Abstract
Streptomyces species are known to produce various bioactive metabolites that can prevent plant diseases. Previously, the Streptomyces strain TFH56 was found to inhibit the gray mold pathogen, Botrytis cinerea, in tomato flower. In this study, the genome sequence of strain TFH56 was acquired using the Pacific Biosciences RS II platform. Three linear sequences (7.67 Mbp in total) were obtained. Based on average nucleotide identity, strain TFH56 was classified as Streptomyces globisporus, which is consistent with the presence of a linear chromosome and linear plasmids. Moreover, as with other examples of S. globisporus, the genome of strain TFH56 included a caryolan-1-ol synthase gene, a conprimycin synthetic gene cluster, and a lidamycin synthetic gene cluster
Keywords: Streptomyces complete genome, linear chromosome, bioactive compound
Members of the genus Streptomyces are Gram-positive bacteria that resemble filamentous fungi. Streptomyces spp. typically inhabit soil, but are also known to interact with plants and animals. The complex life cycle of Streptomyces includes spore germination, vegetative hyphal growth, and programmed cell death in vegetative hyphae accompanying the growth of aerial spore-bearing hyphae (Yagüe et al. 2013). The genomes of Streptomyces spp. have large amounts of G + C content, and some species contain linear chromosomes and plasmids (Bentley et al. 2002; Ikeda et al. 2003; Li et al. 2016). Streptomyces spp. are well known as producers of numerous bioactive compounds. According to Bérdy (2012), in 2010 roughly 33,500 bioactive compounds had been isolated from organisms, and 10,400 of these were reported in Streptomyces spp. This finding suggests that interactions between Streptomyces spp. and other organisms have affected the expression of biosynthetic gene clusters and the evolution and diversity of secondary metabolites. Streptomyces spp. have been recognized to interact with >500 other species (Euzeby 2008), and, because of its diversity, the genus Streptomyces has been considered as a great potential source of new bioactive compounds. Some Streptomyces strains have been identified as antagonists of plant pathogens (Taechowisan et al. 2005; Cha et al. 2016; Kwon et al. 2018). In one study, Streptomyces strain TFH56 was isolated from tomato flowers in Jinju, Republic of Korea. This strain was found to be symbiotic with its host in a metagenome study, and showed outstanding antagonism against Botrytis cinerea—a causal pathogen of gray mold in tomato (Kwon et al. 2018).
The aims of the present study were to identify the known Streptomyces species that is related most closely to strain TFH56, to identify genes associated with the biosynthesis of bioactive compounds in this strain, and to clarify the taxonomy and diversity of these genes. These aims were accomplished through complete genome sequencing, followed by the assessment of average nucleotide identity (ANI) with whole genomes of other Streptomyces spp., and comparison of the biosynthetic genes in strain TFH56 with their homologs in other Streptomyces spp.
Materials and Methods
Genome sequencing and identification
Genome sequencing for strain TFH56 was conducted with the Pacific Biosciences RS II platform of Macrogen Inc. (Daejeon, Republic of Korea). The same platform was used to acquire the complete genome of Streptomyces globisporus strain S4-7 (Kim et al. 2019). Sequence reads were assembled with Canu (version 1.7), and the assembled sequences were polished with the Arrow algorithm in SMRT link (version 5.1.0). The Arrow polish was based on aligned subread BAM files derived from converted bax.h5 files; these served as the raw data files provided by the platform. The genome sequence was annotated via the RASTtk pipeline (Brettin et al. 2015) on the RAST web server (version 2.0; http://rast.nmpdr.org/). The RASTtk pipeline was set as the default, and it functioned by turning on domain bacteria and automatically fixed error options. To identify the species of strain TFH56, 16S rRNA sequences of Actinobacteria were analyzed for maximum likelihood after MUSCLE alignment in MEGA X (version 10.0.5). Furthermore, the genome sequence was compared with previously reported genomes from other Streptomyces spp. with Pyani (version 0.2.8)—a Python 3 module that can calculate ANI with MUMmer (version 3.23). In this study, 24 complete genome-sequenced strains of Actinobacteria were analyzed. The Ubuntu 18.04 shell commands of Pyani with MUMmer were noted in the Pyani heatmap described in the Supplemental Data. To confirm the ANI results, genome sequences were aligned and visualized with dot plots using DECIPHER (version 2.10.0).
