Abstract
Objectives:
Benzodiazepine (BZD) use is common in patients who are engaged in methadone as a treatment for opioid use disorder. BZD prescribing is generally discouraged for this patient population due to the increased risk of BZD dependence and BZD use disorder, medication-assisted treatment (MAT) discontinuation, and opioid-overdose death. However, some patients have concurrent mental health disorders, where BZD use may be clinically indicated. This study evaluates the impact of prescribed BZD on MAT outcomes.
Methods:
Linking urine drug screening data (UDS) and prescribing information from single-payer health records, we conducted a retrospective Kaplan–Meier analysis between patients using prescribed and nonprescribed BZD with methadone treatment retention as the primary outcome. Data are from a network of 52 outpatient clinics in Ontario, Canada, between January 1, 2006 and June 30, 2013.
Results:
We identified 3692 patients initiating methadone-assisted treatment for the first time; 76% were BZD−/UDS− (no BZD prescription and <30% screens positive for BZD); 13% were BZD+/UDS−; 6% BZD−/UDS+; and 6% BZD+/UDS+. Using 1-year treatment retention as a primary outcome, patients using nonprescribed BZD (BZD−/UDS+) were twice as likely (adjusted odds ratio 0.38, 95% confidence interval 0.27–0.53) to discontinue treatment as those not using BZD (BZD−/UDS−), or those using BZD in a prescribed manner (BZD+/UDS+).
Conclusions:
Our findings suggest that prescribed BZD can be used during methadone MAT without impacting a patient's retention in MAT, but nonprescribed BZD use is predictive of treatment discontinuation. Importantly, we urge both the physician and patient to seek alternative clinical options to BZD prescribing, due to the potential for developing physical dependence (and BZD use disorder) to BZD and the risks of negative interactions with opioids.
Keywords: benzodiazepine, methadone maintenance therapy, opioid use disorder, patient retention
Opioid use disorder (OUD) is a major healthcare issue in North America, and opioid-related overdose is the primary preventable cause of death in both urban and rural regions of the continent (Volkow et al., 2014). For patients with OUD, opioid substitution therapy including methadone-assisted treatment is the standard of care (CPSO, 2011). MAT is a harm reduction model of care where opioid agonists (e.g. methadone or buprenorphine/naloxone) are substituted in place of more harmful opioids, including oxycodone, heroin, and fentanyl. Medication-assisted treatment (MAT) is a maintenance and stabilization model of care that can improve patients’ psychosocial functioning as patients stabilize and reduce use of nonprescribed opioids in treatment (CPSO, 2011).
It is common for patients who are receiving MAT to use other prescribed or nonprescribed substances while in treatment (Nielsen, 2007; Roux et al., 2016), and for this reason, frequent urine screening is performed in concert with MAT administration. One such substance that is often screened for is benzodiazepines (BZDs)—a pharmaceutical class that is used by as many as 66% of patients on MAT (Nielsen, 2007). BZD class molecules (eg, diazepam, clonazepam, lorazepam) act on the GABAergic system and are respiratory depressants. BZDs generally provide sedative-like effects and have a high potential for abuse and dependence (Nutt and Malizia, 2001). Due to compounding respiratory depressant actions, BZD use during MAT drastically increases patients’ risk of overdose-related death (Gomes et al., 2014). Approximately 60% of opioid-related overdose deaths in Ontario involve BZD (Dhalla et al., 2009). However, when managed in a controlled treatment setting, the available evidence suggests patients may benefit from BZD maintenance with little risk of increased mortality (Bakker and Streel, 2017).
Benzodiazepines are indicated to manage acute behavioral symptoms such as anxiety, depression, and insomnia (Brands et al., 2000). Due to the abuse potential of BZDs, clinical guidelines generally advocate for short-term BZD prescribing. Long-term prescribed BZD use among a minority of MAT patients with mental health issues may have therapeutic potential. The evidence suggests that BZD use during MAT has a negative impact on treatment outcomes (Brands et al., 2008; Franklyn et al., 2017); however, an important caveat to these studies is whether patients were using prescribed or nonprescribed BZD. It is not yet clear whether prescribed BZD use during MAT can improve outcomes for patients with co-occurring mental health disorders, where BZD may be clinically indicated.
