Abstract
Heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) are very common conditions, particularly in the elderly. However, the mechanisms underlying the two disorders, including their intricate interaction have not been fully resolved. Here, our aim is to review the evidence on the role of the two types of senile amyloidosis in this connection. Two types of senile amyloidosis can be identified: wild-type transthyretin (TTR)-derived amyloidosis (ATTRwt) and isolated atrial amyloidosis (IAA). ATTRwt is an underlying condition that is being increasingly recognized in patients with HFpEF and often accompanied by AF. IAA is an established cause of AF, adding to the mechanism problem. New diagnostic and therapeutic possibilities have emerged that may facilitate clinical management of (senile) amyloidosis, which in turn may have implications for the management of HFpEF and AF.
Keywords: Amyloidosis , Transthyretin , Isolated atrial amyloidosis , HFpEF , Atrial fibrillation
Introduction
Heart failure is a rising epidemic in cardiovascular medicine, especially heart failure with preserved ejection fraction (HFpEF) and the same holds true for atrial fibrillation (AF).1 Importantly, both these disorders often occur together, due to common associated conditions and comorbidities. Moreover, there is an intricate interaction between the two disorders. On the one hand, HFpEF may cause AF by increasing left atrial pressure, thereby atrial stretch/enlargement and eventually fibrosis. On the other hand, AF may precipitate overt heart failure in the setting of diastolic dysfunction (‘HFpEF’), due to whatever cause. However, this is a simplification of the mechanisms involved in the complex interplay between HFpEF and AF and new insights are urgently needed.
Amyloidosis is a protein-misfolding disease characterized by extracellular deposition of a soluble precursor protein that aggregates in the form of insoluble fibrils, causing cell/tissue damage and ultimately organ dysfunction. Over 30 different amyloidogenic proteins have been identified, some of which affect the heart and cause ‘cardiac amyloidosis’. In brief, the clinical hallmark of cardiac amyloidosis is marked thickening and stiffening of the walls of the left and right ventricles leading to diastolic dysfunction (‘restrictive physiology’). A common type of amyloidosis is immunoglobulin light chain-derived (AL amyloidosis), but we will not deal with this separately here. In recent years, another type of amyloidosis has gained increasing attention: transthyretin (TTR)-derived amyloidosis (ATTR), which can be divided into a hereditary type (ATTRm) and a wild-type (ATTRwt). Whereas ATTRm is a rare disease, ATTRwt is relatively common and is increasingly recognized as a cause of HFpEF in the elderly. Moreover, ATTRwt is often accompanied by AF. Another type of amyloidosis affecting the heart at advanced age is so-called isolated atrial amyloidosis (IAA), which also sets the stage for AF. Our aim is to review the evidence on the role of the two types of senile amyloidosis (ATTRwt and IAA) in HFpEF and AF.
Transthyretin
Transthyretin is a naturally occurring protein produced mainly by the liver. It functions as a transport protein for thyroxine and retinol binding protein. Transthyretin is a tetramer rich in ß strands and it has an innate ability to aggregate into insoluble amyloid fibres. The first step is dissociation of TTR into its monomers, followed by accumulation of these monomers into oligomers, composed of 6–10 monomers. These oligomers may aggregate into amyloid fibres, which in turn may be deposited in the extracellular matrix of various tissues and organs, including the heart. Alterations in TTR due to mutations in TTR, the gene encoding TTR, may increase the likelihood of dissociation of TTR into its monomers—and hence their aggregation—and the development of amyloidosis (ATTRm), but we will not deal separately with this disease here.
