Supplemental Digital Content is available in the text.
Keywords: brain edema; humans; infarction, middle cerebral artery; odds ratio; prognosis
Abstract
Background and Purpose—
Predicting malignant middle cerebral artery (MCA) infarction can help to identify patients who may benefit from preventive decompressive surgery. We aimed to investigate the association between the ratio of intracranial cerebrospinal fluid (CSF) volume to intracranial volume (ICV) and malignant MCA infarction.
Methods—
Patients with an occlusion proximal to the M3 segment of the MCA were selected from the DUST (Dutch Acute Stroke Study). Admission imaging included noncontrast computed tomography (CT), CT perfusion, and CT angiography. Patient characteristics and CT findings were collected. The ratio of intracranial CSF volume to ICV (CSF/ICV) was quantified on admission thin-slice noncontrast CT. Malignant MCA infarction was defined as a midline shift of >5 mm on follow-up noncontrast CT, which was performed 3 days after the stroke or in case of clinical deterioration. To test the association between CSF/ICV and malignant MCA infarction, odds ratios and 95% CIs were calculated for 3 multivariable models by using binary logistic regression. Model performances were compared by using the likelihood ratio test.
Results—
Of the 286 included patients, 35 (12%) developed malignant MCA infarction. CSF/ICV was independently associated with malignant MCA infarction in 3 multivariable models: (1) with age and admission National Institutes of Health Stroke Scale (odds ratio, 3.3; 95% CI, 1.1–11.1), (2) with admission National Institutes of Health Stroke Scale and poor collateral score (odds ratio, 7.0; 95% CI, 2.6–21.3), and (3) with terminal internal carotid artery or proximal M1 occlusion and poor collateral score (odds ratio, 7.7; 95% CI, 2.8–23.9). The performance of model 1 (areas under the receiver operating characteristic curves, 0.795 versus 0.824; P=0.033), model 2 (areas under the receiver operating characteristic curves, 0.813 versus 0.850; P<0.001), and model 3 (areas under the receiver operating characteristic curves, 0.811 versus 0.856; P<0.001) improved significantly after adding CSF/ICV.
Conclusions—
The CSF/ICV ratio is associated with malignant MCA infarction and has added value to clinical and imaging prediction models in limited numbers of patients.
Development of malignant edema (ME) is a life-threatening complication and typically occurs in younger patients with a large middle cerebral artery (MCA) infarction.1 Such a malignant MCA infarction occurs in ≤10% of the patients with large supratentorial stroke.2 No official definition exists for ME, but stroke researchers often use the combination of clinical deterioration and midline shift on computed tomography (CT) imaging, although some only use the imaging definition.3,4 Usually, the edema develops between the second and fifth day after the stroke, although onset of symptoms before 24 hours after the stroke is not uncommon. Before surgical intervention was introduced, reported mortality rates associated with malignant MCA infarction ranged between 70% and 80%.1,5 In a pooled analysis of 3 randomized trials, early decompressive surgery has been shown to be effective in patients with malignant MCA infarction in terms of improving clinical outcome and reducing mortality rate.6 Anticipating on development of ME is important, so that the patient can be treated on time. Therefore, prediction of ME is helpful.
Single predictors of malignant MCA infarction have been investigated previously and include both clinical and imaging factors. Clinical features that are present on admission have been related to ME and include age, vomiting, National Institutes of Health Stroke Scale (NIHSS), and coma.7–11 However, the predictive value of clinical parameters regarding malignant MCA infarction is limited, and, therefore, imaging factors are an important addition to prediction models. Imaging factors that have been associated with ME independently of age and NIHSS include early signs of ischemia on noncontrast CT (NCCT), larger volume of deficits on cerebral blood volume maps, higher blood-brain permeability estimates on CT perfusion (CTP), more proximal thrombus location, higher clot burden score, and poor collateral scores on CT angiography (CTA).10–13 Similarly, larger size of the MCA infarction on diffusion-weighted imaging has been found to be associated with ME.8,14
Another potential predictor of ME may be the volume of intracranial cerebrospinal fluid (CSF) on admission. Theoretically, the brain has more space to swell, without herniating, when more CSF volume is present. To assess the predictive value of CSF volume in relation to ME, we evaluated a large prospective cohort of patients with MCA infarction.
