Women with endometriosis had higher probabilities of prolonged use of opioids and concomitant use with benzodiazepines in comparison with a matched cohort of women without endometriosis.
Abstract
OBJECTIVE:
To examine opioid use, opioid prescribing patterns, and timing of the first opioid prescription in endometriosis patients compared with matched women in the control group without endometriosis.
METHODS:
We conducted a retrospective analysis of the Clinformatics Datamart database. Women diagnosed with endometriosis from January 2006 through December 2016 and aged 18–49 years were compared with women in the control group matched on age, region, race, insurance payer, and plan type. Key outcomes included: filled prescription for an opioid, multiple opioid prescriptions, number of days' supply, daily dose (morphine milligram equivalents), and concomitant opioid and benzodiazepine prescriptions. Cohorts were descriptively analyzed using t- and χ2 statistics and multivariable regression analyses yielded adjusted relative risk (RR) ratios and 95% CI.
RESULTS:
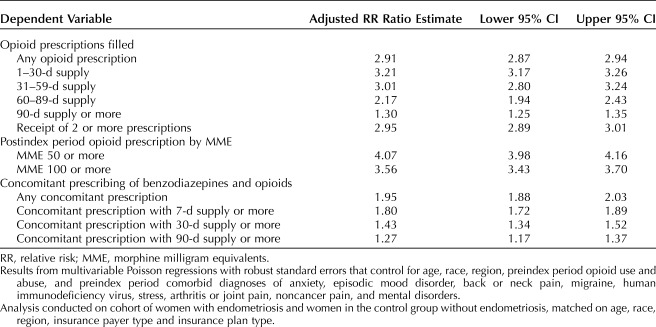
The study sample included 53,847 endometriosis patients and 107,694 patients in the control group. The mean age was 38 years, 62.4% of patients were white, and 51.6% lived in the South. Women in the endometriosis case group, compared with women in the control group, were more likely to fill an opioid prescription (42,705 [79.3%] women in the case group vs 26,106 [24.2%] women in the control group; adjusted RR ratio 2.91; 2.87–2.94), had higher likelihood of filling prescriptions with a dose of 50 morphine milligram equivalents or more (24,544 [45.6%] vs 10,463 [9.7%]; adjusted RR ratio 4.07; 3.98–4.16) or 100 morphine milligram equivalents or more (8,013 [14.9%] vs 3,582 [3.3%]; adjusted RR ratio 3.56; 3.43–3.70). Women in the case group were more likely to have concomitant opioid and benzodiazepine prescriptions (5,453 [10.1%] vs 3,711 [3.5%]; adjusted RR ratio 1.95; 1.88–2.03) and to have used these drugs concurrently for at least 30 days (1,596 [3.0%] vs 1,265 [1.2%]; adjusted RR ratio 1.43; 1.34–1.52) or at least 90 days (875 [1.6%] vs 777 [0.7%]; adjusted RR ratio 1.27; 1.17–1.37). Similar results were obtained after excluding opioid prescriptions received during a 30-day postsurgery window.
CONCLUSION:
Women with endometriosis had higher probabilities of prolonged use of opioids and concomitant use with benzodiazepines compared with women without this condition.
FUNDING SOURCE:
This study was funded by AbbVie, Inc.
Endometriosis is a chronic gynecologic disorder that affects approximately 10% of women of reproductive age.1,2 Endometriosis is associated with pain3,4 and substantial indirect costs (eg, lost work time and productivity).5–7 Up to 87% of women with chronic pelvic pain,8 defined as pain that persists for more than 6 months, have endometriosis.9 Over the past 20 years, chronic pain has increasingly been treated with opioid therapy.10 Furthermore, as endometriosis patients commonly undergo surgical procedures for diagnostic and therapeutic purposes, opioids are also commonly used for postoperative pain management.
Prescription drug abuse, particularly opioid misuse and abuse, has been on the rise and is reported to be the fastest growing drug problem in the United States.10,11 For instance, of the more than 350,000 deaths that occurred since 1999 as a result of opioid overdose, an estimated 40% involved prescription opioids.12 The majority of women in the case group involving opioid abuse reportedly begin with a medical prescription, used primarily to treat chronic pain or acute postsurgical pain. Given the associations between endometriosis, surgical interventions, and pain (potentially both chronic and postoperative), as well as recent concerns regarding medically inappropriate opioid use, it is surprising that there is little published on opioid use in this patient population.