Comparison of bioactive compound biosynthetic gene clusters
Secondary metabolite biosynthetic gene clusters in strain TFH56 were identified with the bacterial version of antiSMASH 4.1.0 (https://antismash.secondarymetabolites.org/). Homologous regions on each genome were identified using NCBI Blastn (https://blast.ncbi.nlm.nih.gov/). These gene clusters were compared with their homologs in S. globisporus via sequence alignment with DECIPHER. The homologous regions on each genome were identified using NCBI Blastn (https://blast.ncbi.nlm.nih.gov/).
Visualization
Almost all figures were created with ggplot2 (version 3.1.0), gplots (version 3.0.1.1), and DECIPHER in R. The R code is described in detail in Supplemental Material, File S1. The completed figures from R were edited with respect to font size and type, and layout.
Data availability
Sequence data are available at GenBank; the accession numbers are listed in Table 1. Strains are available upon request. Supplemental material available at FigShare: https://doi.org/10.25387/g3.8030795.
Table 1. Streptomyces globisporus TFH56 genome assembly and annotation data.
Results and Discussion
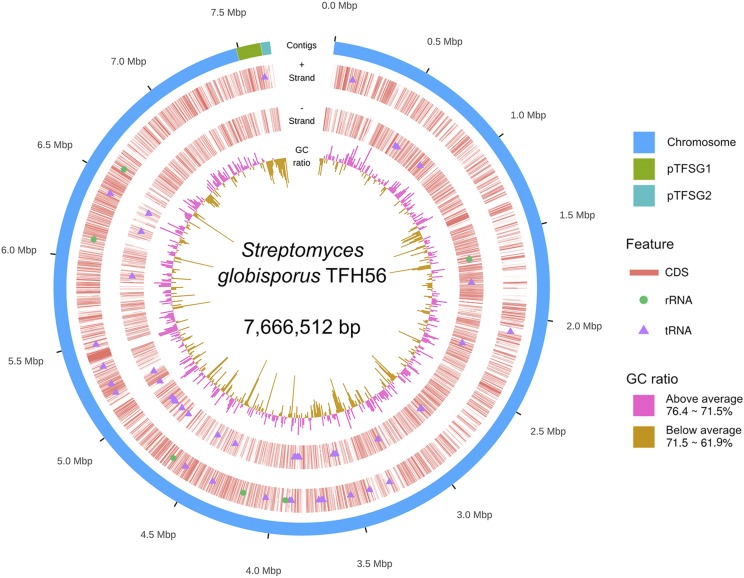
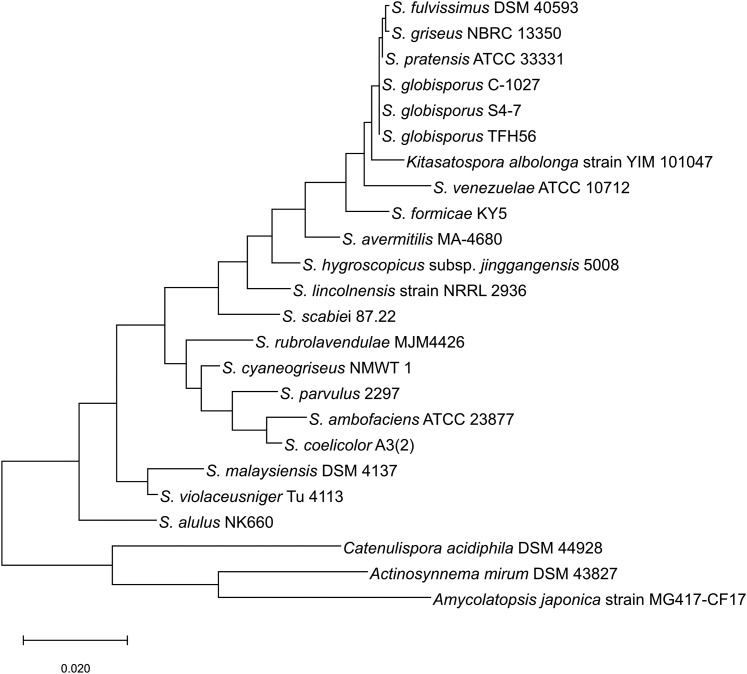
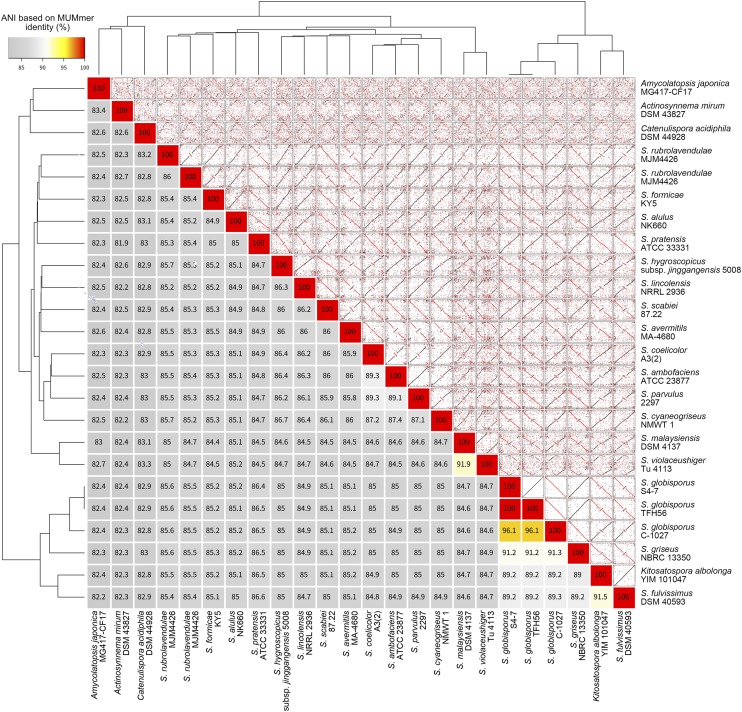
This study was designed to acquire a whole genome sequence for strain TFH56, which was isolated from tomato flower, to classify this strain within the genus Streptomyces, and to identify genes in strain TFH56 that are homologous to sequences associated with the biosynthesis of bioactive compounds in other Actinobacteria genomes. The acquired genome sequence was assembled in three contigs totaling 7.67 Mbp with 71.5% G + C content, 7172 CDS, 66 tRNA, and 18 rRNA (Figure 1 and Table 1). In evolutionary terms, TFH56 was closest to S. globisporus in terms of 16S rRNA (Figure 2). ANI calculations performed with MUMmer indicated that the genome of strain TFH56 had 96.11% sequence similarity with that of S. globisporus C-1027, and 99.99% similarity with that of S. globisporus S4-7 (Cha et al. 2016; Kim et al. 2019), which was isolated from strawberry rhizosphere (Figure 3). Unexpectedly, Kitasatospora was closely related to Streptomyces in the analysis of ANI and 16S rRNA, both of which were associated