Methods
Descriptive data that support the findings of this study are available from the corresponding author on reasonable request.
Patient Selection
Patients were selected from a prospective multicenter observational cohort study, the DUST (Dutch Acute Stroke Study), between May 2009 and August 2013.15 Patients, who participated in DUST, were adult (≥18 years), had suspected ischemic stroke based on clinical signs, presentation within 9 hours after onset of neurological deficits, and NIHSS ≥2, or 1 if an indication for intravenous administration of tPA (tissue-type plasminogen activator) was present.15 Exclusion criteria were contraindications for undergoing CT at admission including contrast allergy and renal failure or the presence of other causes for the neurological deficits on brain CT. Patient characteristics were collected, and all patients underwent CT imaging including NCCT, CTP, and CTA on hospital admission. This study was approved by the medical ethics committees of the participating hospitals. Signed informed consent was taken from all participants or their families. In case the patient died, the need for informed consent was waived by the medical ethics committee.
For the current study, we selected patients from DUST who had an occlusion proximal to the M3 segment of the MCA. Furthermore, patients were excluded if thin-slice NCCT at baseline was unavailable. Because we were only interested in midline shift caused by ME as outcome, patients were excluded if hemorrhagic transformation causing mass effect was present on follow-up CT.
Baseline Data
The following patient characteristics were collected at baseline: age, sex, NIHSS, time from symptom onset to CT scan, intravenous administration of tPA, endovascular treatment, cardiovascular risk factors, and previous medical history of cardiovascular disease.
Imaging Protocol
The imaging protocols have been described previously.15 In short, NCCT, CTP, and CTA scans were acquired as part of the acute stroke protocol. Follow-up NCCTs were planned on the third day after the stroke or at the moment of clinical deterioration. CT scanners (Philips, Siemens, Toshiba, and General Electric Company) with varying collimation widths, ranging from 40 to 320 slices, were used in this study. The tube settings for the NCCT were 120 kVp and 300 to 375 mAs per rotation. Slices were reconstructed with a thickness of 1 mm.
CTP was performed with 80 kVp and 150 mAs with a slice thickness of 5 mm. In dynamic mode, successive image frames were acquired (every 2 seconds for the duration of 50 seconds), while nonionic contrast material and saline were administered.15 Both Alberta Stroke Program Early CT Score levels were included in the CTP coverage.16
CTA covered the head and neck from aortic arch to cranial vertex. The scan delay after intravenous injection of contrast was calculated from time to peak arterial enhancement on CTP or by trigger-based Hounsfield unit threshold measurement of contrast enhancement in the aortic arch.
Imaging Analysis
Imaging data were evaluated by 1 of 3 observers, who all have >5 years of experience in stroke imaging.17 The observers were only informed about the side of symptoms before evaluation.
Baseline NCCT scans were evaluated for presence of hyperdense vessel sign and early signs of ischemia, which were quantified by using the Alberta Stroke Program Early CT Score.16
Fully automated intracranial CSF volume estimation was based on gray value histograms of the NCCT brain parenchyma. To this end, first, the brain was coarsely segmented into 3 tissue regions (gray matter, white matter, and CSF) by registering the International Consortium for Brain Mapping 152 nonlinear atlas18,19 to the NCCT scan using penalized elastic deformation.20 Aided by this segmentation, 3 gaussian mixture models were fitted to the histograms of coarsely segmented tissue regions (Figure 1). The use of a mixture model allows for volume measurement in noisy data, without the need for precise segmentation. The area under each gaussian curve reflects the volume of the particular tissue region.
Figure 1.
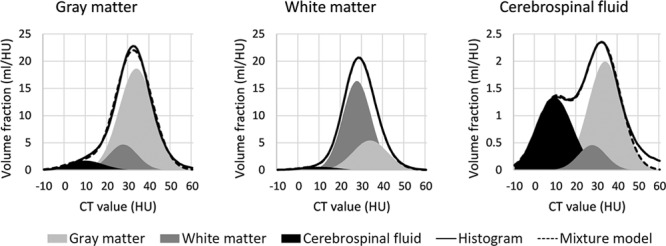
Gaussian mixture models fitted to noncontrast computed tomography (CT) histograms. An example of gaussian mixture models fitted to 3 noncontrast CT histograms: coarsely segmented gray matter, white matter, and cerebrospinal fluid (CSF). The solid line represents the measured intensity histogram, whereas the dashed line represents the mixture model. The mixture model consists of 3 gaussian distributions: gray matter (light gray; mean, 33.6 HU), white matter (dark gray; mean, 27.0 HU), and CSF (black; mean, 9.5 HU). Note that the gray matter histogram is dominated by the gray matter peak, the white matter histogram by the white matter peak, and the CSF histogram by both the CSF and gray matter peaks.