The purpose of this study was to examine the prevalence of opioid use in U.S. patients diagnosed with endometriosis compared with women without endometriosis. In light of evidence that opioid-related risks increase with higher doses,13,14 longer use,15–17 or concomitant use of benzodiazepines,14 the study also assessed the frequency of such prescribing patterns. Finally, the analyses explored the timing of the first opioid prescription in relation to events such as the initial endometriosis diagnosis, outpatient visits, or endometriosis related surgery.
METHODS
Data for this retrospective, matched cohort study were obtained from the Optum Clinformatics Data Mart. The database contains administrative claims submitted for payment by providers (eg, physicians, hospitals, pharmacies, ambulance services) to be reimbursed for a service or product provided. The data covers more than 150 million individuals from different geographic areas in the United States, including demographics, insurance plan enrollment, inpatient and outpatient medical resource use, and filled prescriptions. The claims were verified, adjudicated, adjusted, and deidentified before inclusion. The study included data from May 2000 through December 2017 and complied fully with the Health Insurance Portability and Accountability Act. Because this was a retrospective analysis of deidentified data, Institutional Review Board approval was not required.
For women with endometriosis, the index date was defined as the first date from January 2006 through December 2016 that an endometriosis diagnosis was recorded using International Classification of Diseases, 9th Revision, Clinical Modification (617.xx) or International Classification of Diseases, 10th Revision, Clinical Modification (N80.xx) coding. Women were required to have received one or more inpatient or two or more outpatient claims with this diagnosis, on distinct dates, for inclusion in the endometriosis cohort. Patients were excluded if they were younger than age 18 years or older than age 49 at the index date, received a diagnosis of endometriosis before January 2006, or a diagnosis of cancer or codes for surgeries typically done for cancer, such as radical hysterectomy or resection at any time. Women with endometriosis were also required to have continuous insurance coverage from 1 year before the index date (ie, the preindex period) through 1 year after the index date (ie, the postindex period).
Women without endometriosis at any time were identified, however, women with any diagnosis of cancer or codes for radical hysterectomy or resection were excluded. From this group, women were randomly selected and matched 2-to-1 to women in the case group. The match was based on age, geographic region of residence, race, payer type, and insurance plan type. Each patient in the control group was assigned the same index date as her match. Women in the control group were also required to have continuous insurance coverage from the start of the preindex period through the end of the postindex period. A sensitivity analysis was conducted that was based on a more extensive matching algorithm. In addition to the above variables, women with endometriosis were also matched to women in the control group based on the following common, preindex period comorbidities that might be associated with opioid use: anxiety, episodic mood disorder, back or neck pain, arthritis or joint pain, headache or migraine, and mental disorder.
The analyses compared the outcomes of women with endometriosis relative to women in the control group. Outcomes of interest included filling of a prescription for an opioid medication, filling multiple prescriptions for opioids, total number of days' supply of opioid medication, and the opioid's daily dose, which was based on morphine milligram equivalents. In addition, the analyses examined concomitant prescription fills for opioids and benzodiazepines as well as number of days of such overlapping prescriptions. Because surgery can lead to opioid use, the above outcomes were re-analyzed to compare opioid use in patients with endometriosis and women in the control group after excluding opioid prescriptions filled in the 30 days after the first postindex period surgery date. Finally, for patients with endometriosis, analyses also examined when opioid prescriptions were filled in relation to medical events. Specifically, we determined when opioid prescription fills occurred relative to the initial endometriosis diagnosis, visits to an obstetrician–gynecologist (ob-gyn), or surgery for endometriosis.
Descriptive comparisons between women in the case group and control group were conducted using t-statistics for continuous, normally distributed variables and χ2 statistics for categorical variables. Relative risk (RR) ratios on key outcomes variables were also presented. The main analysis involved a multivariable Poisson regression with robust standard errors that estimated the adjusted RR ratio for the outcomes while controlling for patient demographics (age, race, region, insurance payer type, and insurance plan type) and the following preindex period comorbid diagnoses: anxiety, back or neck pain, episodic mood disorder, headache or migraine, human immunodeficiency virus, noncancer pain, opioid abuse, stress, arthritis or joint pain, and mental disorders. For women with endometriosis, the timing of initiation on opioids was also described. Results from the sensitivity analysis were presented as RR ratio as well. All analyses were conducted using SAS 9.4. All statistical tests were two-sided and P-values <0.05 were considered statistically significant.