with the former classification of Kitasatospora albolonga as Streptomyces albolongus (Labeda et al. 2017). Moreover, consistent with the linear chromosome and two linear plasmids (pTFSG1 and pTFSG2) identified in this study, linear chromosomes and plasmids have been reported for S. globisporus (Li et al. 2016). In strain TFH56, the plasmids had less G + C content than did the chromosome (Figure 1 and Table 1).
Figure 1.
Genome map of Streptomyces globisporus TFH56. The outermost open ring shows the linear chromosome and linear plasmids. The innermost open ring shows the GC ratio, calculated from 10,000 bp. The remaining two open rings indicate CDS, rRNA, and tRNA on each strand.
Figure 2.
Evolutionary history tree for the 16S rRNA sequence, inferred using the Tamura-Nei mode and maximum likelihood method. The tree involved 24 strains of Actinobacteria, and its sequences are ∼1500 bp. Taxonomically, TFH56 is related most closely to Streptomyces globisporus.
Figure 3.
Average nucleotide identity based on MUMmer (ANIm) identification and alignment of 21 Streptomyces strains and three Actinobacteria members. The lower left heatmap shows ANIm identity. The trees show similarity according to ANIm identity scores. The upper right subplots are dot plots of whole genome alignments. Black dots represent same-direction alignments, and red dots represent reverse-direction alignments.
antiSMASH predicted 27 secondary metabolite gene clusters in the genome of strain TFH56 (Table 2). Caryolan-1-ol synthase was identified in cluster 9, and a thiopeptide sequence associated with conprimycin biosynthesis in S. globisporus S4-7 (Cha et al. 2016) was identified in cluster 15. Lidamycin biosynthesis genes were also detected in cluster 27. S. globisporus S4-7 is known to produce the antifungal compounds conprimycin (Cha et al. 2016) and caryolan-1-ol (Cho et al. 2017), and S. globisporus C-1027 is known to produce the antitumor agent lidamycin (originally called C-1027) (Hu et al. 1988; Liu et al. 2002; Li et al. 2016). Lidamycin biosynthesis gene sequences could not be found in other Streptomyces spp., and sequences associated with caryolan-1-ol and conprimycin biosynthesis could be found only among close relatives of S. globisporus based on the ANI phylogeny.
