Abstract
Most cellular processes descend into failure during aging. While a large collection of longevity pathways has been identified in the past decades, the mechanism for age-related decline of cellular homeostasis and organelle function remains largely unsolved. It is known that many organelles undergo structural and functional changes during normal aging, which significantly contributes to the decline of tissue function at old ages. Since recent studies have revealed an emerging role of organelles as regulatory hubs in maintaining cellular homeostasis, understanding of organelle aging will provide important insights into the cellular basis of organismal aging. Here we review current progress on the characterization of age-dependent structural and functional alterations in the more well-studied organelles, as well as the known mechanisms governing organelle aging in model organisms, with a special focus on the fruit fly Drosophila melanogaster.
Keywords: Longevity, Mitochondria, Nucleus, Autophagosome, Lysosome, Proteasome, Cell membrane
1. Introduction
Aging is acknowledged as a complex trait that is influenced by both intrinsic and extrinsic factors (Sutphin and Korstanje, 2016). Over the past two decades, hundreds of longevity genes and pathways have been identified in model organisms (Kenyon, 2010). These longevity factors are involved in nutrition and metabolic pathways (Kenyon et al., 1993; Tatar et al., 2001; Kaeberlein et al., 2005), stress response (Hsu et al., 2003; Morley and Morimoto, 2004; Tullet et al., 2008), inflammatory signaling (Zhang et al., 2013), and epigenetic regulation (Rogina et al., 2002). It has become clear that these factors do not work alone. For example, the cross-talks between insulin/insulin-like growth factor (IGF) and SKN-1 (Skinhead family member 1 )/NFE2-related factor 2 (Nrf2) detoxification pathways (Tullet et al., 2008), and between FOXO (forkhead box protein O) and TGF-beta signaling pathways (Bai et al., 2013), have been shown to play an important role in longevity control. These longevity factors also contribute significantly to cellular maintenance and tissue homeostasis. In particular, they play crucial roles in coordinating organelle functions under stress and normal aging. Advances in organelle biology have revealed an emerging role of organelles as regulatory hubs in maintaining cellular homeostasis, and the coordination among different cellular compartments plays an important role in tissue aging (Gough, 2016). It is evident that distinct structural and functional changes can be observed in aging organelles. Interestingly, the age-related organelle impairments are often cell-type- and tissue-specific. Due to their key role in cellular homeostasis, understanding the mechanisms underlying age-related alterations in organelle function will help decipher the cause of organismal aging. In this review, we will summarize the age-related structural and functional changes of several well-studied organelles in Drosophila and other model organisms.
2. The mitochondria
2.1. Mitochondrial ROS
Mitochondria are the power house of the cell, which produce most of the ATP in non-photosynthetic eukaryotes through oxidative phosphorylation (Fig. 1). As the primary site for oxygen consumption, mitochondria are also the major source of the reactive oxygen species (ROS) in many cell types, due to the leakage of electrons from electron transport chain (ETC) complexes. Thus, it is not surprising that mitochondrial dysfunction, increased superoxide radical production, and elevated oxidative stress are the major hallmarks of aging (López-Otín et al., 2013).
Fig. 1.
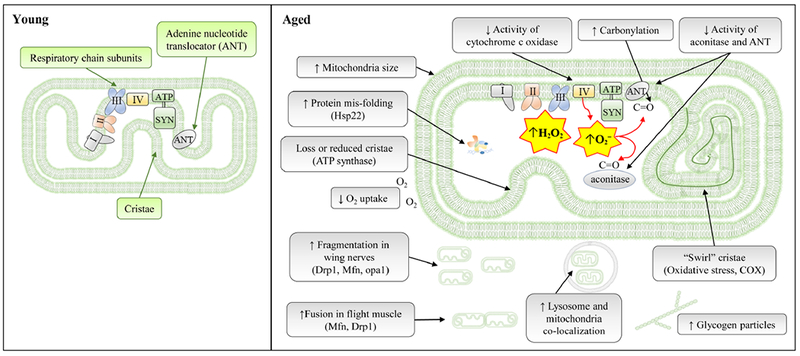
Schematic diagram to summarize the structural and functional changes in aging mitochondria. Signaling pathways responsible for age-related changes are indicated inside parentheses. See text for details.
The generation of ROS in the mitochondrial matrix has been assumed to be a major contributor to oxidative stress and age-related pathology (Harman, 1956; Liochev, 2013). However, the causal link between the average level of mitochondrial ROS and aging is not direct. Many types of ROS, such as hydrogen peroxide (H2O2), play important roles in redox signaling, independently of oxidative damage. Thus, accumulation of oxidative damage is due to changes in protection, damage repair, turnover, and compromised redox signaling, instead of increases in ROS levels. There is an age-dependent increase in mitochondrial H2O2 levels in aged female and male Drosophila (56-day-old) (Ferguson et al., 2005; Cocheme et al., 2011), detected by an engineered ratiometric mass spectrometry probe targeted to mitochondrial matrix. (Cocheme et al., 2011; Cocheme et al., 2012). The possible explanation for increased H2O2 is defective ETC activity (discussed in the following section), or decreased physical activities of elderly flies (Murphy, 2009; Cocheme et al., 2011). However, despite of its lifespan extension effect, dietary restriction did not affect H2O2 (Cocheme et al., 2011). ROS restricted to certain locations, such as cytosol and peroxisome, may play a more important role in lifespan. In addition, tissue-specific mitochondrial changes may be critical during the aging process. To detect tissue-dependent changes in H2O2 levels, new H2O2 and glutathione reporter lines have been generated by fusing redox-sensitive GFPs (roGFPs) to either H2O2 sensor oxidant receptor peroxidase 1 (Orp1) or glutaredoxin (Grx) in Drosophila (Albrecht et al., 2011). These reporter lines allow actual measurement of intracellular redox states at different ages in a tissue-specific manner, and a significant variation of mitochondrial H2O2 levels was detected within patches of adult adipose tissue. However, these studies are unable to identify the causal link between mitochondrial H2O2 levels and lifespan.
Although the well-known free radical theory of aging predicts that age-dependent increase in ROS production can be detrimental to tissue function and organism survival, the contribution of ROS-mediated oxidative damage to aging has been challenged by several studies (Gems and Doonan, 2009; Van Raamsdonk and Hekimi, 2009; Yang and Hekimi, 2010). In Drosophila, adult-specific over-expression of mitochondrial Mn-superoxide dismutase (MnSOD) or Cu/Zn-SOD extends the lifespan (Sun and Tower, 1999; Sun et al., 2002), whereas constitutive expression of MnSOD and catalase has no apparent effects, or in some cases shortens lifespan (Bayne et al., 2005; Mockett et al., 2010). Studies in Caenorhabditis elegans also show that low doses of ROS-generating chemical reagents (e.g., paraquat) extend lifespan (Yang and Hekimi, 2010). An explanation for this seemingly contradictory observation is that the mild oxidative insults might stimulate mitohormesis, an adaptive response associated with prolonged lifespan and healthspan. Further investigations are needed to illustrate the specific mechanism for ROS-mediated lifespan regulation.
2.2. ETC function is compromised during aging
The idea that mitochondrial wellness plays the central role in aging is not new (Harman, 1972). Several aspects of mitochondrial morphology, function, and activity are compromised during aging, and one signature is the decrease of electron transport activity (Fig. 1). In Drosophila, there is a significant reduction in the activity of cytochrome c oxidase (COX; also known as complex IV) by 35% during aging (Ferguson et al., 2005). Interestingly, the expression of complex IV does not change with age, suggesting that the reduction of complex IV activity may be due to the protein conformation changes or decreased turn-over rates (Benzi et al., 1991; Shiva et al., 2001). In addition, the state 3 respiration rate and uncoupled respiration of isolated muscle mitochondria decrease significantly with age. Potential causes for decreased state 3 respiration rate include the impairment of electron flow in ETC and reduced substrate availability. A decrease in complex IV activity could impair electron flow within the ETC system. In addition, activities of aconitase (an enzyme that converts citrate to isocitrate) and adenine nucleotide translocator (ANT) decrease in aged flies (Yan and Sohal, 1998; Das et al., 2001). Such changes will have devastating consequences to the cell, including reduced production of NADH and FADH2, thus providing less reducing substrates for electron transport. Together, these cellular changes lead to impaired state 3 respiration rate during aging. The reduction of complex IV activity also is associated with an increase in H2O2 production (Ferguson et al., 2005). In addition, similar reduction of complex IV activity is found in D. subobscura and mammals (Ferrandiz et al., 1994; Morel et al., 1995; Andreu et al., 1998; Kwong and Sohal, 2000; Navarro and Boveris, 2004), suggesting that age-dependent decrease in mitochondrial respiratory activity is evolutionarily conserved. The initial cause of reduced complex IV activity is still unclear. It has been suggested that oxidoreductases of complex IV are sensitive to increased ROS damage during aging (Ferguson et al., 2005). Besides altered ETC activity, the transcription of mitochondrial genes in complex I–IV are also repressed during aging in both Drosophila and C. elegans (McCarroll et al., 2004). Consistent with these findings, through Ribo-tag translatomic profiling, we recently found that genes in oxidative phosphorylation pathway are down-regulated in aged Drosophila oenocytes (Huang et al., 2019). The dampened expression of these genes can result in a significant increase in ROS production (Owusu-Ansah et al., 2013; Lopez-Fabuel et al., 2016).
Despite the fact that ETC activity declines with age, several early studies from C. elegans and mice indicate that moderate knockdown of ETC genes can promote lifespan (Dillin et al., 2002; Liu et al., 2005; Rea et al., 2007; Lapointe and Hekimi, 2008). In Drosophila, knockdown of five components in mitochondrial complexes I, III, IV, and V extends lifespan, probably through mitohormesis (Copeland et al., 2009). Muscle-specific knockdown of complex I protein ND-75, a NADH dehydrogenase, preserves muscle function and prolongs lifespan, linking muscle mitohormesis to longevity control (Owusu-Ansah et al., 2013). Such extension of lifespan is likely the results of compensatory responses which further induce oxygen consumption through mitochondria biogenesis, and upregulate cellular-defense and metabolic genes (Cristina et al., 2009). Nevertheless, mitochondrial respiratory activity is pivotal for lifespan regulation. Dietary restriction prolongs lifespan in part through the regulation of mitochondrial respiration. Down-regulation of mitochondrial complex I, IV and V diminishes dietary restriction-mediated longevity (Zid et al., 2009; Bahadorani et al., 2010). In addition, up-regulation of the Drosophila PGC-1α homolog (dPGC-1/spargel), an important regulator for mitochondria biogenesis and metabolism, can prolong lifespan (Rera et al., 2011). In summary, ETC function is compromised during aging, which might be a result of accumulated ROS elevation from weakened antioxidant defense and altered metabolic process. Manipulation of ETC activity through pharmaceutics to extend lifespan in late life, or to induce mitohormesis at young age to prevent age-related diseases, will be a promising avenue to pursue.