ROLE OF THE FUNDING SOURCE
AbbVie participated in data analysis; in interpretation of data; and in review, approval of, and decision to submit the manuscript. The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data table, and clinical study report) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors' personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
RESULTS
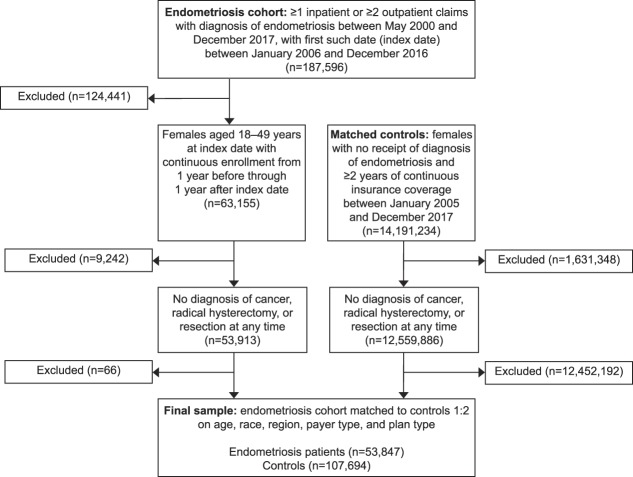
The final sample consisted of 53,847 women with endometriosis and 107,694 women without endometriosis. Figure 1 illustrates how the inclusion and exclusion criteria and the matching algorithm affected sample size. Under the expanded matching algorithm employed in the sensitivity analysis, the 2-to-1 sample consisted of 44,566 women in the case group and 89,132 women in the control group.
Fig. 1. Inclusion and exclusion criteria and sample size.
Lamvu. Opioid Use in Women With Endometriosis. Obstet Gynecol 2019.
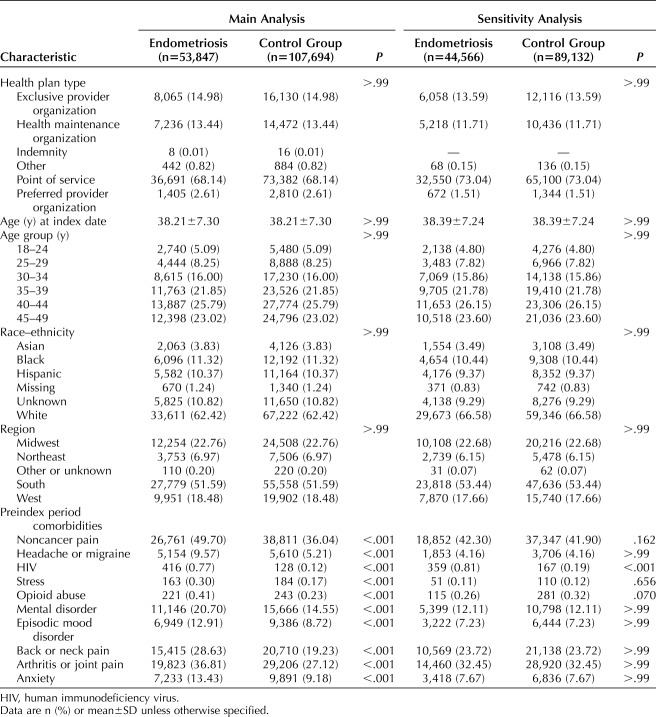
For the main analysis, in this sample of health-insured women with endometriosis, the average age was 38 years (SD=7.3), and most patients were white (62.4%) and residents of the South (51.6%). In addition, most had point-of-service insurance coverage (68.1%) (Table 1). Both endometriosis patients and matched patients in the control group were most frequently diagnosed with noncancer pain (49.7% vs 36.0%), arthritis or joint pain (36.8% vs 27.1%), or back or neck pain (28.6% vs 19.2%) in the preindex period, although endometriosis patients had significantly higher rates of each of these conditions (all P<.001). After matching on patient demographic characteristics, women with endometriosis also had statistically higher rates of mental disorders (20.7% vs 14.6%), anxiety (13.4% vs 9.2%), and episodic mood disorders (12.9% vs 8.7%) compared with women in the control group (all P<.001).
Table 1.
Patient Characteristics
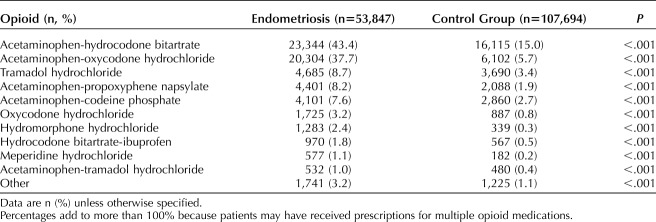
Table 2 illustrates the distribution of filled opioid prescriptions across the cohorts in the postindex period. For both endometriosis patients and patients in the control group, acetaminophen-hydrocodone bitartrate (43.2% vs 14.8%) and acetaminophen-oxycodone hydrochloride (36.4 vs 5.5%) were the opioids with the most commonly filled prescriptions. Women with endometriosis were more likely to have filled a prescription for those opioids compared with the women without endometriosis.
