding, splitting, or viral-like bursts [109]. PGCCs near colon cancer stroma displayed budding of progeny, and the daughter cells that budded from PGCCs expressed epithelial-to-mesenchymal transition (EMT)-related proteins that are likely are associated with lymph node metastasis [127]. PGCCs have been postulated to function as stem-like cells that are capable of generating daughter progeny via endomitosis following mitotic catastrophe [106,107,128].
Fig. 2.
Histopathologic view of polyploid giant cells at the tumor initiation site and low- and high-grade serous carcinomas. A. Continuous sections of damaged ovarian epithelial cells with emergence of invasive high-grade serous carcinoma. A multinucleated giant cancer cell can be seen at the nidus where the invasive carcinoma (f) originates (arrow head, c, d, and e). Both in situ carcinoma and giant cancer cells are stained positive for p53. Arrows indicate the giant cells during transition from in situ carcinoma to invasive carcinoma. B. Left, an example of low-grade serous carcinoma, the nuclei of homogeneous size and absence of nuclear atypia and pleomorphism. Right, an example of a high-grade serous carcinoma with multinucleated giant cells and high nuclear atypia.
The role of polyploidy in vivo has been investigated in Drosophila, and these experiments suggest that polyploidy plays an important role in generating stem cells involved in wound healing and tumor initiation. Endoreplication also occurs in the injured/repairing Drosophila hindgut pylorus and ovarian follicle epithelium [129], which strikingly resembles the processes by which the mammalian liver fully regenerates under conditions where cell division is impaired [130]. In response to starvation of Drosophila, polyploidy can give arise to new stem cells in the intestine via amitosis [131]. Interestingly, genetic analyses of rab5-defective cells in Drosophila revealed that cooperative activation of the morphogenetic driver JNK and Yorkie (a Hippo pathway effector and YAP homolog) generates PGCCs via endoreplication [132]. The YAP activation has been shown in the formation of PGCCs and the blastomere-stage embryogenesis in humans [68,133].
Taken together, the above data suggest that PGCCs are not only capable of division but also play important roles in cancer initiation, drug resistance, and metastasis both in vitro and in vivo as well as in model organisms.
5. The giant cell cycle
Despite of the above efforts, however, it has been unclear about how PGCCs can escape chemotherapy-induced stress in order to generate diploid progeny to support tumor initiation, drug resistance, and metastasis. In order to address this question, we used fluorescence-labeled single-cell time-lapse images to track the dynamic process of mitotic division and cell cycle progression. Using green fluorescent protein–-tracked α-tubulin, red fluorescent protein–tracked histone 2B, and a fluorescent ubiquitination cell cycle indicator (FUCCI), we temporo-spatially tracked the dynamics of cell growth and division. We observed the dynamics of mitotic spindles, and chromatin formation of giant cells and observed that these cells gave rise to mitotically competent daughter cells. We described this process as the giant cell cycle and delineated four distinct but overlapping phases: initiation, self-renewal, termination, and stability (Fig. 3) [113].
Fig. 3.
The giant cell cycle mimics blastomere division. Following initiation by either intrinsic genetic or external stresses, the somatic cell enters a self-renewal endoreplication phase and starts dedifferentiation (reprogramming). The reprogrammed cell enters a termination stage to start differentiation. During this time, giant cells use multiple modes of primitive cell division to generate diploid daughter cells, including the following: (1) horizontal genetic transfer, the DNA migrates horizontally into adjacent cells via the branch of cytoplasm and then followed by budding; (2) formation of an elongated cell with two giant nuclei followed by (3) splitting in the middle of the giant cell or (4) budding; (5) direct budding from a mononucleated giant cell; (6) direct budding from a multinucleated giant cell. During the stability phase, the differentiated cells are grown out of chaos and arrested at a specific developmental level. The dominant clones grow out of this chaos and form a visible tumor, which can behave as benign, malignant, resistant, metastasis or death (cured). The cells that have immediately budded off from the giant cells have a high level of stemness (red triangle) and gradually achieve stability during differentiation (blue triangle).
The initiation phase is characterized by extensive mitotic catastrophe and cell death among nearly all cells, regardless of their stage in the cell cycle. Following this massive, chemotherapy-mediated cell death, a subset of cancer cells survive and transition to tetraploidy and polyploidy. Senescence is a critical step for initiation of the giant cell cycle and is also a critical step in tissue remodeling in normal development and physiology as well as in multiple pathologic conditions [134]. The polyploid cells respond asynchronously to growth-inhibitory factors, successfully evading apoptosis/senescence. A small subset of diploid (2n) cells in the G2 phase display a persistent survival response evidenced by mitotic uncoupling to instigate endoreplication, disabled G1/S or G2/M checkpoint constraints, and down-regulation of mitotic mediators like Aurora A kinase, to evade programmed cell death or senescence. This allows amitotic endocycling, in which surviving cells uncouple the steps of interphase. In this amitotic mode, cells transition from endo-S back to endo-G1; from endo-G2 back to endo-G1; or from endo-prophase, endo-metaphase, or endo-anaphase back to endo-G1. We think this facilitates dual microevolutionary purposes: repetitive endo-G1 and endo-G2 phases allow for a giant cell size to resist cell death and acquire all available extracellular nutrients; and endocycling cells in endo-S generate multiple genome copies within an intact giant nucleus to resist differentiation and induce genomic reprogramming and mutational adaptation.
The self-renewal phase occurs as diploid cancer cells are dying and surviving cells that have transitioned to tetraploidy (4n) continue endocycling or endomitosis to produce mononucleated or multinucleated polyploid (pn) cancer cells. Some multinucleated cells undergo cyto-fission in order to generate smaller, slow-growing polyploid cells that persist through chemotherapeutic treatment via re-entering the initiation phase of amitotic endocycling. FUCCI visualization revealed that PGCC formation was via endocycling (between a G phase and S phase), which produced polytene, mononucleated or multinucleated PGCCs, all of which are truncated forms of the endoreplication cell cycle. The self-renewal stage of the giant cell cycle facilitates reprogramming and allows the somatic cells to achieve dedifferentiation and to maintain an undifferentiated state.
The termination phase occurs when some PGCCs initiate the process of depolyploidization to diploid cells, also known as genome-reductive division, using mechanisms similar to bacterial, fungal, and protozoan routes of cell reproduction. Some PGCCs spawn diploid nuclei via massive protozoan or viral-like budding to yield many smaller stem-like cells with minimal cytoplasm. In the meantime, some PGCCs form a reproductive cyst-like structure, analogous to the cysts formed by Entamoeba [135], capable of disseminating microcell progeny that may be totipotent or pluripotent, while other PGCCs employ a modified version of prokaryotic-like fission, elongating axially to produce smaller elongated polyploid progeny that can themselves begin budding diploid progeny. Still other PGCCs initiate a fungus-like sporulation, bursting and fragmenting into many diploid and aneuploid progeny; this process can also be achieved via horizontal genetic transfer, as previously described by our group in Figs. 2B and 4B [109]. It has been reported that small fragments of sheared tumor cell DNA can indiscriminately enter other cells, suggesting an evolutionarily mobile aspect of tumor-related horizontal gene transfer [136].
Fig. 4.
The model for dualistic stem cell origin of human tumors. Left panel (A), normal fertilization (1n/1n) triggers normal embryogenesis and gametogenesis followed by organ and adult development. The genetic and epigenetic changes lead to failure of stem cell maturation and results in their accumulation along the developmental hierarchy to give rise to a tumor. BM, blastomere; GCs, germ cells; Meso, mesoderm; Ecto, ectoderm; Endo, endoderm; ICM, inner cell mass; Undiff tumors, undifferentiated tumors; LG tumors, low grade tumor; BN tumors, benign tumors. The tumors are more common among younger people (< 50 years). Right panel (B), damaged or aged mature somatic cells and tissue undergo de novo transformation. The transformation process is initiated via the formation of polyploid giant cells, which can partially or completely recapitulate blastomere-like process for dedifferentiation and leads to formation of completely or partially reprogrammed stem cells and to be arrested at different levels of the developmental hierarchy. BM-like, blastomere-like; MMMT, malignant mixed Müllerian tumor; HG serous, high-grade serous ovarian carcinomas; TNBC, triple-negative breast cancers; GBM, glioblastomas; HG dysplasia, high-grade dysplasia. These tumors are more common among elderly people (> 50 years).
In many tumors, both maturation arrest and dedifferentiation are simultaneously involved during their initiation and progression.
Finally, in the stability phase, spawned diploid progeny cells with novel or altered genotypes, evidenced by genomic remodeling, point mutations, or altered karyotype (i.e., chromothripsis), continue to gain cytoplasm and become more stable cells that are capable of mitosis. These spawned diploid progeny cells can go on to differentiate into a variety of cell types with variable levels of developmental potency. The resultant daughter cells with newly acquired chromosomal alterations display long-term proliferation, that can further develop into a new organ, which can behave as benign, malignant, metastatic or resistant phenotype to the original inducers [113].
In addition to endoreplication, cell fusion appears to be capable of initiating the formation of PGCCs. Cell fusion appears to be involved in generating cancer cell heterogeneity, chemoresistance, and metastasis [137,138]. In our study, treatment of cancer cells with a hypoxia mimetic led to cell fusion in approximately 10% of the cells [109]. Incomplete cytokinesis failure followed by re-fusion of small Hodgkin’s cells appears to be the mechanism by which multinucleated giant Hodgkin’s cells are generated in Hodgkin’s lymphoma [139]. The cell fusion event, together with the endoreplication described above, may represent an additional driving force to initiate formation of polyploidy and further tumor evolution.
Taken together, the experiments described here have allowed us to track the origin of resistant cancer in a stepwise programmed manner and to allow a visualization of the birth of therapy-resistant cancer cells from conventional cancer cells via formation of PGCCs [140].
6. The giant cell cycle as a novel mechanism for somatic cell dedifferentiation (or reprogramming)
Supporting the view that PGCCs can be dedifferentiated into cells with embryonic structure and function, we demonstrated [68] that paclitaxel-induced PGCCs are senescent. The development of single PGCCs recapitulated the development of the blastomere into morula and blastocysts. PGCCs are morphologically indistinguishable from natural blastomeres by an scanning electron microscope. The PGCCs also showed time- and location-dependent expressions of embryonic stem cell markers OCT3/4, NANOG, SOX2, and SSEA-1 and three germ layers in vitro [68]. Injection of PGCC-derived spheroids into immunodeficient mice generated mixed carcinoma and germ cell tumor and tumors of different grades [68]. In addition, hypoxic mimetic CoCl2-induced PGCCs were able to survive, cycle slowly, and grow back into regular diploid cells, similar to asexual division in yeast or protozoans [109]. PGCCs were capable of differentiating into adipose tissue, fibroblasts, bone, and hematopoietic cells, including neutrophils, lymphocytes, endothelial cells, and red blood cells [141–144]. Blastocyst- like strutures have been also observed in colorectal cancer cells following treatment of chemotherapy agents by Was et al. [145].