2.3. Carbonylation of mitochondrial proteins increases with age
ROS is known to structurally modify intracellular proteins, including the formation of disulfide cross-links and carbonyls (Stadtman, 1992; Dean et al., 1997). Carbonyl formation can occur on the side chain of amino acids by direct oxidation, and carbonyl group can be added to proteins through the reaction with carbonyl derivatives or aldehydes that are produced during lipid peroxidation (Berlett and Stadtman, 1997). In Drosophila, the carbonylation of mitochondrial proteins of thoracic flight muscle is elevated during aging, independent of protein abundance (Toroser et al., 2007) (Fig. 1). Carbonylation of mitochondrial proteins can be detrimental and influence mitochondrial respiratory activity (Hillered and Ernster, 1983; Stadtman, 1992; Yan et al., 1997). Interestingly, mitochondrial aconitase and ANT are specifically targeted for carbonylation modification during aging, which is thought to be related to the decline of their catalytic activities (Yan and Sohal, 1998; Das et al., 2001; Nystrom, 2005). The increased carbonylation of aconitase and ANT may be due to their sensitive to oxidative damage. Aconitase contains a metal-binding site which is a key feature to its susceptibility to oxidation (Stadtman, 1992). An additional factor for carbonylation is the decline of anti oxidative defense along with the increased protein oxidative damage during aging (Yan and Sohal, 1998). A study has uncovered a crosstalk between the circadian clock and carbonylation in Drosophila. The disruption of circadian rhythms by the mutation of period (per01) accelerates aging and induces high levels of protein carbonyls and lipid peroxidation, especially at old ages (Krishnan et al., 2009). Based on current evidence, elevated carbonylation of mitochondria proteins could be attributable to imbalanced prooxidant generation and antioxidant defenses.
2.4. Structural changes of mitochondrial inner membrane under aging
Mitochondrial inner membrane folds to form cristae, which provide a large surface area to house enzymes that aid in aerobic cellular respiration. Early electron microscopy studies have revealed the enlarged mitochondria with intramitochondrial glycogen particles, as well as reduced cristae (often oriented circumferentially) in aged Drosophila (Sohal, 1970) (Fig. 1). Walker and Benzer (2004) discovered the formation of “swirl”, a rearrangement of mitochondria cristae, under oxidative stress and normal aging in Drosophila. The cristae involved in a swirl are deficient in respiratory enzyme COX activity, possibly due to altered conformation. In addition, the presence of the swirl is associated with apoptotic cell death in flight muscle tissue of Drosophila by a conformational change in cytochrome c (Cho et al., 2011). A genetic screen in Drosophila also identified a hyperoxia sensitive mutant, hyperswirl (hys), which is short-lived and exhibits increased mitochondrial swirls in flight muscle (Walker and Benzer, 2004).
An electron cryo-tomography analysis suggests that the ultrastructure of mitochondria is highly heterogeneous in aged Drosophila. The mitochondrial inner membrane in old flies exhibits various non-standard shapes, such as spherical or concentric shapes. Some mitochondria lose cristae entirely or have minimally developed cristae, indicating a dissociation of mitochondrial ATP synthase dimers, which are important to maintain highly curved cristae ridges by reducing membrane elastic energy (Davies et al., 2012). Such drastic changes in mitochondrial morphology correlate with decreased oxygen uptake and increased peroxide yield in old mitochondria. Thus, the formation of the swirl and the altered ultrastructure of mitochondria suggest the damaged and deformed mitochondria during aging, which can eventually result in apoptosis by releasing cytochrome c (Wang and Youle, 2009). Unlike in Drosophila, the correlation of mitochondrial morphology and activity is less evident in mouse (Brandt et al., 2017).
2.5. Mitochondrial fission and fusion show tissue-dependent changes during aging
Mitochondrial morphology is hardly static, instead, it is undergoing constant changes through fission and fusion processes. Mitochondrial fission and fusion are tightly linked to mitophagy, a selective autophagy for mitochondrial degradation (reviewed in autophagy section) (Hoppins et al., 2007; Smirnova et al., 2001). Mitochondrial fission can separate impaired daughter units thus assisting autophagic processes. In contrast, fusion serves to diffuse damaged mitochondrial proteins, so that functional proteins can compensate to prevent their removal (Smirnova et al., 2001). Mitochondrial fission and fusion processes are mediated by large guanosine triphosphatases (GTPases) in the dynamin family, which are well conserved between yeast, flies, and mammals (Rana et al., 2017). Mitofusin (Mfn) proteins mediate fusion, whereas mitochondrial fission is regulated by dynamin-related protein 1 (Drp1) (Cao et al., 2017; Rana et al., 2017). Studies have indicated that proceeding to mitophagy, Parkin, an E3 ubiquitin ligase, mediates turnover of Mfn to favor fission over fusion (Youle and van der Bliek, 2012; Ashrafi and Schwarz, 2013).
Mitochondrial fusion increases significantly under normal aging (Sun et al., 2015) (Fig. 1), which is correlated with increased Mfn protein abundance (Rana et al., 2013). The midlife shift toward more elongated form of mitochondria is associated with mitochondrial dysfunction and loss of proteostasis (protein homeostasis) (Rana et al., 2013). Short-term over-expression of Drp1 in midlife can restore mitochondrial fragmentation, oxidative phosphorylation, and electron transport system capacity in old flight muscle. Both Drp1 over-expression and Mfn knockdown extend lifespan and restore proteostasis in Drosophila (Rana et al., 2017), supporting the view that accumulation of elongated dysfunctional mitochondria contributes to age-induced proteotoxicity. One possible explanation for the shift of mitochondrial dynamics towards fusion is to adapt to age-onset mitochondrial damage, because fusion can mitigate the damage by complementing the dysfunctional proteins and lipids with other mitochondria. Drp1-mediated lifespan extension is related to autophagy, as researchers have found that disrupting Atgl can suppress the beneficial effects of midlife Drp1 induction (Rana et al., 2017). In contrast to the findings mentioned above, age-dependent increased mitochondrial fragmentation is found in Drosophila wing nerves. The proportion of long axonal mitochondria (tubular and hyperfused) decreases in aged wing nerves (Cao et al., 2017). Mitochondrial fission (Drp1) and fusion (Mfn and Opa1) factors, but not mitophagy proteins (Parkin and PINK1), are involved in the morphology regulation of axonal mitochondria, suggesting mitophagy is dispensable in maintaining axonal mitochondrial morphology in wing nerves (Cao et al., 2017). Together, these findings suggest that age-associated mitochondrial fusion might be tissue-specific. Tissues that require high levels of energy production such as muscle and liver, may heavily rely on mitophagy to remove dysfunctional organelles. As their high-level ROS production will accelerate the damage rate for mitochondria, mitophagy is an effective way to recycle. However, peripheral organs, such as wing nerves, deploy other mechanisms to recycle damaged mitochondria.
2.6. Mitochondrial chaperone Hsp22
In face of increasing amount of protein aggregation, molecular chaperones, such as heat shock proteins (HSPs), are up-regulated in Drosophila. In Drosophila, 7 out of 12 small HSPs (sHSPs) are known to be up-regulated under normal aging. Among them, Hsp22 is localized in the mitochondrial matrix (King and Tower, 1999). During aging Hsp22 is induced 60-fold in Drosophila heads (Landis et al., 2004; Morrow et al., 2004; Tower et al., 2014) (Fig. 1). Other fly tissues, like oenocytes, also exhibit similar induction of Hsp22 (Landis et al., 2004). Over-expression of Hsp22 either ubiquitously or in motor neurons has been demonstrated to preserve locomotor activity and increase lifespan by up to 30% in Drosophila (Dabbaghizadeh et al., 2018). This can be achieved by preventing protein aggregation and damage through Hsp22’s chaperone activity (Morrow et al., 2006). In oenocytes, over-expression of Hsp22 and MnSOD significantly decreases age-induced pigments (Pickles et al., 2018). The targets of Hsp22 in mitochondria are currently unclear. Using immune-affinity conjugation and mass spectrometry, factors involved in ATP synthase pathway are identified as Hsp22-interacting proteins in HeLa cells. Expression of Drosophila Hsp22 in HeLa cells significantly increases ETC respiration and ATP levels (Cornelissen et al., 2018). It is likely that increased Hsp22 expression during aging is a compensatory response to maintain cellular homeostasis by protecting mitochondrial proteins from mis-folding and enhancing proteostasis in mitochondria (Acunzo et al., 2012; Morrow and Tanguay, 2015). Together, Hsp22 could act as a chaperone by preventing mitochondrial protein aggregation (Fig. 1). The effectiveness of Hsp22 to increase lifespan reflects the importance to maintain mitochondrial proteostasis and integrity during aging. How Hsp22 is up-regulated under aging remains to be addressed in the future.
In summary, aging mitochondria exhibit several functional and structural changes, such as decreased complex IV activity, increased protein carbonylation, reduced inner membrane cristae, and increased fusion. These changes are often accompanied with defective ETC (reduced ATP production) and increased superoxide radical production. Although further work is needed to determine the cause of age-related mitochondrial dysfunction, it is evident that many of these alterations are tissue-specific. Different quality control systems (mitophagy vs. fusion-fission) might be used by different tissues to cope with mitochondrial damage during aging. In addition, while ETC activities are significantly compromised during aging, mild mitochondrial stress (mitohormesis) early in life can prolong lifespan through induction of cytoprotective pathways (Yun and Finkel, 2014). The anti-aging signals triggered by mitochondrial stress, especially those systemic factors mediating inter-organ communication, have been the major focus in mitochondrial aging field (Taylor et al., 2014).
3. The nucleus
3.1. Changes in nuclear lamins and nuclear membrane structure during aging
There are distinct features that differentiate young from aged nuclei (Fig. 2).. The nuclear lamina, the interior structure of the nuclear envelope, contributes to both structural support and transcriptional regulation in the nucleus. The major components of the nuclear lamina are nuclear lamins (also known as Class V intermediate filaments).
Fig. 2.
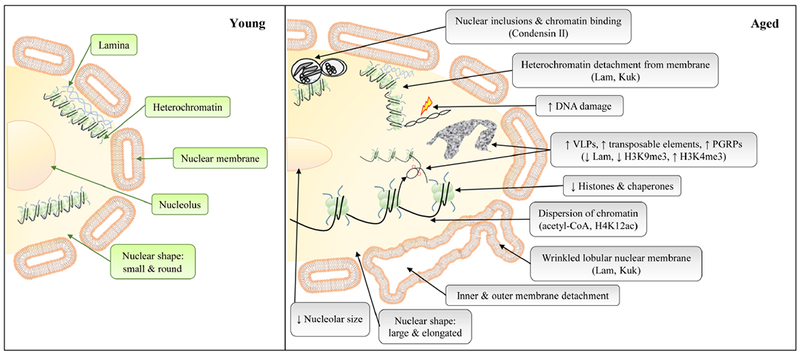
Schematic diagram to show the key nuclear alterations during normal aging. Signaling pathways responsible for age-related changes are indicated inside parentheses. See text for details.
In Drosophila, the expression of B-type lamin (Lam) is decreased in brain and fat bodies with age, but the underlying cause is unclear (Chen et al., 2014; Tran et al., 2016). Age-dependent reduction of Lam in Drosophila fat bodies induces inflammation and hyperplasia in aged gut through altered peptidoglycan recognition proteins (PGRPs) (Chen et al., 2014). The decline of Drosophila Lam in fat bodies correlates with decreased H3K9me3 and increased H3K4me3 which leads to an increase in retrotransposons. The corresponding increase in transposon activity may result in increased double stranded DNA breaks (suggested by antibodies against γ-H2AvD – a histone that becomes phosphorylated with DNA damage) (Chen et al., 2016). Alternatively, while reduced Lam in specific cell types is associated with aging, the induced expression of farnesylated lamina proteins Lam and Kugelkern (kuk) leads to a detachment of heterochromatin from the nuclear membrane, production of nuclear membrane lobulations, and shortened lifespan (Brandt et al., 2008; Petrovsky et al., 2018) Unifying the results from Drosophila to humans, truncated Drosophila Lam or kuk can induce senescence phenotypes in mammalian cells (Brandt et al., 2008). It is important to note that the Lam decline with age is predicted to occur post-transcriptionally (Petrovsky et al., 2018), which could involve lamin modifications including phosphorylation, SUMOylation, and glycosylation (de Leeuw et al., 2018). These results demonstrate that loss of the delicate Lam balance is upstream of specific types of DNA damage and cell senescence during aging, as well as age-related changes in heterochromatin.