Table 2.
Opioid Medications Prescribed in the Postindex Period: Women With Endometriosis and Women in the Control Group
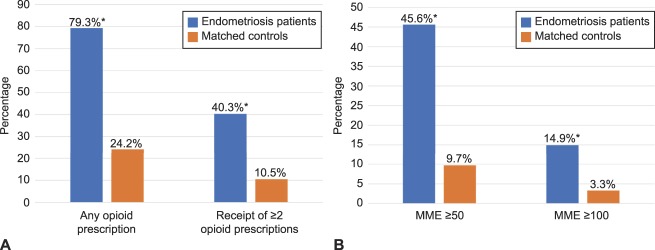
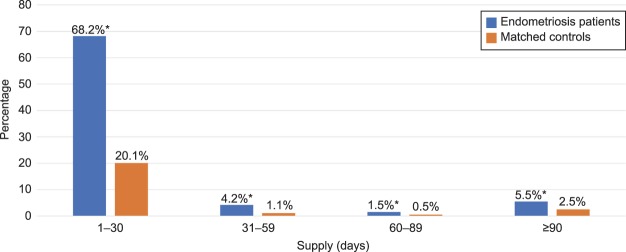
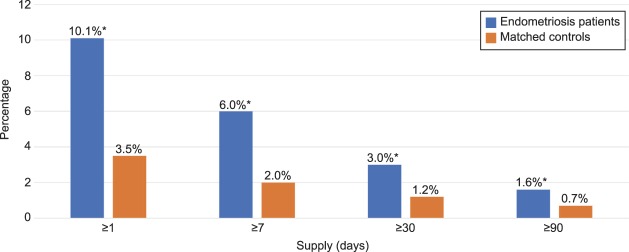
Figure 2 reveals that women with endometriosis, compared with women without, were more likely in the postindex period to have filled at least one prescription for an opioid (79.3% vs 24.2%; P<.001) and to have filled multiple prescriptions for opioids (40.3% vs 10.5%; P<.001). Consistent with filling multiple prescriptions, women with endometriosis were also more likely to have had opioids available for a longer duration than women in the control group. Specifically, in the postindex period, the endometriosis cohort was more likely to have filled opioid prescriptions with total days supplied extending for 31–59 days (4.2% vs 1.1%), 60–89 days (1.5% vs 0.5%), or 90 days or more (5.5% vs 2.5%) (all P<.001; Fig. 3). In addition, as Figure 4 reveals, women with endometriosis were more likely than women without it to have filled prescriptions for opioids and benzodiazepines concomitantly (10.1 vs 3.5%), and such concurrent supply extended for at least 7 days (6.0% vs 2.0%), 30 days (3.0% vs 1.2%), or 90 days (1.6% vs 0.7%) (all P<.001).
Fig. 2. Opioid prescription fills and dosage in postindex period. Receipt of opioids (A) and dosage of opioids (B). Morphine milligram equivalents (MME) based on receipt of dosage (50 morphine milligram equivalents or more or 100 morphine milligram equivalents or more, respectively) for at least 1 day in the postindex period. Unadjusted differences between endometriosis patients (n=53,847) and patients in the control group (n=107,694) matched to endometriosis patients based on age, race, region, insurance payer type, and insurance plan type. *P<.001.
Lamvu. Opioid Use in Women With Endometriosis. Obstet Gynecol 2019.
Fig. 3. Days' supply of opioids in postindex period. Unadjusted differences between endometriosis patients (n=53,847) and patients in the control group (n=107,694) matched to endometriosis patients based on age, race, region, insurance payer type, and insurance plan type. *P<.001.
Lamvu. Opioid Use in Women With Endometriosis. Obstet Gynecol 2019.
Fig. 4. Days' supply of opioids and benzodiazepines concomitantly filled prescriptions in postindex period. Unadjusted differences between endometriosis patients (n=53,847) and patients in the control group (n=107,694) matched to endometriosis patients based on age, race, region, insurance payer type, and insurance plan type. *P<.001.
Lamvu. Opioid Use in Women With Endometriosis. Obstet Gynecol 2019.