The intracranial volume (ICV) was defined as the sum of the gray matter, white matter, and CSF atlas regions, that is, the sum of the areas under the 3 histograms. The intracranial CSF volume was defined as the sum of the areas under the curve of the CSF distributions of the gaussian mixtures inside those masks.
ME was defined as a midline shift of >5 mm. Types of hemorrhagic transformation including hemorrhagic transformation with mass effect (type PH-2) were evaluated by using the ECASS (European Cooperative Acute Stroke Study) criteria.21
For CTP, Alberta Stroke Program Early CT Score was evaluated on perfusion maps, which included cerebral blood volume and mean transit time and were calculated by using commercially available CTP software (Extended Brilliance Workstation 4.5; Philips Healthcare).
On baseline CTA, intracranial artery occlusions and collateral status were evaluated.22–24 The most proximal occlusion was used if multiple occlusions were present, with the exception of a tandem lesion of the extracranial internal carotid artery (ICA) and MCA, in which case the MCA occlusion was used.25 The collateral score was categorized as either poor or good (cutoff, 50%) compared with the contralateral hemisphere by visual inspection of the maximum-intensity projection images.17
Outcome Measures
Primary outcome was the presence of ME on follow-up imaging. The secondary outcome measure was clinical outcome after 90 days. Poor clinical outcome was defined as a score of ≥3 on the modified Rankin Scale.
Statistical Analysis
Patient characteristics and outcomes were compared between the patients included for this study and the patients who were excluded because of unavailability of thin-slice NCCT images by using variable-dependent statistical tests (Table I in the online-only Data Supplement). Similarly, patient characteristics were compared between patients with and without malignant MCA infarction. The intracranial CSF volume was adjusted for ICV by calculating the ratio of CSF volume to ICV (CSF/ICV). Odds ratios (ORs) and 95% CIs were calculated by using binary logistic regression. Potential predictors were identified by screening the literature. Complete-case analysis was performed because no missing values were present for the predictors of interest. Because of the limited number of outcomes, we could only select 3 potential predictors per model. Because younger patients have a higher risk of developing ME than older patients, we added age to one of the models. Similarly, we added NIHSS to 2 of the models. The third model contained 2 imaging parameters: terminal ICA or M1 occlusion and poor collateral score. Subgroup analyses were done for the patient group aged from 18 to 60 and for the group with NIHSS of ≥16. Variance inflation factors were calculated to test the collinearity assumption, which was not violated. Receiver operating characteristic curves were plotted from the predicted probabilities. The areas under the receiver operating characteristic curves (AUROC), were calculated and model performances were compared by using the likelihood ratio test, so that the added value of CSF/ICV could be calculated. The described analyses were performed in R (version 3.4.2).
Results
We selected 472 patients with an MCA occlusion proximal to the M3 segment (Figure I in the online-only Data Supplement). We excluded 179 cases because no thin-slice NCCT was available at baseline. At follow-up, 7 patients had hemorrhagic transformation with mass effect (PH-2) and were thus excluded. The final analysis included 286 patients, of whom 35 (12%) developed ME. No more than 10 (3%) missing values were present for the patient characteristics except for smoking (n=24; 8%). Twenty-two (33%) of the 69 patients with terminal ICA or proximal M1 occlusion developed ME. Twelve (6%) of the 217 patients with an occlusion distal to the terminal ICA or proximal M1 segment developed ME.
The patients without baseline thin-slice NCCT and without PH-2 during follow-up are compared with the included patients (Table I in the online-only Data Supplement). In the group that was excluded because of unavailability of thin-slice NCCT images, 19 of 175 (11%) patients developed malignant MCA infarction. This number was not significantly different from the included group (P=0.766).