Mutations of the Drosophila A-type lamin (LamC) cause a defect in nuclear envelope budding. Nuclear envelope budding is critical, because it often involves the envelopment of subsets of mitochondria-associated RNAs (including RNAs of mitochondrial assembly regulatory factor (Mart) and ATP synthase subunits β and α) at the inner nuclear membrane and the release of these RNAs at the outer nuclear membrane. Young LamC mutants exhibit decreased levels of specific mitochondrial protein transcripts (mtRNAs), progressive mitochondrial degradation and increased polyubiquitinated protein aggregates (Li et al., 2016). These findings suggest that defects in nuclear envelope budding and mtRNA delivery could result in defective mitochondria, induction of ROS, and proteostasis collapse during aging.
The cytoskeleton, lamins, and chromatin all serve to balance forces that stabilize the nuclear envelope architecture. Chromatin’s role as a nuclear envelope force mediator has been demonstrated via Condensin II, which is required for compaction of chromosomes and aids in chromatin-lamin linkage. Over-expression of Condensin II leads to nucleoplasmic reticulum invaginations from the nuclear membrane, leaving double membrane structures that lack DNA or histones in their interior (Bozler et al., 2015). This is consistent with a prominent phenotype seen in the vast majority of aged Drosophila nuclei, i.e., increased double membrane structures in the nucleus [107]. These observations strongly indicate that it is not simply direct gene expression being affected by chromatin modifications during aging. Rather, the force interplay between chromatin and the nuclear lamina alters the morphology of the nucleus itself, leading to disruptive nuclear structures that further impact aging. In humans, autophagy protein LC3 has been detected in the nucleus, which interacts with lamin B1 to facilitate lamin B1 degradation upon oncogenic insults (Dou et al., 2015). It will be interesting to see how autophagy contributes to the change of lamina structure during aging.
Thus, defects in lamina homeostasis appear to be a central aging hub as they are linked with chromatin organization, nuclear envelope budding, DNA damage, mitochondrial dysfunction, autophagy impairment, and senescence. The maintenance of nuclear lamina is particularly crucial in Drosophila possibly because most adult cells are post-mitotic.
3.2. Altered chromatin landscape
One of the major age-related changes in nuclei is the alteration of nucleosome structure, such as loss of histones from the DNA (Feser et al., 2010; Hu et al., 2014) (Fig. 2). The loss of histones is mainly due to the decreased expression of core histone proteins and reduced histone chaperones (Feser et al., 2010; Ivanov et al., 2013; Liu et al., 2013). The altered nucleosome structure will have profound impacts on chromatin landscape and transcriptional activity during aging in multiple organisms.
Aside from the changes in core histone protein levels, histone modifications are major players during aging and result in the dispersion of chromatin from its earlier state. For example, specific histone phosphorylation levels are elevated in subsets of neurons in humans during aging as well as in Alzheimer’s neurons (Lardenoije et al., 2015), leading to the question – does reversing or preventing histone modifications affect aging? This in fact has been shown to be true in fly lines that mimic reduced phosphorylation of histone H3 serine 28 through a serine to alanine mutation (H3S28A). The adjustment of this particular histone modification greatly extends lifespan as well as heart health (Joos et al., 2018). The life extension is linked to the unphosphorylated state of H3S28 reduces the binding of Polycomb Repressor Complex 2 (PRC2) to histone tails (Yung et al., 2015), which is crucial for PRC2-mediated H3K27 trimethylation (H3K27me3). H3K27me3 normally becomes dispersed with age and, more importantly, correlates to age-related gene expression changes (Wood et al., 2010; Jeon et al., 2018).
Heterochromatin can be modulated not only to extend or shorten lifespan, but also to recapitulate aging phenotypes. For example, nucleolar heterochromatin markers trimethylated lysine 9 of histone H3 (H3K9me3) and heterochromatin protein 1 (HP1) become dispersed with age (Wood et al., 2010). Consistently, knockdown of Drosophila HP1 or the H3K9me2/3 methyltransferase Su(var)3-9 can mimic the aging dispersion phenotypes (Wood et al., 2010; Jeon et al., 2018). Alternatively, by reducing heterochromatin dispersion through over-expression of HP1 or Su(var)3-9, lifespan is increased and transposable elements (TEs) are repressed (Larson et al., 2012; Wood et al., 2016). Similarly, mutations in PRC2 subunits E(Z) and ESC prevent the dispersion of H3K27me3 with age and extend lifespan (Siebold et al., 2010; Ma et al., 2018). In Drosophila, heterochromatin levels and longevity can be affected simply by methyl group availability. This is demonstrated through knockdown of methionine pathway components dAhcyL1 and dAhcyL2, which results in decreased H3K4me3 levels, prolonged lifespan, and improvement in multiple health indicators (Parkhitko et al., 2016).
While histone phosphorylation and methylation correspond to age-related gene expression, histone acetylation also plays a role. In the midlife of Drosophila, there is an increase in the levels of acetyl-CoA, H4K12 acetylation, and euchromatin formation. Reversal of the H4K12 acetylation trend by reduction of histone acetyltransferase Chameau results in extended lifespan (Peleg et al., 2016). Increasing deacetylation through over-expression of either histone deacetylase 1 (HDAC1/Rpd3) or NAD+-dependent histone deacetylase Silent Information Regulator 2 (Sir2) can also extend lifespan in Drosophila (Rogina et al., 2002; Rogina and Helfand, 2004). Although there has been some controversy over Sir2 and lifespan, it is important to note that the life extension via Drosophila Sir2 is tissue-specific (muscle tissue-specific expression has little effect) and dosage-dependent (2-5 fold induction is efficacious, while excessive expression is fatal) (Whitaker et al., 2013).
Retrotransposons make up ~30% of the Drosophila genome. The expression of TEs, such as R2 and Gypsy, is significantly up-regulated in aged flies (Li et al., 2013), suggesting a potential role of TEs in the aging process. Mutation of Drosophila Argonaute 2 (dAgo2) leads to activation of TEs and shortened lifespan (Li et al., 2013). Adenosine deaminase acting on RNA (ADAR) enzymes catalyze the editing of nucleotides, and as such, ADAR represses the positive effects of Argonaute and RNAi silencing (Savva et al., 2013). Reducing levels of Drosophila ADAR decreases RNA editing and extends lifespan. Cumulatively these results demonstrate that methods which repress TEs generally enhance lifespan. Future development of interventions that maintain TE repression will be needed to prolong healthspan and lifespan.
3.3. Nucleolar size decreases with age
The size of the nucleolus is a significant predictor of lifespan. It has been shown that reduction in nucleolar size is associated with the interventions that increase lifespan (e.g., dietary restriction, inhibition of the Target of Rapamycin (TOR), and mutations in Drosophila insulin signaling and TGF-beta/activin pathways) (Bai et al., 2013; Martins et al., 2017; Tiku et al., 2017) (Fig. 2). Somewhat counterintuitive to these findings is that the nucleolar size often shrinks with age. The reduction of nucleolar size generally corresponds with the reduction of ribosomal proteins, rRNA levels, and nucleolar proteins (e.g., fibrillarin) (Tiku et al., 2017). Interestingly, over-expression of a basic helix-loop-helix transcription factor Mnt in flight muscle prolongs lifespan while concurrently reducing the size of the muscle nucleolus in Drosophila (Demontis et al., 2014).
It appears that nucleolar size may be an important readout in aging process, owing to it being seen in diverse longevity mutants. However, the molecular mechanisms underlying this change remain to be further deciphered. It is likely that longevity pathways act on a common beneficial pathway that limits the production of ribosomal RNAs and nucleolar proteins. Combination of genetic screenings and aging transcriptomes will allow the discovery of candidate pathways involved in the regulation of nucleolar size during aging.
3.4. Nuclear inclusions and virus-like particles
In Drosophila, nuclear inclusions and virus-like particles (VLPs) within the nucleus become much more prominent with age (Miquel et al., 1972; Gartner and Gartner, 1976) (Fig. 2). Nuclear inclusions have several characteristic morphologies, and one is that they can consist of single or double membranes surrounding a cytoplasmic-like material containing varying degrees of aggregated particles. With increasing age the inclusions often begin to contain smooth endoplasmic reticulum-like structures or have properties of other cytoplasmic organelles. Importantly, the membranes of these inclusions have similar properties to the nuclear membrane, and as such chromatin can link to the inclusion membrane surfaces (Gartner and Gartner, 1976). Over-expression of the nuclear lamina protein kuk results in nuclear inclusion-like bodies, with double membranes surrounding what are speculated to be ribosomes (Brandt et al., 2008). Although it appears that laminar defects may be an underlying cause of nuclear inclusions, the fact that inclusions often have structures representative of other cytoplasmic organelles leaves open the possibility of an unexplored alternative explanation.
Nuclear VLPs have been shown to lack DNA, rather they contain RNA. Specifically, VLPs regularly contain RNA sequences homologous to a transposon known as copia (Shiba and Saigo, 1983). This indicates that VLPs may be partly due to age-related retrotransposon silencing defects. Reduction of an important nucleolar phosphoprotein, Noppl40, leads to VLPs in the nucleus (James et al., 2013). Additionally, knockdown of the chromatin modifying enzyme Poly ADP-ribose polymerase (PARP) leads to a ~50-fold increase in the expression of copia. PARP knockdown also alters heterochromatin repeat regions and affects nucleolar density (Tulin et al., 2002). The buildup of nuclear inclusions and VLPs with age likely contributes to a gradual change in phase transition states within the nucleus.
In summary, the aging nucleus displays significant structural and molecular changes, including dispersion of chromatin, nuclear membrane lobulations, detachment of inner from outer nuclear membrane, decrease in nucleolar size, and increases in TE transcripts and nuclear inclusions (Fig. 2). Meanwhile, there are many key questions that are still open. For example, to what extent do age-related changes in nuclear lamins contribute to other nuclear aging phenotypes (e.g., dispersion of chromatin, increases in TE transcripts and nuclear inclusions)? What is the underlying mechanism for age-dependent activation of TEs? Is the alteration of the chromatin landscape the fundamental cause of aging and senescence? Accumulated evidence indicates that structural changes of the nucleus not only alter processes within the nucleus, but also impact the function of other organelles in the cells (e.g., mitochondria (Cannino et al., 2007; Li et al., 2016) and autophagosome/lysosome (Silvestrini et al., 2018). Further studies are needed to determine the specific mechanisms underlying the complex crosstalk between the nucleus and other organelles during aging.
4. The autophagosome and lysosome
4.1. Autophagosome turnover declines with age
Autophagy was originally discovered as a cytoplasmic degradation process and cellular adaptive response to stresses (Hruban et al., 1963; Deter et al., 1967) (Fig. 3). Although degradation-independent roles of autophagy (such as secretory autophagy) have been discovered (Ponpuak et al., 2015), the major function of autophagy is to support cellular quality control and facilitate lysosome-mediated degradation and recycling. Depending on the mode of cargo sequestration, autophagy can be classified into three major types: macroautophagy, microautophagy, and chaperone-mediated autophagy (Hansen et al., 2018). Much of this section will focus on macroautophagy.