Table 2. Secondary metabolite gene clusters in THF56.
| Cluster | Type | From (bp) | To (bp) | Most similar known cluster |
|---|---|---|---|---|
| The following clusters are from record chromosome | ||||
| Cluster 1 | T1pks-Nrps | 66,088 | 117,817 | Daptomycin biosynthetic gene cluster (7% of genes show similarity) |
| Cluster 2 | T3pks | 208,321 | 249,373 | Tetronasin biosynthetic gene cluster (11% of genes show similarity) |
| Cluster 3 | Melanin | 287,733 | 298,206 | Istamycin biosynthetic gene cluster (4% of genes show similarity) |
| Cluster 4 | Bacteriocin-T1pks-Nrps | 495,294 | 568,024 | SGR PTMs biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 5 | Terpene | 622,086 | 648,659 | Hopene biosynthetic gene cluster (69% of genes show similarity) |
| Cluster 6 | Bacteriocin | 1,229,256 | 1,240,782 | — |
| Cluster 7 | T1pks-Nrps | 1,269,703 | 1,347,182 | Phosphinothricin biosynthetic gene cluster (6% of genes show similarity) |
| Cluster 8 | Siderophore | 1,544,907 | 1,559,623 | — |
| Cluster 9 | Terpene | 1,952,004 | 1,973,011 | — |
| Cluster 10 | Ectoine | 2,096,983 | 2,107,357 | Pristinamycin biosynthetic gene cluster (23% of genes show similarity) |
| Cluster 11 | Lantipeptide | 2,332,022 | 2,354,832 | AmfS biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 12 | T1pks-Nrps | 2,427,242 | 2,481,916 | Enduracidin biosynthetic gene cluster (8% of genes show similarity) |
| Cluster 13 | Lantipeptide-Butyrolactone | 3,047,239 | 3,105,744 | Labyrinthopeptin A1, A3/labyrinthopeptin A2 biosynthetic gene (40% of genes show similarity) |
| Cluster 14 | Lassopeptide | 3,205,276 | 3,227,973 | SRO15-2005 biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 15 | Thiopeptide | 4,506,592 | 4,539,162 | Siomycin biosynthetic gene cluster (7% of genes show similarity) |
| Cluster 16 | Nrps | 4,781,622 | 4,826,601 | Bottromycin A2 biosynthetic gene cluster (27% of genes show similarity) |
| Cluster 17 | Siderophore | 4,937,822 | 4,949,600 | Desferrioxamine B biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 18 | Lantipeptide | 5,001,775 | 5,024,819 | — |
| Cluster 19 | Ectoine | 6,040,034 | 6,050,432 | Ectoine biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 20 | Terpene | 6,475,918 | 6,497,021 | Steffimycin biosynthetic gene cluster (19% of genes show similarity) |
| Cluster 21 | Terpene | 6,782,807 | 6,808,026 | Isorenieratene biosynthetic gene cluster (85% of genes show similarity) |
| Cluster 22 | T3pks | 7,102,494 | 7,143,612 | Herboxidiene biosynthetic gene cluster (6% of genes show similarity) |