In this study, we evaluate the impact of prescribed versus nonprescribed BZD use during MAT in patients with OUD, using 1-year treatment retention as a primary outcome.
METHODS
Clinical Context
In the province of Ontario, MAT for OUD is regulated by formal treatment guidelines established by the provincial medical licensing body, the College of Physicians and Surgeons of Ontario (CPSO) (CPSO, 2011), which set out expectations with respect to physician practice and are enforced through peer audits. For patients initiating MAT, daily observed dosing and regular (scheduled) point-of-care urine screening occurs at a clinic, physician office, or pharmacy. Urine screening is typically conducted using point-of-care test strips, and gas chromatography is not commonly used, and the frequency of screening is generally 2 screens per week. Contingency management is part of the treatment strategy, and as a patient is stabilized on an effective dose of methadone, physicians can increase the number of carried doses as a positive reinforcement tool for abstinence from nonprescribed substance use, including BZD. At the physician's discretion, and in alignment with the CPSO methadone practice guidelines, a stabilized patient can progress from 1 take-home dose to a maximum of 6 take-home doses per week over approximately 6 months of sustained psychosocial stability.
The authors believe the study cohort to be representative of patients with OUD in the province of Ontario, Canada. While it was not possible to link specific mental health diagnosis in the study design, other studies our group has conducted estimated that approximately 90% of the patients with OUD in Ontario have a co-occurring mental illness (unpublished data).
Variability of practice within the guidelines is possible, but limited, and treatment was almost exclusively in the outpatient setting at clinical sites focused on MAT for patients with OUD. All treatment records were obtained from the Ontario Addiction Treatment Centers (OATCs) network of clinics. Further consistency within the dataset arises from standardized policies and operating procedures within the clinic network, which limit the likelihood of variability of treatment. To maintain consistency, patients are typically seen by the same physician throughout the course of their treatment.
Cohort Definition
We conducted a retrospective cohort study of patients initiating MAT across a 52-site outpatient OUD clinic network between January 1, 2006 and June 30, 2012 (with 1-year follow-up to June 30, 2013) in the province of Ontario. While all patients started on methadone, we did include patients who transitioned to buprenorphine over the course of treatment. In Ontario, buprenorphine/naloxone was not widely available during the study window (it has recently become more widely available); thus patients initiating treatment on buprenorphine were excluded because of the small number of such treatment episodes within the timeframe studied. All patients were at least 10 years or older (to exclude data entry errors for newborns; patients <18 years of age accounted for <1% of cohort), and were eligible for public drug coverage through the Ontario Drug Benefit (ODB) plan. We excluded patients with missing information regarding place of residence, age, or sex. All patients were followed from their date of MAT initiation to the date of treatment discontinuation (patient did not receive a prescribed dose of methadone or buprenorphine within 30 days of their last methadone or buprenorphine prescribed dose), death, 1-year follow-up, or end of the study period (June 30, 2013). It is important to note that the majority of MAT patients in Ontario qualify for ODB drug coverage; however, patients with ensured health benefits are not captured in the ODB dataset. We estimate that approximately 85% of Ontario's MAT population is captured using ODB database (unpublished data).
Data Sources
The dataset used for this study was derived from linking electronic medical records from a group of 52 addiction treatment centers across the province of Ontario—the OATCs—to the core data holdings at the Institute for Clinical and Evaluative Sciences (ICES). ICES is a prescribed entity with housed anonymized and linked record level administrative health data for Ontario's single-payer health system. The ODB database was used to identify all patients initiating MAT, and to determine their past medication use. The ODB database contains detailed records of all prescriptions dispensed to Ontario residents eligible for public drug coverage. In Ontario, residents are eligible for public drug coverage if they are aged 65 or older, reside in a long-term care facility, are disabled, are receiving social benefits for income support, or have high prescription drug costs relative to their net household income. Emergency department visits were identified using the Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System, and hospital admissions were identified using the CIHI Discharge Abstract Database. All diagnosis information from physician visits was determined using billing data from the Ontario Health Insurance Plan (OHIP) database (OHIP covers physician services for all permanent residents of Ontario). We obtained patient location of residence and demographic information from the Ontario Registered Persons Database, which contains a unique entry for each resident who has ever received insured health services. Patient information was linked anonymously across databases using encrypted 10-digit health card numbers. These datasets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES). The linking protocol has been described extensively elsewhere (Levy et al., 2003; Hall et al., 2006), and is used routinely for health system research in Ontario (Mamdani et al., 2003; Juurlink, 2009; Juurlink et al., 2009).