Fig. 3.
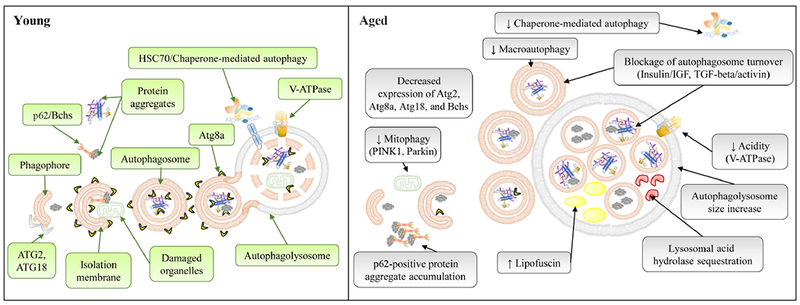
Schematic diagram to show age-dependent changes in the autophagy/lysosome system. Signaling pathways responsible for age-related changes are indicated inside parentheses. See text for details.
Due to its important role in recycling of cellular damage, the autophagy/lysosome system has been implicated in tissue homeostasis and organismal aging (Rubinsztein et al., 2011). Mounting evidence suggests that autophagic activities decline with age along with loss of tissue homeostasis. The evidence comes from three different aspects. First is the decline of mRNA and protein expression of autophagy genes. For example, the transcripts of Atg1, Atg2, Atg5, Atg6, Atg7, Atg8a, Atg18, and bchs decrease in aged flies (Simonsen et al., 2008; Demontis and Perrimon, 2010). The protein level of Atg8a is reduced by 60% in 4-week-old flies (Simonsen et al., 2008). Second, the number of autophagosomes and lysosomes declines with age. In Drosophila flight muscles, the number of autophagosomes (labelled by Atg5-GFP) and lysosomes (labelled by Lampl-GFP) decreases with age (Demontis and Perrimon, 2010). Similarly, the autophagosome formation, indicated by lipidated LC3-II (microtubule-associated protein 1 light chain 3-II), decreases in aged mouse hearts (Taneike et al., 2010). The third line of evidence is the accumulation of damaged organelles (e.g., mitochondria) and p62-positive protein aggregates. p62/Ref(2)P is an adapter protein that recruits polyubiquitinated proteins to autophagosome for degradation (Nezis et al., 2008). Increased levels of p62/Ref(2)P-labelled polyubiquitinated proteins has been observed in aged flight muscles and brains in Drosophila (Demontis and Perrimon, 2010; Bartlett et al., 2011).
However, the above observations do not provide direct evidence for age-dependent changes in autophagic activity. In fact, recent studies show that the number of autophagosomes increases with age, rather than decreases (Chang et al., 2017a; Chang et al., 2017b; Rana et al., 2017; Wilhelm et al., 2017). It seems that the increased autophagosome number is not due to the induction of autophagosome formation, instead it reflects the blockage of autophagosome turnover or degradation. An elegant study conducted in C. elegans shows that autophagic flux declines in aged nematode tissues (Chang et al., 2017a). Reduction of insulin/IGF signaling (IIS) via daf-2 mutation induced autophagic flux throughout adulthood. The autophagic flux assay directly measures autophagosome turnover using an autophagosome/autolysosome reporter (GFP::LGG-1) along with the lysosomal inhibitor Bafilomycin A1 (Baf A1). We recently applied this flux assay to study autophagic activity during cardiac aging in Drosophila. We observed a similar age-dependent reduction of autophagosome turnover in aged fly hearts (Chang et al., 2017b). The longevity paradigms, like mutations in insulin signaling and TGF-beta/activin pathways, can ameliorate the age-dependent decline of autophagosome turnover (Chang et al., 2017a; Chang et al., 2017b). However, not all paradigms show the same effects on age-related changes in autophagic capacity (Chang et al., 2017a). Aside from the defects in autophagosome turnover or degradation, it remains unclear whether autophagosome formation is also dysregulated during aging.
4.2. Changes in lysosomal acidity during aging
The decreased autophagosome turnover can be caused by reduced lysosomal function or blockage of the fusion between autophagosome and lysosome. It is known that lysosomal (or vacuolar) acidity declines during replicative aging in budding yeast Saccharomyces cerevisiae (Hughes and Gottschling, 2012; Carmona-Gutierrez et al., 2016) (Fig. 3). Although the impact on autophagy was not examined in these studies, age-related decline of vacuolar pH significantly impairs mitochondrial morphology and function (e.g., membrane potential). Over-expression of Vma1, a catalytic subunit of the peripheral-membrane-associated V-ATPase, ameliorates age-dependent mitochondrial dysfunction and decreased vacuolar acidity (Hughes and Gottschling, 2012). It remains to be determined whether reduced lysosomal acidity is the major cause for the blockage of autophagosome turnover in other organisms. On the other hand, it has been suggested that lipofuscin, the indigestible oxidized macromolecules (mainly proteins and lipids), accumulates within lysosome with age and impairs lysosomal degradation capacity. Lipofuscin-containing lysosomes can act as sinks to trap newly synthesized lysosomal acid hydrolase to further reduce the normal function of lipofuscin-free lysosomes (Brunk and Terman, 2002; Terman and Brunk, 2004).
4.3. Mitophagy and mitochondrial quality control
As organisms age, ROS-induced mitochondrial damage can trigger either repairing mechanisms through fission and fusion, or clearance of damaged mitochondria through mitophagy (Michaud et al., 2002; Youle and Narendra, 2011). Increased co-localization of lysosome and mitochondria is observed in Drosophila muscles and brain tissues at old age (Cornelissen et al., 2018) (Fig. 3). Using a mitophagy marker mt-Keima, it has been shown that the abundance of lysosome-localizing mitochondria can increase 10-fold in muscles and 2-fold in dopaminergic neurons by 4 weeks of age (Cornelissen et al., 2018). However, increased instances of lysosome and mitochondria co-localization may represent a decreased degradation capacity of lysosome. In fact, using the same reporter, a decrease in mitophagy is found in aged mouse brain tissues (Davies et al., 2001). Further investigation is needed to determine how mitophagy is altered with age.
Mitophagy is known to be regulated by a group of evolutionarily conserved factors (Fiesel et al., 2015), such as PINK1 (PTEN-induced putative kinase 1) and Parkin (an E3 ubiquitin ligase). PINK1 is a mitochondrial kinase and it phosphorylates Parkin upon mitochondrial damage. Phosphorylated Parkin in turn catalyzes ubiquitination on mitochondrial membrane proteins to trigger mitophagy (Twig and Shirihai, 2011; Youle and van der Bliek, 2012). Furthermore, Drosophila parkin mutants are short-lived (Greene et al., 2003), and Parkin deficiency completely blocks the age-induced mitophagy in 4-week-old flies (Cornelissen et al., 2018). In contrast, over-expressing parkin increases both mean and maximum lifespans in female flies (Rana et al., 2013). The extension of lifespan is mediated by reducing protein aggregates during aging and altering mitochondrial fragmentation to favor mitophagy (Ashrafi and Schwarz, 2013; Cao et al., 2017).
In summary, aging is accompanied with decreased autophagic activity, in particular the autophagosome turnover capacity. This is likely due to impaired lysosomal function during aging, such as decreased lysosomal acidity and accumulation of lipofuscin inside lysosome. Although it has become clear that boosting autophagy through drugs or genetic manipulations can prolong healthspan and lifespan, the molecular basis for these robust interventions is poorly understood. In addition, age-related decline of autophagic activity is tissue-specific (Chang et al., 2017a). Further work is needed to determine the cause of tissue-specific impairment of autophagy/lysosome system, and the potential systemic effect of autophagy deficiency in a particular aging tissue.
5. The proteasome
5.1. Proteasome activity and assembly are impaired during aging
The ubiquitin-proteasome system (UPS) is one of the major protein degradation pathways that is responsible for the maintenance of proteostasis and degradation of damaged or misfolded proteins (Amm et al., 2014) (Fig. 4). Protein ubiquitination is the key step in activating UPS degradation process, which involves three major enzymes, E1 (ubiquitin activating enzyme), E2 (ubiquitin conjugating enzyme), and E3 (ubiquitin ligase) (Weissman et al., 2011). Polyubiquitinated protein substrates are recognized and degraded by the 26S proteasome, typically containing one 20S core particle and one or two 19S regulatory particles (Voges et al., 1999; Tanaka, 2009). One of the hallmarks of aging is the accumulation of polyubiquinated proteins or protein aggregates (Lopez-Otin et al., 2013). The age-dependent increase in protein aggregates is tightly associated with the decline of proteasome activity, although the expression of the three ubiquitin enzymes is not altered with age (Gray et al., 2003; Chondrogianni and Gonos, 2005). In Drosophila, 26S proteasome activity declines sharply with age in both female and male flies (more than 89%). Old flies also become more sensitive to proteasome inhibitor treatment than young flies (Vernace et al., 2007). Consistent with these findings, proteasomal peptidase activities are significantly lower in aged flies (Vernace et al., 2007; Tsakiri et al., 2013a). The age-related decline in 26S proteasome activity has also been observed in mammals (Chondrogianni and Gonos, 2005). Several possible mechanisms may contribute to the decline of proteasome activity: decreased expression of proteasome subunits, altered subunit structure, and impaired proteasome assembly (Carrard et al., 2002; Keller et al., 2002; Mao et al., 2010). In Drosophila, the levels of fully assembled 26S holoenzymes are greatly reduced with age, in particular the decrease of 19S regulatory particles (Vernace et al., 2007; Tonoki et al., 2009). Since proteasome assembly is a ATP-dependent process, age-related reduction in oxidative phosphorylation and ATP production might contribute to proteasome disassembly and impaired proteasome activity at old ages (Vernace et al., 2007; Lopez-Otin et al., 2013).
Fig. 4.
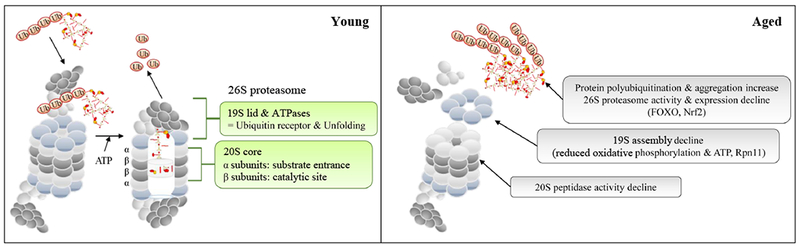
Schematic diagram to summarize the changes in aging proteasome. Signaling pathways responsible for age-related changes are indicated inside parentheses. See text for details.
Many longevity paradigms have been shown to slow proteasome aging. For example, dietary restriction can prevent age-related loss of proteasome function and structure (Gaczynska et al., 2001; Dasuri et al., 2009). In addition, genetic manipulations of proteasome subunits, in particular the 19S regulatory particles, can preserve proteasome function during aging. For example, over-expression of Drosophila Rpn11 (regulatory particle non-ATPase 11), a key component of the lid of 19S particles, prevents age-dependent decline in proteasome assembly and activity (Tonoki et al., 2009). Ectopic expression of Rpn11 also attenuates polyglutamine (polyQ)-induced progressive neurodegeneration, reduces age-related accumulation of misfolded proteins, and prolongs fly lifespan (Tonoki et al., 2009). Over-expression of another 19S component, Rpn6, has been shown to maintain proteostasis, increase resistance to proteotoxic stresses, and extend lifespan in C. elegans (Vilchez et al., 2012). Enhanced proteasome activity can also be achieved by stabilizing UPS-related transcription factor Rpn4 via deletion of E3 ubiquitin ligases Ubr2 and Mubl in budding yeast S. cerevisiae (Kruegel et al., 2011). Although over-expression of 19S components robustly promotes proteasome activity and prolongs lifespan, it remains to be determined whether and how these genetic manipulations directly impact 26S proteasome assembly during normal aging.