Further evidence supporting the view that PGCCs acquire embryonic stemness comes from our high-throughput proteomic profiling, which identified a set of proteins that are differentially expressed in PGCCs and regular cancer cells [146]. The functions represented by the protein set included cell cycle regulation, invasion and metastasis, stem cell generation, chromatin remodeling, and hypoxia. In addition, we showed that the addition of Myc oncogene, a known transcription factor involved in reprogramming could induce formation of polyploidy and convert immortalized ovarian fibroblasts to monomorphic teratoma- like tumors and induce their differentiation toward hematopoietic lineages and generation of red blood cells composed of predominantly embryonic hemoglobins [143]. Recently, additional gene transcripts interacting with Myc were found to be significantly over-represented in polyploid cells compared to diploid cells, suggesting that Myc-induced polyploidy may be an essential precursor to reprogramming and tumorigenesis [147].
Other evidence from different investigators also supports the concept that PGCCs acquire embryonic stemness. It has been shown that genotoxic stress can activate embryonic self-renewal in p53-defective tumor cells [148]. Poorly differentiated aggressive human tumors have an embryonic-like gene expression profile [149,150]. Further, ectopic overexpression of OCT4 in mammary epithelial cells generates tumor-initiating cells [151]. Premature termination of reprogramming leads to tumor development and epigenetic change without mutation in vivo [19]. Cancer cells arose from matured arrested or undifferentiated cancer stem cells via an EMT program [152,153]. The transition of somatic cells to cancer mirrors an epigenetic restriction of extra-embryonic lineages [154]. Senescence has been reported to facilitate reprogramming and cancer stem cell generation [155]. Stem cell pluripotentiality and dedifferentiation have been found across multiple solid and hematopoietic cancers [156]. In a comprehensive analysis of 11,000 tumors and 33 histotypes, dedifferentiated embryonic pheno-types were found in most high grade cancers [157]. Finally, oncogenes have been found to lead to dedifferentiation of neurons and astrocytes into glioma [158], and the introduction of core stem cell factors into mature glial cells generates glioblastoma stem-like cells [159]. Because of numerous similarities between tumors and reprogramming, cancer has been proposed as a pathologic nuclear reprogramming [160].
On the basis of the findings outlined above, we propose that the giant cell cycle represents a stress-induced endogenous mechanism for somatic cell dedifferentiation for generation of stem cells for tumor initiation. Specifically, we propose that the giant cell cycle mimics cleavage division in the embryo and signifies an atavistic, transient cellular reprogramming mechanism that induces genomic reorganization in response to various stresses [113]. Endoreplicative giant cells, in survival mode, generate progeny possessing novel, remodeled genomes that may, depending on the intensity and form of environmental stressors, adaptively dedifferentiate into an embryonic-like cancer stem cell population.
7. Giant cell cycle–induced chaos, germ cells, and germ cell tumors
During the normal development, chaos in blastomeres occurs during the first four days of life, a small group of cells are put aside to form primordial germ cells at the time of gastrulation after formation of the blastocyst. If the giant cell cycle recapitulates the developmental pattern and function of the blastomere then it should, theoretically, be capable of inducing a formation of different lineages, not just somatic cells but also germ cells. Our surprising finding that PGCCs are capable of generating not only three germ layers but also germ cell tumors under the influence of paclitaxel-mitotic failure [68] supports the view that the giant cell cycle recapitulates the blastomere-like program and may be sufficient to induce the formation of germ cells. The idea that cancer cells can recapitulate the most primitive growth mode for their survival has also been proposed by Erenpreisa et al., who referred to the idea as “virgin birth” [49]; Vinnistky, who proposed the “oncogermi-native theory” involving an asexual self-cloning process [48]; and Lineweaver et al, who referred to “derepression atavism” [161,162]. Activation of the primitive transcription program is detected in multiple types of solid tumors, providing molecular evidence to support this concept [163].
The chaos existing at the blastomere stage of human embryonic development must serve a critical purpose to create a new life, whether it is a normal life or a tumor. Chaos eliminates parental-pattern DNA methylation, facilitating novel genomic and epigenomic embryonic patterns. Intrinsic error-prone dual-spindle mechanisms in zygotes can facilitate the generation of multinucleation and chaos in blastomeres [80]. Chaotic aneuploidy is generated from a lack of cell cycle checkpoints, mosaicism, atypical cell division, cellular fragmentation, subchromosomal instability, micro-nucleation, and chromothripsis [81,164]. Intrinsic retrovirus or transposable elements have been shown to be common in human preimplantation embryos and pluripotent cells [165]. Microcells that drive chromothripsis have been observed in both cancer cells and embryos [140,166–168]. Endogenous retroviruses and transposable elements have been found to be activated in both blastomere-stage embryos and PGCCs [115,165], demonstrating an intriguing link between these two processes.
Polyploidy is known to be a potential driver for evolution in plant and animal ecology [169]. Blastomere-mediated chaos also explains McClintock’s observation, that cell stressors could evoke extensive genomic rearrangement to be transmitted via germlines [170–172]. Stresses, especially life-threatening stresses like X-ray irradiation and exposure to potentially lethal chemicals, can lead to genome reorganization and result in a new heritable genome for a new species, a process first described by McClintock seven decades ago [170–172]. A role in genomic chaos in cancer development has also been extensively advocated by Heng et al., [173,174].
Taken together, these findings indicate that chaos may present a robust non-deterministic mechanism to create a system as complex as a human being or a tumor through a nonlinear themodynamic. The giant cell cycle mediated chaos may represent an evolutionarily conserved fundamental mechanism in response to stresses for generating new species or rejuvenation via the formation of a germline, which is conserved through evolution from McClintock’s plants to mammals, and to tumors.
8. The dualistic stem cell origin of human tumors
On the basis of clinical and pathological observation as well as the experimental data described, I propose a new model to explain the origin of tumors, named “the dualistic origin of human tumors”, which includes stem cells derived from the blastomeres in normal embryogenesis and from mature somatic cells via blastomere-like mediated dedifferentiation.
The details of the dualistic model are outlined in Fig. 4, modified from Fig. 8 [68]. During sexual reproduction (Fig. 4A, left), the fertilized egg initiates cleavage division to generate blastomeres. At the 8-cell stage, the blastomere starts to compact and further divisions generate morula with the first differentiation event in the life of a human, namely blastocyst formation, in which the compacted blastomere differentiates into the trophoectoderm and the inner cell mass. Following normal development, epigenetic and genetic alterations during game-togenesis and blastomeres will be distributed into various stem cells through embryogenesis and adulthood to generate the diversity of an individual human being. The parthenogenesis of oocytes, germ cells or arrested early embryonic cells can go through full or abbreviated embryogenesis to generate all the three germ layers, including a fetiform teratoma (homunculus) resembling a malformed fetus before gastrulation [21], variable forms of monomorphic teratomas with tissue from one or two germ layers or immature teratoma if neuroectodermal tissue fails to mature after gastrulation. Tissue immaturation within teratomas or secondary transformation from mature cells generate various somatic tumors associated with teratoma.
During development and adulthood, additional genetic or epigenetic mutations or non-mutational mechanisms [175] can be introduced during organogenesis. Under environmental stresses, including chronic inflammation, virus infection, chemical carcinogens exposure, irradiation, or chemotherapy drugs, the specific stem cell prone to proliferation can be decoupled from the differentiation program to gain cell autonomy for a tumor development. As uterine implantation serves as the filter to selectively eliminate the highly genomically abnormal embryo from further development, the tumors in this group largely show immature tissue resembling that from primitive organs without identifiable mutations [18,19,176,177]. Examples of tumors belonging to this group include immature teratoma, Wilms’ tumor, ependymoma, small lymphocytic lymphoma, well-differentiated carcinomas of different organs, benign tumors, and low-grade dysplasia. As the tetraploid cells that lead to such transformation are largely transient, the tumor tissues in this group usually have minimal cytologic atypia without forming morphologically recognizable giant cells, a lack of PGCCs and gross genomic alterations.
The second source of tumor origin is achieved via the reprogramming or dedifferentiation of aged and damaged somatic cells or benign tumors following the various genetic and environmental inducers. During asexual reproduction (Fig. 4B, right), various environmental stresses together with intrinsic genetic or epigenetic alterations, can lead to relaxed cell cycle control in aged or damaged somatic cells or benign tumor cells, which in turn can lead to de novo dedifferentiation via the giant cell cycle. The transformation process may involve two to six endoreplication cycles to generate 4n, 8n, 16n, 32n, 64n cells respectively similar to cleavage division to generate stem cells with variable developmental potency. The higher the number of the endoreplication cycle the more of infidelity of DNA replication, the more complete the dedifferentiation, which can be up to the morulae stage embryo for teratomas. Similar to stem cell arrest from normal embryogenesis, the level of malignancy corresponds to the level of developmental hierarchy that these stem cells arrest. The more primitive the stage of developmental hierarchy at which the arrest occurs, the more malignant the tumor is likely to be.
Unlike tumors generated from sexual reproduction, there is no uterine implantation involved in tumors generated from asexual reproduction. Depending on the pre-existing epigenetic and genetic alterations or organismal structures, tumors generated along this asexual reproduction pathway display high nuclear pleomorphism with variable numbers of PGCCs. These tumors usually occur among elderly patients and have numerous genomic and epigenetic alterations at the chromosomal levels. Examples include mixed carcinoma and germ cell tumors, malignant mixed Müllerian tumor, high-grade ovarian serous carcinoma, triple-negative breast cancer, glioblastoma, anaplastic lymphoma, and high-grade dysplasia, and post-chemotherapy or radiation therapy-treated resistant tumors.
This dualistic model has incorporated multiple elements from early models proposed by multiple investigators. The blastomere origin for teratomas was proposed by Marchand and Bonnet more than a century ago [30], maturation arrest was proposed by Pierce [178], although dedifferentiation was considered as unnecessary in his model. A model to explain the malignancy based on stem cell potency was also proposed by Biava and Bonsignorio [179]. Asexual clonal reproduction was proposed by Vinnisky and was further refined by Erenpreisa et al. to incorporate polyploidy in the process [48,49]. However, tumors derived from normal development via sexual reproduction, and the concept of maturation arrest generating different malignant and benign tumors along developmental hierarchies was not considered in their model. As the giant cell cycle recapitulated many features of the life cycle of single celled organism like Amoeba [135]. This model also incorporates the atavistic model from single celled organisms to multicellular organisms via activation of ancestral gene networks [161,163,180]. Thus, the dualistic model provides a unified model for all human tumors. The detailed similarities and differences between the stem cells from blastomere and blastomere-like origins are shown in Table 1.
