Abstract
Studies of environmental exposures and childhood cancers that rely on records often only use maternal address at birth or address at cancer diagnosis to assess exposures in early childhood, possibly leading to exposure misclassification and questionable validity due to residential mobility during early childhood.
Our objective was to assess patterns and identify factors that may predict residential mobility in early childhood, and examine the impact of mobility on early childhood exposure assessment for agriculturally applied pesticides and childhood cancers in California.
We obtained the addresses at diagnosis of all childhood cancer cases born in 1998–2011 and diagnosed at 0–5 years of age (n=6,478) from the California Cancer Registry (CCR), and their birth addresses from linked birth certificates. Controls were randomly selected from California birth records and frequency matched (20:1) to all cases by year of birth. We obtained residential histories from a public-record database LexisNexis for both case (n=3,877 with age at diagnosis 1–5 years) and control (n=99,262) families. Logistic regression analyses were conducted to assess the socio-demographic factors in relation to residential mobility in early childhood. We employed a Geographic Information System (GIS)-based system to estimate children’s first year of life exposures to agriculturally applied pesticides based on birth vs diagnosis address or residential histories based upon Lexis-Nexis Public Records and assessed agreement between exposure measures using Spearman correlations and kappa statistics.
Over 20% of case and control children moved in their first year of life, and 55% of children with cancer moved between birth and diagnosis. Older age at diagnosis, younger maternal age, lower maternal education, not having a Hispanic ethnic background, use of public health insurance, and non-metropolitan residence at birth were predictors of higher residential mobility. There was moderate to strong correlation (Spearman correlation=0.76–0.83) and good agreement (kappa=0.75–0.81) between the first year of life exposure estimates for agricultural pesticides applied within 2 km of a residence relying on an address at birth or at diagnosis or LexisNexis addresses; this did not differ by outcome status, but agreement decreased with decreasing buffer size, and increasing distance moved or age at diagnosis.
These findings suggest that residential addresses collected at one point in time may represent residential history in early childhood to a reasonable extent; nevertheless, they exposure misclassification in the first year of life remains an issue. Also, the highest proportion of women not captured by LexisNexis were Hispanic women born in Mexico and those living in the lowest SES neighborhoods, i.e. possibly those with the higher environmental exposures, as well as younger women and those with less than high school education.
Though LexisNexis only captures a sub-population, its data may be useful for augmenting address information and assessing the extent of exposure misclassification when estimating environmental exposures in large record linkage studies. Future research should investigate how to correct for exposure misclassification introduced by residential mobility that is not being captured by records.
Keywords: Pesticide exposure, Residential mobility, Early childhood, Exposure misclassification, Childhood cancers
1. Background
Large-scale record-linkage studies of environmental exposures in early childhood can avoid selection and recall bias that impacts studies with active subject recruitment. However, they often need to assign exposures based upon one maternal residential address, which is readily available from birth certificates (Carozza et al., 2009; Heck et al., 2014; Lavigne et al., 2017; Reynolds et al., 2005; von Ehrenstein et al., 2016) and/or residential address at diagnosis, as was done in some childhood cancer studies (Carozza et al., 2008; García-Pérez et al., 2016, 2015). The reliance on one address implicitly makes the assumption that a child’s residence remains the same throughout early childhood, or if they move, that the exposure levels remain the same. Consequently, exposure misclassification may result from moving in early childhood, especially for exposures with high spatial heterogeneity such as pesticides and air pollution. This is a ubiquitous problem encountered by nearly all record-based studies that lack a complete residential history for each child.
In a 2003–2007 California statewide representative survey, only 14% of women moved in the 2–7 months post-partum (Margerison-Zilko et al., 2016), but the frequency of residential moves increases with a child’s age. For more than 50% of childhood cancer cases under age 5 diagnosed in California between 1988 and 2005, address at birth differed from the address at cancer diagnosis (Reynolds et al., 2004; Urayama et al., 2009), which raises concerns about using residence at birth to assess exposures in early childhood. While previous studies that examined residential proximity to environmental exposures have referred to exposure misclassification when solely using address at delivery or conception instead of a full residential history (Fell et al., 2004; Hodgson et al., 2015; Pennington et al., 2016; Schulman et al., 1993) and the potential bias resulting from residential mobility during pregnancy (Pennington et al., 2016), they rarely focused on early childhood. To date, only a few studies investigated the impact of residential mobility in early childhood on exposure assessment or effect estimates (Brokamp et al., 2016; Danysh et al., 2017; Nikkilä et al., 2018; Tee Lewis et al., 2019). Two Texas studies reported exposure estimates for two air toxics at diagnosis to be lower than at birth among young children (0–4 years) diagnosed with leukemia or central nervous system tumor (Danysh et al., 2017; Tee Lewis et al., 2019). An Ohio study examined traffic-related air pollution, greenspace, and community-level characteristics among children from birth through age seven in relation to asthma (Brokamp et al., 2016) and a Finnish study examined background radiation exposure among children in relation to childhood leukemia (Nikkilä et al., 2018). Both studies suggested that using the birth address or the last known address instead of the full address history may result in exposure misclassification leading to a bias toward the null.
It is often not feasible to acquire complete residential histories from interviews for subjects in record-based studies to generate a gold standard against which to compare recorded birth or diagnosis addresses. However, databases containing public records of individuals collected by commercial companies have become available in recent years allowing us to trace individuals without a self-reported residential history. For example, LexisNexis® Public Records (https://www.lexisnexis.com/en-us/products/public-records.page, hereinafter referred to as LexisNexis), a commercial credit reporting company, provides all known addresses for a set of individuals upon request. Earlier studies have shown that addresses acquired from LexisNexis are useful for reconstructing residential histories for subjects in epidemiological studies with an overall match rate of ~70–85% when detailed address histories were obtained in interviews (Hurley et al., 2017; Jacquez et al., 2011; Wheeler and Wang, 2015). However, subjects from these studies were mostly middle-aged or older, and their residential mobility may differ from that of women at child-bearing age.
The degree of exposure misclassification due to mobility depends on the distance moved, the spatial heterogeneity of the exposure (Bell and Belanger, 2012), and the method of exposure assessment in each study. For example, ecological measures of agricultural activity at the county level (Carozza et al., 2008) would not be altered by moving within a county. In contrast, individual-level measures of proximity to pesticide applications or crops as a proxy within a ~800–1000 m buffer of a child’s residence (Cockburn et al., 2011; Gómez-Barroso et al., 2016; Rull et al., 2009) may be subject to considerable misclassification due to residential mobility. Thus far, there is no literature reporting the impact of residential mobility in children’s first year of life at a spatial resolution that would capture fine-scale variation in pesticide exposures from agricultural applications.
The objectives of the present study are to assess patterns of mobility and identify maternal and child characteristics that may predict residential mobility in early childhood between birth and diagnosis before age 6, and to examine the impact of mobility on first year of life exposure assessment for agricultural pesticides applied near residences in California.
2. Methods
2.1. Study Population
We obtained information on all childhood cancer cases diagnosed before 6 years of age born in 1998–2011 from the California Cancer Registry (http://www.ccrcal.org). This study was conducted as part of the Air Pollution and Childhood Cancers (APCC) study, a large case-control investigation of children ages 0 to 5 years in CA, described previously (Heck et al., 2013). In brief, cases were linked to birth certificates using first and last names, date of birth, and social security number when available, using a probabilistic linkage program (LinkPlus, CDC) (89% matching rate); it is likely most of the remaining 11% of cases were born out of state (Urayama et al., 2009). From among all cases (n=7,160), we excluded 682 cases without address at diagnosis listed on cancer reports, mostly diagnosed in 2012 and 2013. For other years, less than 1–2% were missing address at diagnosis. The final case dataset included 6,478 childhood cancer cases. Controls free of cancer by age of 6 were randomly selected from birth certificates and frequency matched (20:1) by year of birth to all childhood cancer cases. Controls who were likely nonviable births (birth weight <500 g or birth before 20 weeks of gestation) (n=461), with unknown sex (n=2), those with a birth address outside of California (n=494), with missing census tract information (n=319), or who died before age of 6 according to a death certificate (n=1,599) were excluded. When analyzing LexisNexis addresses and related exposure estimates, we excluded all subjects born before 2001 or in 2007 due to an error that occurred during the delivery of a dataset containing address information to LexisNexis, leaving for analyses 99,262 controls. This project was approved by the human subjects protection boards of the University of California, Los Angeles, and the California Committee for the Protection of Human Subjects.