BZD Use
Based on scheduled bi-weekly urine drug screening and physician prescriptions through OHIP, we categorized patients into 4 groups based on whether the patient received a BZD prescription (BZD +/−) after initiating MAT, within the study window, and whether the patient screened positive for BZD use (urine drug screening data [UDS] +/−). We chose an a priori definition of ≥30% of positive BZD urine screens to attribute a patient to the UDS+ group. A definition of >30% was chosen to reflect use patterns which account for a material number of positive BZD urine drug screens. Urine toxicology screening was performed via an enzyme immunoassay, which has the ability to detect BZD in the urine (Handford et al., 2011). Standardized urine test strips were used across the clinic network, and gas chromatographic mass spectroscopy testing was not used. The detection period and sensitivity differs for each BZD, ranging from a few hours to several days (Handford et al., 2011).
Definition of Treatment Retention
All patients were followed for at least 1 year, to a maximum follow-up date of June 17, 2013. Continuous MAT was assessed on the basis of a prescription refill within 30 days of the previous prescription. We defined a patient as having been retained in treatment if they completed at least 1 year of continuous and uninterrupted MAT. In the event that a patient transitioned to a non-OATC clinic, was incarcerated, hospitalized, or was otherwise prevented from refilling their prescription for 30 consecutive days, they were considered to have dropped out of treatment. However, if the patient continued treatment elsewhere, it is possible for the patient to be classified as having ended treatment, thereby artificially inflating the rate of attrition. For the purpose of treatment retention analysis, patient's treatment retention and urine drug screening in MAT was followed for up to 1 year after entry in MAT so long as the patient was retained in treatment at the clinic network being studied.
Statistical Analysis
Descriptive statistics were summarized for baseline characteristics of patients, and standardized differences were used to compare patients in the various BZD use groups. For the purpose of this study, only a patient's first treatment episode was considered. For the primary analysis, logistic regression analysis was used to characterize the time to treatment discontinuation across the 4 patient groups. Statistical analysis was performed using SAS Enterprise Guide 7.1.
Ethics Review
This study was approved by the Research Ethics Boards of Laurentian University, Sudbury, Ontario and Sunnybrook Hospital, Toronto, Ontario.
RESULTS
Patient Demographic
We identified 3692 patients who initiated MAT for OUD across a network of 52 clinics in the province of Ontario, Canada, between January 1, 2006 and June 30, 2012. Across the BZD use groups, the BZD+/UDS+ group were older (median 38 years), more likely to be in the lowest income quintile, and had more frequent interaction with primary health care, mental health care, and emergency department visits, medical comorbidities, and diagnosis for a depression or anxiety related disorder (Table 1). All groups had clinically similar MAT methadone peak dose of >60 mg.
TABLE 1.