Table 1.
Comparison of Human Blastomere and Blastomere-like Program for Dedifferentiation in Dualistic Model.
| Blastomere | Blastomere-like | |
|---|---|---|
| Mode of reproduction | Sexual | Asexual |
| Starting cells | Haploid, genomic imprinted sperm & egg (1n & 1n) | Specialized diploid somatic cells (2n) |
| Initiation | Cell fusion (2n,2c) | Endoreplication or cell fusion Tetraploid, polyploidy (≥4n,4c) |
| Potency | Totipotent zygote | Specialized somatic cells |
| Division | Nuclear Cleavage | The giant cell cycle (≥4n,4c); Endoreplication, amitosis [113] |
| Microenvironment | Zona pellucida | Somatic environment |
| Nuclear to cytoplasmic ratio | Progressive increase | Progressive increase [113] |
| Genomic instability (chaos) | Failed/asymmetrical cytokinesis, microcell, nuclear fusion, aneuploidy [81,164,208] | failed/asymmetrical cytokinesis, microcell, nuclear fusion, chromothripsis, aneuploidy |
| Horizontal DNA transfer | Yes (via microcell) | Yes (branching) |
| Retrovirus activation | Yes [165,209] | Yes [115] |
| Loss of Xist expression | Yes [210] | Yes [68] |
| OCT4/NANOG/SOX2 Expression | Yes | Yes [68] |
| Level of dedifferentiation | Complete | Complete or partial |
| Time of dedifferentiation | 4–5 days | Variable, stress type and strength, cell type dependent |
| Out of dedifferentiation | Resume mitosis | Budding, splitting, viral burst, and horizontal genetic transfer, followed resumption of mitosis [113] |
| Next step in Development | Blastocyst (embryoblasts + trophoblasts) | Blastocyts-like structures [68] (embryoblast-like) + (trophoblasts?) |
| Three germ layers | Yes | Yes |
| Germ cells | Yes | Yes (germ cell tumor) [68] |
| Outcome | Fetus (+uterine implantation); teratoma and other tumors (−uterine implantation) | Teratoma and other tumors |
| Common tumor types | Immature tissue with no or minimal nuclear atypia; low grade tumor (Type 1 tumors) [21,201] | Marked nuclear atypia, anaplasia, PGCCs; high grade tumors (type II tumors) [21,201] |
| Tumor examples | Wilms’s tumor, immature teratoma, ependymoma, fibroma, small lymphocytic lymphoma [21,201] | High grade serous carcinoma, glioblastoma, triple negative breast cancer; anaplastic lymphoma [21,201] |
However, it must be emphasized that the type of tumor generated via sexual and asexual pathways is most likely dependent on the preexisting genetic change in the genome, inducers, and the micro-environments. For example, BRCA1 and BRCA2 germline mutations are always associated with high grade carcinomas in the breast and ovary [181]; Reprogramming in vivo using transcription factors from normal healthy somatic cells, presumably without previous tissue damage in these animals, can generate Wilms’ tumors, uroepthelial carcinoma, and skin papilloma [18], similarly to those generated from sexual reproduction. In many tumors, both sexual and asexual mechanisms are used in their initiation and progression.
9. Relationship between the dualistic model and other models of cancer origin
The dualistic stem cell model provides rational explanations for several existing models of the origins of cancer: the somatic mutation model, the multistep progression model, and the cancer stem cell model.
9.1. Somatic mutation model
In the somatic mutation model, cancer arises from an uncontrolled proliferation of cells due to oncogene activation or loss of tumor suppressor genes from quiescent somatic cells. This gene-centric view of cancer development has recently been challenged by the wide range in the number of mutations per tumor identified in the pan-TCGA project [44,45]. Some tumors acquired thousands of mutations, including mega-genomic deletions and duplications, while others lack any mutations at all. Furthermore, many mutations have been reported in normal tissues, arguing against the driver role of somatic mutations in tumor initations.
In the dualistic model proposed here, the mutations, per se, are not required for development of cancer as reprogramming can be achieved via activation of normal embryonic transcription factors. The genetic mutations, however, particularly in inherited cancers, like p53 in Li-Fraumeni syndrome, retinoblastoma genes in retinal cancer, or BRCA genes in familial breast cancer, can prime the somatic cells for dedifferentiation. Other mutations, such as KRAS or BRAF, may uncouple proliferation from differentiation and can lead to inhibition of stem cell maturation. The other genetic changes may directly or indirectly affect the epigenetic mediated programming and reporgramming and lead to stem cell maturation arrest [182].
9.2. Multistep progression model
Another commonly described model of cancer origin is the progression from benign to malignant tumors via the accumulation of mutations. In the dualistic stem cell model, benign and malignant tumors can be explained by the arrest of stem cells at different developmental hierarchies. Depending on the severity of the intrinsic genetic or external insults and the microenvironment in which particular somatic cells reside, dedifferentiation may proceed toward a primitive stage of embryogenesis, resulting in a malignant tumor; or dedifferentiate to a less primitive stage of embryogenesis, resulting in a benign tumor. Supporting this interpretation, it has been reported that many malignant tumors, including tumors of the colon, breast, and pancreas, are initiated via a single catastrophic event. This phenomenon is called the “big bang” model of development [183–185], most likely due to high number of endoreplication cell cycles required to achieve the higher level of dedifferentiation. These cells then arrest at the primitive stage of the developmental hierarchy for these organs to grow into a malignant organ. On the other hand, some benign tumors, including colon polyps, which may be low level of dedifferentiation by low number of endoreplicaton cell cycle. However, benign tumor can be further dedifferentiated by additional endorereplication cell cycles in a stepwise manner to be transformed either into invasive cancer or completely regress.
9.3. Cancer stem cell model
The concept of cancer stem cells can be neatly explained in the dualistic model. Batlle and Clevers proposed that cancer stem cell hierarchies in many types of cancer are not fixed and that stochastic or chaotic tissue micro-environmental contexts might yield interconversion among cancer stem cells and non-stem cancer cells [62,186].
In the dualistic model presented here, some cancer stem cells arise during normal embryonic and adult development, while others arise from PGCCs during de novo transformation. The immediately budded daughter cells from PGCCs show activated expression of cancer stem cell markers, including ALDH1a, CD133, and CD44 [68,109,115,187], indicating that at least some cancer stem cells may be daughter cells that have immediately budded from PGCCs. However, it is difficult to compare the stem cells in different experimental systems. For example, hair-follicle stem cells are clearly different from stem cells in highly malignant tumors like glioblastoma and high-grade serous carcinoma, which are very plastic and highly prone to dedifferentiation. PGCCs provide a stable experimental system to track these cells expressing these markers, which should help further clarify the relationship between marker-defined cancer stem cells and PGCCs.
10. The relationship between dualistic stem cell model and other cancer phenomena
In addition to the several popular cancer origin models described above, there are several cancer-related phenomena that are currently under active investigation. The dualistic stem cell model also provides rational explanations for several of these phenomena.
10.1. Epithelial-to-mesenchymal transition
Epithelial to mesenchymal transition (EMT) plays an important role in multiple stages of development. Improper balance between epithelial-to-mesenchymal ratios leads to tissue maturation arrest and tumor development, either sarcoma or carcinoma. In recent years, EMT has been intensively studied as a potential mechanism for invasion and metastasis because of the increased mobility of mesenchymal cells [152,188]. However, recent data suggest that EMT may not be required for metastasis, at least in some cancer models [189,190]. EMT promoting transcription factor Snail has been shown to be capable of actviating expression of the filament forming protein septin-6, resulting in the midboy persistence, abscission failure, and multinucleation of tumor cells, and tissue stiffening, which can promote the genomic instability and cancer progression [191]. EMT is involved in multiple stages of human development, and the role of EMT is clearly different in the development of tumors arising from stem cells arrested at a high level of development (malignant) and tumors arising from stem cells arrested at a late stage of development (benign). Therefore, the role of EMT in tumor metastasis must be interpreted within the context of development with great caution: A highly malignant tumor may not need to undergo EMT for dissemination; Similarly, a benign tumor may not possess sufficient stemness to be able to spread to distant sites regardless of whether it has acquired a mesenchymal phenotype or not. In addition, therapeutic stress may trigger differentiation toward benign lineages via EMT rather than promoting the proliferation and migration associated with the metastatic phenotype [144].
10.2. Senescence, immortalization, and transformation
The dualistic model also provides a rational explanation for the long-known paradox that senescence is associated with stemness and also an explanation for the senescent-associated secretory phenotype [134, 192]. Senescence has been shown to be a major source of cancer stemness via reprogramming [155]. In addition, senescence is a prerequisite for cells to achieve immortalization or transformation from primary cells: crisis usually follows senescence and is associated with massive karyotypic changes, among them, that an immortalized or transformed cell can grow indefinitely [193,194]. Shortening of telomeres leads to a crisis, and cancer development is associated with telomere stability through re-expression of telomerase. Following the anti-telomerase therapy, the cancer cells re-enter crisis and grow into fit, stable clones expressing alternative lengthening of telomerase pathways [195,196]. All of these phenomena can be explained by the giant cell cycle mediated dedifferentiation and reprogramming.
10.3. Warburg effect
Warburg et al. observed that tumors use aerobic glycolysis to generate lactate from glucose in the presence of O2 [197]. Thousands of papers over the past ten years have delineated the underlying mechanisms and functions [198]. Cancer has been considered as a mitochondria metabolic disease [199]. We have reported that embryonic hemoglobin and embryonic red blood cells can be generated from fibroblasts in the presence of hypoxic mimetic CoCl2 [143], and the embryonic hemoglobins can also be observed in high-grade cancer cells [143]. Loss of CSL, a component in Notch signaling, unlocks the hypoxic response and allows cancer cells to acquire a PGCC phenotype [200]. The ability to generate embryonic hemoglobins for O2 also explains the failure of some clinical trials of angiogenic therapy and resistance to such therapy: anti-angiogenetic therapies can only stabilize particular subsets of cancers, as angiogenesis occurs late in development.
10.4. Clinical and pathologic features of patients’ tumors
Two types of tumors that have long been observed by pathologists are (i) immature tumors lacking PGCCs and nuclear atypia or heterogeneity and (ii) tumors associated with anaplasia (backward growth). Tumors in the first group show overgrowth corresponding to the tissue in early embryonic and adult tissue development and lack of PGCCs without nuclear atypia or heterogeneity. Examples include immature teratoma, Wilms tumor, low-grade serous carcinoma, small lymphocytic leukemia, and cystadenoma. Tumors in second group are derived from transformation of dedifferentiation from somatic cells via a blastomere-like mechanisms. These tumors show multiple PGCCs with marked heterogeneity and anaplasia. Examples include dedifferentiated liposarcoma, high-grade ovarian serous carcinoma, triple-negative breast cancer, and glioblastoma. In the female reproductive system, type I epithelial tumors belong to the first group from sexual reproduction, and type II tumors belong to the second group from asexual reproduction [201]. The additional details can be found in Table 1.