| Cluster 23 | Nrps | 7,185,040 | 7,270,761 | Griseobactin biosynthetic gene cluster (100% of genes show similarity) |
| Cluster 24 | Terpene-Nrps | 7,290,141 | 7,356,887 | — |
| Cluster 25 | Butyrolactone | 7,380,909 | 7,391,850 | Coelimycin biosynthetic gene cluster (16% of genes show similarity) |
| Cluster 26 | Terpene | 7,431,928 | 7,457,471 | Isorenieratene biosynthetic gene cluster (100% of genes show similarity) |
| The following clusters are from pTFSG1 | ||||
| Cluster 27 | T1pks-Nrps | 16,475 | 112,894 | Lidamycin biosynthetic gene cluster (93% of genes show similarity) |
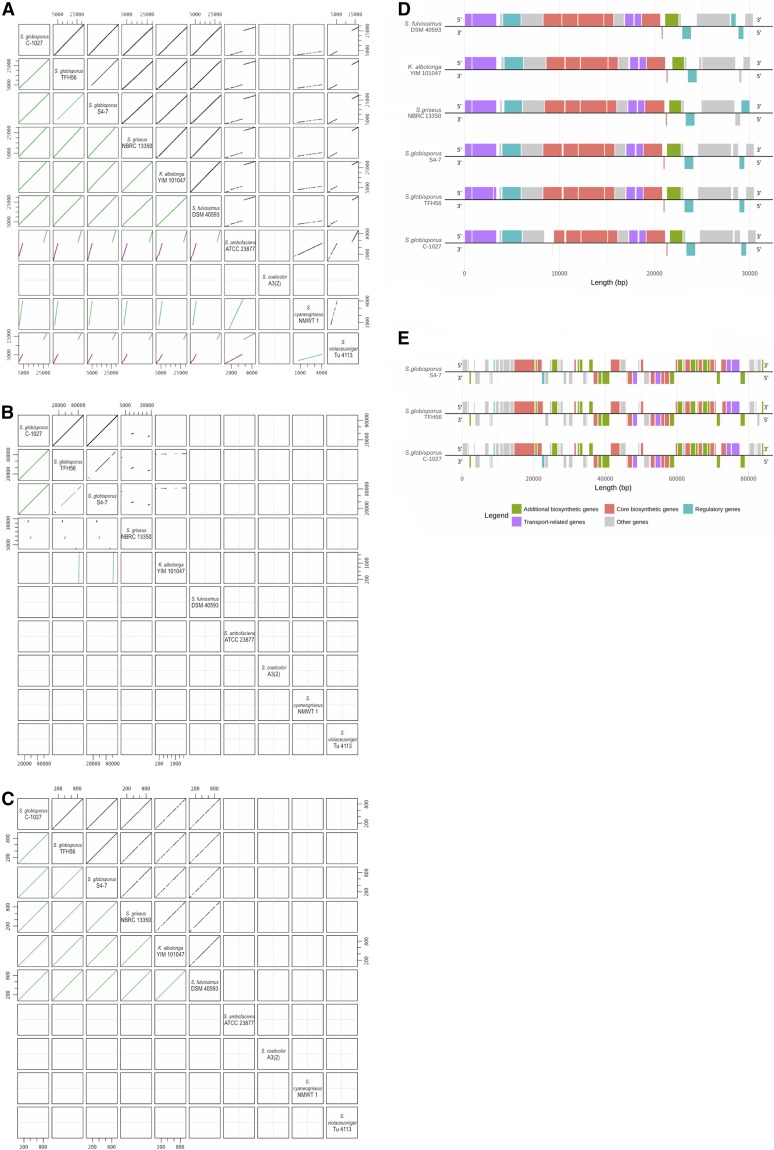
The sequences of these biosynthetic genes were compared among 10 strains in ANI species-similarity order (Figure 4). The caryolan-1-ol synthase sequence in strain TFH56 comprised 978 bp. This sequence was identical to that in strain S4-7, but differed by 24 bp from the sequence in strain C-1027. Streptomyces griseus NBRC 13350, K. albolonga YIM 101047, and Streptomyces fulvissimus DSM 40593 all had similar caryolan-1-ol synthase sequences (Figure 4C). Based on the antiSMASH analyses of CDS structure (Figure 4, D and E), which produced results consistent with the ANI results, the conprimycin biosynthesis sequence in strain TFH56 was 99–100% similar to the sequence in strain S4-7 (Table S1), and was also similar to the homologous sequences in strain C-1027, S. griseus, K. albolonga, and S. fulvissimus (Figure 4, A and D). The homolog of the S. globisporus lidamycin biosynthetic cluster in strain TFH56 was located in pTFSG1. This finding corresponded to a previous report that this cluster is located in the linear plasmid SGLP1 in strain C-1027 (Li et al. 2016). The lidamycin cluster in strain TFH56 differed from that in strain S4-7 in the 0–25 and >65 kbp regions (Figure 4, B and E). However, the lidamycin CDS structure in strain TFH56 was similar to that in strain C-1027, with the exception of the regulator gene, which differed by roughly 22 kbp in the most similar sequence (Figure 4E and Table S2). These results indicate that genes involved in the biosynthesis of caryolan-1-ol, conprimycin, and lidamycin are unique to S. globisporus and its closest relatives among sequenced Streptomyces strains (Figure 4 and Figure S1). This finding could improve our understanding of the diversity and evolution of biosynthetic gene clusters among plant probiotic Streptomyces spp. strains.
Figure 4.