2.2. Maternal and Child Characteristics
Based on previous literature (Canfield et al., 2006; Margerison-Zilko et al., 2016; Urayama et al., 2009), we considered factors that potentially influence mobility in pregnancy or early childhood including age at diagnosis (0, 1, ≥2 years), year of birth (1998–2004 vs 2005–2011), maternal age at delivery (≤19, 20–24, 25–29, 30–34, ≥35), maternal race/ethnicity (non-Hispanic White, Hispanic, Black, Asian/Pacific islander, others), maternal birthplace (California, other U.S. states, Mexico, other foreign countries), maternal education (<12 years, 12 years, 13–15 years, ≥16 years), parity (1, 2, ≥3), rural/urban classification of residence at birth (metropolitan vs non-metropolitan), and several socioeconomic variables including payment source for prenatal care as a proxy for family income (private/HMO/ Blue Cross Blue Shield vs MediCal/government/ self-pay) and neighborhood level socioeconomic status (SES).
2.3. Source of Address Data
Maternal residence at birth of each case and control was collected from birth certificates and each case’s residence at diagnosis (latitude and longitude only) was obtained from the California Cancer Registry (CCR). Additional addresses were acquired from LexisNexis, through a combination of sources including Gramm-Leach-Bliley-compliant proprietary data, bankruptcy filings, court filings, incorporation documents, judgments, jury verdicts and settlements, real estate property records, sanctions, Uniform Commercial Code-1 liens, motor vehicle and driver’s license records, professional licenses and voter registration, liquor licenses, IRS enrolled agents, and inactive business directory contacts. We securely provided LexisNexis with a data file containing personal identifiers including child’s first and last names, child’s date of birth, parental first and last names, parental date of birth, and mother’s residential address (i.e., street, city, zip code, state) listed on birth certificate for all subjects and requested batch search services; after matching, the requested data were provided to UCLA and permanently destroyed at LexisNexis. LexisNexis returned a dataset containing all known addresses and the first seen and last seen dates associated with each address. We geocoded birth certificate addresses and LexisNexis addresses using an automated approach (Goldberg et al., 2008).
Following previously developed methods (Hurley et al., 2017; Wheeler and Wang, 2015), we cleaned and processed all addresses: first, we removed all post office box (P.O. Box) addresses that are not residential locations and are believed to introduce substantial geographically-based exposure misclassification (Hurley et al., 2003). Second, we identified and removed duplicate addresses compiled from multiple sources using geographic distance between a set of geocoded addresses. Third, we created a residential history timeline for each individual. We limited the LexisNexis data to the time period from the date of birth to the date of diagnosis for each case and the relevant early life period (<age of 5 years) for each control. We then defined the earliest known date of each address as the “start” date, and used the “start” date of the next sequential address as the “end” date of the previous address. This was recommended by LexisNexis, who informed us that “start” dates are more accurate than “end” dates. The final address was assigned an artificial “end” date corresponding to date of diagnosis for cases or the end of the fifth year of life for each control. For analyses below that focused on first year of life only, we restricted the residential history to the first year.
2.4. Geographic Measures
We calculated distance (kilometers) between geocoded birth and diagnosis addresses. Birth and diagnosis residences cannot be directly compared in terms of street number and name because only geocoded address at diagnosis (i.e., latitude and longitude) was available from the CCR. Previous geographic studies suggested that generally 70–80% of the addresses during automated geocoding have a positional error of 100 m or less depending on geocoding platforms, although this could be up to a few kilometers in rural areas (Cayo and Talbot, 2003; Faure et al., 2017). To differentiate between potential positional error occurring in geocoding and moving within neighborhoods, we defined any address change of >100m as a move, and considered alternative distance cutoff of 200m in sensitivity analyses.
Among cases who moved between birth and diagnosis of cancer, we examined the distribution of distance between the two residences, categorized into five levels (≤ 500 m, >500m – 2 km, >2 km – 4 km, >4 km – 10 km, >10 km) by child and maternal characteristics. These cutoffs were primarily selected for estimating the level of misclassification in buffer-based exposure assessment, adopted by our group (500 m or 2 km) (Costello et al., 2009; Ling et al., 2018; Wang et al., 2011) and other studies using buffer sizes between 500 m and 8 km radius (Bell et al., 2001; Gemmill et al., 2013; Gunier et al., 2011; Shaw et al., 2018; Shelton et al., 2014; Wofford et al., 2013) to estimate exposures to agricultural pesticide applied in proximity to residences. For example, with a distance of 2 km between the two addresses, a 2 km-radius buffer yields an intersection of ~ 40% of the size of the entire buffer; while 2 km-radius buffers would not overlap if the residences at their centers were 4 km apart. Similarly, the distribution of distance among cases who moved in the first year of life was examined using LexisNexis addresses. We also calculated the total number of different addresses and the distance between addresses, as listed on LexisNexis records, for cases and controls separately in their first year of life. Those who moved more than once were counted multiple times in calculating the distance moved, and in estimating the timing of moves. In addition, we conducted a sensitivity analysis excluding controls who left California after the child’s birth according to LexisNexis records, because our cases most likely were born and stayed in California by design.
2.5. Pesticide Exposures
We estimated agricultural pesticide exposures using a GIS-based Residential Ambient Pesticide Estimation System, as previously described (Cockburn et al., 2011; Rull and Ritz, 2003). In brief, since 1974 agricultural pesticide applications for commercial use are recorded in Pesticide Use Reports (PUR) mandated by the CA Department of Pesticide Regulation (CDPR). Each PUR record includes the name of the pesticide’s active ingredient, the poundage applied, the crop type, and the location and the date of application. The California Department of Water Resources (CDWR) performs countywide, large-scale surveys of land use and crop cover every 7–10 years. Land use maps increase spatial resolution because they provide more detailed land use geography that allows us to refine the location of pesticide applications (Rull and Ritz, 2003). We then combined PUR records, land use maps, and geocoded birth and diagnosis addresses to produce estimates of pesticide exposure in each child’s first year of life. Annual exposure estimates (pounds per acre) were calculated by adding the poundage of pesticide applied in a 2 km buffer around each address and weighting the total poundage by the proportion of acreage treated within the buffer.
We selected eight known or probable carcinogens from the top 200 frequently applied pesticides in our study population, while also considering the summary rating provided by the Pesticide Action Network (PAN) pesticide database (http://www.pesticideinfo.org/) that incorporates the most carcinogenic ranking assigned by organizations including the International Agency for Research on Cancer (IARC), the U.S. Environmental Protection Agency (EPA) and the U.S. National Institutes of Health (NIH). For each of the carcinogens examined in this study, we summed the annual pounds applied per acre to obtain exposure values for each calendar year using the 2km-buffer around each address. In the children’s first year of life, we used weighted averages with weights representing the proportions (in days) of the relevant exposure period falling into each calendar year. Three estimates for exposures in the first year of life were calculated accordingly, using 1) address at birth, 2) address at diagnosis, and 3) LexisNexis addresses (in available years). We then dichotomized children’s exposures in their first year of life as ever/never exposed to a specific carcinogen.