Cohort Statistics
| BZD Rx + UDS + | BZD Rx + UDS− | BZD Rx − UDS + | BZD Rx − UDS − | |
| Variable | (n = 208) | (n = 464) | (n = 219) | (n = 2801) |
| Age, mean (SD) | 37.5 (10.8) | 35.5 (11.3) | 35.2 (10.7) | 31.3 (9.7) |
| Median (IQR) | 37 (28.5–46) | 33 (26–44) | 33 (27–42) | 29 (24–37) |
| Sex | ||||
| Male | 104 (50.0) | 234 (50.4) | 123 (56.2) | 1604 (57.3) |
| Female | 104 (50.0) | 230 (49.6) | 96 (43.8) | 1197 (42.7) |
| Income quintile | ||||
| Q1 (lowest) | 93 (44.7) | 195 (42.0) | 77 (35.2) | 1031 (36.8) |
| Q2 | 47 (22.6) | 98 (21.1) | 47 (21.5) | 649 (23.2) |
| Q3 | 29 (13.9) | 83 (17.9) | 34 (15.5) | 497 (17.7) |
| Q4 | 23 (11.1) | 54 (11.6) | 30 (13.7) | 337 (12.0) |
| Q5 (highest) | 16 (7.7) | 33 (7.1) | 31 (14.2) | 283 (10.1) |
| Health system use | ||||
| Number of hospitalizations mean (SD) | 0.30 (0.76) | 0.24 (0.79) | 0.25 (0.81) | 0.14 (0.49) |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Number of psychiatric hospitalizations mean (SD) | 0.27 (0.75) | 0.22 (0.64) | 0.16 (0.49) | 0.07 (0.36) |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Number of GP/FP visits mean (SD) | 24.1 (21.6) | 19.6 (18.5) | 19.5 (22.5) | 13.2 (14.0) |
| Median (IQR) | 19 (10–32) | 15 (8–25) | 15 (7–23) | 9 (4–17) |
| Number of psych visits mean (SD) | 3.1 (6.4) | 2.1 (4.6) | 1.8 (4.6) | 1.0 (3.7) |
| Median (IQR) | 0 (0–3) | 0 (0–2) | 0 (0–2) | 0 (0–0) |
| Number of ED visits mean (SD) | 5.6 (7.8) | 3.9 (7.4) | 3.4 (5.4) | 2.3 (3.8) |
| Median (IQR) | 3 (1–8) | 2 (0–4.5) | 2 (0–4) | 1 (0–3) |
| Peak dose mean (SD) | 67.0 (31.3) | 68.6 (30.5) | 63.6 (29.8) | 63.7 (27.1) |
| Median (IQR) | 67.8 (42.1–91.4) | 67.3 (46.6–89.4) | 65.0 (37.5–84.8) | 62.3 (41.9–83.8) |
| Charlson comorbities | ||||
| No hospitalizations | 123 (59.1) | 288 (62.1) | 143 (65.3) | 2037 (72.7) |
| 0 comorbidities | 66 (31.7) | 136 (29.3) | 63 (28.8) | 685 (24.5) |
| 1 comorbidity | 13 (6.3) | 27 (5.8) | 9 (4.1) | 55 (2.0) |
| 2+ comorbidities | 6 (2.9) | 13 (2.8) | 4 (1.8) | 24 (0.9) |
| Diagnosis of mood/anxiety disorder | 168 (80.8) | 325 (70.0) | 133 (60.7) | 993 (35.5) |
BZD, benzodiazepine; CI, confidence interval; ED, emergency department; FP, family physician; GP, general practitioner; IQR, interquartile range; ODB, Ontario Drug Benefit; OR, odds ratio; SD, standard deviation; UDS, urine drug screening data.
Prevalence of BZD Use
The majority of patients in the cohort did not receive a BZD prescription, nor did they have positive BZD urine drug screens >30% the time during their treatment episode (BZD−/UDS−; 76%; 2801/3692). Approximately 13% (BZD+/UDS−; 12%; 464/3692) of patients received a BZD prescription, yet had fewer than 30% of their urine screens test positive for BZD over their treatment window. Six per cent (BZD−/UDS+; 6%; 219/3692) of patients screened positive for BZD in greater than 30% of their urine screens during treatment while not having a prescription for BZD. A minority of patients (BZD+/UDS+; 6%; 208/3692) had a prescription for BZD and had greater than 30% BZD urine drug screens.
Treatment Retention
Logistic regression demonstrated that the BZD−/UDS+ group had 2-fold greater likelihood of discontinuing MAT (adjusted odds ratio [aOR] 0.38, 95% confidence interval [CI] 0.27–0.53) as compared with the BZD−/UDS− group. Other BZD use groups were not statistically different than the BZD−/UDS− group with respect to treatment retention (Table 2). A Kaplan–Meier analysis demonstrates probability of 1-year treatment retention across all groups (Fig. 1).
TABLE 2.