Ageing may also destabilize TTR, eventually also leading to amyloidosis (ATTRwt), which was formerly known as senile systemic amyloidosis (SSA). Following smaller studies in 1983, Cornwell et al.2 reported a study of 85 autopsies in patients ≥80 years of age. They showed the presence of TTR amyloid in as many as 25% of the (left) ventricles. More recently, 25% was also reported in a Finnish group of patients aged ≥85 years (256 autopsies), supporting the notion that ATTRwt is a very common finding in the very elderly.3 The underlying mechanisms are still being debated, but may involve age-related post-transcriptional biochemical alterations in TTR or its chaperones.4,5
Amyloidosis transthyretin-derived wild-type and heart failure with preserved ejection fraction
Elaborating on the above data, Mohammed et al.6 investigated the frequency of amyloid in ventricular specimens from patients with an antemortem diagnosis of HFpEF. The presence of TTR (wild-type) was associated with advanced age and male sex, and age- and sex-adjusted prevalence was higher in HFpEF patients (n = 109) than in control subjects (n = 131). Among the HFpEF patients, moderate or severe interstitial amyloid deposition was present in 5% of them, while mild interstitial and/or intramural coronary vascular deposition was present in 12% of them.6 This landmark pathology study was followed by a clinical study in HFpEF patients aged >60 years and with left ventricular hypertrophy. All patients underwent 99mTc-DPD scintigraphy to diagnose ATTR.7 A total of 16 patients (13%) showed moderate-to-severe uptake on the scintigram, indicative of ATTR.7 In addition, a longitudinal study in 121 patients with ATTRwt was recently reported by Connors et al.,8 supporting the notion that ATTRwt is associated with HFpEF. Over 20 years, they enrolled patients with biopsy-proven ATTRwt in a prospective, observational study. Age at enrolment was 76 years and 98% were male. Left ventricular ejection fraction at baseline was on average preserved, but the vast majority already had symptoms of heart failure. Median survival was 47 months and 78% of all these patients died from heart failure or sudden death.
Theoretically, the mechanism underlying the occurrence of HFpEF in the setting of ATTRwt would be advanced diastolic left ventricular dysfunction secondary to the deposition of amyloid fibrils in the extracellular matrix. Indeed, like in other types of cardiac amyloidosis, ATTRwt is characterized by myocardial thickening and reduced diastolic tissue velocities on echocardiography, often amounting to a restrictive filling pattern,9 which was confirmed by right-sided catheterization. Conversely, it cannot be excluded that HFpEF per se promotes amyloid deposition due to the associated pro-oxidative state leading to increased TTR oxidation.
Amyloidosis transthyretin-derived wild-type and atrial fibrillation
We identified five studies reporting the prevalence of AF in the setting of cardiac amyloidosis due to ATTRwt (Table 1).10–14 Overall, although the studies vary in terms of entry criteria, clinical characteristics and numbers of subjects, cardiac amyloidosis due to ATTRwt appears to be associated with a high prevalence of AF. Moreover, the prevalence of AF in ATTRwt also appears to be higher than the prevalence of AF in AL amyloidosis or ATTRm. Conversely, the study by Longhi et al. also showed that AF may also precipitate haemodynamic deterioration in the setting of amyloid cardiomyopathy. Indeed, despite comparable wall thickness, end-diastolic diameter and atrial size in patients presenting with heart failure, cardiac index was reduced in the subgroup of patients with AF, most likely due to loss of atrial kick.11
Table 1.
Frequency of atrial fibrillation in different types of cardiac amyloidosis
| N | AL amyloidosis (%) | ATTRm (%) | ATTRwt (%) | |
|---|---|---|---|---|
| Rapezzi et al.10 | 233 | 12 | 5 | 27 |
| Longhi et al.11 | 262 | 9 | 11 | 40 |
| Pinney et al.12 | 138 | 16 | NA | 43 |
| Kristen et al.13 | 216 | 16 | 18 | 30 |
| Grogan et al.14 | 360 | NA | NA | 62 |
The study by Longhi et al. was the only one to focus on atrial fibrillation. Another limitation is that all five studies were retrospective.
AL, amyloidosis immunoglobulin light chain-derived; ATTRm, amyloidosis transthyretin (TTR)-derived hereditary type; ATTRwt, amyloidosis transthyretin (TTR)-derived wild-type; NA, not applicable.
The mechanism underlying the high prevalence of AF in ATTRwt most likely involves atrial (ultra)structural remodelling, secondary to diastolic left ventricular dysfunction and increased filling pressures, even without overt HFpEF.15,16 Indeed, in the studies by Rapezzi et al.10 and Longhi et al.11 atrial pressure and atrial size were independent risk factors for the development of AF. Adding to these studies, Barbhaiya et al.17 investigated the electrophysiological characteristics of patients with cardiac amyloidosis and AF compared with control patients with AF due to other aetiologies. The amyloid group consisted of 18 patients with either AL amyloidosis (n = 4) or ATTRwt (n = 14), and almost all patients had advanced diastolic dysfunction on echocardiography. Among other findings, the amyloid patients showed prolonged conduction intervals (PR, AH, HV) compared with the control group. In addition, in a subgroup (n = 7) endocardial atrial voltage mapping was performed, showing extensive areas of low voltage and more so than in the control patients (Figure 1).17 Finally, arrhythmia-free survival after catheter ablation was significantly worse in the amyloid patients compared with the control patients. These remarkable findings would suggest that amyloid deposition in ATTRwt is perhaps not confined to the ventricles, but this important point will be further discussed below.