2.6. Statistical Analysis
To evaluate maternal and child characteristics associated with the cases’ residential mobility (moved versus did not move) between birth and diagnosis, we conducted logistic regression analysis and estimated odds ratios (ORs) and 95% confidence intervals (CIs). We compared the residential mobility in the first year of life based on LexisNexis addresses among subgroups of cases and controls (“movers”, “non-movers”, “not captured”) using Chi-square tests. We also applied Chi-square tests to compare the distance moved among subgroups of cases. To compare the impact of mobility on misclassification of pesticide exposures assessed for buffers of different sizes (i.e., 500 m and 2 km), we examined the agreement between exposures assigned to birth residence and those assigned to LexisNexis addresses for cases (age at diagnosis 1–5 years) and all controls. Restricting to cases diagnosed at or after 1 year of age, we assessed the level of agreement between exposures within 2 km of residences during the first year of life assigned to birth address and those assigned to diagnosis address, by age at diagnosis, year of birth, and by distance moved (>100 m - 2km, >2 km – 4 km, >4 km). These comparisons used: 1) continuous cumulative exposure estimates (pounds per acre) using Spearman correlation coefficients, since the exposure estimates were not normally distributed and, 2) dichotomous exposure indicators using Cohen’s kappa statistics, a robust agreement measure that takes the possibility of agreement by chance into account.
3. Results
Table 1 shows the child and maternal characteristics by residential mobility from birth to diagnosis based on addresses listed on birth certificates and in the CCR. Of 6,478 childhood cancer cases born in 1998–2011, 3,548 (54.8%) had a residential location at diagnosis at least 100 m distance away from their address at birth. Cases diagnosed at an older age compared with those diagnosed within the first year of life were more likely to move between birth and diagnosis. Mothers of younger age, with lower education, not having a Hispanic ethnic background, those who used public health insurance or resided in non-metropolitan areas at delivery, or those whose addresses are only partially captured by LexisNexis were more likely to move. Using a 200m distance moved as an alternative cutoff, we classified 3,297 (50.1%) cases as movers; the factors predicting residential mobility remained the same.
Table 1.
Case characteristics associated with residential mobilitya between birth and diagnosis dates (born 1998–2011) based on addresses listed on birth certificates and in the CCR.
| Moversa |
Non-moversa |
Crude OR | Adjusted ORb | |||
|---|---|---|---|---|---|---|
| N= 3,548 |
% | N= 2,930 |
% | (95% CI) | (95% CI) | |
| Year of birth | ||||||
| 1998–2004 | 2,201 | 56.8 | 1,676 | 43.2 | 1.00 | 1.00 |
| 2005–2011 | 1,347 | 51.8 | 1,254 | 48.2 | 0.83 (0.75, 0.92) | 0.94 (0.85, 1.05) |
| Age at diagnosis | ||||||
| 0 | 550 | 37.2 | 929 | 62.8 | 1.00 | 1.00 |
| 1 | 664 | 49.6 | 676 | 50.4 | 1.65 (1.42, 1.93) | 1.70 (1.45, 1.99) |
| 2–5 | 2,334 | 63.8 | 1,325 | 36.2 | 3.00 (2.64, 3.41) | 3.12 (2.73, 3.56) |
| Maternal Age | ||||||
| 19 or less | 416 | 69.3 | 184 | 30.7 | 3.28 (2.66, 4.06) | 2.57 (1.98, 3.33) |
| 20–24 | 861 | 64.2 | 480 | 35.8 | 2.54 (2.16, 2.98) | 2.19 (1.81, 2.65) |
| 25–29 | 959 | 57.4 | 713 | 42.6 | 1.92 (1.65, 2.24) | 1.87 (1.59, 2.21) |
| 30–34 | 798 | 49.1 | 826 | 50.9 | 1.36 (1.17, 1.59) | 1.39 (1.18, 1.62) |
| 35 and older | 514 | 41.4 | 727 | 58.6 | 1.00 | 1.00 |
| Maternal Education | ||||||
| <12 years | 1,034 | 59.8 | 694 | 40.2 | 1.83 (1.59, 2.10) | 1.35 (1.10, 1.66) |
| 12 years | 1,049 | 58.2 | 752 | 41.8 | 1.71 (1.49, 1.96) | 1.24 (1.04, 1.48) |
| 13–15 years | 676 | 54.6 | 563 | 45.4 | 1.47 (1.27, 1.71) | 1.24 (1.05, 1.47) |
| 16+ years | 697 | 45.0 | 851 | 55.0 | 1.00 | 1.00 |
| Missing | 92 | 56.8 | 70 | 43.2 | - | - |
| Maternal Race/Ethnicity | ||||||
| White, non-Hispanic | 1,166 | 55.1 | 949 | 44.9 | 1.00 | 1.00 |
| Hispanic, any race | 1,795 | 55.6 | 1,433 | 44.4 | 1.02 (0.91, 1.14) | 0.70 (0.60, 0.82) |
| Black | 181 | 61.8 | 112 | 38.2 | 1.30 (1.01, 1.68) | 1.00 (0.76, 1.31) |
| Asian/PI | 289 | 47.1 | 324 | 52.9 | 0.74 (0.62, 0.89) | 0.82 (0.65, 1.03) |
| Other/Refused | 117 | 51.1 | 112 | 48.9 | 0.84 (0.60, 1.17) | 0.77 (0.54, 1.11) |
| Parity | ||||||
| 1 | 1,469 | 58.3 | 1,049 | 41.7 | 1.00 | 1.00 |
| 2 | 1,045 | 52.5 | 945 | 47.5 | 0.78 (0.69, 0.88) | 0.88 (0.77, 1.01) |
| 3 or more | 1,034 | 52.5 | 936 | 47.5 | 0.78 (0.69, 0.88) | 0.93 (0.80, 1.08) |
| Payment type of prenatal care | ||||||
| Private/HMO/BCBS | 1,740 | 48.9 | 1,817 | 51.1 | 1.00 | 1.00 |
| MediCal/Govt/self-pay | 1,784 | 61.9 | 1,097 | 38.1 | 1.67 (1.51, 1.85) | 1.53 (1.34, 1.75) |
| Missing | 24 | 60.0 | 16 | 40.0 | - | - |
| Maternal birthplace | ||||||
| California | 1,634 | 56.7 | 1,246 | 43.3 | 1.00 | 1.00 |
| Mexico | 931 | 56.0 | 731 | 44.0 | 0.95 (0.84, 1.08) | 1.00 (0.85, 1.18) |
| Other US States | 412 | 52.5 | 373 | 47.5 | 0.84 (0.71, 0.98) | 1.02 (0.85, 1.21) |
| Other foreign countries | 567 | 49.6 | 577 | 50.4 | 0.75 (0.65, 0.86) | 0.96 (0.80, 1.15) |
| Missing | 4 | 57.1 | 3 | 42.9 | - | - |
| Urban/rural status at birth | ||||||
| Metropolitan | 3,256 | 53.7 | 2,803 | 46.3 | 1.00 | 1.00 |
| Non-metropolitan | 292 | 69.7 | 127 | 30.3 | 1.98 (1.59, 2.46) | 1.74 (1.38, 2.19) |
| Quintiles of neighborhood SES at birth | ||||||
| 1 (Lowest) | 929 | 56.5 | 715 | 43.5 | 1.45 (1.23, 1.71) | 0.88 (0.72, 1.08) |
| 2 | 884 | 57.7 | 647 | 42.3 | 1.54 (1.30, 1.82) | 0.96 (0.79, 1.17) |
| 3 | 719 | 56.0 | 565 | 44.0 | 1.45 (1.22, 1.72) | 0.98 (0.81, 1.19) |
| 4 | 590 | 53.2 | 519 | 46.8 | 1.28 (1.07, 1.53) | 1.03 (0.85, 1.24) |
| 5 (Highest) | 426 | 46.8 | 484 | 53.2 | 1.00 | 1.00 |
| For cases born 2001–2011 (excluding 2007) | ||||||
| LexisNexis datac | ||||||
| Fully captured | 1,269 | 48.2 | 1,362 | 51.8 | - | - |
| Partially captured | 948 | 67.7 | 453 | 32.2 | - | - |
| Missing | 184 | 47.9 | 200 | 52.1 | - | - |
Residential mobility was defined by comparing address at birth vs at diagnosis; Movers (55%): distance between addresses at birth and diagnosis > 100m; Non-movers (45%): distance ≤ 100m
Adjusted for all variables in the table
Based on all address information from birth to diagnosis provided by LexisNexis
The information from LexisNexis suggested more than 20% of case (age at diagnosis 1–5 years) and control mothers moved in the child’s first year of life, with less than 4% moving multiple times (Figure 1). Noticeably, for 42% of case mothers and 45% of control mothers LexisNexis provided no or partial residential information only at best during the child’s first year of life and these children were more likely to be born in earlier years (2001–2005), born to mothers of younger age, with lower education, having a Hispanic background or originating from Mexico, using public health insurance, and residing in lower SES neighborhoods (Table 2). Using LexisNexis information, we identified similar predictors for residential mobility among cases and controls in their first year of life, and these predictors did not differ substantially when examining cases’ residential mobility from birth to diagnosis comparing birth and diagnosis addresses.
