Abstract
Learning and memory are indisputably key features of animal success. Using information about past experiences is critical for optimal decision-making in a fluctuating environment. Those abilities are usually believed to be limited to organisms with a nervous system, precluding their existence in non-neural organisms. However, recent studies showed that the slime mould Physarum polycephalum, despite being unicellular, displays habituation, a simple form of learning. In this paper, we studied the possible substrate of both short- and long-term habituation in slime moulds. We habituated slime moulds to sodium, a known repellent, using a 6 day training and turned them into a dormant state named sclerotia. Those slime moulds were then revived and tested for habituation. We showed that information acquired during the training was preserved through the dormant stage as slime moulds still showed habituation after a one-month dormancy period. Chemical analyses indicated a continuous uptake of sodium during the process of habituation and showed that sodium was retained throughout the dormant stage. Lastly, we showed that memory inception via constrained absorption of sodium for 2 h elicited habituation. Our results suggest that slime moulds absorbed the repellent and used it as a ‘circulating memory’.
This article is part of the theme issue ‘Liquid brains, solid brains: How distributed cognitive architectures process information’.
Keywords: learning, memory, habituation, Physarum polycephalum, slime moulds
1. Introduction
All biological systems face similar challenges due to the nature of their living state. To survive they must adjust their internal structure and/or their behaviour in response to a fluctuating environment. The topic of the present issue ‘Solid brains, liquid brains’ questions the structure supporting these abilities. Whether it is a true brain or a brain-like structure (e.g. root-brains [1–3], nanobrains [4], swarm brain [5,6], immune brain [7,8]), these systems allow organisms to cope with environmental changes more quickly than genetic evolution would. Every organism needs to acquire information about both the environmental and its internal state and use that information to adapt its behaviour. Abilities such as sensing, learning, memory, decision making, etc. are often considered cognitive abilities and believed to be restricted to neural organisms. Yet, a growing number of researchers are now questioning this restriction and defending the idea that cognitive abilities might extend to non-neural organisms (e.g. [9–13]). Godfrey-Smith [14, p. 238] argues for a continuity between animal cognition and mechanisms for behavioural control observed in non-neural organisms. He defines cognition as ‘a collection of capacities which, in combination, allow organisms to achieve certain kinds of coordination between their actions and the world’. Yet, can we reach in ‘liquid brains’ the nature of computations and cognitive complexity that can be achieved by ‘solid brains’?
A ‘solid brain’ is capable of storing rich memories and learning complex associations; do we find such capacities in ‘liquid brains’? Learning is often considered the holy grail of cognitive abilities. Functionally, learning allows organisms to adjust their behaviours to the local environment through individual experience. Learning has often been defined in a way that automatically rules out non-neural organisms, for example learning has been defined as ‘the acquisition of neuronal representations of new information’ [15, p. 7]. However, some authors agreed that in theory non-neural organisms might be equipped to manifest simple forms of learning [11,16]. Indeed, non-neural organisms possess sensory systems capable of detecting various cues in the environment and information processing systems able to encode past exposures and direct differential behaviour [16]. In addition, although non-neural organisms lack the complex hardware of a true brain, they live in environments that are no less complex and face the same decision-making challenges: they must acquire resources, adapt to changing conditions and choose suitable microclimates to inhabit. Thus, in order to truly understand the most fundamental mechanisms necessary and sufficient for learning, it is essential to study early organisms such as unicellulars that implement learning in a non-neural substrate.
There are several reliable reports documenting learning and memory in single celled organisms such as ciliates [17–21] and more recently slime moulds [22,23]. The acellular slime mould Physarum polycephalum is an ideal biological model to investigate the mechanisms supporting learning in non-neural organisms. First, P. polycephalum is a macroscopic unicellular organism easy to manipulate and to observe, while retaining similar characteristics to other neuron-less, microscopic creatures. Second, genetically identical individuals can be obtained by cutting a single cell into multiple viable cells. Third, and most importantly, it demonstrates key aspects of complex decision-making. Slime moulds can find their way through a maze [24], construct efficient transport networks [25], interact and cooperate with congeners [26], anticipate periodic events [27], navigate complex environment [28] and optimize nutrient-intake [29]. However, despite their intriguing cognitive abilities little is known about slime moulds' learning abilities. Yet, two recent studies showed that slime moulds are capable of habituation, a simple form of learning [22,23].
Habituation is defined as a progressive decrease in the magnitude of a behavioural response to an iterative stimulus [30,31]. Habituation differs from sensory adaptation, sensory or motor fatigue in that the response recovers to its original state when the stimulus is withheld. Habituation might for example enable an organism to ignore unpleasant stimuli in its environment while still allowing the organism to respond to novel and potentially harmful stimuli that would fall in the same sensory modality [31]. Using locomotion as the behavioural output and diverse chemical repellents as stimuli (quinine, caffeine and salt), recent studies [22,23] demonstrated that P. polycephalum learned to ignore a repellent when it was encountered repeatedly (habituation), but could respond again when the repellent was withheld for a certain time (recovery). By using diverse chemicals, it was shown that habituated slime moulds were still able to produce an aversive response toward a new stimulus (stimulus specificity), ruling out sensory and motor fatigue [22]. It was also revealed that slime moulds habituated to a repellent could transfer this adaptive response by cell fusion to individuals that had never encountered the repellent [23]. This transfer of an adaptive response required cytoplasm mixing between both slime moulds, suggesting that the substrate of habituation circulated within the cytoplasm.