Patient characteristics of the selected study population are summarized in Table 1. Crude ORs are shown in Table II in the online-only Data Supplement. Age was significantly lower in the ME group than in the non-ME group (OR, 1.5; 95% CI, 1.2–2.0) as was increase in admission NIHSS (OR, 1.2; 95% CI, 1.1–1.3). No large differences were observed between the 2 groups regarding treatment or cardiovascular risk factors. Specific imaging findings on admission that were more prevalent in the ME group than in the non-ME group included hyperdense vessel sign (OR, 3.5; 95% CI, 1.7–8.0), lower Alberta Stroke Program Early CT Score (OR, 1.8; 95% CI, 1.5–2.1), terminal ICA, or proximal M1 occlusion (OR, 7.3; 95% CI, 3.5–16.0) and poor collateral score (OR, 7.3; 95% CI, 3.4–16.7). Decrease in CSF/ICV was significantly associated with malignant MCA infarction (OR, 4.5; 95% CI, 1.9–11.7). Examples illustrating the association between CSF/ICV and ME are shown in Figure 2. Prevalence of poor clinical outcome 90 days after the stroke was higher in the ME group than in the non-ME group (97% versus 47%, respectively; P<0.001).
Table 1.
Patient Characteristics
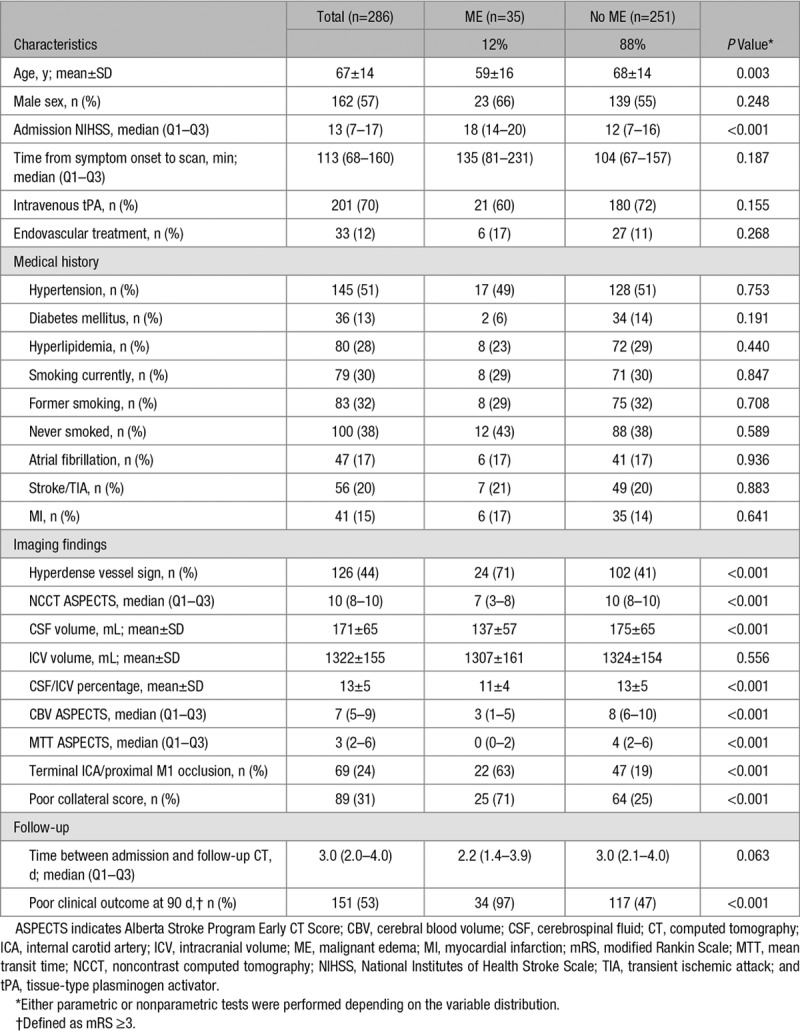
Figure 2.