Figure 1.
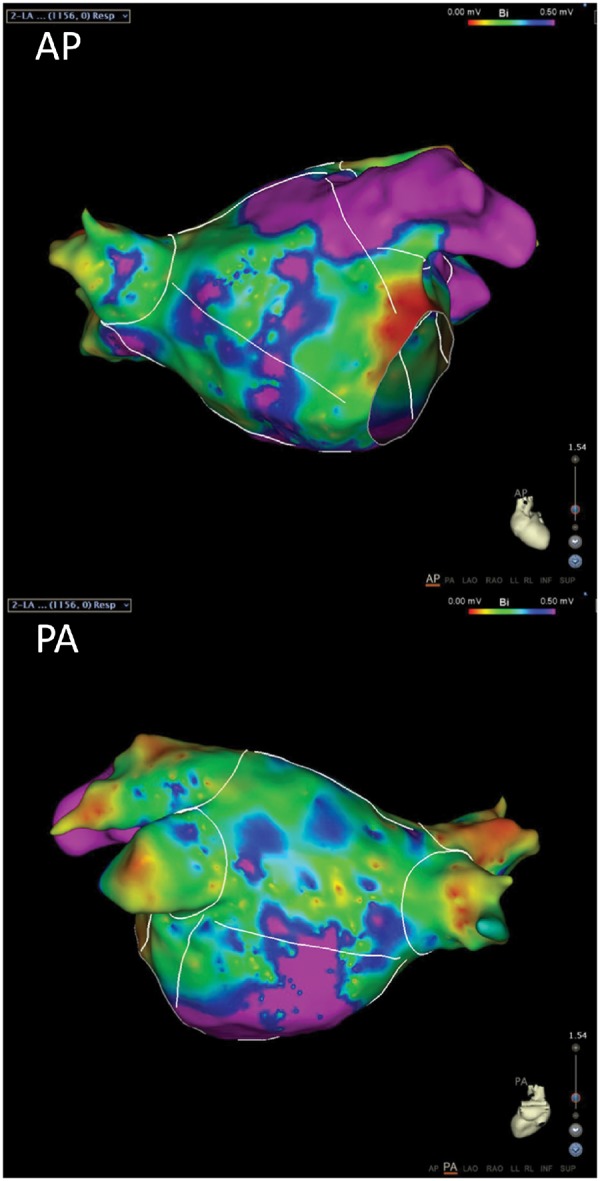
Representative electroanatomic bipolar voltage maps of the left atrium of a patient with cardiac amyloidosis. White lines indicate boundaries and analysed left atrial segments. The voltage scale indicates all purple areas as normal voltage; all others are low voltage. Note the great extent of low-voltage area. AP, anteroposterior; PA, posteroanterior. Reprinted with permission of Barbhaiya et al.17
Isolated atrial amyloidosis and atrial fibrillation
Most commonly, amyloidosis (including ATTRwt) is a systemic disease affecting various organs and tissues, which may include the heart, but there is one particular type of amyloidosis which only affects the heart. More specifically, this type of localized amyloidosis is confined to the atria, hence its name isolated atrial amyloidosis. The incidence of IAA increases with age, reaching >90% in the ninth decade,18–20 and it is therefore also considered to be a form of senile amyloidosis, such as ATTRwt. The fibril protein that is deposited in IAA is atrial natriuretic peptide (ANP), a naturally occurring peptide hormone synthesized by atrial cardiomyocytes. A potent stimulus for ANP secretion is AF, probably secondary to atrial stretch and perhaps also due to fibrillatory activity per se. It is essentially still unknown why ANP may deposit as amyloid. In this connection, IAA does not appear to be more common in patients with cardiovascular risk factors (hypertension or diabetes mellitus), suggesting that IAA is a relatively autonomic process. Nevertheless, IAA is a clinically relevant condition, in particular given its association with AF. In the first study that systematically addressed this issue, Röcken et al.21 investigated right atrial appendages from 245 patients undergoing open heart surgery (bypass surgery, valve surgery). Their mean age was 63 years, 174 were males, and persistent AF was present in 15% of the patients. Amyloid was found in 16% of the patients and all deposits were immunoreactive for ANP.21 The presence of amyloid was associated with age, sex, P-wave duration, (mitral) valve disease, and the presence of AF. Moreover, patients with AF also had larger amounts of amyloid in their appendages than patients in sinus rhythm. Regarding the observation that patients undergoing mitral valve surgery had a high prevalence of amyloid, Röcken et al. speculated that high ANP levels secondary to the valvular disease and atrial dilatation (stretch) may have played a role.