In the re-analyses that excluded opioid prescriptions filled within 30 days of the first postindex period surgery, women with endometriosis were more likely to have filled an opioid prescription than women without endometriosis (45.1% vs 23.6%; P<.001) and to have filled multiple prescriptions (23.6% vs 10.2%; P<.001). Also excluding opioid prescriptions filled during the 30 days of the first postindex period surgery, women with endometriosis were more likely to have filled an opioid prescription extending for 31–59 days (2.8% vs 1.1%), 60–89 days (1.2% vs 0.5%) or 90 days or more (5.1% vs 2.5%), all P<.001. Concomitant use of opioids and benzodiazepines was also higher in these endometriosis patients than patients in the control group (8.1% vs 3.4%; P<.001) and the overlap extended for at least 7 days (5.0% vs 2.0%), at least 30 days (2.7% vs 1.2%), or at least 90 days (1.6% vs 0.7%) (all P<.001).
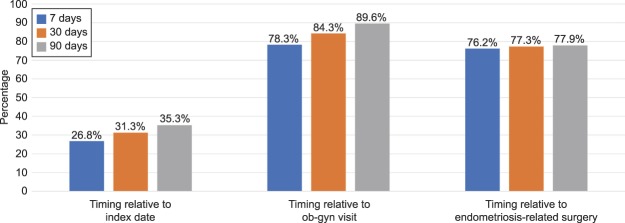
Figure 5 shows the timing of opioid use for women with endometriosis; more than one quarter of patients (26.8%) filled their first opioid prescription within 7 days of their earliest recorded claim for endometriosis. Furthermore, among endometriosis patients who initiated on opioids in the postindex period, 76.2% filled an opioid prescription within 7 days of an endometriosis-related surgery.
Fig. 5. Timing of first opioid prescription fills for women with endometriosis in postindex period.
Lamvu. Opioid Use in Women With Endometriosis. Obstet Gynecol 2019.
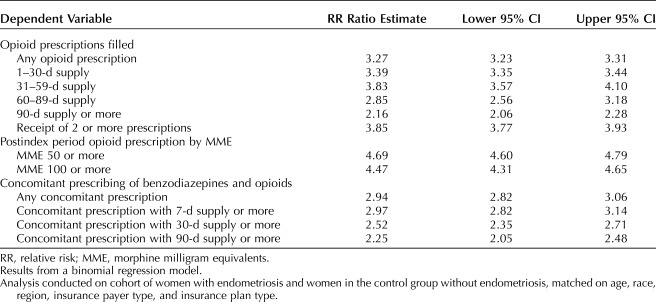
From the main analysis, Table 3 presents crude RR ratio and Table 4 presents results of the multivariable regressions estimating the adjusted RR of the outcome of interest. These results are generally consistent with the unadjusted results presented in Figures 1–4. For example, women with endometriosis, compared with women in the control group, were more likely to fill an opioid prescription (42,705 [79.3%] vs 26,106 [24.2%], adjusted RR ratio 2.91; 95% CI 2.87–2.94). In addition, patients with endometriosis were more likely to have filled an opioid prescription with a dose of 50 morphine milligram equivalents or more (24,544 [45.6%] vs 10,463 [9.7%], adjusted RR ratio 4.07; 95% CI 3.98–4.16) or 100 morphine milligram equivalents or more (8,013 [14.9%] vs 3,582 [3.3%], adjusted RR ratio 3.56; 95% CI 3.43–3.70). Endometriosis patients were also more likely to have filled opioid prescriptions with a total supply of at least 90 days in the postindex period (2,956 [5.5%] vs 2,733 [2.5%], adjusted RR ratio 1.30; 95% CI 1.25–1.35). Similarly, the women with endometriosis were more likely to have filled opioid and benzodiazepine prescriptions concomitantly (5,453 [10.1%] vs 3,711 [3.5%], adjusted RR ratio 1.95; 95% CI 1.88–2.03) and to have a concurrent supply of these medicines for at least 30 days (1,596 [3.0%] vs 1,265 [1.2%], adjusted RR ratio 1.43; 95% CI 1.34–1.52) or at least 90 days (875 [1.6%] vs 777 [0.7%], adjusted RR ratio 1.27; 95% CI 1.17–1.37).
Table 3.
Main Analysis: Crude Relative Risk Ratios Associated With Endometriosis Compared With Women in the Control Group Without Endometriosis, Postindex Period
Table 4.
Main Analysis: Adjusted Relative Risk Ratios Associated With Endometriosis Compared With Women in the Control Group Without Endometriosis, Postindex Period
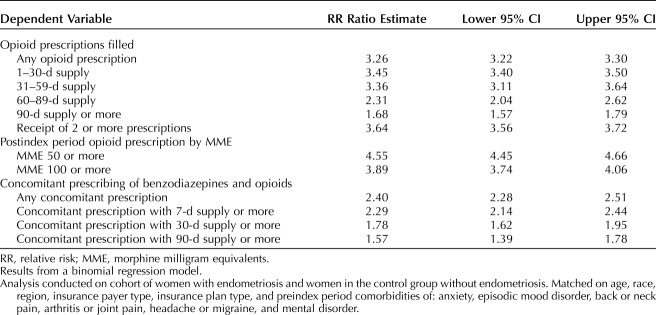
From the sensitivity analysis, Table 5 shows the RR ratio for the key outcomes. Results are consistent with those presented above. Relative to women without endometriosis, in this study, women with endometriosis were at higher risk of filling an opioid prescription, filling opioid prescriptions associated with a greater number of days' supply and with higher doses, and more likely to fill concomitant prescriptions for opioids and benzodiazepines.