Examples illustrating the association between the ratio of intracranial cerebrospinal fluid volume (CSF) and intracranial volume (ICV) and malignant middle cerebral artery (MCA) infarction. First example of a baseline noncontrast computed tomography (CT) image of an 81-y-old man with a large MCA infarction due to an occlusion of the proximal M1 segment (A). The ratio between intracranial CSF/ICV was 0.19. On follow-up, noncontrast CT demarcation of the infarction is visible, but no midline shift has occurred (B). Second example of a baseline noncontrast CT image of a 52-y-old woman with a large MCA infarction due to an occlusion of the proximal M1 segment (C). The CSF/ICV was 0.08. On follow-up, noncontrast CT malignant edema has developed leading to a midline shift of >5 mm (D).
The results of the multivariable analysis are shown in Table 2 and details of the receiver operating characteristic curves in Table 3 and Figure 3. In model 1, CSF/ICV was associated with ME independent of age and admission NIHSS (OR, 3.3; 95% CI, 1.1–11.3). The model with CSF/ICV had a significantly better performance than the model without CSF/ICV when comparing the AUROCs (0.824 versus 0.795, respectively; P=0.033). In model 2, CSF/ICV was associated with ME independent of admission NIHSS and poor collateral score (OR, 7.0; 95% CI, 2.6–21.3). A significant difference was observed between the performance of the model with CSF/ICV and the model without CSF/ICV (AUROC, 0.850 versus 0.813, respectively; P<0.001). In model 3, CSF/ICV was associated with ME independent of the presence of a terminal ICA or proximal M1 occlusion and poor collateral score (OR, 7.7; 95% CI, 2.8–23.9). Furthermore, a significant difference was found between the performance of model 3 with CSF/ICV and without CSF/ICV (AUROC, 0.856 versus 0.811, respectively; P<0.001).
Table 2.
Multivariable Prediction Models and the Association With Malignant Middle Cerebral Artery Infarction
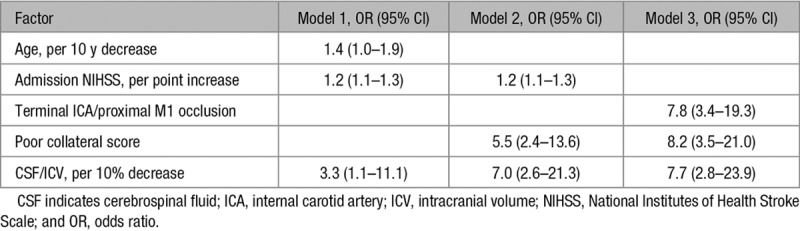
Table 3.
Comparison of Clinical and Imaging Models With and Without the Ratio Between Intracranial CSF Volume and ICV
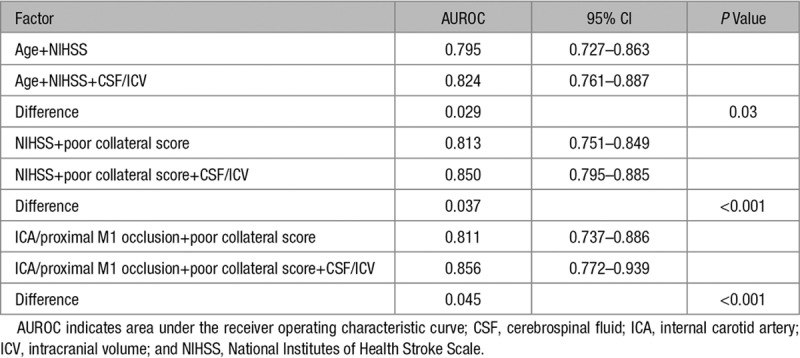
Figure 3.
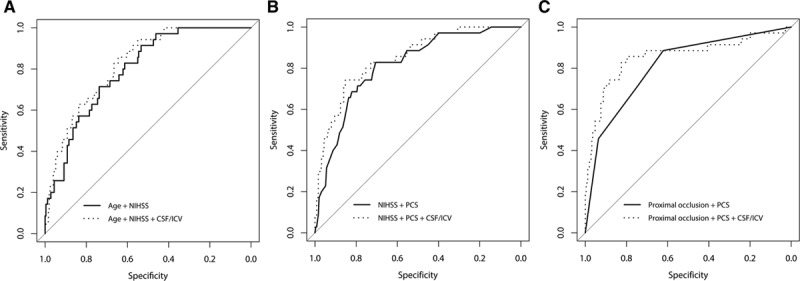
Performance of prediction models with and without the ratio between intracranial cerebrospinal fluid volume (CSF) and intracranial volume (ICV). Clinical prediction model (A), prediction model with clinical and imaging predictors (B), and imaging prediction model (C) with and without the ratio between intracranial CSF/ICV, respectively. Proximal occlusion indicates terminal internal carotid artery or proximal M1 occlusion. NIHSS indicates National Institutes of Health Stroke Scale; and PCS, poor collateral score.