5.2. 20S proteasome activation and proteostasis
20S catalytic core particle of the eukaryote proteasome consists of two outer α-rings and two inner β-rings. Each α-ring or β-ring contains seven different subunits, and three β subunits (β1, β2, and β5) are responsible for the proteolytic activity of the proteasome (Tanaka, 2009). In Drosophila, the levels of 20S proteasome increase upon H2O2 treatment. Knockdown of the β subunit Prosβ1 reduces tolerance to oxidative stress (Pickering et al., 2013) (Fig. 4). Similar to 19S components, ectopic expression of 20S subunits has been implicated in longevity regulation. It has been shown that PBS-5 (an ortholog of mammalian β5 subunit) is the only 20S subunit that is up-regulated in long-lived germline-deficient glp-1 mutants in C. elegans (Vilchez et al., 2012). Over-expression of PBS-5 extends lifespan in C. elegans (Chondrogianni et al., 2015). Ectopic expression of PBS-5 enhances proteasome activity, increases the number of 26S proteasome, and prevents age-related increases in autofluorescence pigments. Additionally, over-expression of PBS-5 increases resistance to oxidative stress induced by paraquat and juglone, and protects worms from polyQ- or Aβ-induced proteotoxicity (Chondrogianni et al., 2015). Although it remains to be determined whether over-expression of 20S subunits can enhance proteasome activity in higher organisms, it seems that proteasome activation, by over-expression of either 19S or 20S subunits, can serve as an anti-aging approach by enhancing proteasome function and reducing age-related proteotoxicity.
5.3. The regulation of proteasome aging
It is known that many longevity pathways target the proteasome and directly regulate the transcription of proteasome subunit genes. In C. elegans, the FOXO transcription factor Daf-16 is required for the induction of Rpn6 expression in glp-1 mutants (Vilchez et al., 2012) (Fig. 4). Rpn6 is the only 19S component up-regulated in glp-1 mutants. Knockdown of Rpn6 can have a compensatory effect and induces the expression of other components in 26S proteasome (Vilchez et al., 2012). Drosophila FOXO and its target 4E-BP have also been implicated in the regulation of proteostasis. Ectopic expression of FOXO and 4E-BP delays age-dependent increases in polyubiquitinated protein aggregates in flight muscle and preserves muscle function (Demontis and Perrimon, 2010). Impaired ubiquitin-proteasome activity has been linked to age-related decline of cardiac performance in Drosophila. Knockdown of UPS genes disrupts normal cardiac function in young flies. Modest over-expression of Drosophila FOXO increases the expression of UPS genes (including several genes encoding E2 and E3 ubiquitin enzymes) in fly hearts, prevents age-induced ubiquitinated proteins in hearts, and alleviates cardiac functional decline with age (Blice-Baum et al., 2017).
Proteasome function is crucial for cell survival under aging and stresses (e.g., oxidative stress). Mild oxidative stress can induce the ubiquitin-proteasome activity, while severe and chronic oxidative stresses may impair proteasome function (Aiken et al., 2011; Shang and Taylor, 2011; Lefaki et al., 2017). The Nrf2/Kelch-like ECH-associated protein 1 (Keap1) signaling pathway is the key player in regulating cellular oxidative stress response (Sykiotis and Bohmann, 2008, 2010). It has been shown that short-term inhibition of proteasome function in Drosophila induces Nrf2 signaling and the expression of glutathione S-transferase Delta (gstD1). In aged flies, proteasome dysfunction-mediated induction of Nrf2 signaling is significantly reduced. Knockdown of CncC (cap ‘n’ collar isoform C), the key transcription factor in Drosophila Nrf2 signaling, reduces proteasome activities and induces the accumulation of ubiquitinated proteins at young ages. Blocking Nrf2 signaling by CncC knockdown also reduces the resistance to proteotoxic stress and shortens lifespan (Tsakiri et al., 2013a,b). In contrast, induction of Nrf2 signaling by over-expressing CncC increases the expression of proteasome subunits and promotes proteasome activity (Tsakiri et al., 2013b). Although it is likely that Nrf2/CncC signaling regulates proteasome activity through the activation by proteasome gene expression, future studies are necessary to validate this hypothesis and elucidate the specific roles of Nrf2/CncC signaling in maintaining proteostasis and proteasome function during normal aging.
In summary, both proteasome assembly and proteasomal peptidase activities are significantly decreased during normal aging. As a consequence, aging tissues exhibit accumulated protein aggregates and loss of proteostasis. Age-dependent reduction in oxidative phosphorylation and ATP production has been proposed as the major cause of impaired proteasome function. However, how aging alters proteasome activity is not fully understood. Longevity pathways, such as FOXO and Nrf2, have already been implicated in the regulation of proteasome aging, mainly through transcriptional activation of proteasome subunit genes. Nevertheless, interventions that can trigger proteasome activation late in life are promising strategies to preserve proteasome function and alleviate age-related proteotoxicity.
6. The cell membrane
6.1. Changes in lipid composition of cell membrane
The lipid composition of cell membrane can alter membrane fluidity, peroxidation index, lipid raft microdomains, and the optimal function of proteins embedded in the membrane (Brenner, 1984; Diaz et al., 2018) (Fig. 5). Additionally, the membrane and its properly functioning proteins mediate molecular exchange with the environment. Therefore, it is important to examine the changes in lipid composition that occur with age in Drosophila. The percentage of polyunsaturated fatty acids, which are prone to oxidation stress, increases with age, resulting in a negative impact on membrane fluidity (Magwere et al., 2006). Decreased membrane fluidity has also been previously identified in aged rats (Hashimoto et al., 1999). In long-lived fly mutants, the unsaturation vs. saturation index is greater than that of wild-type flies. Long-lived flies also exhibit lower melting points of individual fatty acids, indicating the impact of the changes in number and position of double bonds in the lipids (Moghadam et al., 2013). The higher proportion of monounsaturated fatty acids (C:16:1 and C:18:1) enhances membrane fluidity and affects other physiochemical properties of the membrane and the function of membrane proteins (Moghadam et al., 2015). The findings mentioned above indicate that the activity of membrane desaturases and their post-translational regulators (likely the caplains) may play a pivotal role in dampening the effects of age-related ROS via the cell membrane and preserving proper fluidity-related functions (Murakami et al., 2017).
Fig. 5.
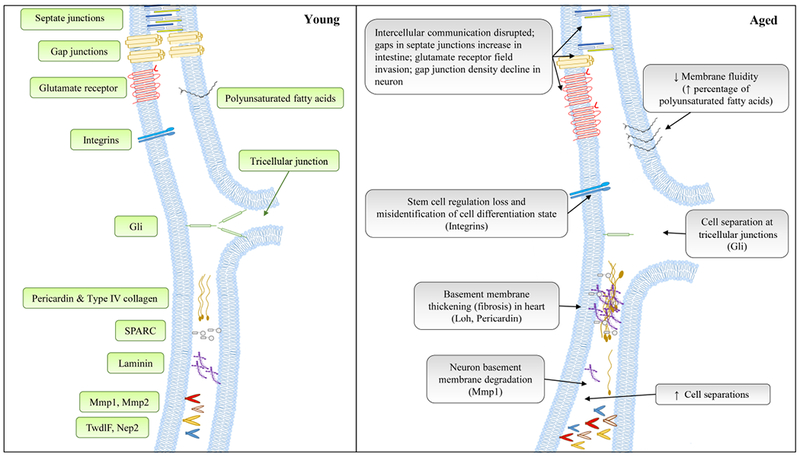
Schematic diagram to show the major changes of cell membrane during normal aging. Signaling pathways responsible for age-related changes are indicated inside parentheses. See text for details.
6.2. Intercellular junction density
Aging is associated with a loss of intercellular junction density and dysregulation of communication between neighboring cells (Augustin et al., 2017; Resnik-Docampo et al., 2017). As demonstrated in Drosophila, gaps in septate junctions increase with age in intestinal enterocytes (Resnik-Docampo et al., 2017) (Fig. 5). The transmembrane protein Gliotactin (Gli) localizes to tricellular junctions, and its depletion leads to gap patterns analogous to that in aged flies, as well as increased stem cell proliferation, changes in differentiation states, and weakened barrier function (Resnik-Docampo et al., 2017). However, dietary restriction can prevent the decline of Gli at tricellular junctions during aging (Resnik-Docampo et al., 2017), possibly through post-translational modification of Gli (Padash-Barmchi et al., 2010). Another type of junction, the gap junction (composed of innexins in Drosophila), displays a density decline in specific neuronal types with age. This can cause a decrease in electrical transmission speed. The reduction of IIS results in the transport of the innexin shaking B to the membrane (mediated by Rab4 and Rab11 GTPases), rather than to the lysosome, leading to the rescue of the loss of density phenotype and the restoration of the transmission speeds (Augustin et al., 2017). Intercellular communication is also disrupted at Drosophila neuromuscular junctions (NMJs) with age. This occurs as the distribution of glutamate receptors at NMJs is altered during aging, which results in reduced synaptic transmission (Wagner et al., 2015). The density of multiple cellular junctions declines with age, largely due to transport and recycling deficiencies (Wagner et al., 2015; Augustin et al., 2017). The recycling of many junction proteins is altered by nutrient signaling pathways, indicating that some of the lifespan benefits from dietary restriction and IIS inhibition are due in part to the maintenance of cell junction balance. Proper cell junction protein recycling ultimately preserves barrier function, intercellular signaling, and proper stem cell differentiation during aging.
6.3. Extracellular matrix
The extracellular matrix (ECM) is essential for cell adhesion, differentiation, and cell-to-cell communication (Theocharis et al., 2016). In Drosophila, a type IV collagen-like protein Pericardin increases in hearts with age (Vaughan et al., 2018) (Fig. 5), which is consistent with cardiac fibrosis in humans (Burkauskiene et al., 2006). The thickening of the basement membrane in cardiac tissue with age decreases cardiac functions, such as fractional shortening (Sessions et al., 2017). In contrast to cardiac tissue, the basement membrane of neuronal tissue is degraded with age, demonstrating tissue-specific effects on ECM aging (DeVault et al., 2018). Nevertheless, loss of cardiac ECM integrity with age can result in heart failure (Wilmes et al., 2018). Heart-specific knockdown of Pericardin, Laminin A (proteoglycan), Viking (a type IV collagen subunit), and SPARC (a collagen-binding protein) extends lifespan and improves cardiac functions (Sessions et al., 2017; Vaughan et al., 2018). Pericardin is recruited to the cardiac ECM by the ADAMTS-like protein Lonely heart (Loh) to maintain heart structure and function, while mutations of Loh or Pericardin shorten lifespan (Drechsler et al., 2013). Pericardin is mislocalized during aging, which can lead to loss of cell-to-cell connectivity in hearts. Glycosylation, oxidation, and multimerism of Pericardin are also altered with age, consequently affecting heart integrity (Wilmes et al., 2018). Pericardin produced by fat body can be recruited to cardiac ECM, demonstrating the intricacy of multi-tissue ECM maintenance in a localized environment (Drechsler et al., 2013). Thus, the loss of connectivity due to modifications of ECM components in one tissue leads to further defective communication in other tissues.