11. Therapeutic implications of the dualistic model
Definition of the giant cell cycle not only helps us to understand tumor initiation, acquisition of resistance to therapy, and disease relapse, but is also offers several previously unappreciated targets for cancer therapy.
First of all, senescence represents the first step in the initiation of the giant cell cycle. Thus, anti-inflammatory agents that induce senescence or the senescence-associated secretory phenotype could be employed as a tumor-prevention strategy. Therapeutic targeting of senes-cent cells, or pro-senescent therapy, may induce a tumorigenic reversion or anti-malignant tissue microenvironment. The role of senescence in the giant cell cycle also provides a rational explanation for the long-established chemo-preventive effect of aspirin and also suggests the use of anti-inflammatory agents to prevent formation of polyploidy to enhance the therapeutic effect and prevent disease relapse [202].
Second, self-renewal and termination stages of giant cells are associated with primitive division. Drugs that are antibacterial, antifungal, or anti-protozoan may be effective [126]. In addition, a recent study that utilized chemical inhibitors against centromere-associated protein CENP-E and kinesin-related protein Eg5 to study cell fates following mitotic slippage may have implications for anti-microtubule drug treatment [203].
Third, human embryogenesis and fetal development are immunoprivileged; similarly, the giant cell cycle recapitulates embryonic reprogramming, which is also immune privileged. Therefore, targeting PGCCs may require combinations of immunomodulatory drugs. In addition, early embryo-like structures derived from PGCCs may represent a potential tumor vaccine. Supporting this view, induced pluripotent stem cells have been reported to elicit anti-tumor response [204].
Finally, successful treatment of cancer must be directed toward blocking the different stages of the giant cell cycle and differentiating arrested malignant tumors toward becoming benign tumors, the so-called “differentiation therapy”, first advocated by Pierce et al. in 1970 [205]. Differentiation therapy has been successfully used in treatment of acute myelogenous leukemia [206], although it has had only limited success with solid tumors. The PGCCs offer new targets for differentiation therapy, and differentiation agents should be induced concurrently with chemotherapy, not afterwards.
12. Conclusion
Looking back on the history of cancer research, the understanding of the origins of cancer has taken multiple turns over the past two centuries. The embryonic theory of cancer, based on the intuition of pathologists, was first proposed nearly 150 years ago. At the beginning of the 20th century, two parallel theories of cancer origin, the somatic mutation theory and the cancer stem cell theory, were proposed [186]. In the past 50 years, the somatic mutation theory has been dominant, and the cancer stem theory has fluctuated in popularity. In the past two decades, the understanding of cancer has moved from the view that cancer is caused by a single or a few genes to the current concept of it being an enormous, genetically heterogeneous collection of diseases caused by differential mutations [2–4], that require individualized therapy based on specific mutations.
In my view, cancers should be viewed as a group of defined diseases arrested at different stages of development from stem cells generated via sexual and asexual reproduction. Efforts should be directed toward targeting the giant cell cycle and to make the arrested stem cells and to differentiate again into mature benign cells in addition to cytotoxic study and immunotherapy.
It has been about half a century since Presdient Nixon launched War on cancer. Billions of dollars have been invested in fighting this disease but no insight has been gained on the initation and progresssion of this disease. Maybe it is time for cancer researchers to re-examine some of the established concepts in cancer biology and make a new conceptual turn again.
Acknowledgements
I am a member of the scientific advisory board for Novintum. This work was supported by the University of Texas MD Anderson Cancer Center’s Ovarian Cancer SPORE grant from the National Institutes of Health, MD Anderson Ovarian Cancer Moon Shot®. I would like to thank many of my colleagues, students, and trainees for their helpful input, and to Stephanie Deming for editing the article.
References
- [1].Weinberg RA, Coming full circle-from endless complexity to simplicity and back again, Cell 157 (1) (2014) 267–271. [DOI] [PubMed] [Google Scholar]
- [2].Nowell PC, The clonal evolution of tumor cell populations, Science 194 (4260) (1976) 23–28. [DOI] [PubMed] [Google Scholar]
- [3].McGranahan N, Swanton C, Clonal heterogeneity and tumor evolution: past, present, and the future, Cell 168 (4) (2017) 613–628. [DOI] [PubMed] [Google Scholar]
- [4].Greaves M, Maley CC, Clonal evolution in cancer, Nature 481 (7381) (2012) 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kumar V, Abbas AK, Fausto N, Aster J, Robbins and Contran Pathologic Basis of Disease, Sauders Elsevier, 2010, pp. 262–270 Chapter 7. [Google Scholar]
- [6].Willis RA, Pathology of Tumors, 4th edition, Butterworth & Co. Ltd, 1967. [Google Scholar]
- [7].Sadler T, Langman’s Medical Embryology, 12th edition, Lippincott Willians & Wilkins, 2012. (Chapter 3, p37). [Google Scholar]
- [8].Hemberger M, Dean W, Reik W, Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal, Nat. Rev. Mol. Cell Biol. 10 (8) (2009) 526–537. [DOI] [PubMed] [Google Scholar]
- [9].Turner S, Wong HP, Rai J, Hartshorne GM, Telomere lengths in human oocytes, cleavage stage embryos and blastocysts, Mol. Hum. Reprod 16 (9) (2010) 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ledbetter DH, Chaos in the embryo, Nat. Med 15 (5) (2009) 490–491. [DOI] [PubMed] [Google Scholar]
- [11].Chazaud C, Yamanaka Y, Lineage specification in the mouse preimplantation embryo, Development 143 (7) (2016) 1063–1074. [DOI] [PubMed] [Google Scholar]
- [12].Waddington CH, The strategy of the Gene, A Discussion of Some Aspects of Theorectical Biology, George Allen & Unwin, London, 1957. [Google Scholar]
- [13].Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, Truckenmuller RK, van Oudenaarden A, van Blitterswijk CA, Geijsen N, Blastocyst-like structures generated solely from stem cells, Nature 557 (7703) (2018) 106–111. [DOI] [PubMed] [Google Scholar]
- [14].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126 (4) (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [15].Gurdon JB, Elsdale TR, Fischberg M, Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei, Nature 182 (4627) (1958) 64–65. [DOI] [PubMed] [Google Scholar]
- [16].Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH, Viable offspring derived from fetal and adult mammalian cells, Nature 385 (6619) (1997) 810–813. [DOI] [PubMed] [Google Scholar]
- [17].Liu Z, Cai Y, Wang Y, Nie Y, Zhang C, Xu Y, Zhang X, Lu Y, Wang Z, Poo M, Sun Q, Cloning of macaque monkeys by somatic cell nuclear transfer, Cell 172 (4) (2018) 881–887 e7. [DOI] [PubMed] [Google Scholar]
- [18].Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, Grana O, Megias D, Dominguez O, Martinez D, Manzanares M, Ortega S, Serrano M, Reprogramming in vivo produces teratomas and iPS cells with totipotency features, Nature 502 (7471) (2013) 340–345. [DOI] [PubMed] [Google Scholar]
- [19].Ohnishi K, Semi K, Yamamoto T, Shimizu M, Tanaka A, Mitsunaga K, Okita K, Osafune K, Arioka Y, Maeda T, Soejima H, Moriwaki H, Yamanaka S, Woltjen K, Yamada Y, Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation, Cell 156 (4) (2014) 663–677. [DOI] [PubMed] [Google Scholar]
- [20].Mosteiro L, Pantoja C, Alcazar N, Marion RM, Chondronasiou D, Rovira M, Fernandez-Marcos PJ, Munoz-Martin M, Blanco-Aparicio C, Pastor J, Gomez-Lopez G, De Martino A, Blasco MA, Abad M, Serrano M, Tissue damage and senescence provide critical signals for cellular reprogramming in vivo, Science 354 (6315) (2016). [DOI] [PubMed] [Google Scholar]
- [21].Kurman RJ, Carcangiu ML, Herrington CS, Young RH, WHO Classification of Tumours of Female Reproductive Organ, 4th edition, Publisher: International Agency for Reseach on Cancer (IARC), Lyon, 2014. 2014. [Google Scholar]
- [22].Recamier J, Recherches Sur the Traitement Du Cancer: par La Compression Methodique Simple Ou Combinee, Er Sur I’Histoire General De La Meme Maladie, Paris, Gabon (1829). [Google Scholar]
- [23].Remak R, Ein beitrag zur entwickelungsgeschichte der krebshaften geschwulste, Deut Klin 6 (1854) 70–174. [Google Scholar]
- [24].Durente FN, Nesso fisio-pathologico tra la structtura dei nei materni e la genesi di alcuni tumori maligni, Arch Memori eed Osservazioni di Chirugi Practica 11 (1874) 217–226. [Google Scholar]
- [25].Cohnheim J, Ueber entzundung und eiterung, Pathologische Anatomie und Klinishe Medizin 40 (1867) 1–79 1867. [Google Scholar]
- [26].Bignold LP, Coghlan BL, Jersmann HP, Hansemann, Boveri, chromosomes and the gametogenesis-related theories of tumours, Cell Biol. Int 30 (7) (2006) 640–644. [DOI] [PubMed] [Google Scholar]
- [27].Bignold LP, Coghlan B, Jersmann H, David Paul Hansemann: chromosomes and the origin of the cancerous features of tumor cells, Cell. Oncol 31 (1) (2009) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Virchow R, Ueber Perlgeschwuelste (Cholesteratoma Joh. Mueller’s), Archie fur patholgische Anatomie und Physiologie und fur klinksche Medizin (1855) 371–415. [Google Scholar]
- [29].Beard J, Embryological aspects and etiology of carcinoma, Lancet 165 (4249) (1902) 281–283. [Google Scholar]
- [30].Maehle AH, Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries, Notes Rec. R. Soc. Lond 65 (4) (2011) 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bainbridge WS, The Cancer Problem, The Mcmillan Co., New York, 1914. [Google Scholar]