Of the 87 patients aged from 18 to 60, 20 (23%) developed ME (Table III in the online-only Data Supplement). In this group of patients, no significant associations between CSF/ICV and ME were observed in the 3 multivariable models (Table IV in the online-only Data Supplement). The 3 models did not improve significantly after CSF/ICV was added (Table V in the online-only Data Supplement).
Of the 95 patients with an NIHSS of ≥16, 21 (22%) developed ME (Table VI in the online-only Data Supplement). In this group of patients, no associations between CSF/ICV and ME were observed in the 3 multivariable models (Table VII in the online-only Data Supplement). Only model 2 improved significantly (P=0.047) after CSF/ICV was added (Table VIII in the online-only Data Supplement).
Discussion
In this study, we evaluated the added value of the ratio of intracranial CSF volume to ICV in predicting ME. By building 3 statistical models, we showed that CSF/ICV is a predictor of malignant MCA infarction independent of (1) age and NIHSS, (2) NIHSS and poor collateral score, and (3) terminal ICA or proximal M1 occlusion and poor collateral score. When comparing performances of the models with and without CSF/ICV, the 3 models improved significantly after CSF/ICV was added.
Our results are in accordance with the only previous study that investigated the association between intracranial CSF volume and ME.26 In the previous study, which had a retrospective design, half of the 52 patients with terminal carotid or proximal M1 occlusion developed ME, whereas in our study, 12% developed ME. This difference can be explained by the different use of selection criteria. In the previous study, patients with an ICA or proximal M1 occlusion were included, whereas in our study, we also included patients with a distal M1 or M2 occlusion. In our study, 33% (22 of 69) of the patients with terminal ICA or proximal M1 occlusion developed ME. As expected, the presence of a terminal ICA or proximal M1 occlusion was associated with the development of ME as compared with the presence of a more distal occlusion. Nonetheless, 6% of the patients with an occlusion of the distal M1 segment or M2 segment of the MCA also developed ME. This implies that future studies should not only address the most proximal MCA occlusions, although these patients face the highest risk of developing malignant MCA infarction.
As brain volume shrinks with increasing age, it is not surprising that patients who develop ME are typically younger than patients who do not develop ME because there is less space for the brain to swell without causing herniation.1 Similar to atrophy, previous stroke, which is typically a disease of the elderly, may lead to an increase of the ratio of intracranial CSF volume to ICV. In our study, patients with malignant MCA infarction were indeed younger than the patients without malignant MCA infarction. We did not formally test the correlation between age and CSF/ICV, but the assumption of collinearity between predictors was not violated. Moreover, when adjusted for age and admission NIHSS, CSF/ICV was still significantly related to ME, and the clinical model improved significantly after CSF/ICV was added. The observed associations did not hold in the subgroup analyses of patients aged from 18 to 60 and patients with an NIHSS of ≥16. Although the ORs indicated a positive association between CSF/ICV and malignant MCA infarction for these subgroups, the power was too low for the associations to reach significance.
Similarly to the clinical model, CSF/ICV was of added value to the imaging prediction model. In fact, the AUROC of the imaging model was even higher than the AUROC of the clinical model. However, we did not formally test the difference between the performances of the clinical and imaging models because this was not our primary research question. Still, these results emphasize the need for the use of imaging findings for the prediction of malignant MCA infarction and, perhaps, other complications of stroke.
One strength of this study was the prospective design. As a consequence, we only had few missing data and a low number of dropouts, which minimizes the risks of information bias and selection bias, respectively. Another strong point of this study was the quantification of the intracranial CSF volume and ICV by applying a brain atlas on the CT scans. Because this is an entirely automated technique for quantifying brain volumes, neither observation bias nor interrater reliability is an issue for this measurement of interest. Furthermore, this method is robust to CT noise, loss of gray-white differentiation, or other early ischemic changes on NCCT as the volumes are derived from mixture model histograms and not directly from segmentations.