Figure 1.
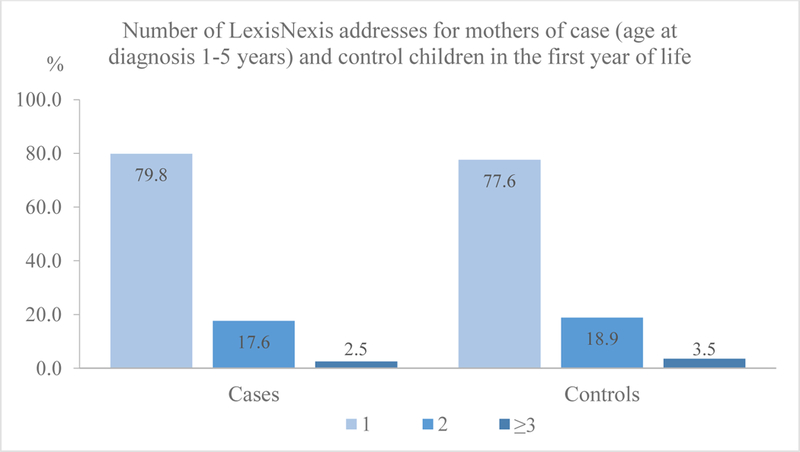
Number of LexisNexis addresses for mothers of case (age at diagnosis 1–5 years) and control children
Table 2.
Child and maternal characteristics associated with residential mobility in child’s first year of life based on LexisNexis (for children born 2001–2011a).
| Cases, age at diagnosis 1–5 years | Controls | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moversb | Non-moversb | Not capturedb | P for Chi- square |
Moversb | Non-moversb | Not capturedb | P for Chi- square |
|||||||
| N= 482 |
Row % |
N= 1,907 |
Row % |
N= 1,488 |
Row % |
N= 13,007 |
Row % |
N= 45,035 |
Row % |
N= 41,220 |
Row % |
|||
| Year of birth | ||||||||||||||
| 2001–2004 | 122 | 6.5 | 871 | 46.3 | 888 | 47.2 | <.0001 | 3,099 | 6.7 | 19,017 | 41.1 | 24,183 | 52.2 | <.0001 |
| 2005–2011a | 360 | 18.0 | 1,036 | 51.9 | 600 | 30.1 | 9,908 | 18.7 | 26,018 | 49.1 | 17,037 | 32.2 | ||
| Age at diagnosis | ||||||||||||||
| 1 | 132 | 12.4 | 539 | 50.7 | 392 | 36.9 | 0.461 | - | - | - | - | - | - | - |
| 2–5 | 350 | 12.4 | 1,368 | 48.6 | 1,096 | 38.9 | - | - | - | - | - | - | ||
| Maternal Age | ||||||||||||||
| 19 or less | 35 | 10.4 | 71 | 21.1 | 230 | 68.5 | <.0001 | 1,068 | 11.5 | 1,925 | 20.7 | 6,324 | 67.9 | <.0001 |
| 20–24 | 152 | 18.8 | 359 | 44.5 | 296 | 36.7 | 3,916 | 17.5 | 8,890 | 39.8 | 9,539 | 42.7 | ||
| 25–29 | 136 | 13.7 | 498 | 50.1 | 361 | 36.3 | 3,807 | 14.4 | 12,252 | 46.4 | 10,367 | 39.2 | ||
| 30–34 | 91 | 9.2 | 543 | 55.2 | 350 | 35.6 | 2,660 | 10.9 | 12,553 | 51.6 | 9,104 | 37.4 | ||
| 35 and older | 68 | 9.0 | 436 | 57.7 | 251 | 33.2 | 1,556 | 9.2 | 9,415 | 55.9 | 5,875 | 34.9 | ||
| Missing | - | - | - | - | - | - | - | - | - | - | 11 | 100.0 | ||
| Maternal Education | ||||||||||||||
| <12 years | 99 | 9.7 | 328 | 32.1 | 596 | 58.3 | <.0001 | 2,761 | 9.9 | 7,768 | 27.9 | 17,276 | 62.1 | <.0001 |
| 12 years | 164 | 15.4 | 500 | 46.9 | 401 | 37.7 | 4,126 | 16.0 | 11,537 | 44.7 | 10,136 | 39.3 | ||
| 13–15 years | 130 | 17.1 | 437 | 57.3 | 195 | 25.6 | 3,327 | 16.6 | 10,814 | 53.9 | 5,938 | 29.6 | ||
| 16+ years | 72 | 7.8 | 592 | 64.3 | 256 | 27.8 | 2,446 | 10.7 | 13,815 | 60.2 | 6,675 | 29.1 | ||
| Missing | 17 | 15.9 | 50 | 46.7 | 40 | 37.4 | 347 | 13.1 | 1,101 | 41.7 | 1,195 | 45.2 | ||
| Maternal Race/Ethnicity | ||||||||||||||
| White, non-Hispanic | 150 | 12.4 | 694 | 57.5 | 363 | 30.1 | <.0001 | 3,896 | 13.5 | 15,980 | 55.3 | 9,016 | 31.2 | <.0001 |
| Hispanic, any race | 229 | 11.7 | 817 | 41.7 | 911 | 46.6 | 5,920 | 11.7 | 18,966 | 37.5 | 25,739 | 50.8 | ||
| Black | 30 | 16.7 | 90 | 50.0 | 60 | 33.3 | 1,276 | 23.1 | 2,647 | 47.8 | 1,609 | 29.1 | ||
| Asian/PI | 49 | 13.0 | 229 | 60.9 | 98 | 26.1 | 1,318 | 12.8 | 5,646 | 54.7 | 3,356 | 32.5 | ||
| Other/Refused | 24 | 15.3 | 77 | 49.0 | 56 | 35.7 | 597 | 15.3 | 1,796 | 46.1 | 1,500 | 38.5 | ||
| Parity | ||||||||||||||
| 1 | 191 | 12.5 | 727 | 47.6 | 610 | 39.9 | 0.0413 | 5,206 | 13.5 | 16,633 | 43.1 | 16,737 | 43.4 | <.0001 |
| 2 | 144 | 12.4 | 613 | 52.8 | 404 | 34.8 | 4,028 | 12.9 | 15,073 | 48.1 | 12,237 | 39.0 | ||
| 3 or more | 147 | 12.4 | 567 | 47.7 | 474 | 39.9 | 3,767 | 12.9 | 13,303 | 45.4 | 12,223 | 41.7 | ||
| Missing | - | - | - | - | - | - | 6 | 10.9 | 26 | 47.3 | 23 | 41.8 | ||
| Payment type of prenatal care | ||||||||||||||
| Private/HMO/BCBS | 239 | 11.4 | 1,210 | 57.9 | 641 | 30.7 | <.0001 | 5,907 | 12.2 | 27,497 | 56.7 | 15,130 | 31.2 | <.0001 |
| MediCal/Govt/self-pay | 243 | 13.7 | 690 | 38.9 | 839 | 47.3 | 6,991 | 14.0 | 17,289 | 34.6 | 25,684 | 51.4 | ||
| Missing | - | - | 7 | 46.7 | 8 | 53.3 | 109 | 14.3 | 249 | 32.6 | 406 | 53.1 | ||
| Maternal birthplace | ||||||||||||||
| California | 270 | 15.2 | 994 | 55.8 | 517 | 29.0 | <.0001 | 7,175 | 16.8 | 22,890 | 53.7 | 12,530 | 29.4 | <.0001 |
| Mexico | 79 | 8.0 | 313 | 31.6 | 597 | 60.4 | 1,939 | 7.3 | 7,310 | 27.4 | 17,452 | 65.4 | ||
| Other US States | 51 | 12.3 | 258 | 62.3 | 105 | 25.4 | 1,754 | 15.9 | 5,949 | 53.8 | 3,347 | 30.3 | ||
| Other foreign countries | 82 | 11.9 | 342 | 49.6 | 265 | 38.5 | 2,132 | 11.3 | 8,848 | 47.0 | 7,832 | 41.6 | ||
| Missing | - | - | - | - | 4 | 100.0 | 7 | 6.7 | 38 | 36.5 | 59 | 56.7 | ||
| Urban/rural status at birth | ||||||||||||||
| Metropolitan | 444 | 12.3 | 1,804 | 50.0 | 1,363 | 37.7 | 0.0018 | 12,130 | 13.1 | 42,367 | 45.9 | 37,825 | 41.0 | <.0001 |