Another study also investigated the presence of amyloid in atrial appendages obtained from 72 patients during valve replacement surgery.22 All patients had rheumatic valve disease and chronic AF, and the findings in these patients were compared with patients with heart failure and sinus rhythm. Amyloid was found in 46% of the valvular patients compared with only 12% of the controls, and amyloid was also related to the duration of AF. In addition, a study was performed in heart specimens obtained from consecutive autopsied subjects, mean age 75 years, 41 males.23 In 37 heart specimens either Grade 0 (no/trivial deposits) or Grade 3 (dense network/solid foci) was found. Of these heart specimens, 27 had corresponding premortem ECGs, with Grade 3 IAA being present in 14 cases and Grade 0 in 13 cases (Figure 2). The ECG in as many as 10 of the 14 cases with Grade 3 IAA showed AF/atrial flutter (71%), whereas only 4 of the 13 cases with Grade 0 IAA showed AF/atrial flutter (31%).
Figure 2.
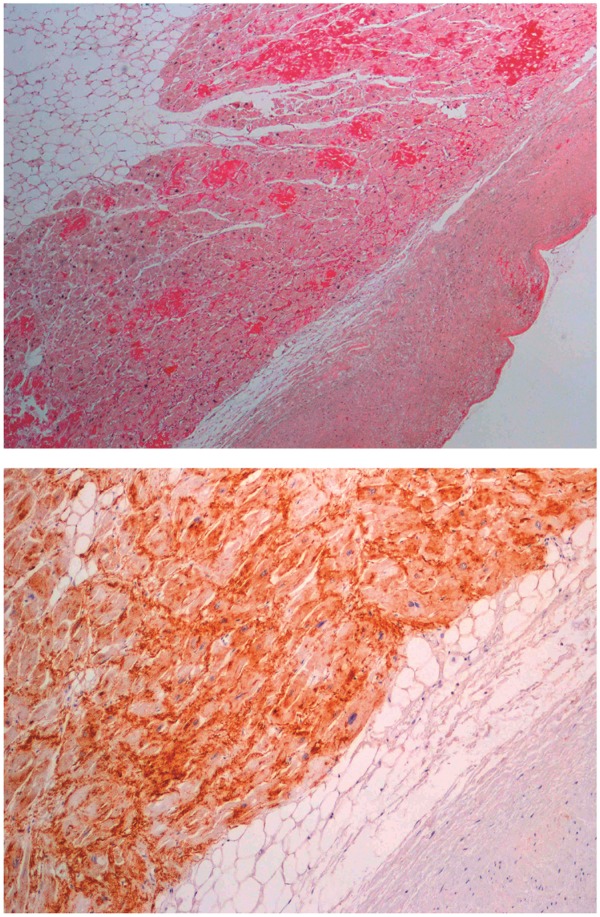
Sirius red staining of left atrial tissue showing high grade isolated atrial amyloidosis (x4) (upper panel). Immunostaining of left atrial tissue in a patient with isolated atrial amyloidosis demonstrating the presence of atrial natriuretic peptide (x4) (lower panel). Reprinted with permission of Ariyarajah et al.23
The mechanisms underlying AF in general are complex and involve electrical, structural, and autonomic atrial remodelling processes. Together, the processes affect the electrophysiological properties of the atria, in particular conduction, thus setting the stage for AF. IAA is likely operative in all these processes, but the most prominent aspect is probably the effect on atrial conduction. Indeed, as demonstrated by Röcken et al.21 the deposition of amyloid fibrils impedes atrial conduction (prolonged P-waves). Conversely, they also provide data that AF in turn may affect amyloid deposition, since AF was associated with significantly higher amounts of amyloid compared with sinus rhythm. Röcken et al. thus speculate that AF is perhaps not an initiator of amyloid deposition in IAA, but that it may promote further deposition once the process has started.