One of the limitations of this study was the large number of exclusions because of the unavailability of thin-slice NCCT images, which was not a standard procedure for the DUST study. We chose to exclude those patients because volume measurements would be less precise on thick-slice images and for the sake of the uniformity of the measurements. However, we do not think that excluding patients in this manner influences the results because the collection of thin-slice data is a random feature and neither related to CSF volume nor the development of ME. Furthermore, no large relevant differences were observed between the characteristics of the included and excluded patient groups. In general, thin-slice NCCT data are nowadays routinely acquired as part of stroke imaging protocols. Another drawback of this study was the limited number of outcomes. As a consequence, we could not add >3 variables to the prediction models, and we were not able to include CTP variables or build a large prognostic model. Unfortunately, we did not have a sufficient large sample size either for developing a new clinically usable prediction model or for validating a previously developed model.10 In the future, larger cohorts with MCA infarction should be evaluated for the purpose of developing a prediction model that can be readily used in clinical practice. We used a dichotomized measure of midline shift as the primary outcome. Although dichotomizing this measure may lead to loss of information, we think that interrater variability is lower than when a continuous measure was used, but we were not able to formally test this assumption.
We did not use clinical information to define malignant MCA infarctions. Although some previous studies used both clinical and imaging information for defining ME, we think that solely using the quantitative measure of midline shift is sufficient to identify malignant MCA infarction as has been done previously.4 We did not collect data on treatment of malignant MCA infarction. As a result, we were not able to evaluate whether patients, who have been treated, would have been treated sooner, or patients, who have not been treated, would have been treated after taking into account the results of this study. Still, we found that CSF/ICV significantly improves 3 types of prediction models. As a consequence, patients at risk for malignant MCA infarction can be recognized and treated earlier. In the future, larger prediction models need to be developed, and their influence on patient management and clinical outcome should be evaluated.
In conclusion, the CSF/ICV ratio is associated with malignant MCA infarction and has added value to clinical and imaging prediction models in limited numbers of patients.
Acknowledgments
The DUST (Dutch Acute Stroke Study) investigators are as follows: Academic Medical Center, Amsterdam: Majoie C.B. and Roos Y.B.; Catharina Hospital, Eindhoven: Duijm L.E. and Keizer K.; Erasmus Medical Center, Rotterdam: van der Lugt A. and Dippel D.W.; Gelre Hospitals, Apeldoorn: Droogh-de Greve K.E. and Bienfait H.P.; Leiden University Medical Center, Leiden: van Walderveen M.A. and Wermer M.J.; Medical Center Haaglanden, The Hague: Lycklama à Nijeholt G.J. and Boiten J.; Onze Lieve Vrouwe Gasthuis, Amsterdam: Duyndam D. and Kwa V.I.; Radboud University Nijmegen Medical Centre, Nijmegen: Meijer F.J. and van Dijk E.J.; Rijnstate Hospital, Arnhem: Kesselring F.O. and Hofmeijer J.; St. Antonius Hospital, Nieuwegein: Vos J.A. and Schonewille W.J.; St. Elisabeth Hospital, Tilburg: van Rooij W.J. and de Kort P.L.; St. Franciscus Hospital, Rotterdam: Pleiter C.C. and Bakker S.L.; VU Medical Center, Amsterdam: Bot J. and Visser M.C.; University Medical Center Utrecht, Utrecht: Velthuis B.K., van der Schaaf I.C., Dankbaar J.W., Mali W.P., van Seeters T., Horsch A.D., Niesten J.M., Biessels G.J., Kappelle L.J., Luitse M.J., and van der Graaf Y., all from the Netherlands.
Sources of Funding
This study was supported by grants from the Dutch Heart Foundation (grant numbers 2008 T034 and 2012 T061) and the Nuts Ohra Foundation (grant number 0903–012). This research has been made possible by the Dutch Heart Foundation and the Netherlands Organization for Scientific Research (NWO), domain Applied and Engineering Sciences (TTW), as part of their joint strategic research program: Earlier Recognition of Cardiovascular Diseases (grant number 14732).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.024882.