| Non-metropolitan | 38 | 14.3 | 103 | 38.7 | 125 | 47.0 | 877 | 12.6 | 2,668 | 38.4 | 3,395 | 48.9 | ||
| Quintiles of neighborhood SES at birth | ||||||||||||||
| 1 (Lowest) | 115 | 11.8 | 425 | 43.5 | 437 | 44.7 | <.0001 | 3,459 | 12.9 | 10,185 | 38.1 | 13,067 | 48.9 | <.0001 |
| 2 | 127 | 13.7 | 422 | 45.5 | 379 | 40.8 | 3,295 | 14.0 | 9,997 | 42.3 | 10,328 | 43.7 | ||
| 3 | 102 | 13.1 | 390 | 50.3 | 284 | 36.6 | 2,694 | 13.6 | 9,115 | 46.0 | 8,004 | 40.4 | ||
| 4 | 86 | 12.6 | 358 | 52.6 | 237 | 34.8 | 2,121 | 13.4 | 8,029 | 50.9 | 5,638 | 35.7 | ||
| 5 (Highest) | 52 | 10.1 | 312 | 60.6 | 151 | 29.3 | 1438 | 10.8 | 7709 | 57.8 | 4183 | 31.4 | ||
Except 2007
Not captured: with missing or only partial residential information in the first year of life from LexisNexis
Among the cases who moved between birth and diagnosis, the median distance moved was 5.05 km. Overall, 30.1% of all cases moved less than 2 km, while more than half moved more than 4 km (Table 3). Cases with an older age at diagnosis, born to younger or Black mothers, or born in metropolitan areas were more likely to move further between birth and diagnosis. Based on LexisNexis addresses, cases diagnosed at age 1 and those diagnosed at ages 2–5 became more similar in distance moved in the first year of life (Supplementary Table 1); the median distance moved in a child’s first year of life was 6.97 km for cases (age at diagnosis 1–5), and 9.81 km for all controls.
Table 3.
Distance (kilometers) between birth address and diagnosis address among cases born 1998–2011 who moved before diagnosis.
| Distance ≤ 500 m |
500 m < Distance ≤ 2 km |
2 km < Distance ≤ 4 km |
4 km < Distance ≤ 10 km |
Distance > 10 km |
P for Chi- square test |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| All | 459 | 12.9 | 609 | 17.2 | 523 | 14.7 | 730 | 20.6 | 1,227 | 34.6 | - |
| Age at diagnosis | |||||||||||
| 0 | 148 | 26.9 | 102 | 18.5 | 76 | 13.8 | 81 | 14.7 | 143 | 26.0 | <.0001 |
| 1 | 98 | 14.8 | 122 | 18.4 | 103 | 15.5 | 126 | 19.0 | 215 | 32.4 | |
| 2–5 | 213 | 9.1 | 385 | 16.5 | 344 | 14.7 | 523 | 22.4 | 869 | 37.2 | |
| Maternal Age | |||||||||||
| 19 or less | 39 | 9.4 | 73 | 17.5 | 68 | 16.3 | 106 | 25.5 | 130 | 31.3 | <.0001 |
| 20–24 | 73 | 8.5 | 161 | 18.7 | 137 | 15.9 | 188 | 21.8 | 302 | 35.1 | |
| 25–29 | 118 | 12.3 | 148 | 15.4 | 141 | 14.7 | 196 | 20.4 | 356 | 37.1 | |
| 30–34 | 129 | 16.2 | 134 | 16.8 | 109 | 13.7 | 146 | 18.3 | 280 | 35.1 | |
| 35 and older | 100 | 19.5 | 93 | 18.1 | 68 | 13.2 | 94 | 18.3 | 159 | 30.9 | |
| Maternal Race/Ethnicity | |||||||||||
| White, non-Hispanic | 168 | 14.4 | 179 | 15.4 | 155 | 13.3 | 219 | 18.8 | 445 | 38.2 | 0.0006 |
| Hispanic, any race | 228 | 12.7 | 347 | 19.3 | 286 | 15.9 | 373 | 20.8 | 561 | 31.3 | |
| Black | 14 | 7.7 | 29 | 16.0 | 27 | 14.9 | 38 | 21.0 | 73 | 40.3 | |
| Asian/PI | 36 | 12.5 | 34 | 11.8 | 38 | 13.1 | 70 | 24.2 | 111 | 38.4 | |
| Other/Refused | 13 | 11.1 | 20 | 17.1 | 17 | 14.5 | 30 | 25.6 | 37 | 31.6 | |
| Urban/rural status at birth | |||||||||||
| Metropolitan | 407 | 12.5 | 544 | 16.7 | 485 | 14.9 | 683 | 21.0 | 1137 | 34.9 | 0.0035 |
| Non-metropolitan | 52 | 17.8 | 65 | 22.3 | 38 | 13.0 | 47 | 16.1 | 90 | 30.8 | |
Exposures to specific carcinogens during the first years of life calculated for birth address were compared with those calculated for diagnosis address or LexisNexis addresses among cases diagnosed between ages of 1 to 5, and birth residence was compared with LexisNexis addresses among all controls. Overall, the correlations between continuous exposure estimates (pounds per acre) within a 2 km buffer of birth vs diagnosis address were moderate to strong (0.67–0.76), and those between birth residence and LexisNexis addresses were even stronger (0.76–0.83); additionally, these correlations did not differ by outcome status (Table 4); however, these correlations weakened when the buffer size decreased from 2 km to 500 m. When stratifying by age at diagnosis, the correlations between exposure estimates within a 2 km buffer of birth vs diagnosis address were stronger for those with a younger age at diagnosis, born in a later year (2005–2011), and who moved shorter distances (Table 5). The kappa statistics between dichotomous exposures (ever vs. never exposed) were similar, also suggesting overall good agreement (Table 4).
Table 4.