Dot plots of aligned secondary metabolite related sequences and comparisons of their CDS, performed with antiSMASH. (A and D) C-1027 lidamycin synthetic gene cluster and homologs. (B and E) S4-7 conprimycin synthetic gene cluster and homologs. (C) Caryolan-1-ol synthase. All axes at dot plots are lengths of DNA sequences. Black and red diagonals in the upper right block correspond to forward and reverse strands, respectively. In the lower left block, the gradient from green to blue to magenta indicates alignment from the highest to the lowest score. Strain or species axis order depends on the ANI results. In (D and E), each filled block indicates a CDS.
Acknowledgments
This research was supported by the Next-Generation BioGreen 21 Program (PJ013250) and by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry, and Fisheries, Republic of Korea (project no. 315004-5).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25387/g3.8030795.
Communicating editor: R. Kulathinal
Literature Cited
- Bentley S. D., Chater K. F., Cerdeño-Tárraga A. M., Chalis G. L., Thomson N. R., et al. , 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3 (2). Nature 417: 141–147. 10.1038/417141a [DOI] [PubMed] [Google Scholar]
- Bérdy J., 2012. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. (Tokyo) 65: 385–395 (erratum: J. Antibiot. (Tokyo) 65: 441). 10.1038/ja.2012.27 [DOI] [PubMed] [Google Scholar]
- Brettin T., Davis J. J., Disz T., Edards R. A., Robert A., et al. , 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 5: 8365 10.1038/srep08365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J. Y., Han S., Hong H. J., Cho H., Kim D., et al. , 2016. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 10: 119–129. 10.1038/ismej.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho G., Kim J., Park C. G., Nislow C., Weller D. M., et al. , 2017. Caryolan-1-ol, an antifungal volatile produced by Streptomyces spp., inhibits the endomembrane system of fungi. Open Biol. 7: 170075 10.1098/rsob.170075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euzeby J. P., 2008. Genus Streptomyces List of prokaryotic names with standing in nomenclature. Available at: http://www.bacterio.net/streptomyces.html. Accessed: January 18, 2019.
- Hu J. L., Xue Y. C., Xie M. Y., Zhang R., Otani T., et al. , 1988. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J. Antibiot. (Tokyo) 41: 1575–1579. 10.7164/antibiotics.41.1575 [DOI] [PubMed] [Google Scholar]
- Ikeda H., Ishikakwa J., Hanamoto A., Shinose M., Kikuchi H., et al. , 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21: 526–531. 10.1038/nbt820 [DOI] [PubMed] [Google Scholar]
- Kim D. R., Jeon C. W., Shin J. H., Weller D. M., Thomashow L., et al. , 2019. Function and distribution of a lantipeptide in strawberry Fusarium wilt disease-suppressive soils. Mol. Plant Microbe Interact. 32: 306–312. 10.1094/MPMI-05-18-0129-R [DOI] [PubMed] [Google Scholar]
- Kwon Y., Lee J. T., Kim H. S., Jeon C., Kwak Y. S., 2018. Comparative tomato flower and pollinator hive microbial communities. J. Plant Dis. Prot. 125: 115–119. 10.1007/s41348-017-0090-z [DOI] [Google Scholar]
- Labeda D. P., Dunlap C. A., Rong X., Huang Y., Doroghazi J. R., et al. , 2017. Phylogenetic relationships in the family Streptomycetaceae using multi-locus sequence analysis. Antonie van Leeuwenhoek 110: 563–583. 10.1007/s10482-016-0824-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Lei X., Zhang C., Jiang Z., Shi Y., et al. , 2016. Complete genome sequence of Streptomyces globisporus C-1027, the producer of an enediyne antibiotic lidamycin. J. Biotechnol. 222: 9–10. 10.1016/j.jbiotec.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Liu W., Christenson S. D., Standage S., Shen B., 2002. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 297: 1170–1173. 10.1126/science.1072110 [DOI] [PubMed] [Google Scholar]
- Taechowisan T., Lu C., Shen Y., Lumyong S., 2005. Secondary metabolites from endophytic Streptomyces aureofaciens CMUAc130 and their antifungal activity. Microbiology 151: 1691–1695. 10.1099/mic.0.27758-0 [DOI] [PubMed] [Google Scholar]
- Yagüe P., López-García M. T., Rioseras B., Sánchez J., Manteca A., 2013. Pre-sporulation stages of Streptomyces differentiation: state-of-the-art and future perspectives. FEMS Microbiol. Lett. 342: 79–88. 10.1111/1574-6968.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data are available at GenBank; the accession numbers are listed in Table 1. Strains are available upon request. Supplemental material available at FigShare: https://doi.org/10.25387/g3.8030795.