- [32].Stevens LC, Little CC, Spontaneous testicular teratomas in an inbred strain of mice, Proc. Natl. Acad. Sci. U. S. A 40 (11) (1954) 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stevens LC, Experimental production of testicular teratomas in mice, Proc. Natl. Acad. Sci. U. S. A 52 (1964) 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kleinsmith LJ, Pierce GB Jr., Multipotentiality of single embryonal carcinoma cells, Cancer Res 24 (1964) 1544–1551. [PubMed] [Google Scholar]
- [35].Pierce GB, Speers WC, Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation, Cancer Res 48 (8) (1988) 1996–2004. [PubMed] [Google Scholar]
- [36].Pierce GB, Nakane PK, Martinez-Hernandez A, Ward JM, Ultrastructural comparison of differentiation of stem cells of murine adenocarcinomas of colon and breast with their normal counterparts, J. Natl. Cancer Inst 58 (5) (1977) 1329–1345. [DOI] [PubMed] [Google Scholar]
- [37].Pierce GB, Wallace C, Differentiation of malignant to benign cells, Cancer Res 31 (2) (1971) 127–134. [PubMed] [Google Scholar]
- [38].Sell S, Pierce GB, Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers, Lab. Invest 70 (1) (1994) 6–22. [PubMed] [Google Scholar]
- [39].Sell S, On the stem cell origin of cancer, Am. J. Pathol 176 (6) (2010) 2584–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mintz B, Illmensee K, Normal genetically mosaic mice produced from malignant teratocarcinoma cells, Proc. Natl. Acad. Sci. U. S. A 72 (9) (1975) 3585–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Illmensee K, Mintz B, Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts, Proc. Natl. Acad. Sci. U. S. A 73 (2) (1976) 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Beutner R, Uber die Ursache der Neoplasie, J. Cancer Res. Oncol 24 (1926) 99–116. [Google Scholar]
- [43].Mintz B, Cronmiller C, Custer RP, Somatic cell origin of teratocarcinomas, Proc. Natl. Acad. Sci. U. S. A 75 (6) (1978) 2834–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Soto AM, Sonnenschein C, Emergentism as a default: cancer as a problem of tissue organization, J. Biosci 30 (1) (2005) 103–118. [DOI] [PubMed] [Google Scholar]
- [45].Baker SG, A cancer theory kerfuffle can lead to new lines of research, J. Natl. Cancer Inst 107 (2) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ, Cancer/testis antigens, gametogenesis and cancer. Nature reviews, Cancer 5 (8) (2005) 615–625. [DOI] [PubMed] [Google Scholar]
- [47].Old LJ, Cancer is a somatic cell pregnancy, Cancer Immun 7 (2007) 19. [PMC free article] [PubMed] [Google Scholar]
- [48].Vinnitsky V, The development of a malignant tumor is due to a desperate asexual self-cloning process in which cancer stem cells develop the ability to mimic the genetic program of germline cells, Intrinsically Disord. Proteins 2 (1) (2014) e29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Erenpreisa J, Salmina K, Huna A, Jackson TR, Vazquez-Martin A, Cragg MS, The “virgin birth”, polyploidy, and the origin of cancer, Oncoscience 2 (1) (2015) 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ratajczak MZ, Shin DM, Kucia M, Very small embryonic/epiblast-like stem cells: a missing link to support the germ line hypothesis of cancer development? Am. J. Pathol 174 (6) (2009) 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu C, Xu S, Ma Z, Zeng Y, Chen Z, Lu Y, Generation of pluripotent cancer-initiating cells from transformed bone marrow-derived cells, Cancer Lett 303 (2) (2011) 140–149. [DOI] [PubMed] [Google Scholar]
- [52].Liu C, Ma Z, Xu S, Hou J, Hu Y, Yu Y, Liu R, Chen Z, Lu Y, Activation of the germ-cell potential of human bone marrow-derived cells by a chemical carcinogen, Sci. Rep 4 (2014) 5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu C, Ma Z, Hou J, Zhang H, Liu R, Wu W, Liu W, Lu Y, Germline traits of human hepatoblastoma cells associated with growth and metastasis, Biochem. Biophys. Res. Commun 437 (1) (2013) 120–126. [DOI] [PubMed] [Google Scholar]
- [54].Becker AJ, Mc CE, Till JE, Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells, Nature 197 (1963) 452–454. [DOI] [PubMed] [Google Scholar]
- [55].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Cacerescortes J, Minden M, Paterson B, Caligiuri MA, Dick JE, A cell initiating human acute myeloid-leukemia after transplantation into scid mice, Nature 367 (6464) (1994) 645–648. [DOI] [PubMed] [Google Scholar]
- [56].Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM, Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells, Cancer Res 66 (19) (2006) 9339–9344. [DOI] [PubMed] [Google Scholar]
- [57].Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ, Efficient tumour formation by single human melanoma cells, Nature 456 (7222) (2008) 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ, Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized, Cancer Cell 18 (5) (2010) 510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Meacham CE, Morrison SJ, Tumour heterogeneity and cancer cell plasticity, Nature 501 (7467) (2013) 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kreso A, Dick JE, Evolution of the cancer stem cell model, Cell Stem Cell 14 (3) (2014) 275–291. [DOI] [PubMed] [Google Scholar]
- [61].Nguyen LV, Vanner R, Dirks P, Eaves CJ, Cancer stem cells: an evolving concept. Nature reviews, Cancer 12 (2) (2012) 133–143. [DOI] [PubMed] [Google Scholar]
- [62].Batlle E, Clevers H, Cancer stem cells revisited, Nat. Med 23 (2017) 1124. [DOI] [PubMed] [Google Scholar]
- [63].Beck B, Blanpain C, Unravelling cancer stem cell potential. Nature reviews, Cancer 13 (10) (2013) 727–738. [DOI] [PubMed] [Google Scholar]
- [64].Blanpain C, Simons BD, Unravelling stem cell dynamics by lineage tracing, Nat. Rev. Mol. Cell Biol 14 (8) (2013) 489–502. [DOI] [PubMed] [Google Scholar]
- [65].Yang T, Rycaj K, Liu ZM, Tang DG, Cancer stem cells: constantly evolving and functionally heterogeneous therapeutic targets, Cancer Res 74 (11) (2014) 2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Maenhaut C, Dumont JE, Roger PP, van Staveren WC, Cancer stem cells: a reality, a myth, a fuzzy concept or a misnomer? An analysis, Carcinogenesis 31 (2) (2010) 149–158. [DOI] [PubMed] [Google Scholar]
- [67].Antoniou A, Hebrant A, Dom G, Dumont JE, Maenhaut C, Cancer stem cells, a fuzzy evolving concept: a cell population or a cell property? Cell Cycle 12 (24) (2013) 3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Niu N, Mercado-Uribe I, Liu J, Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells, Oncogene 36 (2017) 4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Orr-Weaver TL, When bigger is better: the role of polyploidy in organogenesis, Trends Genet 31 (6) (2015) 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Scholes DR, Paige KN, Plasticity in ploidy: a generalized response to stress, Trends Plant Sci 20 (3) (2015) 165–175. [DOI] [PubMed] [Google Scholar]
- [71].Fox DT, Duronio RJ, Endoreplication and polyploidy: insights into development and disease, Development 140 (1) (2013) 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Edgar BA, Zielke N, Gutierrez C, Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth, Nat. Rev. Mol. Cell Biol 15 (3) (2014) 197–210. [DOI] [PubMed] [Google Scholar]
- [73].Comai L, The advantages and disadvantages of being polyploid, Nat. Rev. Genet 6 (11) (2005) 836–846. [DOI] [PubMed] [Google Scholar]
- [74].Carbone L, Chavez SL, Mammalian pre-implantation chromosomal instability: species comparison, evolutionary considerations, and pathological correlations, Syst. Biol. Reprod. Med 61 (6) (2015) 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Niakan KK, Han J, Pedersen RA, Simon C, Pera RA, Human pre-implantation embryo development, Development 139 (5) (2012) 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kligman I, Benadiva C, Alikani M, Munne S, The presence of multinucleated blastomeres in human embryos is correlated with chromosomal abnormalities, Hum. Reprod 11 (7) (1996) 1492–1498. [DOI] [PubMed] [Google Scholar]
- [77].Pickering SJ, Taylor A, Johnson MH, Braude PR, An analysis of multi-nucleated blastomere formation in human embryos, Hum. Reprod 10 (7) (1995) 1912–1922. [DOI] [PubMed] [Google Scholar]
- [78].Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, Debrock S, Amyere M, Vikkula M, Schuit F, Fryns JP, Verbeke G, D’Hooghe T, Moreau Y, Vermeesch JR, Chromosome instability is common in human cleavage-stage embryos, Nat. Med 15 (5) (2009) 577–583. [DOI] [PubMed] [Google Scholar]
- [79].Vera-Rodriguez M, Chavez SL, Rubio C, Reijo Pera RA, Simon C, Prediction model for aneuploidy in early human embryo development revealed by single-cell analysis, Nat. Commun 6 (2015) 7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Reichmann J, Nijmeijer B, Hossain MJ, Eguren M, Schneider I, Politi AZ, Roberti MJ, Hufnagel L, Hiiragi T, Ellenberg J, Dual-spindle formation in zygotes keeps parental genomes apart in early mammalian embryos, Science 361 (6398) (2018) 189–193. [DOI] [PubMed] [Google Scholar]
- [81].Daughtry BL, Chavez SL, Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences, Cell Tissue Res 363 (1) (2016) 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Levan A, Hauschka TS, Endomitotic reduplication mechanisms in ascites tumors of the mouse, J. Natl. Cancer Inst 14 (1) (1953) 1–43. [PubMed] [Google Scholar]
- [83].Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhang CZ, Wala J, Mermel CH, Sougnez C, Gabriel SB, Hernandez B, Shen H, Laird PW, Getz G, Meyerson M, Beroukhim R, Pan-cancer patterns of somatic copy number alteration, Nat. Genet 45 (10) (2013) 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Barrett MT, Pritchard D, Palanca-Wessels C, Anderson J, Reid BJ, Rabinovitch PS, Molecular phenotype of spontaneously arising 4N (G2-tetraploid) intermediates of neoplastic progression in Barrett’s esophagus, Cancer Res 63 (14) (2003) 4211–4217. [PubMed] [Google Scholar]
- [85].McCluggage WG, Lyness RW, Atkinson RJ, Dobbs SP, Harley I, McClelland HR, Price JH, Morphological effects of chemotherapy on ovarian carcinoma, J. Clin. Pathol 55 (1) (2002) 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Illidge TM, Cragg MS, Fringes B, Olive P, Erenpreisa JA, Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage, Cell Biol. Int 24 (9) (2000) 621–633. [DOI] [PubMed] [Google Scholar]