The balance of regeneration and degradation of ECM is critical. A transcriptional comparison between Drosophila and rodent hearts reveals that the regulators of ECM show a general expression increase with age. These regulators include matrix metalloproteinase 1 (Mmp1), Mmp2, neprilysin 2 (Nep2; a β-amyloid degrading enzyme), and TweedleF (TwdlF) (Cannon et al., 2017). The knockdown of Drosophila Mmp2 (dMmp2) in epidermal tissue rescues the decline of dendrite regenerative capacity with age by preventing the degradation of the basement membrane (DeVault et al., 2018). Drosophila Mmp1 (dMmp1) increases in motor neurons with age, and over-expression of dMmp1 in young flies decreases neuromuscular function. Longevity paradigms, such as dietary restriction and insulin receptor (InR) mutants, prevent age-dependent increase in dMmp1 expression. Additionally, over-expression of dTimp (Drosophila tissue inhibitor of matrix metalloproteinases) in motor neurons rescues the decline of motor function with age but does not extend lifespan (Azpurua et al., 2018). These experiments indicate that healthy regeneration in nervous tissue relies on the preservation of ECM proteins laid down before injury; however, the dendritic regeneration cues within the ECM remain unknown and would constitute an important finding. Additionally, the increase of modifiers of ECM composition that occurs with age could be the primary driver of brain and neuromuscular dysfunction, as very long-lived neurons become increasingly unable to respond to a muted signaling environment induced by neighboring tissue types.
Integrins function as transmembrane linkers between ECM and actin cytoskeleton. Reduction of integrin-linked kinase (Ilk) and β1-integrin (encoded by myospheroid, mys) prevents cardiac arrhythmia and increases lifespan in Drosophila (Nishimura et al., 2014). A mutation of βν integrin (βint-ν), one of the integrin β subunits, leads to increased intestinal stem cell proliferation and detrimentally increased c-Jun N-terminal kinase (JNK) signaling (Okumura et al., 2014). βint-ν mutants also exhibit enhanced midgut epithelium defects during aging, suggesting an important role of integrin in the maintenance of midgut homeostasis in Drosophila (Okumura et al., 2014).
In summary, with increasing age the saturation levels of fatty acids in the cell membrane change, thus altering the cell’s ability to absorb ROS and dampen its cascading effects. Meanwhile, intercellular junction recycling becomes dysregulated and cells can become physically separated. Critically, the delicate ECM balance is often disrupted by outside tissue types in the local microenvironment or even in distant locales. This miscommunication exacerbates the situation within a cell as the environment no longer stimulates proper responses to insults. Therefore, the above evidence indicates that “support” tissues of cardiomyocytes and neurons may be the initiators of aging processes in “major” tissues like heart and brain.
7. Perspectives
It is evident that aging significantly impacts and alters many aspects of organelles’ structure and function, although cell type- and tissue-specific changes are often observed. Aside from their essential roles in maintaining normal cellular operations, organelles are also the regulatory hubs that produce signals and process information in response to extrinsic and intrinsic stresses and insults. Therefore, age-related impairment of organelle function not only impacts organelle-specific processes, but also disrupts cellular homeostasis and even the function of neighboring cells or distal tissues. However, our understanding of organelle aging is far from complete. There are several important questions that remain unanswered.
7.1. Inter-organelle communication during aging
Breakthroughs in recent years reveal that inter-organelle communication plays a key role in tissue homeostasis and adaptive/compensatory responses to age-related stresses (Dakik and Titorenko, 2016; Gottschling and Nystrom, 2017). As mentioned above, impaired vacuolar acidity can lead to mitochondrial dysfunction during replicative aging in budding yeast (Hughes and Gottschling, 2012). Mitochondrial-nuclear interactions play a crucial role in stress response and longevity control (Nargund et al., 2012; Zhu et al., 2014). Further investigation of specific signals (including metabolites) that are used by organelles to coordinate the adaptive responses and maintain cellular homeostasis during aging is an exciting and important future direction in aging research. The identification of these organelle-specific signals can also facilitate the development of aging biomarkers. Due to the complexity of the organelle interactive network, it remains challenging to map every possible interaction that occurs during normal aging. Nevertheless, the newly identified organelle-specific makers and the development of super-resolution microscopy will greatly advance our understanding of the role of inter-organelle communication in cell senescence and tissue aging.
7.2. Age-related changes in understudied organelles
During normal aging, all organelles are subjected to change, yet we know very little about age-related alterations of several understudied organelles, such as the peroxisome. It has been shown that in rat liver peroxisomal enzyme activities decrease during normal aging (Perichon et al., 1998). Recent studies find that the expression of most peroxisomal enzymes (e.g., peroxisomal β-oxidation enzymes and peroxisomal biogenesis enzymes) are down-regulated in aged C. elegans and Drosophila (Narayan et al., 2016; Huang et al., 2019). Consistently, human fibroblasts exhibit decreased peroxisome import with advancing age (Legakis et al., 2002). Nevertheless, how peroxisome import function declines with age still remains elusive. As reported previously (Narayan et al., 2016; Weir et al., 2017), one possible cause of impaired peroxisome import is the down-regulation of Peroxin 5 (Pex5), the key peroxisome importing factor. Another potential cause could be the reduction of the affinity between Pex5 and peroxisomal matrix proteins (the cargo). Further development of in vivo peroxisome import assays and peroxisome proteomics will be the important areas of future investigation. Nevertheless, Drosophila will remain to be a powerful genetic tool to tackle these unanswered questions in the field of organelle aging.
Acknowledgments
This work was supported by NIH/NIA R00 AG048016 and R01AG058741 to H.B., Glenn/AFAR Scholarships for Research in the Biology of Aging to K.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acunzo J, Katsogiannou M, Rocchi P, 2012. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol 44, 1622–1631. [DOI] [PubMed] [Google Scholar]
- Aiken CT, Kaake RM, Wang X, Huang L, 2011. Oxidative stress-mediated regulation of proteasome complexes. Mol. Cell. Proteomics 10, R110 006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht SC, Barata AG, Grosshans J, Teleman AA, Dick TP, 2011. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829. [DOI] [PubMed] [Google Scholar]
- Amm I, Sommer T, Wolf DH, 2014. Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim. Biophys. Acta 1843, 182–196. [DOI] [PubMed] [Google Scholar]
- Andreu AL, Arbos MA, Perez-Martos A, Lopez-Perez MJ, Asin J, Lopez N, Montoya J, Schwartz S, 1998. Reduced mitochondrial DNA transcription in senescent rat heart. Biochem. Biophys. Res. Commun 252, 577–581. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Schwarz TL, 2013. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H, McGourty K, Allen MJ, Madem SK, Adcott J, Kerr F, Wong CT, Vincent A, Godenschwege T, Boucrot E, 2017. Reduced insulin signaling maintains electrical transmission in a neural circuit in aging flies. PLoS Biol. 15, e2001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpurua J, Mahoney RE, Eaton BA, 2018. Transcriptomics of aged Drosophila motor neurons reveals a matrix metalloproteinase that impairs motor function. Aging Cell 17, e12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadorani S, Hur JH, Lo T Jr., Vu K, Walker DW, 2010. Perturbation of mitochondrial complex V alters the response to dietary restriction in Drosophila. Aging Cell 9, 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Kang P, Hernandez AM, Tatar M, 2013. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 9, e1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, Harris GL, Nezis IP, Schubert DR, Simonsen A, Finley KD, 2011. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy 7, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne AC, Mockett RJ, Orr WC, Sohal RS, 2005. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem. J 391, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Curti D, Pastoris O, Marzatico F, Villa RF, Dagani F, 1991. Sequential damage in mitochondrial complexes by peroxidative stress. Neurochem. Res 16, 1295–1302. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER, 1997. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem 272, 20313–20316. [DOI] [PubMed] [Google Scholar]
- Blice-Baum AC, Zambon AC, Kaushik G, Viswanathan MC, Engler AJ, Bodmer R, Cammarato A, 2017. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 16, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler J, Nguyen HQ, Rogers GC, Bosco G, 2015. Condensins exert force on chromatin-nuclear envelope tethers to mediate nucleoplasmic reticulum formation in Drosophila melanogaster. G3 5, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Krohne G, GroBhans J, 2008. The farnesylated nuclear proteins KUGELKERN and LAMENT B promote aging - like phenotypes in Drosophila flies. Aging Cell 7, 541–551. [DOI] [PubMed] [Google Scholar]
- Brandt T, Mourier A, Tain LS, Partridge L, Larsson NG, Kuhlbrandt W, 2017. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. eLife 6, e24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner RR, 1984. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog. Lipid Res 23, 69–96. [DOI] [PubMed] [Google Scholar]
- Brunk UΤ, Terman A, 2002. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. FEBS 269, 1996–2002. [DOI] [PubMed] [Google Scholar]
- Burkauskienė A, Mackiewicz Z, Virtanen I, Konttinen YT, 2006. Age-related changes in myocardial nerve and collagen networks of the auricle of the right atrium. Acta Cardiol. 61, 513–518. [DOI] [PubMed] [Google Scholar]
- Cannino G, Di Liegro CM, Rinaldi AM, 2007. Nuclear-mitochondrial interaction. Mitochondrion 7, 359–366. [DOI] [PubMed] [Google Scholar]
- Cannon L, Zambon AC, Cammarato A, Zhang Z, Vogler G, Munoz M, Taylor E, Cartry J, Bernstein SI, Melov S, 2017. Expression patterns of cardiac aging in Drosophila. Aging Cell 16, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Wang H, Wang Z, Wang Q, Zhang S, Deng Y, Fang Y, 2017. In vivo imaging reveals mitophagy independence in the maintenance of axonal mitochondria during normal aging. Aging Cell 16, 1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Gutierrez D, Hughes AL, Madeo F, Ruckenstuhl C, 2016. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev 32, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrard G, Bulteau AL, Petropoulos I, Friguet B, 2002. Impairment of proteasome structure and function in aging. Int. J. Biochem. Cell Biol 34, 1461–1474. [DOI] [PubMed] [Google Scholar]
- Chang JT, Kumsta C, Heilman AB, Adams LM, Hansen M, 2017a. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. eLife 6, e18459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Kang P, Liu Y, Huang K, Taylor E, Sagona A, Nezis I, Bodmer R, Ocorr K, Bai H, 2017b. Activin signaling regulates autophagy and cardiac aging through mTORC2. bioRxiv, doi: 10.1101/139360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zheng X, Xiao D, Zheng Y, 2016. Age-associated de-repression of retrotransposons in the Drosophila fat body, its potential cause and consequence. Aging Cell 15, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zheng X, Zheng Y, 2014. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell 159, 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Hur JH, Walker DW, 2011. The role of mitochondria in Drosophila aging. Exp. Gerontol 46, 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, Gonos ES, 2015. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J. 29, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Gonos ES, 2005. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp. Gerontol 40, 931–938. [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Logan A, Prime TA, Abakumova I, Quin C, McQuaker SJ, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Hartley RC, Partridge L, Murphy MP, 2012. Using the mitochondria-targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila. Nat. Protoc 7, 946–958. [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carre JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP, 2011. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland JM, Cho J, Lo T Jr., Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW, 2009. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol 19, 1591–1598. [DOI] [PubMed] [Google Scholar]