Agreement between first year of life exposures assigned to address at birth (from the birth certificate) vs address at diagnosis (from the CCR) or LexisNexis addresses among cases (age at diagnosis 1–5 years) and controls born 2001–2011a using different buffer sizes.
| Buffer = 2000 m |
Buffer = 500 m |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth certificate address vs LexisNexis Addresses |
Birth certificate address vs Diagnosis address |
Birth certificate address vs LexisNexis Addresses |
Birth certificate address vs Diagnosis address |
|||||||||
| Controls |
Cases |
Cases |
Controls |
Cases |
Cases |
|||||||
| Chemical | rb | κc | rb | κc | rb | κc | rb | κc | rb | κc | rb | κc |
| Petroleum oil, unclassified | 0.78 | 0.76 | 0.78 | 0.77 | 0.69 | 0.67 | 0.73 | 0.72 | 0.66 | 0.64 | 0.58 | 0.57 |
| Mineral oil | 0.79 | 0.78 | 0.80 | 0.79 | 0.69 | 0.68 | 0.72 | 0.71 | 0.65 | 0.64 | 0.59 | 0.58 |
| Diuron | 0.79 | 0.78 | 0.80 | 0.80 | 0.70 | 0.69 | 0.71 | 0.71 | 0.63 | 0.62 | 0.60 | 0.59 |
| Thiophanate-methyl | 0.77 | 0.75 | 0.76 | 0.75 | 0.67 | 0.66 | 0.69 | 0.69 | 0.67 | 0.66 | 0.54 | 0.53 |
| Permethrin | 0.79 | 0.77 | 0.78 | 0.76 | 0.70 | 0.68 | 0.73 | 0.72 | 0.68 | 0.67 | 0.58 | 0.57 |
| Oxyfluorfen | 0.83 | 0.81 | 0.83 | 0.81 | 0.76 | 0.74 | 0.75 | 0.74 | 0.76 | 0.75 | 0.62 | 0.61 |
| Hexythiazox | 0.77 | 0.76 | 0.79 | 0.78 | 0.67 | 0.65 | 0.69 | 0.69 | 0.63 | 0.63 | 0.45 | 0.45 |
| Pyrethrins | 0.77 | 0.76 | 0.78 | 0.77 | 0.71 | 0.69 | 0.70 | 0.70 | 0.66 | 0.66 | 0.54 | 0.54 |
Except 2007
Spearman correlation coefficients were calculated using continuous exposure estimates in child’s first year of life
Kappa statistics were calculated using binary exposure indicators for child’s first year of life
Table 5.
Agreement between first year of life exposures assigned to address at birth (from the birth certificate) vs address at diagnosis (from the CCR) among cases born 1998–2011 and diagnosed at age 1–5 years, using a 2000 m buffer.
| Address at birth (from the birth certificate) vs Address at diagnosis (from the CCR, cases only) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| By age at diagnosis |
By year of birth |
By distance (D) movedc |
||||||||||||||
| Age = 1 (N=1,340) |
Age = 2 (N=1,262) |
Age ≥ 3 (N=2,397) |
1998–2004 (N=3,141) |
2005–2011 (N=1,858) |
100 m < D ≤ 2 km (N=818) |
2 km < D ≤ 4 km (N=447) |
D > 4 km (N=1,733) |
|||||||||
| Chemical | ra | κb | ra | κb | ra | κb | ra | κb | ra | κb | ra | κb | ra | κb | ra | κb |
| Petroleum oil, unclassified | 0.76 | 0.74 | 0.70 | 0.68 | 0.64 | 0.61 | 0.67 | 0.65 | 0.70 | 0.69 | 0.91 | 0.89 | 0.67 | 0.62 | 0.24 | 0.20 |
| Mineral oil | 0.76 | 0.76 | 0.69 | 0.67 | 0.65 | 0.63 | 0.68 | 0.67 | 0.70 | 0.69 | 0.91 | 0.89 | 0.67 | 0.63 | 0.29 | 0.27 |
| Diuron | 0.79 | 0.77 | 0.66 | 0.66 | 0.67 | 0.67 | 0.69 | 0.68 | 0.71 | 0.70 | 0.93 | 0.92 | 0.74 | 0.72 | 0.33 | 0.31 |
| Thiophanate-methyl | 0.74 | 0.76 | 0.69 | 0.67 | 0.62 | 0.59 | 0.66 | 0.65 | 0.68 | 0.67 | 0.88 | 0.85 | 0.54 | 0.51 | 0.22 | 0.20 |
| Permethrin | 0.77 | 0.75 | 0.71 | 0.68 | 0.65 | 0.64 | 0.67 | 0.65 | 0.74 | 0.72 | 0.93 | 0.92 | 0.63 | 0.55 | 0.29 | 0.25 |
| Oxyfluorfen | 0.80 | 0.79 | 0.77 | 0.75 | 0.74 | 0.71 | 0.74 | 0.72 | 0.79 | 0.77 | 0.94 | 0.91 | 0.81 | 0.77 | 0.43 | 0.39 |
| Hexythiazox | 0.74 | 0.73 | 0.68 | 0.67 | 0.62 | 0.60 | 0.66 | 0.65 | 0.68 | 0.66 | 0.87 | 0.85 | 0.60 | 0.56 | 0.25 | 0.22 |
| Pyrethrins | 0.76 | 0.75 | 0.73 | 0.71 | 0.65 | 0.64 | 0.67 | 0.65 | 0.75 | 0.74 | 0.92 | 0.91 | 0.64 | 0.61 | 0.24 | 0.22 |
Spearman correlation coefficients were calculated using continuous exposure estimates in child’s first year of life
Kappa statistics were calculated using binary exposure indicators for child’s first year of life
Non-movers (D ≤ 100 m) had almost perfect correlation and therefore are not shown here
4. Discussion
This study found overall moderate to strong (correlation/kappa=0.7~0.8) agreement between first year of life exposures to agricultural pesticides 2 km surrounding the birth residence and diagnosis address or reconstructed LexisNexis residential history. However, exposure misclassification might vary by buffer size, age at diagnosis (cases only), and distance moved. We identified several demographic and socioeconomic factors that may predict residential mobility in early childhood.
As with previous studies that focused on residential mobility during pregnancy, higher residential mobility in early childhood was associated with a range of maternal socio-demographic characteristics such as lower maternal age, lower maternal education, and lower individual or neighborhood level SES (Amoon et al., 2018; Bell and Belanger, 2012; Canfield et al., 2006; Margerison-Zilko et al., 2016; Urayama et al., 2009). We found cancer cases born to Hispanic mothers to be less likely to move; however, findings for maternal race/ethnicity vary across studies at different geographic locations, years, and population compositions (Bell and Belanger, 2012). The likelihood of moving between birth and diagnosis of cancer increases with case’s age at diagnosis, suggesting that birth residence becomes a less representative proxy for true exposures as children grow up. Movers captured by LexisNexis more often only had a partial residential histories compared with non-movers. Using address information obtained from LexisNexis, we found similar predictors for mobility in cases and controls; however, we observed that maternal and child characteristics of those with complete address information in the first year of life from LexisNexis were quite different from those with missing or partially missing address information, i.e., the highest proportion of women not captured by LexisNexis were Hispanic women born in Mexico and those living in the lowest SES neighborhoods, i.e. possibly those with the higher environmental exposures, as well as young women and those with less than high school education.
The use of LexisNexis has a remarkable advantage in identifying the timing of moves, providing important information for future research that assesses time-sensitive exposures or involves outcomes with a susceptible period after a child’s birth. In our study mothers of case and control children were more likely to move in the child’s first year of life, compared with the following years in early childhood. Control mothers seemed to have slightly higher mobility than the case mothers throughout the entire study period, likely because the case families (by definition) were diagnosed within California and therefore their residences were California-based at least at birth and at diagnosis, while control families could have left California but were still captured in the LexisNexis database with the nationwide search ability. After excluding those families who left California after the index child’s birth, mobility of cases and controls was more similar.
A small literature previously examined patterns of residential mobility in early childhood to identify the potential for environmental exposure misclassification (Brokamp et al., 2016; Margerison-Zilko et al., 2016; Nikkilä et al., 2018) but none focused on pesticide exposures. In general, we had good agreement between exposures to agricultural pesticides within a 2 km buffer of birth residence and LexisNexis addresses for cases and controls in their first year of life. The level of misclassification introduced by residential mobility may be acceptable if our study period of interest is the child’s first year of life, since only around 20% of cases (diagnosed at age 1–5 years) and control mothers changed their residence during this period and this did not differ by disease status. Similarly, for all cases who moved between birth and diagnosis, we have moderate to good agreement between exposures to agricultural pesticides within a 2 km buffer of birth residence and diagnosis residence. The degree of exposure misclassification is a function of the distance moved and the buffer size used in relation to the spatial heterogeneity of the exposure. For example, for those who moved within a short distance (≤ 2 km), misclassification is minimal; yet a longer distance (> 4 km) could be problematic, in particular when the buffer radius is smaller than the distance between locations. Thus, estimated exposures to agricultural pesticides within 500 m of a residence are more sensitive to the variation of exposures due to mobility compared with those using 2 km or even larger buffer radii, as shown in our study. Similarly, other environmental epidemiologic studies of child health outcomes that used a smaller buffer radius or a shorter distance (typically within several hundred meters) to exposure sources such as traffic-related air pollution, electronic magnetic fields, or powerlines might also be vulnerable to inaccurate location assignments.