- [87].Wang Y, Wang Y, Zheng W, Cytologic changes of ovarian epithelial cancer induced by neoadjuvant chemotherapy, Int. J. Clin. Exp. Pathol 6 (10) (2013) 2121–2128. [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang L, Ding P, Lv H, Zhang D, Liu G, Yang Z, Li Y, Liu J, Zhang S, Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor, Biomed Res. Int (2014) 903542 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qu Y, Zhang L, Rong Z, He T, Zhang S, Number of glioma polyploid giant cancer cells (PGCCs) associated with vasculogenic mimicry formation and tumor grade in human glioma, J. Exp. Clin. Cancer Res 32 (2013) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fei F, Zhang D, Yang Z, Wang S, Wang X, Wu Z, Wu Q, Zhang S, The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer, J. Exp. Clin. Cancer Res 34 (1) (2015) 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mu Z, Wang C, Ye Z, Rossi G, Sun C, Li L, Zhu Z, Yang H, Cristofanilli M, Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer, Breast Cancer Res. Treat 165 (3) (2017) 733–741. [DOI] [PubMed] [Google Scholar]
- [92].Clawson GA, Matters GL, Xin P, Imamura-Kawasawa Y, Du Z, Thiboutot DM, Helm KF, Neves RI, Abraham T, Macrophage-tumor cell fusions from peripheral blood of melanoma patients, PLoS One 10 (8) (2015) e0134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu H, Sun B, Wang S, Liu C, Lu Y, Li D, Liu X, Circulating tumor cells as a biomarker in pancreatic ductal adenocarcinoma, Cell. Physiol. Biochem 42 (1) (2017) 373–382. [DOI] [PubMed] [Google Scholar]
- [94].Rantala JK, Edgren H, Lehtinen L, Wolf M, Kleivi K, Vollan HK, Aaltola AR, Laasola P, Kilpinen S, Saviranta P, Iljin K, Kallioniemi O, Integrative functional genomics analysis of sustained polyploidy phenotypes in breast cancer cells identifies an oncogenic profile for GINS2, Neoplasia (New York N.Y.) 12 (11) (2010) 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vitale I, Galluzzi L, Senovilla L, Criollo A, Jemaa M, Castedo M, Kroemer G, Illicit survival of cancer cells during polyploidization and depolyploidization, Cell Death Differ 18 (9) (2011) 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Walen KH, Human diploid fibroblast cells in senescence; cycling through polyploidy to mitotic cells, In Vitro Cell. Dev. Biol. Anim 42 (7) (2006) 216–224. [DOI] [PubMed] [Google Scholar]
- [97].Vakifahmetoglu H, Olsson M, Zhivotovsky B, Death through a tragedy: mitotic catastrophe, Cell Death Differ 15 (7) (2008) 1153–1162. [DOI] [PubMed] [Google Scholar]
- [98].Ganem NJ, Storchova Z, Pellman D, Tetraploidy, aneuploidy and cancer, Curr. Opin. Genet. Dev 17 (2) (2007) 157–162. [DOI] [PubMed] [Google Scholar]
- [99].Ganem NJ, Pellman D, Limiting the proliferation of polyploid cells, Cell 131 (3) (2007) 437–440. [DOI] [PubMed] [Google Scholar]
- [100].Wen Q, Goldenson B, Silver SJ, Schenone M, Dancik V, Huang Z, Wang LZ, Lewis TA, An WF, Li X, Bray MA, Thiollier C, Diebold L, Gilles L, Vokes MS, Moore CB, Bliss-Moreau M, Verplank L, Tolliday NJ, Mishra R, Vemula S, Shi J, Wei L, Kapur R, Lopez CK, Gerby B, Ballerini P, Pflumio F, Gilliland DG, Goldberg L, Birger Y, Izraeli S, Gamis AS, Smith FO, Woods WG, Taub J, Scherer CA, Bradner JE, Goh BC, Mercher T, Carpenter AE, Gould RJ, Clemons PA, Carr SA, Root DE, Schreiber SL, Stern AM, Crispino JD, Identification of regulators of polyploidization presents therapeutic targets for treatment of AMKL, Cell 150 (3) (2012) 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ewald JA, Desotelle JA, Wilding G, Jarrard DF, Therapy-induced senescence in cancer, J. Natl. Cancer Inst 102 (20) (2010) 1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhang S, Zhou K, Luo X, Li L, Tu HC, Sehgal A, Nguyen LH, Zhang Y, Gopal P, Tarlow BD, Siegwart DJ, Zhu H, The polyploid state plays a tumor-suppressive role in the liver, Dev. Cell 44 (4) (2018) 447–459 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D, Cytokinesis failure triggers hippo tumor suppressor pathway activation, Cell 158 (4) (2014) 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Solari F, Domenget C, Gire V, Woods C, Lazarides E, Rousset B, Jurdic P, Multinucleated cells can continuously generate mononucleated cells in the absence of mitosis: a study of cells of the avian osteoclast lineage, J. Cell. Sci 108 (10) (1995) 3233–3241. [DOI] [PubMed] [Google Scholar]
- [105].Erenpreisa JA, Cragg MS, Fringes B, Sharakhov I, Illidge TM, Release of mitotic descendants by giant cells from irradiated Burkitt’s lymphoma cell line, Cell Biol. Int 24 (9) (2000) 635–648. [DOI] [PubMed] [Google Scholar]
- [106].Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R, Neosis: a novel type of cell division in cancer, Cancer Biol. Ther 3 (2) (2004) 207–218. [DOI] [PubMed] [Google Scholar]
- [107].Walen KH, Spontaneous cell transformation: karyoplasts derived from multi-nucleated cells produce new cell growth in senescent human epithelial cell cultures, In Vitro Cell. Dev. Biol.- Anim 40 (5–6) (2004) 150–158. [DOI] [PubMed] [Google Scholar]
- [108].Walen KH, The origin of transformed cells: studies of spontaneous and induced cell transformation in cell cultures from marsupials, a snail, and human amniocytes, Cancer Genet. Cytogenet 133 (1) (2002) 45–54. [DOI] [PubMed] [Google Scholar]
- [109].Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J, Generation of cancer stem-like cells through the formation of polyploid giant cancer cells, Oncogene 33 (1) (2014) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Weihua Z, Lin Q, Ramoth AJ, Fan D, Fidler IJ, Formation of solid tumors by a single multinucleated cancer cell, Cancer 117 (17) (2011) 4092–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rohnalter V, Roth K, Finkernagel F, Adhikary T, Obert J, Dorzweiler K, Bensberg M, Muller-Brusselbach S, Muller R, A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype, Oncotarget (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Puig PE, Guilly MN, Bouchot A, Droin N, Cathelin D, Bouyer F, Favier L, Ghiringhelli F, Kroemer G, Solary E, Martin F, Chauffert B, Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy, Cell Biol. Int 32 (9) (2008) 1031–1043. [DOI] [PubMed] [Google Scholar]
- [113].Niu N, Zhang J, Zhang N, Mercado-Uribe I, Tao F, Han Z, Pathak S, Multani AS, Kuang J, Yao J, Bast RC, Sood AK, Hung MC, Liu J, Linking genomic reorganization to tumor initiation via the giant cell cycle, Oncogenesis 5 (12) (2016) e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mirzayans R, Andrais B, Scott A, Wang YW, Kumar P, Murray D, Multinucleated giant cancer cells produced in response to ionizing radiation retain viability and replicate their genome, Int. J. Mol. Sci 18 (2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Díaz-Carballo D, Saka S, Klein J, Rennkamp T, Acikelli AH, Malak S, Jastrow H, Wennemuth G, Tempfer C, Schmitz I, Tannapfel A, Strumberg D, A distinct oncogenerative multinucleated cancer cell serves as a source of stemness and tumor heterogeneity, Cancer Res 78 (9) (2018) 2318–2331. [DOI] [PubMed] [Google Scholar]
- [116].Zheng L, Dai H, Zhou M, Li X, Liu C, Guo Z, Wu X, Wu J, Wang C, Zhong J, Huang Q, Garcia-Aguilar J, Pfeifer GP, Shen B, Polyploid cells rewire DNA damage response networks to overcome replication stress-induced barriers for tumour progression, Nat. Commun 3 (2012) 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Leikam C, Hufnagel AL, Otto C, Murphy DJ, Muhling B, Kneitz S, Nanda I, Schmid M, Wagner TU, Haferkamp S, Brocker EB, Schartl M, Meierjohann S, In vitro evidence for senescent multinucleated melanocytes as a source for tumor-initiating cells, Cell Death Dis 6 (2015) e1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D, Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells, Nature 437 (7061) (2005) 1043–1047. [DOI] [PubMed] [Google Scholar]
- [119].Davoli T, de Lange T, The causes and consequences of polyploidy in normal development and cancer, Annu. Rev. Cell Dev. Biol 27 (2011) 585–610. [DOI] [PubMed] [Google Scholar]
- [120].Vitale I, Senovilla L, Jemaa M, Michaud M, Galluzzi L, Kepp O, Nanty L, Criollo A, Rello-Varona S, Manic G, Metivier D, Vivet S, Tajeddine N, Joza N, Valent A, Castedo M, Kroemer G, Multipolar mitosis of tetraploid cells: inhibition by p53 and dependency on Mos, EMBO J 29 (7) (2010) 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Erenpreisa J, Salmina K, Huna A, Kosmacek EA, Cragg MS, Ianzini F, Anisimov AP, Polyploid tumour cells elicit paradiploid progeny through depolyploidizing divisions and regulated autophagic degradation, Cell Biol. Int 35 (7) (2011) 687–695. [DOI] [PubMed] [Google Scholar]
- [122].Mittal K, Donthamsetty S, Kaur R, Yang C, Gupta MV, Reid MD, Choi DH, Rida PCG, Aneja R, Multinucleated polyploidy drives resistance to docetaxel chemotherapy in prostate cancer, Br. J. Cancer 116 (2017) 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dewhurst SM, McGranahan N, Burrell RA, Rowan AJ, Grönroos E, Endesfelder D, Joshi T, Mouradov D, Gibbs P, Ward RL, Hawkins NJ, Szallasi Z, Sieber OM, Swanton C, Tolerance of whole-genome doubling propagates chromosomal instability and accelerates Cancer genome evolution, Cancer Discov 4 (2) (2014) 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Mirzayans R, Andrais B, Murray D, Roles of polyploid/multinucleated giant cancer cells in metastasis and diesease relapse following anticancer treatment, Cancers 10 (4) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ogden A, Rida PC, Reid MD, Kucuk O, Aneja R, Die-hard survivors: heterogeneity in apoptotic thresholds may underlie chemoresistance, Expert Rev. Anticancer Ther 15 (3) (2015) 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ogden A, Rida PC, Knudsen BS, Kucuk O, Aneja R, Docetaxel-induced polyploidization may underlie chemoresistance and disease relapse, Cancer Lett 367 (2) (2015) 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhang D, Yang X, Yang Z, Fei F, Li S, Qu J, Zhang M, Li Y, Zhang X, Zhang S, Daughter cells and erythroid cells budding from PGCCs and their clinicopathological significances in colorectal cancer, J. Cancer 8 (3) (2017) 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Erenpreisa J, Kalejs M, Cragg MS, Mitotic catastrophe and endomitosis in tumour cells: an evolutionary key to a molecular solution, Cell Biol. Int 29 (12) (2005) 1012–1018. [DOI] [PubMed] [Google Scholar]