Logistic Regression
| Region | Number of Patients | Number Retained, n (%) | Unadjusted OR | Unadjusted 95% CI | Adjusted OR | Adjusted 95% CI |
| Primary outcome: successful completion of OAT (ODB eligible) | ||||||
| BZD Rx + UDS+ | 208 | 96 (46.2) | 0.76 | 0.58–1.01 | 0.80 | 0.57–1.12 |
| BZD Rx + UDS− | 464 | 237 (51.1) | 0.93 | 0.76–1.13 | 0.90 | 0.71–1.14 |
| BZD Rx − UDS+ | 219 | 70 (32.0) | 0.42 | 0.31–0.56 | 0.39 | 0.28–0.55 |
| BZD Rx − UDS− | 2801 | 1481 (52.9) | 1.00 | 1.00 | ||
BZD, benzodiazepine; CI, confidence interval; OAT, opioid agonist treatment; ODB, Ontario Drug Benefit; OR, odds ratio; UDS, urine drug screening data.
FIGURE 1.
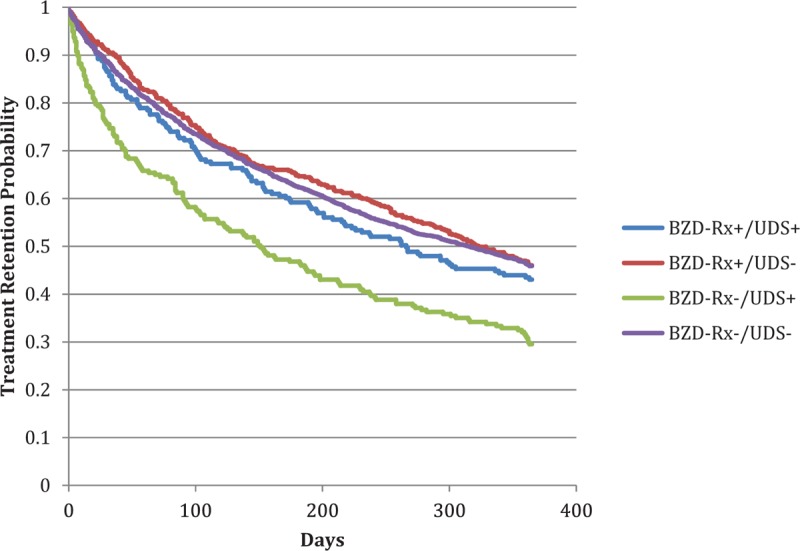
Treatment retention by BZD classification. BZD, benzodiazepine.
As a secondary outcome, we used data from the provincial Discharge Abstract Database to assess likelihood of death during the study window; however, death (overdose or otherwise) was an uncommon outcome (<1%) and therefore could not be reliably assessed across groups.
For regression analysis, covariates for adjusted regression models included patient sex, clinic location, use of Telehealth services, methadone dose, diagnosis of a mood/anxiety disorder in the year before initiation of MAT, and number of general practitioner physician visits in the year before initiation of MAT. Other variables considered were age, number of acute admissions, number of psychiatric admissions, number of ED visits, and number of psychiatric office visits in the year before initiation of MAT.
DISCUSSION
This study addresses an important clinical question in addiction medicine: Does concurrent prescribed BZD use during MAT impact treatment retention? The current literature reveals mixed findings on the impact of BZD use on MAT retention (Kellogg et al., 2006; Schiff et al., 2007; Brands et al., 2008; Specka et al., 2011), and few, if any, studies have resolved the impact of prescribed versus nonprescribed BZD use during MAT. A recent study from our group revealed that both baseline and heavy BZD use increased a patients likelihood of discontinuing MAT (Franklyn et al., 2017); however, this study was limited in that we were unable to resolve the difference in prescribed versus nonprescribed BZD use in the study design. In the present study, we linked clinical data from MAT clinics (including urine toxicology) to Ontario's provincial prescription records in a single-payer healthcare system to address the question of the impact of prescribed BZD use on patients engaged in MAT. We chose >30% of urine drug screens as a cut-off to allow distinction between patients who regularly use BZD versus patients who may have a BZD prescription, but only use as needed. Thus, heavy BZD use patients would be classified as BZD+/UDS+ and patients with intermittent or as needed BZD use would be classified as BZD+/UDS−. It is also possible patients with a BZD prescription who diverted their BZD prescription would be classified as BZD+/UDS−. Similarly, if a patient receives only a short script for BZD once, but then has >30% urines BZD-positive, then would be classified as BZD+/USD+; however, the patient may more appropriately be classified as a BZD−/UDS+ patient.