References
- 1.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 2.Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology. 1995;45:1286–1290. doi: 10.1212/wnl.45.7.1286. [DOI] [PubMed] [Google Scholar]
- 3.Sykora M, Steiner T, Rocco A, Turcani P, Hacke W, Diedler J. Baroreflex sensitivity to predict malignant middle cerebral artery infarction. Stroke. 2012;43:714–719. doi: 10.1161/STROKEAHA.111.632778. doi: 10.1161/STROKEAHA.111.632778. [DOI] [PubMed] [Google Scholar]
- 4.Walcott BP, Miller JC, Kwon CS, Sheth SA, Hiller M, Cronin CA, et al. Outcomes in severe middle cerebral artery ischemic stroke. Neurocrit Care. 2014;21:20–26. doi: 10.1007/s12028-013-9838-x. doi: 10.1007/s12028-013-9838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–623. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 6.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. DECIMAL, DESTINY, and HAMLET Investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 7.Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW. Mass effect and death from severe acute stroke. Neurology. 1997;49:1090–1095. doi: 10.1212/wnl.49.4.1090. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheim C, Samson Y, Manaï R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31:2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- 9.Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32:2117–2123. doi: 10.1161/hs0901.095719. [DOI] [PubMed] [Google Scholar]
- 10.Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS One. 2017;12:e0171425. doi: 10.1371/journal.pone.0171425. doi: 10.1371/journal.pone.0171425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dittrich R, Kloska SP, Fischer T, Nam E, Ritter MA, Seidensticker P, et al. Accuracy of perfusion-CT in predicting malignant middle cerebral artery brain infarction. J Neurol. 2008;255:896–902. doi: 10.1007/s00415-008-0802-1. doi: 10.1007/s00415-008-0802-1. [DOI] [PubMed] [Google Scholar]
- 12.Bektas H, Wu TC, Kasam M, Harun N, Sitton CW, Grotta JC, et al. Increased blood-brain barrier permeability on perfusion CT might predict malignant middle cerebral artery infarction. Stroke. 2010;41:2539–2544. doi: 10.1161/STROKEAHA.110.591362. doi: 10.1161/STROKEAHA.110.591362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horsch AD, Dankbaar JW, Stemerdink TA, Bennink E, van Seeters T, Kappelle LJ, et al. DUST Investigators. Imaging findings associated with space-occupying edema in patients with large middle cerebral artery infarcts. AJNR Am J Neuroradiol. 2016;37:831–837. doi: 10.3174/ajnr.A4637. doi: 10.3174/ajnr.A4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Köhrmann M, et al. Clinical Trial Net of the German Competence Network Stroke. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68:435–445. doi: 10.1002/ana.22125. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 15.van Seeters T, Biessels GJ, van der Schaaf IC, Dankbaar JW, Horsch AD, Luitse MJ, et al. DUST Investigators. Prediction of outcome in patients with suspected acute ischaemic stroke with CT perfusion and CT angiography: the Dutch Acute Stroke Trial (DUST) study protocol. BMC Neurol. 2014;14:37. doi: 10.1186/1471-2377-14-37. doi: 10.1186/1471-2377-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 17.van Seeters T, Biessels GJ, Kappelle LJ, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. Dutch Acute Stroke Study (DUST) Investigators. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis. 2015;40:258–269. doi: 10.1159/000441088. doi: 10.1159/000441088. [DOI] [PubMed] [Google Scholar]
- 18.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. [Google Scholar]
- 20.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 22.Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, et al. Calgary CTA Study Group. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi: 10.1111/j.1747-4949.2008.00221.x. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 23.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 25.El-Mitwalli A, Saad M, Christou I, Malkoff M, Alexandrov AV. Clinical and sonographic patterns of tandem internal carotid artery/middle cerebral artery occlusion in tissue plasminogen activator-treated patients. Stroke. 2002;33:99–102. doi: 10.1161/hs0102.101892. [DOI] [PubMed] [Google Scholar]
- 26.Minnerup J, Wersching H, Ringelstein EB, Heindel W, Niederstadt T, Schilling M, et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke. 2011;42:3403–3409. doi: 10.1161/STROKEAHA.111.619734. doi: 10.1161/STROKEAHA.111.619734. [DOI] [PubMed] [Google Scholar]