- [129].Tamori Y, Deng WM, Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia, Dev. Cell 25 (4) (2013) 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gentric G, Desdouets C, Polyploidization in liver tissue, Am. J. Pathol 184 (2) (2014) 322–331. [DOI] [PubMed] [Google Scholar]
- [131].Lucchetta EM, Ohlstein B, Amitosis of polyploid cells regenerates functional stem cells in the Drosophila intestine, Cell Stem Cell 20 (5) (2017) 609–620 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cong B, Ohsawa S, Igaki T, JNK and Yorkie drive tumor progression by generating polyploid giant cells in Drosophila, Oncogene 37 (23) (2018) 3088–3097. [DOI] [PubMed] [Google Scholar]
- [133].Boroviak T, Nichols J, The birth of embryonic pluripotency, Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 369 (1657) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Munoz-Espin D, Serrano M, Cellular senescence: from physiology to pathology, Nat. Rev. Mol. Cell Biol 15 (7) (2014) 482–496. [DOI] [PubMed] [Google Scholar]
- [135].Niculescu V, Carcinogenesis: recent insights in protist stem cell biology lead to a better understanding of atavistic mechanisms implied in cancer development, MOJ Tumor Res 1 (1) (2018) 18–29. [Google Scholar]
- [136].Raghuram GV, Gupta D, Subramaniam S, Gaikwad A, Khare NK, Nobre M, Nair NK, Mittal I, Physical shearing imparts biological activity to DNA and ability to transmit itself horizontally across species and kingdom boundaries, BMC Mol. Biol 18 (1) (2017) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Noubissi FK, Ogle BM, Cancer cell fusion: mechanisms slowly unravel, Int. J. Mol. Sci 17 (9) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bastida-Ruiz D, Van Hoesen K, Cohen M, The dark side of cell fusion, Int. J. Mol. Sci 17 (5) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rengstl B, Newrzela S, Heinrich T, Weiser C, Thalheimer FB, Schmid F, Warner K, Hartmann S, Schroeder T, Kuppers R, Rieger MA, Hansmann ML, Incomplete cytokinesis and re-fusion of small mononucleated Hodgkin cells lead to giant multinucleated Reed-Sternberg cells, Proc. Natl. Acad. Sci. U. S. A 110 (51) (2013) 20729–20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Pellestor F, Gatinois V, Puechberty J, Genevieve D, Lefort G, Chromothripsis: potential origin in gametogenesis and preimplantation cell divisions. A review, Fertil. Steril 102 (6) (2014) 1785–1796. [DOI] [PubMed] [Google Scholar]
- [141].Zhang S, Mercado-Uribe I, Sood A, Bast RC, Liu J, Coevolution of neoplastic epithelial cells and multilineage stroma via polyploid giant cells during immortalization and transformation of mullerian epithelial cells, Genes Cancer 7 (3–4) (2016) 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zhang S, Mercado-Uribe I, Liu J, Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel, Int. J. Cancer 134 (3) (2014) 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang S, Mercado-Uribe I, Liu J, Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo, Cancer Lett 333 (2) (2013) 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Jia L, Zhang S, Ye Y, Li X, Mercado-Uribe I, Bast RC Jr., J. Liu, Paclitaxel inhibits ovarian tumor growth by inducing epithelial cancer cells to benign fibroblast-like cells, Cancer Lett 326 (2) (2012) 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Was H, Czarnecka J, Kominek A, Barszcz K, Bernas T, Piwocka K, Kaminska B, Some chemotherapeutics-treated colon cancer cells display a specific phenotype being a combination of stem-like and senescent cell features, Cancer Biol. Ther 19 (1) (2018) 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhang S, Mercado-Uribe I, Hanash S, Liu J, iTRAQ-based proteomic analysis of polyploid giant cancer cells and budding progeny cells reveals several distinct pathways for ovarian cancer development, PLoS One 8 (11) (2013) e80120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Vazquez-Martin A, Anatskaya OV, Giuliani A, Erenpreisa J, Huang S, Salmina K, Inashkina I, Huna A, Nikolsky NN, Vinogradov AE, Somatic polyploidy is associated with the upregulation of c-MYC interacting genes and EMT-like signature, Oncotarget 7 (46) (2016) 75235–75260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Salmina K, Jankevics E, Huna A, Perminov D, Radovica I, Klymenko T, Ivanov A, Jascenko E, Scherthan H, Cragg M, Erenpreisa J, Up-regulation of the embryonic self-renewal network through reversible polyploidy in irradiated p53-mutant tumour cells, Exp. Cell Res 316 (13) (2010) 2099–2112. [DOI] [PubMed] [Google Scholar]
- [149].Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA, An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors, Nat. Genet 40 (5) (2008) 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kim J, Orkin SH, Embryonic stem cell-specific signatures in cancer: insights into genomic regulatory networks and implications for medicine, Genome Med 3 (11) (2011) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Beltran AS, Rivenbark AG, Richardson BT, Yuan X, Quian H, Hunt JP, Zimmerman E, Graves LM, Blancafort P, Generation of tumor-initiating cells by exogenous delivery of OCT4 transcription factor, Breast Cancer Res.: BCR 13 (5) (2011) R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Ye X, Weinberg RA, Epithelial-mesenchymal plasticity: a central regulator of Cancer progression, Trends Cell Biol 25 (11) (2015) 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chaffer CL, Weinberg RA, How does multistep tumorigenesis really proceed? Cancer Discov 5 (1) (2015) 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Smith ZD, Shi J, Gu H, Donaghey J, Clement K, Cacchiarelli D, Gnirke A, Michor F, Meissner A, Epigenetic restriction of extraembryonic lineages mirrors the somatic transition to cancer, Nature 549 (7673) (2017) 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Milanovic M, Fan DNY, Belenki D, Dabritz JHM, Zhao Z, Yu Y, Dorr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA, Mendoza-Parra MA, Kanashova T, Metzner M, Pardon K, Reimann M, Trumpp A, Dorken B, Zuber J, Gronemeyer H, Hummel M, Dittmar G, Lee S, Schmitt CA, Senescence-associated reprogramming promotes cancer stemness, Nature 553 (7686) (2018) 96–100. [DOI] [PubMed] [Google Scholar]
- [156].Palmer NP, Schmid PR, Berger B, Kohane IS, A gene expression profile of stem cell pluripotentiality and differentiation is conserved across diverse solid and hematopoietic cancers, Genome Biol 13 (8) (2012) R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kaminska B, Huelsken J, Omberg L, Gevaert O, Colaprico A, Czerwinska P, Mazurek S, Mishra L, Heyn H, Krasnitz A, Godwin AK, Lazar AJ, Cancer Genome Atlas Research, N, J.M. Stuart, K.A. Hoadley, P.W. Laird, H. Noushmehr, M. Wiznerowicz, Machine learning identifies stemness features associated with oncogenic dedifferentiation, Cell 173 (2) (2018) 338–354 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM, Dedifferentiation of neurons and astrocytes by onco-genes can induce gliomas in mice, Science 338 (6110) (2012) 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, Curry WT, Martuza RL, Rivera MN, Rossetti N, Kasif S, Beik S, Kadri S, Tirosh I, Wortman I, Shalek AK, Rozenblatt-Rosen O, Regev A, Louis DN, Bernstein BE, Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells, Cell 157 (3) (2014) 580–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Goding CR, Pei D, Lu X, Cancer: pathological nuclear reprogramming? Nature reviews, Cancer 14 (8) (2014) 568–573. [DOI] [PubMed] [Google Scholar]
- [161].Vincent M, Cancer: a de-repression of a default survival program common to all cells?: a life-history perspective on the nature of cancer, BioEssays 34 (1) (2012) 72–82. [DOI] [PubMed] [Google Scholar]
- [162].Lineweaver CH, Davies PC, Vincent MD, Targeting cancer’s weaknesses (not its strengths): therapeutic strategies suggested by the atavistic model, BioEssays 36 (9) (2014) 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Trigos AS, Pearson RB, Papenfuss AT, Goode DL, Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors, Proc. Natl. Acad. Sci. U. S. A 114 (24) (2017) 6406–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chavez SL, Loewke KE, Han J, Moussavi F, Colls P, Munne S, Behr B, Reijo Pera RA, Dynamic blastomere behaviour reflects human embryo ploidy by the four-cell stage, Nat. Commun 3 (2012) 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Grow EJ, Flynn RA, Chavez SL, Bayless NL, Wossidlo M, Wesche DJ, Martin L, Ware CB, Blish CA, Chang HY, Pera RA, Wysocka J, Intrinsic retroviral reactivation in human preimplantation embryos and pluripotent cells, Nature 522 (7555) (2015) 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D, Chromothripsis from DNA damage in micronuclei, Nature 522 (7555) (2015) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhang CZ, Leibowitz ML, Pellman D, Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements, Genes Dev 27 (23) (2013) 2513–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal S, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Quail MA, Burton J, Swerdlow H, Carter NP, Morsberger LA, Iacobuzio-Donahue C, Follows GA, Green AR, Flanagan AM, Stratton MR, Futreal PA, Campbell PJ, Massive genomic rearrangement acquired in a single catastrophic event during cancer development, Cell 144 (1) (2011) 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Neiman M, Beaton MJ, Hessen DO, Jeyasingh PD, Weider LJ, Endopolyploidy as a potential driver of animal ecology and evolution, Biol. Rev. Camb. Philos. Soc (2015). [DOI] [PubMed] [Google Scholar]
- [170].McClintock B, The significance of responses of the genome to challenge, Science 226 (4676) (1984) 792–801. [DOI] [PubMed] [Google Scholar]