Our results clearly demonstrate that patients who are prescribed BZD during MAT (BZD+/UDS+ or BZD+/UDS−) have similar treatment retention to those patients who are not (BZD−/UDS−). As expected, BZD+/UDS+ patients had the greatest health system usage for emergency department visits, hospitalizations, and mental health hospitalizations; yet this group's treatment retention was clinically similar to the BZD−/UDS− reference group. However, patients with nonprescribed BZD use (BZD−/UDS+) have more than twice the likelihood of MAT discontinuation. It should also be noted that patients prescribed benzodiazepines, but without regularly positive urines (BZD+/UDS−), might have taken the medication only for a short period of time, might not have taken their BZD, or may divert their medication for sale to others. Thus, our data address an important gap in the addiction literature which supports the notion that patients who may benefit from prescribed BZD use are not necessarily more likely to discontinue treatment. While we did not observe many deaths in the study window, physicians and patients should be acutely aware of the dangers of coprescribing BZD to patients with OUD, given the increased risk of overdose and death (Brands et al., 2008), particularly as the availability of synthetic opioids, including fentanyl, has increased dramatically. Moreover, we also advocate, that if patients are going to use BZD during MAT that education about the risks of overdose (including the prescription of accompanying naloxone kit) be provided to the patient (Gladden et al., 2016).
Using a large clinical dataset linked with provincial administrative healthcare data, our study design was able to address the impact of prescribed verses nonprescribed BZD use in MAT. With over 3600 patients in the cohort, this study captures a representative sample of all patients initiating outpatient MAT in Ontario. The fact that all patients in the cohort were from 1 network of clinics adds strength to the comparisons made between patients. Additionally, this study used high-quality data and quantitative metrics rather than patient self-report, which is a limitation of other previous studies.
Conversely, because we used retrospective data from the Ontario Drug Benefit plan, our study cohort would not have included patients with privately ensured health benefits. Similarly, due to the nature of administrative health data, we are unable to assess other important qualitative factors. For example, we are unable to assess change in psychosocial function of patients with prescribed BZD use during MAT. Our study has many strengths and limitations that warrant discussion. Further limitations to the health systems data approach include the potential for patients to lose eligibility for public drug coverage over follow-up (eg, as a result of becoming employed). In such cases, patients could appear to have discontinued therapy when they had instead changed coverage from public to private health insurer. However, we do not believe such cases would have a substantial impact on the data because they are likely to be rare in the first year of treatment. As patients with incomplete health system profiles were excluded from analysis, patients accessing services outside the provincial healthcare funding would have fallen outside the scope of analysis (eg, undocumented immigrants). Thus, the generalizability of our results should be taken in context of the limitations outlined above, and we believe further qualitative study is warranted to address the types of clinical benefit a patient will experience while using prescribed BZD during MAT.
CONCLUSIONS
Our findings suggest that prescribed BZD use during MAT does not materially impact a patient's retention in treatment; but using BZD without a prescription does negatively impact treatment retention. The increased use of healthcare resources and lower income among patients prescribed BZD suggest that this patient population has more complex physical and mental health needs and might be expected to have poorer treatment retention than the comparison group. Therefore, the prescribing of BZD to this group may be a portion of a comprehensive approach to treatment which supports improving retention to be similar to those patients who do not require BZD. We urge both the physician and patient to seek alternative clinical options before BZD prescribing due to the potential for developing BZD-dependence, BZD use disorder, and the risks of respiratory depression and overdose when used in combination with opioids.
Footnotes
Funding: This study was funded by a Clinical Innovation Grant from the Northern Ontario Academic Medicine Association. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC).