Interrelations and potentially vicious circles
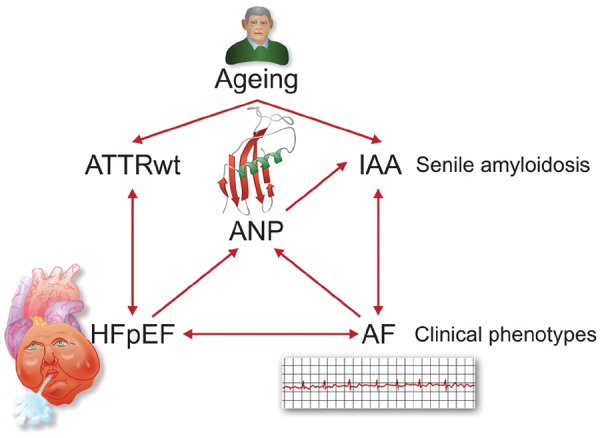
The above data point to a scenario that two naturally occurring peptides (TTR and ANP) with clear beneficial physiological functions somehow begin to exert a detrimental effect on the heart along with ageing, eventually leading to the clinical phenotypes of HFpEF and AF. Moreover, although many of the above studies show associations rather than causation and the data are not final, there appear to be intricate interrelations between the components of the processes involved, potentially leading to detrimental vicious circles.
In brief, ageing is a risk factor for amyloidosis (senile amyloidosis), leading to ATTRwt and/or IAA, depending on the protein involved (TTR or ANP, respectively). In turn, ATTRwt may cause or promote HFpEF, and vice versa, while IAA may cause AF, and vice versa. Moreover, HFpEF and AF facilitate each other and senile amyloidosis may thus also provide another piece of the puzzle explaining the interaction between these two diseases (vicious twins). Finally, an interesting intermediary role might be played by ANP, potentially linking both HFpEF and AF with IAA. However, there are several remaining issues to resolve. First, it is not fully understood how TTR and ANP become amyloidogenic. Second, the same holds true for how HFpEF facilitates ATTRwt and AF facilitates IAA. In addition, ATTRwt and IAA are not mutually exclusive. More specifically, it is conceivable that ATTRwt is not confined to the ventricles but also directly affects the atria. Indeed, in the study by Röcken et al., the amyloid atrial deposits were immunoreactive for ANP in all 40 cases, but four of these cases also showed additional TTR-positive amyloid deposits. In addition, in a Cardiac Magnetic Resonance (CMR) study, late gadolinium enhancement consistent with atrial amyloidosis was observed in the atrial wall in as many as 17 (78%) of 22 patients with cardiac amyloidosis, including eight patients with ATTRwt.24 If anything is to be deduced from these data, it seems rather likely that the two forms of senile amyloidosis will act in concert, once more setting the stage for AF. Finally, although the concept is appealing, there is little clinical evidence for the existence of a vicious circle between AF, ANP and IAA. More work is needed to elucidate these issues, but it is already clear that, besides the ventricles, the atria are an important but underestimated site of action in senile cardiac amyloidosis (ATTRwt, IAA) and its sequelae.
Of note, this review focusses on HFpEF but some patients with ATTR with cardiac involvement ‘progress’ to heart failure with reduced ejection fraction (HFrEF) and the interaction with AF then probably changes, resembling more the interaction between HFrEF and AF in general and AF playing a less prominent role, but this remains to be investigated.