In assessing patterns of and examining factors that may predict residential mobility in early childhood, our strength is that we identified a large number of childhood cancer cases born and diagnosed in California through California birth certificates and the California cancer registry. Unlike smaller questionnaire-based studies, our results were unlikely to be influenced by selection bias introduced by participation or recall bias; though the analysis based on LexisNexis addresses could be subject to potential selection bias, the conclusions drawn from it were comparable to those derived by examining birth vs diagnosis residence in cases only. To our knowledge, only a couple of studies examined the post-partum residential mobility either in a statewide sample of California women (Margerison-Zilko et al., 2016) or in enrolled childhood leukemia cases of a Northern California Study (Urayama et al., 2009) but both focused on factors associated with residential mobility and changes in neighborhood SES. Our study, on the other hand, for the first time calculated distance moved in early life of children and examined the potential for exposure misclassification by age at diagnosis and distance moved. Though pregnancy period is the most critical period for most environmental exposures, first year of life exposures might also contribute substantially to young children’s health outcomes (Ma et al., 2002). Therefore, it is crucial to assess exposures not only during pregnancy but also in the early life of children.
This study also has a few limitations. We were unable to obtain a secondary address from any registry for our population-based controls that would be comparable to cases’ diagnosis residence, limiting our ability to examine the patterns of residential mobility parallel in cases and controls; however, the reconstructed LexisNexis residential histories allowed us to compare moving patterns in cases vs controls. Additionally, only the latitude and longitude of the diagnosis residence on the cancer reports were available, thus we cannot directly compare the street number and names of birth and diagnosis residences to ascertain residential mobility. We had to rely on the geocoded diagnosis address which does not guarantee the same level of geocoding accuracy as for the birth addresses we geocoded using an automated approach. However, the use of 100 m (or 200 m) cutoff likely accounts for the possible positional errors during geocoding. Besides, there might be intended or unintended misreporting of birth residence and diagnosis residence among individuals seeking health care services, particularly for undocumented or uninsured families (Pamela Kempert, personal communication). Address information from LexisNexis has several intrinsic issues including multiple unique addresses for the same time period, inaccurate or missing first seen and last seen dates associated with certain addresses (Hurley et al., 2017), disagreement between LexisNexis addresses and registry-obtained addresses at the time of self-reporting (Supplementary Table 2), inconsistent data quality in different sub-groups of the general population, and time-varying sources of addresses over years, therefore limiting the power to rely solely on this method for large-scale records-based epidemiological studies. Future research attempting to reconstruct residential histories for study subjects using LexisNexis should also be aware of the potential for selection bias, introduced by varying availability of public records within sub-groups of the population.
In conclusion, residential mobility among childhood cancer cases diagnosed in California and their matched controls was associated with a number of child and maternal factors. Unlike adult cancers or other childhood outcomes such as asthma, which have a range of known demographic and lifestyle risk factors, the etiology of most childhood cancers remains underexplored, with few risk factors established for which we would need to control (even for leukemia which has been the most studied childhood cancer to date). The overall agreement between exposures to agricultural pesticides in early life of children assessed using a 2 km buffer around residences at birth and alternative addresses was moderate to good and decreased with the buffer size. These findings suggest that residential addresses collected at one point in time should be used with caution when estimating environmental exposures in early childhood, especially after the first year of life. Future research should consider factors that might help correct for the misclassification introduced by residential mobility. LexisNexis data, or other similar methods for reconstructing residential histories, may be useful for augmenting existing address information and constructing residential histories in estimating environmental exposures for large records-based epidemiological studies. Advancing technologies and additional data sources for improved tracking of people’s residences in the big data era may help us to obtain a more accurate residential history for populations when studying environmental causes of diseases such as childhood cancers.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01ES10544, R21ES019986, R21ES018960, R01CA195218. Dr. Heck was supported by a grant from Alex’s Lemonade Stand Foundation [grant number 17-01882].
Footnotes
Conflict of interest
The authors declare no competing interests.
References
- Amoon AT, Oksuzyan S, Crespi CM, Arah OA, Cockburn M, Vergara X, Kheifets L, 2018. Residential mobility and childhood leukemia. Environ. Res. 164, 459–466. doi: 10.1016/j.envres.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EM, Hertz-Picciotto I, Beaumont JJ, 2001. Case-cohort analysis of agricultural pesticide applications near maternal residence and selected causes of fetal death. Am. J. Epidemiol. 154, 702–710. doi: 10.1093/aje/154.8.702 [DOI] [PubMed] [Google Scholar]
- Bell ML, Belanger K, 2012. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J. Expo. Sci. Environ. Epidemiol. 22, 429–438. doi: 10.1038/jes.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, LeMasters GK, Ryan PH, 2016. Residential mobility impacts exposure assessment and community socioeconomic characteristics in longitudinal epidemiology studies. J. Expo. Sci. Environ. Epidemiol. 26, 428–34. doi: 10.1038/jes.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Ramadhani TA, Langlois PH, Waller ADK, 2006. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J. Expo. Sci. Environ. Epidemiol. 16, 538–543. doi: 10.1038/sj.jes.7500501 [DOI] [PubMed] [Google Scholar]
- Carozza SE, Li B, Elgethun K, Whitworth R, 2008. Risk of childhood cancers associated with residence in agriculturally intense areas in the United States. Environ. Health Perspect. 116, 559–565. doi: 10.1289/ehp.9967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozza SE, Li B, Wang Q, Horel S, Cooper S, 2009. Agricultural pesticides and risk of childhood cancers. Int. J. Hyg. Environ. Health 212, 186–95. doi: 10.1016/j.ijheh.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayo MR, Talbot TO, 2003. Positional error in automated geocoding of residential addresses. Int. J. Health Geogr. 2, 10. doi: 10.1186/1476-072X-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn M, Mills P, Zhang X, Zadnick J, Goldberg D, Ritz B, 2011. Prostate Cancer and Ambient Pesticide Exposure in Agriculturally Intensive Areas in California. Am. J. Epidemiol. 173, 1280–1288. doi: 10.1093/aje/kwr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B, 2009. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am. J. Epidemiol. 169, 919–26. doi: 10.1093/aje/kwp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysh HE, Mitchell LE, Zhang K, Scheurer ME, Lupo PJ, 2017. Differences in environmental exposure assignment due to residential mobility among children with a central nervous system tumor: Texas, 1995–2009. J. Expo. Sci. Environ. Epidemiol. 27, 41–46. doi: 10.1038/jes.2015.63 [DOI] [PubMed] [Google Scholar]
- Faure E, Danjou AM, Clavel-Chapelon F, Boutron-Ruault MC, Dossus L, Fervers B, 2017. Accuracy of two geocoding methods for geographic information system-based exposure assessment in epidemiological studies. Environ. Heal. A Glob. Access Sci. Source 16. doi: 10.1186/s12940-017-0217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell DB, Dodds L, King WD, 2004. Residential mobility during pregnancy. Paediatr. Perinat. Epidemiol. 18, 408–414. doi: 10.1111/j.1365-3016.2004.00580.x [DOI] [PubMed] [Google Scholar]