Financial disclosures: JKE received funding as a successful recipient of the 2014 to 2015 CANOC Scholarship Awards Program (this program is supported through the Centres Grant [CIHR 711314]). CANOC is funded by the Canadian Institutes of Health Research (CIHR) through a Centres Grant (Centres for HIV/AIDS Population Health and Health Services Research [CIHR 711314]); two Operating Grants (HIV/AIDS Priority Announcement [CIHR 711310]; Population and Public Health [CIHR 711319]); the CIHR Canadian HIV Trials Network [CTN 242]; and a Foundation Grant (Expansion of Antiretroviral Therapy and its Impact on Vulnerable Populations in Canada and Global Settings [CIHR #143342]). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
The authors have no conflicts of interest to disclose.
REFERENCES
- Bakker A, Streel E. Benzodiazepine maintenance in opiate substitution treatment: Good or bad? A retrospective primary care case-note review. J Psychopharmacol 2017; 31:62–66. [DOI] [PubMed] [Google Scholar]
- Brands J, Brands B, Marsh DCT. The expansion of methadone prescribing in Ontario, 1996–1998. Addict Res 2000; 8:485–496. [Google Scholar]
- Brands B, Blake J, Marsh DC, et al. The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis 2008; 27:37–48. [DOI] [PubMed] [Google Scholar]
- College of Physicians and Surgeons of Ontario. Methadone Maintenance Treatment Program Standards and Clinical Guidelines. Methadone Program, editor. Toronto, Ontario: The College of Physicians and Surgeons of Ontario; 2011. [Google Scholar]
- Dhalla IA, Mamdani MM, Sivilotti ML, et al. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ 2009; 181:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklyn AM, Eibl JK, Gauthier G, et al. The impact of benzodiazepine use in patients enrolled in opioid agonist therapy in Northern and rural Ontario. Harm Reduct J 2017; 14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths: 27 states, 2013–2014. MMWR Morb Mortal Wkly Rep 2016; 65:837–843. [DOI] [PubMed] [Google Scholar]
- Gomes T, Mamdani MM, Dhalla IA, et al. The burden of premature opioid-related mortality. Addiction 2014; 109:1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S, Schulze K, Groome P, et al. Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol 2006; 59:67–76. [DOI] [PubMed] [Google Scholar]
- Handford CK, Srivastava M, Cirone A, et al. Buprenorphine/Naloxone for Opioid Dependence: Clinical Practice Guideline. Toronto, ON: Centre for Addiction and Mental Health; 2011. [Google Scholar]
- Juurlink DN. Proton pump inhibitors and clopidogrel: putting the interaction in perspective. Circulation 2009; 120:2310–2312. [DOI] [PubMed] [Google Scholar]
- Juurlink DN, Gomes T, Lipscombe LL, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 2009; 339:b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg S, Melia D, Khuri E, et al. Adolescent and young adult heroin patients: drug use and success in methadone maintenance treatment. J Addict Dis 2006; 25:15–25. [DOI] [PubMed] [Google Scholar]
- Levy AR, O’Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003; 10:67–71. [PubMed] [Google Scholar]
- Mamdani M, Rochon P, Juurlink DN, et al. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med 2003; 163:481–486. [DOI] [PubMed] [Google Scholar]
- Nielsen S. Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction 2007; 102:616–622. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 2001; 179:390–396. [DOI] [PubMed] [Google Scholar]
- Roux P, Lions C, Vilotitch A, et al. Correlates of cocaine use during methadone treatment: implications for screening and clinical management (ANRS Methaville study). Harm Reduct J 2016; 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Levit S, Moreno RC. Retention and illicit drug use among methadone patients in Israel: a gender comparison. Addict Behav 2007; 32:2108–2119. [DOI] [PubMed] [Google Scholar]
- Specka M, Bonnet U, Heilmann M, et al. Longitudinal patterns of benzodiazepine consumption in a German cohort of methadone maintenance treatment patients. Hum Psychopharmacol 2011; 26:404–411. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies: tackling the opioid-overdose epidemic. N Engl J Med 2014; 370:2063–2066. [DOI] [PubMed] [Google Scholar]