- [171].McClintock B, The origin and behavior of mutable loci in maize, Proc. Natl. Acad. Sci. U. S. A 36 (6) (1950) 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].McClintock B, The stability of broken ends of chromosomes in Zea Mays, Genetics 26 (2) (1941) 234–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Horne SD, Chowdhury SK, Heng HH, Stress, genomic adaptation, and the evolutionary trade-off, Front. Genet 5 (2014) 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Heng HH, The genome-centric concept: resynthesis of evolutionary theory, BioEssays 31 (5) (2009) 512–525. [DOI] [PubMed] [Google Scholar]
- [175].Huang S, Non-genetic heterogeneity of cells in development: more than just noise, Development 136 (23) (2009) 3853–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Versteeg R, Cancer: tumours outside the mutation box, Nature 506 (7489) (2014) 438–439. [DOI] [PubMed] [Google Scholar]
- [177].Mack SC, Witt H, Piro RM, Gu L, Zuyderduyn S, Stutz AM, Wang X, Gallo M, Garzia L, Zayne K, Zhang X, Ramaswamy V, Jager N, Jones DT, Sill M, Pugh TJ, Ryzhova M, Wani KM, Shih DJ, Head R, Remke M, Bailey SD, Zichner T, Faria CC, Barszczyk M, Stark S, Seker-Cin H, Hutter S, Johann P, Bender S, Hovestadt V, Tzaridis T, Dubuc AM, Northcott PA, Peacock J, Bertrand KC, Agnihotri S, Cavalli FM, Clarke I, Nethery-Brokx K, Creasy CL, Verma SK, Koster J, Wu X, Yao Y, Milde T, Sin-Chan P, Zuccaro J, Lau L, Pereira S, Castelo-Branco P, Hirst M, Marra MA, Roberts SS, Fults D, Massimi L, Cho YJ, Van Meter T, Grajkowska W, Lach B, Kulozik AE, von Deimling A, Witt O, Scherer SW, Fan X, Muraszko KM, Kool M, Pomeroy SL, Gupta N, Phillips J, Huang A, Tabori U, Hawkins C, Malkin D, Kongkham PN, Weiss WA, Jabado N, Rutka JT, Bouffet E, Korbel JO, Lupien M, Aldape KD, Bader GD, Eils R, Lichter P, Dirks PB, Pfister SM, Korshunov A, Taylor MD, Epigenomic alterations define lethal CIMP-positive ependymomas of infancy, Nature 506 (7489) (2014) 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Pierce GB, The cancer cell and its control by the embryo. Rous-Whipple award lecture, Am. J. Pathol 113 (1) (1983) 117–124. [PMC free article] [PubMed] [Google Scholar]
- [179].Biava PMB, Daniele, Cancer and cell differentiation: a model to explain malignancy, J. Tumor Marker Oncol 17 (3) (2002) 47–54. [Google Scholar]
- [180].Bussey KJ, Cisneros LH, Lineweaver CH, Davies PCW, Ancestral gene regulatory networks drive cancer, Proc. Natl. Acad. Sci. U. S. A 114 (24) (2017) 6160–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M, Mulligan AM, Couch FJ, Engel C, McGuffog L, Healey S, Sinilnikova OM, Southey MC, Terry MB, Goldgar D, O’Malley F, John EM, Janavicius R, Tihomirova L, Hansen TV, Nielsen FC, Osorio A, Stavropoulou A, Benitez J, Manoukian S, Peissel B, Barile M, Volorio S, Pasini B, Dolcetti R, Putignano AL, Ottini L, Radice P, Hamann U, Rashid MU, Hogervorst FB, Kriege M, van der Luijt RB, Peock S, Frost D, Evans DG, Brewer C, Walker L, Rogers MT, Side LE, Houghton C, Weaver J, Godwin AK, Schmutzler RK, Wappenschmidt B. Meindl A, Kast K, Arnold N, Niederacher D, Sutter C, Deissler H, Gadzicki D, Preisler-Adams S, Varon-Mateeva R, Schonbuchner I, Gevensleben H, Stoppa-Lyonnet D, Belotti M, Barjhoux L, Isaacs C, Peshkin BN, Caldes T, de la Hoya M, Canadas C, Heikkinen T, Heikkila P, Aittomaki K, Blanco I, Lazaro C, Brunet J, Agnarsson BA, Arason A, Barkardottir RB, Dumont M, Simard J, Montagna M, Agata S, D’Andrea E, Yan M, Fox S, Rebbeck TR, Rubinstein W, Tung N, Garber JE, Wang X, Fredericksen Z, Pankratz VS, Lindor NM, Szabo C, Offit K, Sakr R, Gaudet MM, Singer CF, Tea MK, Rappaport C, Mai PL, Greene MH, Sokolenko A, Imyanitov E, Toland AE, Senter L, Sweet K, Thomassen M, Gerdes AM, Kruse T, Caligo M, Aretini P, Rantala J, von Wachenfeld A, Henriksson K, Steele L, Neuhausen SL, Nussbaum R, Beattie M, Odunsi K, Sucheston L, Gayther SA, Nathanson K, Gross J, Walsh C, Karlan B, Chenevix-Trench G, Easton DF, Antoniou AC, Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA), Cancer Epidemiol. Biomark. Prevent 21 (1) (2012) 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Feinberg AP, The key role of epigenetics in human disease prevention and mitigation, N. Engl. J. Med 378 (14) (2018) 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D, Curtis C, A Big Bang model of human colorectal tumor growth, Nat. Genet 47 (3) (2015) 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Hosseini H, Obradovic MM, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Guzvic M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, Klein CA, Early dissemination seeds metastasis in breast cancer, Nature 540 (22/29) (2016) 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Notta F, Chan-Seng-Yue M, Lemire M, Li Y, Wilson GW, Connor AA, Denroche RE, Liang SB, Brown AM, Kim JC, Wang T, Simpson JT, Beck T, Borgida A, Buchner N, Chadwick D, Hafezi-Bakhtiari S, Dick JE, Heisler L, Hollingsworth MA, Ibrahimov E, Jang GH, Johns J, Jorgensen LG, Law C, Ludkovski O, Lungu I, Ng K, Pasternack D, Petersen GM, Shlush LI, Timms L, Tsao MS, Wilson JM, Yung CK, Zogopoulos G, Bartlett JM, Alexandrov LB, Real FX, Cleary SP, Roehrl MH, McPherson JD, Stein LD, Hudson TJ, Campbell PJ, Gallinger S, A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns, Nature 538 (7625) (2016) 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Clevers H, The cancer stem cell: premises, promises and challenges, Nat. Med 17 (3) (2011) 313–319. [DOI] [PubMed] [Google Scholar]
- [187].Jiang Q, Zhang Q, Wang S, Xie S, Fang W, Liu Z, Liu J, Yao K, A fraction of CD133+ CNE2 cells is made of giant cancer cells with morphological evidence of asymmetric mitosis, J. Cancer 6 (12) (2015) 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Lambert AW, Pattabiraman DR, Weinberg RA, Emerging biological principles of metastasis, Cell 168 (4) (2017) 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H, EMT and MET: necessary or permissive for metastasis? Mol. Oncol 11 (7) (2017) 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Brabletz T, Kalluri R, Nieto MA, Weinberg RA, EMT in cancer. Nature reviews, Cancer 18 (2) (2018) 128–134. [DOI] [PubMed] [Google Scholar]
- [191].Simi AK, Anlas AA, Stallings-Mann M, Zhang S, Hsia M Cichon, D.C. Radisky, C.M. Nelson, A soft microenvironment protects from failure of midbody abscission and multinucleation downstream of the EMT-promoting transcription factor Snail, Cancer Res 78 (9) (2018) 2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Campisi J, Aging, cellular senescence, and cancer, Annu. Rev. Physio 75 (2013) 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Shay JW, Wright WE, Senescence and immortalization: role of telomeres and telomerase, Carcinogenesis 26 (5) (2005) 867–874. [DOI] [PubMed] [Google Scholar]
- [194].Hahn WC, Immortalization and transformation of human cells, Mol. Cells 13 (3) (2002) 351–361. [PubMed] [Google Scholar]
- [195].Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, Johnson S, Ivanova E, Kost-Alimova M, Protopopov A, Wang YA, Shirihai OS, Chin L, DePinho RA, Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer, Cell 148 (4) (2012) 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, Hu J, Wang W, Xiao Y, Zhang H, Zhang J, Gan B, Perry SR, Jiang S, Li L, Horner JW, Wang YA, Chin L, DePinho RA, Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases, Cell 148 (5) (2012) 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Warburg O, On the origin of cancer cells, Science 123 (3191) (1956) 309–314. [DOI] [PubMed] [Google Scholar]
- [198].Liberti MV, Locasale JW, The warburg effect: how does it benefit cancer cells? Trends Biochem. Sci 41 (3) (2016) 211–218.26778478 [Google Scholar]
- [199].Seyfried TN, Cancer as a mitochondrial metabolic disease, Front. Cell Dev. Biol 3 (2015) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Braune EB, Tsoi YL, Phoon YP, Landor S, Silva Cascales H, Ramskold D, Deng Q, Lindqvist A, Lian X, Sahlgren C, Jin SB, Lendahl U, Loss of CSL unlocks a hypoxic response and enhanced tumor growth potential in breast Cancer cells, Stem Cell Rep 6 (5) (2016) 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kurman RJ, Shih Ie M, The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded, Am. J. Pathol 186 (4) (2016) 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Lissa D, Senovilla L, Rello-Varona S, Vitale I, Michaud M, Pietrocola F, Boileve A, Obrist F, Bordenave C, Garcia P, Michels J, Jemaa M, Kepp O, Castedo M, Kroemer G, Resveratrol and aspirin eliminate tetraploid cells for anticancer chemoprevention, Proc. Natl. Acad. Sci. U. S. A 111 (8) (2014) 3020–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Ohashi A, Ohori M, Iwai K, Nakayama Y, Nambu T, Morishita D, Kawamoto T, Miyamoto M, Hirayama T, Okaniwa M, Banno H, Ishikawa T, Kandori H, Iwata K, Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells, Nat. Commun 6 (2015) 7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Kooreman NG, Kim Y, de Almeida PE, Termglinchan V, Diecke S, Shao NY, Wei TT, Yi H, Dey D, Nelakanti R, Brouwer TP, Paik DT, Sagiv-Barfi I, Han A, Quax PHA, Hamming JF, Levy R, Davis MM, Wu JC, Autologous iPSC-Based vaccines elicit anti-tumor responses in vivo, Cell Stem Cell 22 (4) (2018) 501–513 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Pierce GB, Johnson LD, Differentiation and cancer, In Vitro 7 (3) (1971) 140–145. [DOI] [PubMed] [Google Scholar]
- [206].Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, Wiernik PH, Alltrans-retinoic acid in acute promyelocytic leukemia, N. Engl. J. Med 337 (15) (1997) 1021–1028. [DOI] [PubMed] [Google Scholar]
- [207].Takahashi K, Yamanaka S, A developmental framework for induced pluripotency, Development 142 (19) (2015) 3274–3285. [DOI] [PubMed] [Google Scholar]
- [208].Hardy K, Winston RM, Handyside AH, Binucleate blastomeres in preimplantation human embryos in vitro: failure of cytokinesis during early cleavage, J. Reprod. Fertil 98 (2) (1993) 549–558. [DOI] [PubMed] [Google Scholar]
- [209].Meyer TJ, Rosenkrantz JL, Carbone L, Chavez SL, Endogenous retroviruses: with us and against us, Front. Chem 5 (2017) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Briggs SF, Dominguez AA, Chavez SL, Reijo Pera RA, Single-cell XIST expression in human preimplantation embryos and newly reprogrammed female induced pluripotent stem cells, Stem Cells 33 (6) (2015) 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]