Diagnostic considerations
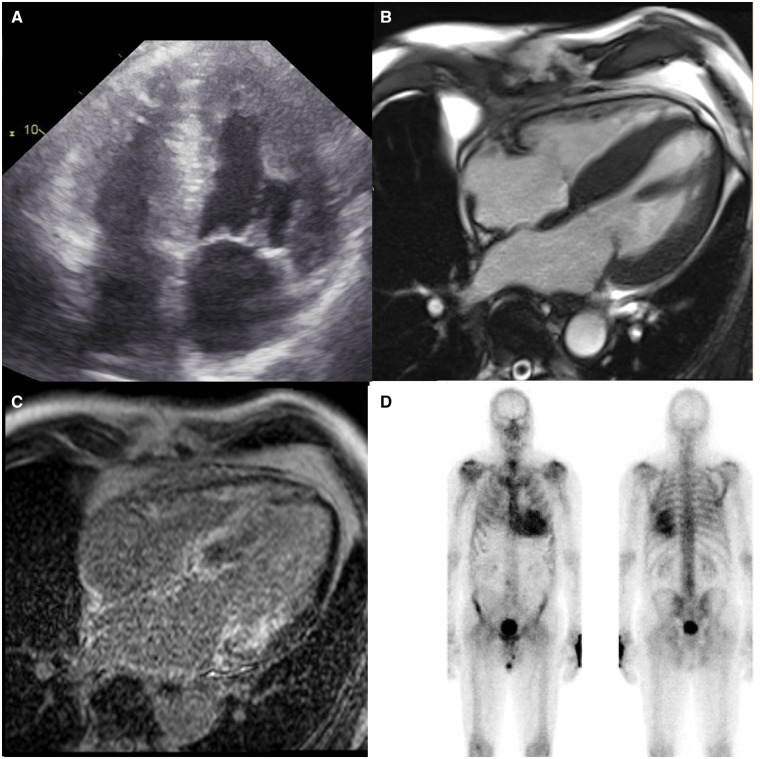
The clinical diagnosis of ATTRwt with cardiac involvement can be challenging. Patients may present with a variety of symptoms and not all patients are elderly men. Moreover, the typical cardiac findings of low voltage ECG and concentric left ventricular hypertrophy on the echocardiogram may not be present.7,25 Brain natriuretic peptide levels are usually significantly elevated and more so than in non-amyloidotic cardiomyopathy, while troponin levels are often also raised. However, these biomarkers only provide circumstantial evidence at best in terms of establishing the diagnosis.14,26. CMR imaging with late gadolinium enhancement and T1 mapping may be helpful but is not able to reliably differentiate between cardiac amyloidosis due to ATTR or other types of amyloidosis.27 Instead, a simple ‘bone-scintigram’ has emerged as a reliable, non-invasive tool to diagnose cardiac amyloidosis due to ATTR (either ATTRm or ATTRwt).28–30 (Figure 3). Importantly, this readily available, non-invasive technique avoids the need for taking a myocardial biopsy. Pros and cons of the above tests are summarized in Supplementary material (Table S1) online.
Figure 3.
Representative echocardiographic (A), cardiac magnetic resonance (B), cardiac magnetic resonance with late gadolinium enhancement (C), and 99mTc-HDP scintigraphy (D) images of a patient with HFpEF due to ATTRwt. Note the hypertrophy, the late gadolinium enhancement (white areas) both in left ventricle and the atria, as well as the pronounced uptake of tracer in the heart on the scintigram.

Take home figure Intricate interrelations exist between HFpEF, AF, and senile amyloidosis, potentially leading to detrimental vicious circles.
Diagnosing IAA in the clinical setting is even more challenging. Electrocardiographic features may raise suspicion, in particular a prolonged and low voltage P-wave, AV-block, and paroxysmal AF, but this is non-specific and clearly not sufficient to establish a definite diagnosis. The role of CMR imaging has not yet been fully defined, but detection of atrial amyloid in patients with cardiac amyloidosis is feasible (Figure 3).24,31,32 However, these data pertain to patients with ATTR and AL amyloidosis, but to our knowledge no CMR imaging studies have been performed in IAA. It is thus not yet possible to ascertain the presence of IAA in the clinical setting.
Pharmacological considerations
Therapeutic management of HFpEF and AF (and the combination thereof) is beyond the scope of this review and for this the reader is referred to the guidelines.33,34 Of note, although these guidelines generally also apply in the setting of concomitant amyloidosis, one issue should be mentioned here and that is that use of digoxin and calcium-antagonists for rate control for AF is not recommended given the risk of toxicity due to possible binding of these agents to amyloid fibrils. The focus here is on the pharmacological treatment of amyloidosis. In general, treatment of ATTR can be divided into three approaches: (i) stabilization of the TTR tetramers, (ii) suppression of the production of TTR by the liver, and (iii) removal of TTR amyloid deposits from tissues. An example of the first approach is the use of Diflunisal. This compound prevents dissociation of TTR into its monomers and has been shown to retard progression of polyneuropathy caused by ATTRm although no effect on cardiac involvement has been demonstrated as yet. The other available TTR tetramer stabilizer is Tafamidis and this agent does not have the NSAID side-effects of Diflunisal. It has already proved to be efficacious for neurological manifestations but very recently, in the ATTR-ACT study, Tafamidis was also shown to reduce all-cause mortality and cardiovascular-related hospitalizations in patients with cardiac involvement.35 Of note, although these are very promising results it is as yet uncertain whether Tafamidis would also be beneficial in patients with concomitant AF. The second approach entails gene-silencing, aimed at suppressing the production of TTR by the liver by harnessing small interfering ribonucleic acids (siRNA) or antisense oligonucleotides (ASO). Two such suppressors are now available: Patisiran (siRNA) and Inotersen (ASO).36,37 Both agents appear to have favourable effects on polyneuropathy, while Patisiran possibly also stops further worsening of the cardiomyopathy, but since this was only a secondary endpoint, it needs to be proven in a new study. Finally, treatment with anti-serum amyloid P (SAP) antibodies (Dezamizumab) after pre-treatment with CPHPC (Miridesap) to remove circulating SAP is a promising third approach, aimed at removing amyloid fibrils from the tissues. Initial results with these removers in patients with systemic amyloidosis with liver, spleen and kidney involvement are promising.38,39 Of note, although there is currently no treatment for IAA, theoretically treatment with anti-SAP antibodies might work in this setting, but this obviously needs to be proven.