Table 5.
Sensitivity Analysis: Crude Relative Risk Ratios Associated With Endometriosis Compared With Women in the Control Group Without Endometriosis, Postindex Period
DISCUSSION
This retrospective, matched cohort study examines short- and long-term opioid use in patients with endometriosis compared with patients without endometriosis. Women with endometriosis were at greater risk (adjusted RR ratio 2.91), compared with similar women without endometriosis, to fill a prescription for an opioid. Furthermore, the patients with endometriosis were at greater risk of filling a longer-term prescription, prescriptions for a higher dose, or a concomitant prescription for a benzodiazepine. Among the patients with endometriosis who filled an opioid prescription, the first such prescription was most often filled around the time of surgery; 76.2% of endometriosis patients filled an initial opioid medication prescription within 7 days of an endometriosis-related surgery. Even after excluding opioid prescriptions filled within 30 days after the first postindex period surgery, these analyses showed that endometriosis patients had higher rates of filling opioid prescriptions, for longer duration and concomitantly with benzodiazepines than did a matched cohort of women without endometriosis. Robustness of the main results was demonstrated by similar findings from a sensitivity analysis that used an extensive matching process that included not only significant demographic, region, and insurance plan variables but also a large number of preindex period comorbidities that are believed to be associated with opioid use and were common in both cohorts.
In the present study, most of the endometriosis patients (79.3%) received at least one day's supply of opioids in the postindex period. This evidence is suggestive of extensive opioid use and is generally consistent with the results of a 2016 survey, conducted by fellows of the American College of Obstetricians and Gynecologists (ACOG),18 which indicated that patients with endometriosis receive nearly one quarter (24%) of the opioid mediation prescribed by U.S. ob-gyns.18
In addition to having a higher prevalence of opioid use, patients with endometriosis in this study were also more likely to use the medication for an extended period or at a higher dose. For instance, 11.1% of the patients with endometriosis received more than a month's supply of opioids compared with 4.2% of patients in the control group and 5.5% of endometriosis patients received more than a 3-month's supply compared with 2.5% of women in the control group. This is a concerning finding because research has shown no therapeutic benefit of long-term opioid use in the treatment of noncancer pain.15 Furthermore, long-term opioid use has been associated with physical dependence, increased tolerance, opioid addiction, and adverse events.15–17 Increased risk of overdose and fatality has been demonstrated when opioid dosing exceeds 20 morphine milligram equivalents/d, with 20–50 morphine milligram equivalents/d dosing associated with a greater risk of inadvertent opioid overdose and more than 50 morphine milligram equivalents/d dosing linked to a higher risk of fatality.13 In our study, we found that filled prescriptions with opioid dosages 50 morphine milligram equivalents or more and 100 morphine milligram equivalents or more were more prevalent among the endometriosis patients.