- García-Pérez J, López-Abente G, Gómez-Barroso D, Morales-Piga A, Pardo Romaguera E, Tamayo I, Fernández-Navarro P, Ramis R, 2015. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 140, 542–553. doi: 10.1016/j.envres.2015.05.014 [DOI] [PubMed] [Google Scholar]
- García-Pérez J, Morales-Piga A, Gómez J, Gómez-Barroso D, Tamayo-Uria I, Pardo Romaguera E, Fernández-Navarro P, López-Abente G, Ramis R, 2016. Association between residential proximity to environmental pollution sources and childhood renal tumors. Environ. Res. 147, 405–414. doi: 10.1016/j.envres.2016.02.036 [DOI] [PubMed] [Google Scholar]
- Gemmill A, Gunier RB, Bradman A, Eskenazi B, Harley KG, 2013. Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environ. Health Perspect. 121, 737–43. doi: 10.1289/ehp.1205682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DW, Wilson JP, Knoblock CA, Ritz B, Cockburn MG, 2008. An effective and efficient approach for manually improving geocoded data. Int. J. Health Geogr. 7, 60. doi: 10.1186/1476-072X-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Barroso D, García-Pérez J, López-Abente G, Tamayo-Uria I, Morales-Piga A, Pardo Romaguera E, Ramis R, 2016. Agricultural crop exposure and risk of childhood cancer: new findings from a case–control study in Spain. Int. J. Health Geogr. 15, 18. doi: 10.1186/s12942-016-0047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB, Ward MH, Airola M, Bell EM, Colt J, Nishioka M, Buffler PA, Reynolds P, Rull RP, Hertz A, Metayer C, Nuckols JR, 2011. Determinants of agricultural pesticide concentrations in carpet dust. Environ. Health Perspect. 119, 970–6. doi: 10.1289/ehp.1002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Lombardi CA, Cockburn M, Meyers TJ, Wilhelm M, Ritz B, 2013. Epidemiology of rhabdoid tumors of early childhood. Pediatr. Blood Cancer 60, 77–81. doi: 10.1002/pbc.24141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Park AS, Qiu J, Cockburn M, Ritz B, 2014. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int. J. Hyg. Environ. Health 217, 662–668. doi: 10.1016/j.ijheh.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S, Lurz PWW, Shirley MDF, Bythell M, Rankin J, 2015. Exposure misclassification due to residential mobility during pregnancy. Int. J. Hyg. Environ. Health 218, 414–421. doi: 10.1016/j.ijheh.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Hurley S, Hertz A, Nelson DO, Layefsky M, Von Behren J, Bernstein L, Deapen D, Reynolds P, 2017. Tracing a Path to the Past: Exploring the Use of Commercial Credit Reporting Data to Construct Residential Histories for Epidemiologic Studies of Environmental Exposures. Am. J. Epidemiol. 185, 238–246. doi: 10.1093/aje/kww108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SE, Saunders TM, Nivas R, Hertz A, Reynolds P, 2003. Post office box addresses: a challenge for geographic information system-based studies. Epidemiology 14, 386–91. doi: 10.1097/01.EDE.0000073161.66729.89 [DOI] [PubMed] [Google Scholar]
- Jacquez GM, Slotnick MJ, Meliker JR, AvRuskin G, Copeland G, Nriagu J, 2011. Accuracy of commercially available residential histories for epidemiologic studies. Am. J. Epidemiol. 173, 236–243. doi: 10.1093/aje/kwq350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne É, Bélair M-A, Do MT, Stieb DM, Hystad P, Van Donkelaar A, Martin RV, Crouse DL, Crighton E, Chen H, Brook JR, Burnett RT, Weichenthal S, Villeneuve PJ, To T, Cakmak S, Johnson M, Yasseen Iii A.S., Johnson KC, Ofner M, Xie L, Walker M, 2017. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 100, 139–147. doi: 10.1016/j.envint.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Ling C, Liew Z, von Ehrenstein O, Heck J, Park A, Cui X, Cockburn M, Wu J, Ritz B, 2018. Prenatal Exposure to Ambient Pesticides and Preterm Birth and Term Low Birthweight in Agricultural Regions of California. Toxics 6, 41. doi: 10.3390/toxics6030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, Reynolds P, 2002. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ. Health Perspect. 110, 955–960. doi: 10.1289/ehp.02110955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerison-Zilko C, Cubbin C, Jun J, Marchi K, Braveman P, 2016. Post-partum Residential Mobility Among a Statewide Representative Sample of California Women, 2003–2007. Matern. Child Health J. 20, 139–148. doi: 10.1007/s10995-015-1812-0 [DOI] [PubMed] [Google Scholar]
- Nikkilä A, Kendall G, Raitanen J, Spycher B, Lohi O, Auvinen A, 2018. Effects of incomplete residential histories on studies of environmental exposure with application to childhood leukaemia and background radiation. Environ. Res. 166, 466–472. doi: 10.1016/J.ENVRES.2018.06.035 [DOI] [PubMed] [Google Scholar]
- Pennington AF, Strickland MJ, Klein M, Zhai X, Russell AG, Hansen C, Darrow LA, 2016. Measurement error in mobile source air pollution exposure estimates due to residential mobility during pregnancy. J. Expo. Sci. Environ. Epidemiol. doi, 513–520. doi: 10.1038/jes.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Harnly M, Hertz A, 2005. Agricultural pesticide use and childhood cancer in California. Epidemiology 16, 93–100. doi: 10.1097/01.ede.0000147119.32704.5c [DOI] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Behren J Von, Gunier RB, Goldberg DE, Hertz A, 2004. Residential Exposure to Traffic in California and Childhood Cancer. Epidemiology 15, 6–12. doi: 10.1097/01.ede.0000101749.28283.de [DOI] [PubMed] [Google Scholar]
- Rull RP, Gunier R, Von Behren J, Hertz A, Crouse V, Buffler PA, Reynolds P, 2009. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ. Res. 109, 891–9. doi: 10.1016/j.envres.2009.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull RP, Ritz B, 2003. Historical pesticide exposure in California using pesticide use reports and land-use surveys: an assessment of misclassification error and bias. Environ. Health Perspect. 111, 1582–9. doi: 10.1289/ehp.6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J, Selvin S, Shaw GM, Malcoe LH, 1993. Exposure misclassification due to residential mobility during pregnancy in epidemiologic investigations of congenital malformations. Arch. Environ. Health. doi: 10.1080/00039896.1993.9938404 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Yang W, Roberts EM, Kegley SE, Stevenson DK, Carmichael SL, English PB, 2018. Residential Agricultural Pesticide Exposures and Risks of Spontaneous Preterm Birth. Epidemiology 29, 8–21. doi: 10.1097/EDE.0000000000000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I, 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The charge study. Environ. Health Perspect. 122, 1103–1109. doi: 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee Lewis PG, Chen T-Y, Chan W, Symanski E, 2019. Predictors of residential mobility and its impact on air pollution exposure among children diagnosed with early childhood leukemia. J. Expo. Sci. Environ. Epidemiol. 1. doi: 10.1038/s41370-019-0126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA, 2009. Factors Associated With Residential Mobility in Children With Leukemia: Implications For Assigning Exposures. Ann. Epidemiol. 19, 834–840. doi: 10.1016/j.annepidem.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Heck JE, Park AS, Cockburn M, Escobedo L, Ritz B, 2016. In Utero and Early-Life Exposure to Ambient Air Toxics and Childhood Brain Tumors: A Population-Based Case–Control Study in California, USA. Environ. Health Perspect. 124, 1093–1099. doi: 10.1289/ehp.1408582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B, 2011. Parkinson’s disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 26, 547–55. doi: 10.1007/s10654-011-9574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DC, Wang A, 2015. Assessment of Residential History Generation Using a Public-Record Database. Int. J. Environ. Res. Public Health 12, 11670–82. doi: 10.3390/ijerph120911670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford P, Segawa R, Schreider J, Federighi V, Neal R, Brattesani M, 2013. Community air monitoring for pesticides. Part 3: using health-based screening levels to evaluate results collected for a year. Environ. Monit. Assess. 186, 1355–1370. doi: 10.1007/s10661-013-3394-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