- Cornelissen T, Vilain S, Vints K, Gounko N, Verstreken P, Vandenberghe W, 2018. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. eLife 7, e35878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C, 2009. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 5, e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbaghizadeh A, Morrow G, Amer YO, Chatelain EH, Pichaud N, Tanguay RM, 2018. Identification of proteins interacting with the mitochondrial small heat shock protein Hsp22 of Drosophila melanogaster: Implication in mitochondrial homeostasis. PLoS One 13, e0193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakik P, Titorenko VI, 2016. Communications between mitochondria, the nucleus, vacuoles, peroxisomes, the endoplasmic reticulum, the plasma membrane, lipid droplets, and the cytosol during yeast chronological aging. Front. Genet 7, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Levine RL, Orr WC, Sohal RS, 2001. Selectivity of protein oxidative damage during aging in Drosophila melanogaster. Biochem. J 360, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasuri K, Zhang L, Ebenezer P, Liu Y, Fernandez-Kim SO, Keller JN, 2009. Aging and dietary restriction alter proteasome biogenesis and composition in the brain and liver. Mech. Ageing Dev 130, 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Anselmi C, Wittig I, Faraldo-Gomez JD, Kuhlbrandt W, 2012. Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A 109, 13602–13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP, 2001. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic. Biol. Med 31, 181–190. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Gruenbaum Y, Medalia O, 2018. Nuclear lamins: thin filaments with major functions. Trends Cell Biol 28, 34–45. [DOI] [PubMed] [Google Scholar]
- Dean RT, Fu S, Stocker R, Davies MJ, 1997. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J 324 (Pt 1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Patel VK, Swindell WR, Perrimon N, 2014. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 7, 1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Perrimon N, 2010. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter RL, Baudhuin P, De Duve C, 1967. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol 35, C11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault L, Li T, Izabel S, Thompson-Peer KL, Jan LY, Jan YN, 2018. Dendrite regeneration of adult Drosophila sensory neurons diminishes with aging and is inhibited by epidermal-derived matrix metalloproteinase 2. Genes Dev. 32, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Fabelo N, Ferrer I, Marin R, 2018. “Lipid raft aging” in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer’s disease. Neurobiol. Aging 67, 42–52. [DOI] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C, 2002. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401. [DOI] [PubMed] [Google Scholar]
- Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, Ivanov A, Capell BC, Drake AM, Shah PP, Catanzaro JM, Ricketts MD, Lamark T, Adam SA, Marmorstein R, Zong WX, Johansen T, Goldman RD, Adams PD, Berger SL, 2015. Autophagy mediates degradation of nuclear lamina. Nature 527, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler M, Schmidt AC, Meyer H, Paululat A, 2013. The conserved ADAMTS-like protein lonely heart mediates matrix formation and cardiac tissue integrity. PLoS Genet. 9, e1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS, 2005. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J 390, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz ML, Martinez M, De Juan E, Diez A, Bustos G, Mi quel J, 1994. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res. 644, 335–338. [DOI] [PubMed] [Google Scholar]
- Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK, 2010. Elevated histone expression promotes life span extension. Mol. Cell 39, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesel FC, Ando M, Hudec R, Hill AR, Castanedes-Casey M, Caulfield TR, Moussaud-Lamodiere EL, Stankowski JN, Bauer PO, Lorenzo-Betancor O, Ferrer I, Arbelo JM, Siuda J, Chen L, Dawson VL, Dawson TM, Wszolek ZK, Ross OA, Dickson DW, Springer W, 2015. (Patho-)physiological relevance of PINK1-dependent ubiquitin phosphorylation. EMBORep. 16, 1114–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaczynska M, Osmulski PA, Ward WF, 2001. Caretaker or undertaker? The role of the proteasome in aging. Mech. Ageing Dev 122, 235–254. [DOI] [PubMed] [Google Scholar]
- Gartner LP, Gartner RC, 1976. Nuclear inclusions: a study of aging in Drosophila. J. Gerontol 31, 396–404. [DOI] [PubMed] [Google Scholar]
- Gems D, Doonan R, 2009. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle 8, 1681–1687. [DOI] [PubMed] [Google Scholar]
- Gottschling DE, Nystrom T, 2017. The upsides and downsides of organelle interconnectivity. Cell 169, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough NR, 2016. Emerging roles for organelles in cellular regulation. Sci. Signal 9, eg11. [DOI] [PubMed] [Google Scholar]
- Gray DA, Tsirigotis M, Woulfe J, 2003. Ubiquitin, proteasomes, and the aging brain. Sci. Aging Knowledge Environ 2003, RE6. [DOI] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ, 2003. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. U. S. A 100, 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Rubinsztein DC, Walker DW, 2018. Autophagy as a promoter of longevity: insights from model organisms. Nat. Revi. Mol. Cell Biol 19, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D, 1956. Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11, 298–300. [DOI] [PubMed] [Google Scholar]
- Harman D, 1972. The biologic clock: the mitochondria? J. Am. Geriatr. Soc 20, 145–147. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Masumura S, 1999. Effect of aging on plasma membrane fluidity of rat aortic endothelial cells. Exp. Gerontol 34, 687–698. [DOI] [PubMed] [Google Scholar]
- Hillered L, Ernster L, 1983. Respiratory activity of isolated rat brain mitochondria following in vitro exposure to oxygen radicals. J. Cereb. Blood Flow Metab 3, 207–214. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J, 2007. The machines that divide and fuse mitochondria. Annu. Rev. Biochem 76, 751–780. [DOI] [PubMed] [Google Scholar]
- Hruban Z, Spargo B, Swift H, Wissler RW, Kleinfeld RG, 1963. Focal cytoplasmic degradation. Am. J. Pathol 42, 657–683. [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C, 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145. [DOI] [PubMed] [Google Scholar]
- Hu Z, Chen K, Xia Z, Chavez M, Pal S, Seol JH, Chen CC, Li W, Tyler JK, 2014. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes Dev. 28, 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Chen W, Zhu F, Li PW, Kapahi P, Bai H, 2019. RiboTag translatomic profiling of Drosophila oenocytes under aging and induced oxidative stress. BMC Genomics 20, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Gottschling DE, 2012. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 492, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson DM, Rai TS, Shah PP, Hewitt G, Korolchuk VI, Passos JF, Wu H, Berger SL, Adams PD, 2013. Lysosome-mediated processing of chromatin in senescence. J. Cell Biol 202, 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Cindass R Jr, Mayer D, Terhoeve S, Mumphrey C, DiMario P, 2013. Nucleolar stress in Drosophila melanogaster. RNAi-mediated depletion of Noppl40. Nucleus 4, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HJ, Kim YS, Kim JG, Heo K, Pyo JH, Yamaguchi M, Park JS, Yoo MA, 2018. Effect of heterochromatin stability on intestinal stem cell aging in Drosophila. Mech. Ageing Dev 173, 50–60. [DOI] [PubMed] [Google Scholar]
- Joos J, Saadatmand A, Schnabel C, Viktorinová I, Brand T, Kramer M, Nattel S, Dobrev D, Tomancak P, Backs J, 2018. Ectopic expression of S28A-mutated Histone H3 modulates longevity, stress resistance and cardiac function in Drosophila. Sci. Rep 8, 2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK, 2005. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196. [DOI] [PubMed] [Google Scholar]
- Keller JN, Gee J, Ding Q, 2002. The proteasome in brain aging. Ageing Res. Rev 1, 279–293. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ, 2010. The genetics of ageing. Nature 464, 504–512. [DOI] [PubMed] [Google Scholar]
- King V, Tower J, 1999. Aging-specific expression of Drosophila hsp22. Dev. Biol 207, 107–118. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Kretzschmar D, Rakshit K, Chow E, Giebultowicz JM, 2009. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging 1, 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel U, Robison B, Dange T, Kahlert G, Delaney JR, Kotireddy S, Tsuchiya M, Tsuchiyama S, Murakami CJ, Schleit J, Sutphin G, Carr D, Tar K, Dittmar G, Kaeberlein M, Kennedy BK, Schmidt M, 2011. Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genet. 7, e1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS, 2000. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys 373, 16–22. [DOI] [PubMed] [Google Scholar]
- Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavare S, Tower J, 2004. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A 101, 7663–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S, 2008. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J. Biol. Chem 283, 26217–26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, Coleman P, Lemere CA, Hof PR, van den Hove DL, 2015. The epigenetics of aging and neurodegeneration. Prog. Neurobiol 131, 21–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson K, Yan S-J, Tsurumi A, Liu J, Zhou J, Gaur K, Guo D, Eickbush TH, Li WX, 2012. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 8, e1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaki M, Papaevgeniou N, Chondrogianni N, 2017. Redox regulation of proteasome function. Redox Biol. 13, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legakis JE, Koepke JI, Jedeszko C, Barlaskar L, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR, 2002. Peroxisome senescence in human fibroblasts. Mol. Biol. Cell 13, 4243–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prazak L, Chatterjee N, Griininger S, Krug L, Theodorou D, Dubnau J, 2013. Activation of transposable elements during aging and neuronal decline in Drosophila. Nat. Neurosci 16, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hassinger L, Thomson T, Ding B, Ashley J, Hassinger W, Budnik V, 2016. Lamin mutations accelerate aging via defective export of mitochondrial mRNAs through nuclear envelope budding. Curr. Biol 26, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev SI, 2013. Reactive oxygen species and the free radical theory of aging. Tree Radic. Biol. Med 60, 1–4. [DOI] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA, 2013. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 4, 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S, 2005. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 19, 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP, 2016. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. U. S. A 113, 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wang H, Cai Y, Wang H, Niu K, Wu X, Ma H, Yang Y, Tong W, Liu F, 2018. Epigenetic drift of H3K27me3 in aging links glycolysis to healthy longevity in Drosophila. eLife 7, e35368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, Pamplona R, Miwa S, Martinez-Diaz P, Portero-Otin M, Brand MD, Partridge L, 2006. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci 61, 136–145. [DOI] [PubMed] [Google Scholar]
- Mao L, Romer I, Nebrich G, Klein O, Koppelstatter A, Hin SC, Hard D, Zabel C, 2010. Aging in mouse brain is a cell/tissue-level phenomenon exacerbated by proteasome loss. J. Proteome Res 9, 3551–3560. [DOI] [PubMed] [Google Scholar]
- Martins T, Eusebio N, Correia A, Marinho J, Casares F, Pereira PS, 2017. TGFbeta/Activin signalling is required for ribosome biogenesis and cell growth in Drosophila salivary glands. Open Biol. 7, 160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, Kenyon C, Bargmann CI, Li H, 2004. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat. Genet 36, 197–204. [DOI] [PubMed] [Google Scholar]
- Michaud S, Morrow G, Marchand J, Tanguay RM, 2002. Drosophila small heat shock proteins: cell and organelle-specific chaperones? Prog. Mol. Subcell. Biol 28, 79–101. [DOI] [PubMed] [Google Scholar]
- Miquel J, Bensch KG, Philpott DE, 1972. Viruslike particles in the tissues of normal and gamma-irradiated Drosophila melanogaster. J. Invertebr. Pathol 19, 156–159. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal BH, Sohal RS, 2010. Expression of multiple copies of mitochondrially targeted catalase or genomic Mn superoxide dismutase transgenes does not extend the life span of Drosophila melanogaster. Free Radic. Biol. Med 49, 2028–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam NN, Holmstrup M, Manenti T, Loeschcke V, 2015. Phospholipid fatty acid composition linking larval-density to lifespan of adult Drosophila melanogaster. Exp. Gerontol 72, 177–183. [DOI] [PubMed] [Google Scholar]
- Moghadam NN, Holmstrup M, Pertoldi C, Loeschcke V, 2013. Age-induced perturbation in cell membrane phospholipid fatty acid profile of longevity-selected Drosophila melanogaster and corresponding control lines. Exp. Gerontol 48, 1362–1368. [DOI] [PubMed] [Google Scholar]
- Morel F, Mazet F, Touraille S, Alziari S, 1995. Changes in the respiratory chain complexes activities and in the mitochondrial DNA content during ageing in D. subobscura. Mech. Ageing Dev 84, 171–181. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI, 2004. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 15, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM, 2006. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones 11, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM, 2004. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 18, 598–599. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM, 2015. Drosophila melanogaster Hsp22: a mitochondrial small heat shock protein influencing the aging process. Front. Genet 6, 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Nagao K, Juni N, Hara Y, Umeda M, 2017. An N-terminal di-proline motif is essential for fatty acid-dependent degradation of Delta9-desaturase in Drosophila. J. Biol. Chem 292, 19976–19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, 2009. How mitochondria produce reactive oxygen species. Biochem. J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan V, Ly T, Pourkarimi E, Murillo AB, Gartner A, Lamond AI, Kenyon C, 2016. Deep proteome analysis identifies age-related processes in C. elegans. Cell Syst. 3, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM, 2012. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A, 2004. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am. J. Physiol. Regul. Integr. Comp. Physiol 287, R1244–R1249. [DOI] [PubMed] [Google Scholar]