Our analysis also showed that patients with endometriosis were more likely, relative to women in the control group (RR ratio 1.95), to have filled concomitant prescriptions for an opioid and benzodiazepine. Evidence suggests that benzodiazepines may reinforce the effect of opioids,19,20 and this effect may explain why these drugs are used concomitantly.21,22 However, opioids and benzodiazepines are some of the most frequently abused psychoactive drug classes in the world,23 and patients taking opioids and benzodiazepines concurrently are an estimated 10 times more likely to die an overdose death relative to those taking opioids alone.14 Although such risks raise concerns about the frequency of opioid and benzodiazepine overlap in this study (10.1% of the endometriosis patients vs 3.5% of patients in the control group; P<.001), a higher frequency of opioid and benzodiazepine concomitance was observed in a 2011 investigation, which looked at patients from U.S. pain clinics and reported that 15.7% of oral fluid specimens were positive for benzodiazepines.24 The lower rate in the present study may represent more responsible opioid prescribing in recent years, consistent with some research,25 or less opioid prescribing by ob-gyns relative to other doctors, as evidenced in other studies.26,27
Among women with endometriosis, the timing of opioid initiation suggests that opioids were most frequently prescribed to treat postsurgical pain. This finding is consistent with the fact that surgery is a common diagnostic test for endometriosis and treatment modality for endometriosis-related infertility or pain28 and the status of opioid-based therapy as a cornerstone of pain management for surgery.29 However, the longer-term, higher-dose prescribing observed among this population with endometriosis stands in contrast to ACOG guidelines recommending that clinicians use the fewest opioid pills appropriate.18 Moreover, surveys have reported that 40% of patients felt they received more opioids than needed after a minimally invasive hysterectomy,30 and that the majority of patients report unused opioids after surgery.29,31
Finally, based on the finding that more than one quarter (26.8%) of endometriosis patients filled opioid prescriptions within 7 days of their earliest recorded claim for endometriosis, our study suggests that opioids have been prescribed frequently to treat the pain of endometriosis. This treatment pattern stands in contrast to the research recommending against the use of opioids in treating noncancer, chronic pain.15–17 However, it is consistent with a 2016 ACOG survey that suggested additional guidance to appropriate pain management may be helpful. For instance, the median rate of opioid prescribing among the ACOG members who responded to that survey was 26 opioid pills per-patient for any cause; less than 20% of the respondents said that they adhered to ACOG recommendations for prescribing pain medication.18
The findings of this study must be considered within the context of the following limitations. Every patient in the population was well-insured, and results may not be generalizable to all U.S. women. Additionally, patients were identified as having endometriosis based on diagnostic codes, which may or may not have been the product of rigorous diagnostic procedures such as pelvic examinations, ultrasound scans, and laparoscopy. Furthermore, the index date for patients with endometriosis, defined as the first recorded date with a diagnosis of endometriosis, may not have reflected the actual initial diagnosis if that diagnosis was recorded before our data study period of 2005 through 2017. Our study also did not control for whether the endometriosis diagnosis was surgically confirmed. In addition, although we could determine that prescriptions for opioids or benzodiazepines were filled, we could not confirm how the drugs were taken (eg, whether they were taken orally, intranasally, or intravenously; whether opioids were taken at the same time with benzodiazepines; whether the drugs were taken by the patient or someone else). By examining prescribed opioids and filled prescriptions, the study did not consider illicit opioid use. Further, the study did not control for pain status or the severity of pain. Patients with moderate to severe pain may have been more likely to use opioids more frequently. Despite these limitations, our study had several strengths, including the use of a large study sample that resembles other endometriosis populations in terms of demographics, presence of other chronic pain and psychiatric comorbidities, indicating that our results may be generalizable. The addition of a large matched control group and multivariable analysis adjusting for potential differences between the two study cohorts helps reduce the possibility that our findings are the result of chance alone. We also had the ability to study short- (30 days) and long- term (90 days) prescription patterns without input from health care providers or patients, thus reducing the potential for reporting bias.
In conclusion, these analyses indicate that opioid use is prevalent in this population of patients with endometriosis. Compared with women without endometriosis, women with endometriosis had higher odds of prolonged use, and concomitant use with benzodiazepines. Our findings emphasize the need for better health care provider education and appropriate patient selection and counseling before selecting opioids for pain management. Although some opioid use may be unavoidable in endometriosis, medical management should be aggressively optimized, and multiple treatment modalities pursued (surgery and involvement of pain specialists) to minimize inappropriate or excessive use. Because this was an initial examination comparing opioid prescribing and use patterns between women with endometriosis and those without, future research would examine subgroups of patients such as those presenting with chronic pain or infertility. The effect of concomitant medical therapy on opioid use in these populations could also be studied. Further work is also needed to explore whether opioid prescribing differs among endometriosis patients without medical insurance. Moreover, although we suspect that opioid use in patients with endometriosis may lead to similar negative consequences as in other chronic pain patients, this assumption has not yet been specifically confirmed.
Footnotes
This study was funded by AbbVie, Inc. The Open Access fee was funded by AbbVie, Inc. The authors followed Good Publication Practice (GPP3) for manuscripts that are supported by pharmaceutical companies.
Financial Disclosure Ahmed M. Soliman, Shivaji R. Manthena, Keith Gordon, and Julie Knight are employees and shareholders of AbbVie, Inc. Georgine Lamvu and Hugh S. Taylor received financial compensation from AbbVie, Inc., for their work on this research project. Editorial assistance in the preparation of this manuscript was provided by Dr. Maureen Lage and Dr. Michael Treglia of HealthMetrics Outcomes Research, LLC. Support for this assistance was funded by AbbVie, Inc.
Presented at the Academy of Managed Care Pharmacy conference, April 23–26, 2018, Boston, Massachusetts.
Peer reviews are available at http://links.lww.com/AOG/B366.