Conclusions
Heart failure with preserved ejection fraction and AF are very common diseases that also often occur in combination, further aggravating each other. Senile amyloidosis, either due to TTR (ATTRwt) or ANP (IAA) appears to play an important role in both diseases and in their interaction. In terms of diagnostics, bone scintigraphy has become available and affords an easy and reliable way to establish the presence of cardiac ATTRwt. Moreover, pharmacological options are now available or under development to treat ATTRwt and possibly also IAA, thereby potentially stopping, or even reversing, the downhill course of some patients with HFpEF and AF.
Supplementary Material
Acknowledgements
We thank Jackie Senior for editing the manuscript
Conflict of interest: P.v.d.M. was collaborator in the ATTR-ACT study. H.L.A.N. is member of an advisory board of Alnylam.
References
- 1. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M.. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016;68:2217–2228. [DOI] [PubMed] [Google Scholar]
- 2. Cornwell GG 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkanen P.. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med 1983;75:618–623. [DOI] [PubMed] [Google Scholar]
- 3. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L.. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med 2008;40:232–239. [DOI] [PubMed] [Google Scholar]
- 4. Zhao L, Buxbaum JN, Reixach N.. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 2013;52:1913–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buxbaum JN, Tagoe C, Gallo G, Walker JR, Kurian S, Salomon DR.. Why are some amyloidoses systemic? Does hepatic “chaperoning at a distance” prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J 2012;26:2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM.. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2014;2:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P.. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 8. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC.. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 2016;133:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Granstam SO, Rosengren S, Vedin O, Kero T, Sorensen J, Carlson K, Flachskampf FA, Wikstrom G.. Evaluation of patients with cardiac amyloidosis using echocardiography, ECG and right heart catheterization. Amyloid 2013;20:27–33. [DOI] [PubMed] [Google Scholar]
- 10. Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, Salvi F, Ciliberti P, Pastorelli F, Biagini E, Coccolo F, Cooke RM, Bacchi-Reggiani L, Sangiorgi D, Ferlini A, Cavo M, Zamagni E, Fonte ML, Palladini G, Salinaro F, Musca F, Obici L, Branzi A, Perlini S.. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203–1212. [DOI] [PubMed] [Google Scholar]
- 11. Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, Bacchi-Reggiani ML, Leone O, Ferlini A, Russo A, Gallelli I, Rapezzi C.. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 2015;22:147–155. [DOI] [PubMed] [Google Scholar]
- 12. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N, McCarthy CA, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ.. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013;2:e000098.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kristen AV, Brokbals E, Aus Dem Siepen F, Bauer R, Hein S, Aurich M, Riffel J, Behrens H-M, Krüger S, Schirmacher P, Katus HA, Röcken C.. Cardiac amyloid load: a prognostic and predictive biomarker in patients with light-chain amyloidosis. J Am Coll Cardiol 2016;68:13–24. [DOI] [PubMed] [Google Scholar]
- 14. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A.. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016;68:1014–1020. [DOI] [PubMed] [Google Scholar]
- 15. De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC.. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res 2011;89:754–765. [DOI] [PubMed] [Google Scholar]
- 16. De Jong AM, Van Gelder IC, Vreeswijk-Baudoin I, Cannon MV, Van Gilst WH, Maass AH.. Atrial remodeling is directly related to end-diastolic left ventricular pressure in a mouse model of ventricular pressure overload. PLoS One 2013;8:e72651.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barbhaiya CR, Kumar S, Baldinger SH, Michaud GF, Stevenson WG, Falk R, John RM.. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm 2016;13:383–390. [DOI] [PubMed] [Google Scholar]
- 18. Westermark P, Johansson B, Natvig JB.. Senile cardiac amyloidosis: evidence of two different amyloid substances in the ageing heart. Scand J Immunol 1979;10:303–308. [DOI] [PubMed] [Google Scholar]
- 19. Steiner I. The prevalence of isolated atrial amyloid. J Pathol 1987;153:395–398. [DOI] [PubMed] [Google Scholar]