- Nezis IP., Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, Brech A, 2008. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell Biol 180, 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Kumsta C, Kaushik G, Diop SB, Ding Y, Bisharat-Kernizan J, Catan H, Cammarato A, Ross RS, Engler AJ, 2014. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 13, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T, 2005. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T, Takeda K, Taniguchi K, Adachi-Yamada T, 2014. βν integrin inhibits chronic and high level activation of INK to repress senescence phenotypes in Drosophila adult midgut. PLoS One 9, e89387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Song W, Perrimon N, 2013. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padash-Barmchi M, Browne K, Sturgeon K, Jusiak B, Auld VJ, 2010. Control of Gliotactin localization and levels by tyrosine phosphorylation and endocytosis is necessary for survival of polarized epithelia. J. Cell Sci 123, 4052–4062. [DOI] [PubMed] [Google Scholar]
- Parkhitko AA, Binari R, Zhang N, Asara JM, Demontis F, Perrimon N, 2016. Tissue-specific down-regulation of S-adenosyl-homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev. 30, 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Feller C, Forne I, Schiller E, Sévin DC, Schauer T, Regnard C, Straub T, Prestel M, Klima C, 2016. Life span extension by targeting a link between metabolism and histone acetylation in Drosophila. EMBO Rep. 17, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perichon R, Bourre JM, Kelly JF, Roth GS, 1998. The role of peroxisomes in aging. Cell. Mol. Life Sci 54, 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky R, Krohne G, Großhans J, 2018. Overexpression of the lamina proteins Lamin and Kugelkern induces specific ultrastructural alterations in the morphology of the nuclear envelope of intestinal stem cells and enterocytes. Eur. J. Cell Biol 97, 102–113. [DOI] [PubMed] [Google Scholar]
- Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJ, 2013. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol 216, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles S, Vigie P, Youle RJ, 2018. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol 28, R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponpuak M, Mandell MA, Kimura T, Chauhan S, Cleyrat C, Deretic V, 2015. Secretory autophagy. Curr. Opin. Cell Biol 35, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Oliveira MP, Khamoui AV, Aparicio R, Rera M, Rossiter HB, Walker DW, 2017. Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat. Commun 8, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Rera M, Walker DW, 2013. Parkin over expression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. U. S. A 110, 8638–8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE, 2007. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 5, e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr., Jones DL, Walker DW, 2011. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14, 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik-Docampo M, Koehler CL, Clark RI, Schinaman JM, Sauer V, Wong DM, Lewis S, D’Alterio C, Walker DW, Jones DL, 2017. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat. Cell Biol 19, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, 2004. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. U. S. A 101, 15998–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S, 2002. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 298, 1745–1745. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Marino G, Kroemer G, 2011. Autophagy and aging. Cell 146, 682–695. [DOI] [PubMed] [Google Scholar]
- Savva YA, Jepson JE, Chang Y-J, Whitaker R, Jones BC, St Laurent G, Tackett MR, Kapranov P, Jiang N, Du G, 2013. RNA editing regulates transposon-mediated heterochromatic gene silencing. Nat. Commun 4, 2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions AO, Kaushik G, Parker S, Raedschelders K, Bodmer R, Van Eyk JE, Engler AJ, 2017. Extracellular matrix downregulation in the Drosophila heart preserves contractile function and improves lifespan. Matrix Biol. 62, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang F, Taylor A, 2011. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic. Biol. Med 51, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T, Saigo K, 1983. Retrovirus-like particles containing RNA homologous to the transposable element copia in Drosophila melanogaster. Nature 302, 119–124. [DOI] [PubMed] [Google Scholar]
- Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM, 2001. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. U. S. A 98, 7212–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ, 2010. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance. Proc. Natl. Acad. Sci. U. S. A 107, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini MJ, Johnson JR, Kumar AV, Thakurta TG, Blais K, Neill ZA, Marion SW, St Amand V, Reenan RA, Lapierre LR, 2018. Nuclear export inhibition enhances HLH-30/TFEB activity, autophagy, and lifespan. Cell Rep. 23, 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD, 2008. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4, 176–184. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM, 2001. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, 1970. Mitochondrial changes in the heart of Drosophila repleta, wollaston with age. Exp. Gerontol 5, 213–216. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, 1992. Protein oxidation and aging. Science 257, 1220–1224. [DOI] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J, 2002. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics 161, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Tower I, 1999. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol 19, 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Yun I, Liu J, Malide D, Liu C, Rovira II, Holmstrom KM, Fergusson MM, Yoo YH, Combs CA, Finkel T, 2015. Measuring in vivo mitophagy. Mol. Cell 60, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Korstanje R, 2016. Chapter 1 - Longevity as a Complex Genetic Trait, in: Kaeberlein MR, Martin GM (Eds.), Handbook of the Biology of Aging (Eighth Edition). Academic Press, San Diego, pp. 3–54. [Google Scholar]
- Sykiotis GP, Bohmann D, 2008. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D, 2010. Stress-activated cap ‘n’ collar transcription factors in aging and human disease. Sci. Signal 3, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, 2009. The proteasome: overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 85, 12–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K, 2010. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS, 2001. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Berendzen KM, Dillin A, 2014. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol 15, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A, Brunk UT, 2004. Lipofuscin. Int. J. Biochem. Cell Biol 36, 1400–1404. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, 2016. Extracellular matrix structure. Adv. Drug Deliv. Rev 97, 4–27. [DOI] [PubMed] [Google Scholar]
- Tiku V, Jain C, Raz Y, Nakamura S, Heestand B, Liu W, Späth M, Suchiman HED, Müller R-U, Slagboom PE, 2017. Small nucleoli are a cellular hallmark of longevity. Nat. Commun 8, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M, 2009. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol. Cell. Biol 29, 1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toroser D, Orr WC, Sohal RS, 2007. Carbonylation of mitochondrial proteins in Drosophila melanogaster during aging. Biochem. Biophys. Res. Commun 363, 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J, Landis G, Gao R, Luan A, Lee J, Sun Y, 2014. Variegated expression of Hsp22 transgenic reporters indicates cell-specific patterns of aging in Drosophila oenocytes. J. Gerontol. A Biol. Sci. Med. Sci 69, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran JR, Chen H, Zheng X, Zheng Y, 2016. Lamin in inflammation and aging. Curr. Opin. Cell Biol 40, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri EN, Sykiotis GP, Papassideri IS, Gorgoulis VG, Bohmann D, Trougakos IP, 2013a. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress. LASEB J. 27, 2407–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri EN, Sykiotis GP, Papassideri IS, Terpos E, Dimopoulos MA, Gorgoulis VG, Bohmann D, Trougakos IP, 2013b. Proteasome dysfunction in Drosophila signals to an Nrf2-dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell 12, 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulin A, Stewart D, Spradling AC, 2002. The Drosophila heterochromatic gene encoding poly (ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development. Genes Dev. 16, 2108–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK, 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Shirihai OS, 2011. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal 14, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S, 2009. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 5, e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L, Marley R, Miellet S, Hartley PS, 2018. The impact of SPARC on age-related cardiac dysfunction and fibrosis in Drosophila. Exp. Gerontol 109, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernace VA, Arnaud L, Schmidt-Glenewinkel T, Figueiredo-Pereira ME, 2007. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 21, 2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues AP, Manning G, Dillin A, 2012. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 489, 263–268. [DOI] [PubMed] [Google Scholar]
- Voges D, Zwickl P, Baumeister W, 1999. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu. Rew. Biochem 68, 1015–1068. [DOI] [PubMed] [Google Scholar]
- Wagner N, Laugks U, Heckmann M, Asan E, Neuser K, 2015. Aging Drosophila melanogaster display altered pre– and postsynaptic ultrastructure at adult neuromuscular junctions. J. Comp. Neurol 523, 2457–2475. [DOI] [PubMed] [Google Scholar]
- Walker DW, Benzer S, 2004. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc. Natl. Acad. Sci. U. S. A 101, 10290–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Youle RJ, 2009. The role of mitochondria in apoptosis. Annu. Rev. Genet 43, 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, Laboy R, Hirschey MD, Mair WB, 2017. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 26, 884–896 e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM, Shabek N, Ciechanover A, 2011. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol 12, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergin WP, Gruber PJ, Newcomb EH, 1970. Fine structural investigation of nuclear inclusions in plants. J. Ultrastruct. Res 30, 533–557. [DOI] [PubMed] [Google Scholar]
- Whitaker R, Faulkner S, Miyokawa R, Burhenn L, Henriksen M, Wood JG, Helfand SL, 2013. Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner. Aging 5, 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm T, Byrne J, Medina R, Kolundzic E, Geisinger J, Hajduskova M, Tursun B, Richly H, 2017. Neuronal inhibition of the autophagy nucleation complex extends life span in post-reproductive C. elegans. Genes Dev. 31, 1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes AC, Klinke N, Rotstein B, Meyer H, Paululat A, 2018. Biosynthesis and assembly of the collagen IV-like protein Pericardin in Drosophila melanogaster. Biol. Open 7, bio030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Hillenmeyer S, Lawrence C, Chang C, Hosier S, Lightfoot W, Mukherjee E, Jiang N, Schorl C, Brodsky AS, Neretti N, Helfand SL, 2010. Chromatin remodeling in the aging genome of Drosophila. Aging Cell 9, 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Jones BC, Jiang N, Chang C, Hosier S, Wickremesinghe P, Garcia M, Hartnett DA, Burhenn L, Neretti N, 2016. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc. Natl. Acad. Sci. U. S. A 113, 11277–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Levine RL, Sohal RS, 1997. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. U. S. A 94, 11168–11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS, 1998. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. U. S. A 95, 12896–12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hekimi S, 2010. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP, 2011. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol 12, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM, 2012. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Finkel T, 2014. Mitohormesis. Cell Metab. 19, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung PYK, Stuetzer A, Fischle W, Martinez A-M, Cavalli G, 2015. Histone H3 serine 28 is essential for efficient polycomb-mediated gene repression in Drosophila. Cell Rep. 11, 1437–1445. [DOI] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D, 2013. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 497, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CT, Ingelmo P, Rand DM, 2014. GxGxE for lifespan in Drosophila, mitochondrial, nuclear, and dietary interactions that modify longevity. PLoS Genet. 10, e1004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P, 2009. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]