REFERENCES
- 1.Bulun SE. Endometriosis. NEJM 2009;360:268–79. [DOI] [PubMed] [Google Scholar]
- 2.Rogers PA, D'Hooghe TM, Fazleabas A, Gargett CE, Guidice LC, Montgomery GW, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci 2009;16:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science 2005;308:1587–9. [DOI] [PubMed] [Google Scholar]
- 4.Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol 2017;209:8–13. [DOI] [PubMed] [Google Scholar]
- 5.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod 2012;27:1292–9. [DOI] [PubMed] [Google Scholar]
- 6.Soliman AM, Yang H, Du EX, Kelley C, Winkel C. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod 2016;31:712–22. [DOI] [PubMed] [Google Scholar]
- 7.Soliman AM, Coyne KS, Gries KS, Castelli-Haley J, Snabes MC, Surrey ES. The effect of endometriosis symptoms on absenteeism and presenteeism in the workplace and at home. J Manag Care Spec Pharm 2017;23:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong C. ACOG updates guideline on diagnosis and treatment of endometriosis. Am Fam Physician 2011;83:84. [Google Scholar]
- 9.Speer L, Mushkbar S, Erbele T. Chronic pelvic pain in women. Am Fam Physician 2016;93:380–7. [PubMed] [Google Scholar]
- 10.Kanouse AB, Compton P. The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother 2015;29:102–14. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep 2012;61:10–3. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Understanding the epidemic|drug overdose|CDC injury center. Available at: https://www.cdc.gov/drugoverdose/epidemic/index.html. Retrieved June 18, 2018. [Google Scholar]
- 13.Adewumi AD, Hollingworth SA, Maravilla JC, Connor JP, Alati R. Prescribed dose of opioids and overdose: a systematic review and meta-analysis of unintentional prescription opioid overdose. CNS Drugs 2018;32:101–16. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016;17:85–98. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009;10:131–46. [DOI] [PubMed] [Google Scholar]
- 16.Fields HL. The doctor's dilemma: opiate analgesics and chronic pain. Neuron 2011;69:591–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Els C, Kunyk D, Lappi VG, Sonnenberg B, Hagtvedt R, Sharma S, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. The Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No. CD012509. 10.1002/14651858.CD012509.pub2. [DOI] [PMC free article] [PubMed]
- 18.Madsen AM, Stark LM, Has P, Emerson JB, Schulkin J, Matteson KA. Opioid knowledge and prescribing practices among obstetrician-gynecologists. Obstet Gynecol 2018;131:150–7. [DOI] [PubMed] [Google Scholar]
- 19.Lintzeris N, Mitchell TB, Bond AJ, Nestor L, Strang J. Pharmacodynamics of diazepam co-administered with methadone or buprenorphine under high dose conditions in opioid dependent patients. Drug Alcohol Depend 2007;91:187–94. [DOI] [PubMed] [Google Scholar]
- 20.Lintzeris N, Nielsen S. Benzodiazepines, methadone and buprenorphine: interactions and clinical management. Am J Addict 2010;19:59–72. [DOI] [PubMed] [Google Scholar]
- 21.Chen KW, Berger CC, Forde DP, D'Adamo C, Weintraub E, Gandhi D. Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry 2011;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66(suppl 9):31–41. [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration (SAMHSA). The TEDS report: substance abuse treatment admissions for abuse of benzodiazepines. Rockville (MD): Center for Behavioral Health Statistics and Quality; 2011. [Google Scholar]
- 24.Heltsley R, DePriest A, Black DL, Robert T, Marshall L, Meadows VM, et al. Oral fluid drug testing of chronic pain patients. I. Positive prevalence rates of licit and illicit drugs. J Anal Toxicol 2011;35:529–40. [DOI] [PubMed] [Google Scholar]
- 25.Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am J Psychiatry 2016;173:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner SG, Baker O, Rodgers AF, Garner C, Nelson LS, Kreiner PW, et al. Opioid prescriptions by specialty in Ohio, 2010-2014. Pain Med 2018;19:978–89. [DOI] [PubMed] [Google Scholar]
- 27.Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med 2015;49:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute for Quality and Efficiency in Health Care. Treatment options for endometriosis. Cologne (Germany): Institute for Quality and Efficiency in Health Care; 2017. [Google Scholar]
- 29.Bartels K, Mayes LM, Dingmann C, Bullard KJ, Hopfer CJ, Binswanger IA. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One 2016;11:e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.As-Sanie S, Till SR, Mowers EL, Lim CS, Skinner BD, Fritsch L, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol 2017;130:1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg 2017;152:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]