- 20. Kawamura S, Takahashi M, Ishihara T, Uchino F.. Incidence and distribution of isolated atrial amyloid: histologic and immunohistochemical studies of 100 aging hearts. Pathol Int 1995;45:335–342. [DOI] [PubMed] [Google Scholar]
- 21. Röcken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A, Goette A.. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106:2091–2097. [DOI] [PubMed] [Google Scholar]
- 22. Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Martin SS, Rapezzi C, Bacchi RML, Marinelli G.. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J 2004;25:1237–1241. [DOI] [PubMed] [Google Scholar]
- 23. Ariyarajah V, Steiner I, Hajkova P, Khadem A, Kvasnicka J, Apiyasawat S, Spodick DH.. The association of atrial tachyarrhythmias with isolated atrial amyloid disease: preliminary observations in autopsied heart specimens. Cardiology 2009;113:132–137. [DOI] [PubMed] [Google Scholar]
- 24. Kwong RY, Heydari B, Abbasi S, Steel K, Al-Mallah M, Wu H, Falk RH.. Characterization of cardiac amyloidosis by atrial late gadolinium enhancement using contrast-enhanced cardiac magnetic resonance imaging and correlation with left atrial conduit and contractile function. Am J Cardiol 2015;116:622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gonzalez-Lopez E, Gagliardi C, Dominguez F, Quarta CC, de Haro-Del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo-Marcos M, Lorenzini M, Lara-Pezzi E, Foffi S, Alonso-Pulpon L, Rapezzi C, Garcia-Pavia P.. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J 2017;38:1895–1904. [DOI] [PubMed] [Google Scholar]
- 26. Klaassen SHC, Tromp J, Nienhuis HLA, van der Meer P, van den Berg MP, Blokzijl H, van Veldhuisen DJ, Hazenberg BPC.. Frequency of and prognostic significance of cardiac involvement at presentation in hereditary transthyretin-derived amyloidosis and the value of N-terminal pro-B-type natriuretic peptide. Am J Cardiol 2018;121:107–112. [DOI] [PubMed] [Google Scholar]
- 27. Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, Kotecha T, Francis R, Hutt DF, Rezk T, Rosmini S, Quarta CC, Whelan CJ, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M.. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017;70:466–477. [DOI] [PubMed] [Google Scholar]
- 28. Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. ( 99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castano A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A, Bettencourt B, Gollob J, Karsten V, Vest JA, Chiuzan C, Maurer MS, Bokhari S.. Serial scanning with technetium pyrophosphate ((99m)Tc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol 2016;23:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, Dierckx RA, Slart RH, Hazenberg BP.. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid 2014;21:35–44. [DOI] [PubMed] [Google Scholar]
- 31. Lyne JC, Petryka J, Pennell DJ.. Atrial enhancement by cardiovascular magnetic resonance in cardiac amyloidosis. Eur Heart J 2008;29:212.. [DOI] [PubMed] [Google Scholar]
- 32. de Gregorio C, Dattilo G, Casale M, Terrizzi A, Donato R, Di Bella G.. Left atrial morphology, size and function in patients with transthyretin cardiac amyloidosis and primary hypertrophic cardiomyopathy—comparative strain imaging study. Circ J 2016;80:1830–1837. [DOI] [PubMed] [Google Scholar]
- 33. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC)Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2016;38:2893–2962. [Google Scholar]
- 34. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 35. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C; ATTR-ACT Study Investigators. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 36. Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang C-C, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin K-P, Vita G, Attarian S, Planté-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB.. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 37. Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Plante-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH 3rd, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceicao I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA, Coelho T.. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 38. Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB.. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med 2015;373:1106–1114. [DOI] [PubMed] [Google Scholar]
- 39. Richards DB, Cookson LM, Barton SV, Liefaard L, Lane T, Hutt DF, Ritter JM, Fontana M, Moon JC, Gillmore JD, Wechalekar A, Hawkins PN, Pepys MB.. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med 2018;10:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.