In this paper, our aim was twofold, first identify a possible mechanism underlying habituation in slime moulds and second demonstrate that this mechanism allows information to be preserved for a very long time.
2. Material and methods
(a). Species studied and rearing conditions
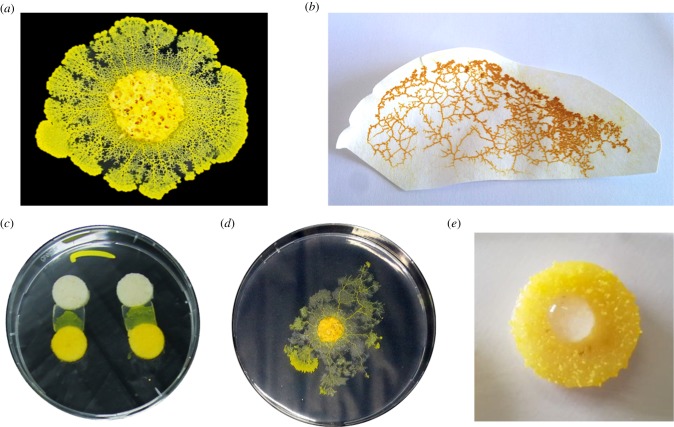
Physarum polycephalum, also called the acellular slime mould, belongs to the Myxomycetes. Its vegetative morph, the plasmodium, a vast multinucleate cell, can grow to cover up to a few square metres and crawl at speeds from 0.1 to a few centimetres per hour [32,33] (figure 1a). In natura, slime moulds are found on organic substrates like tree bark or forest soil where they feed on microorganisms such as bacteria or fungi [34]. In the presence of chemical substances in the environment, P. polycephalum shows directional movements toward or away from the stimulus (i.e. chemotaxis) [32]. When humidity and food availability decrease, the plasmodium turns into an encysted resting stage made of desiccated spherules ranging from 24 to 40 µm: the sclerotium [32]. During the sclerotization process (figure 1b), slime moulds lose 50% of their total protein content together with 40% of their DNA and 65% of their RNA [32]. Plasmodial cultures can be easily reinitiated from sclerotia after up to 3 years but viability decreases dramatically after 1 year [35].
Figure 1.
Physarum polycephalum and experimental set-ups. Physarum polycephalum photographs showing (a) the plasmodial stage with extending pseudopodsl and tubule network and (b) the dormant stage (sclerotial). (c) Bridge crossing test: habituated and control plasmodia were required to cross an agar gel bridge with or without salt to reach a food source (oat gel). (d) Exploration test: habituated and control sclerotia were required to explore an arena with or without salt. (e) Inception experiment: plasmodia were constrained to absorb salt (habituated) or distilled water (control). (Online version in colour.)
We used a strain of P. polycephalum provided by Southern Biological, Victoria, Australia. Large plasmodia were cultured in large Petri dishes (Ø 145 mm) on a 1% agar gel containing 10% of blended oat flakes (Quaker Oats Company). Rearing and experiments took place in the dark at 26°C and 70% humidity in temperature-controlled chambers. Experiments were recorded using digital cameras (EOS 70D, Canon).
(b). Mass habituation
We designed a new and more efficient protocol to habituate slime moulds inspired from [22,23] in which slime moulds encounter a repellent while feeding. Twenty plasmodia (Ø = 145 mm) were cut in half and each part was assigned to one of two training treatments: control (C) or habituation (H). During the training, habituated plasmodia (N = 20) were fed on salt oat gel (10% blended oats in a 1% agar solution to which NaCl was added at 50 mM concentration) in large Petri dishes (Ø = 145 mm) for 6 days (training period) while control plasmodia (N = 20) were fed on a plain oat gel (10% blended oats in a 1% agar solution). Once the training period ended, plasmodia were tested for short-term habituation.
(i). Test for short-term habituation
We tested the habituated plasmodia for short-term habituation using a bridge crossing experiment as in [22] (figure 1c). On day 1, before starting the habituation training, we verified that control and habituated plasmodia showed a clear aversive behaviour toward the NaCl. We cut 10 circular samples (Ø = 18 mm) from 20 control plasmodia and 20 habituated plasmodia. Five of those 10 samples were offered a bridge (1% agar gel, H = 2 mm, L = 13 mm, W = 15 mm) containing NaCl (100 mM) (Bridge S) leading to a patch of oat gel (H = 2 mm, Ø = 18 mm) while the remaining five had to cross a bridge without NaCl (Bridge A). Thus we had four conditions CA, CS, HA and HS (N = 100 for each condition). On day 6, once the habituation training was completed, we repeated this procedure to test the plasmodia for habituation. We recorded the time to reach the food patch for each plasmodial sample. High values of time to reach the food indicate aversion [23].
(ii). Test for repellent uptake
Past studies have shown that slime moulds can pick up and retain sodium within the cytoplasm [36,37]. Thus, we tested if plasmodia accumulated a certain amount of repellent (NaCl) throughout the habituation process. Once we had conducted the short-term habituation test, the remaining parts of the plasmodia were used for sodium analyses. The plasmodia were dried in an oven at 70°C for 48 h before being ground into a powder and weighed to the nearest 0.0001 g (dry weight). We measured the sodium content of each sample with a ROSS™ sodium ion selective electrode (ISE) (N = 20 for each treatment).
(iii). Test for repellent extrusion
As demonstrated in [23], the aversive response toward salt recovers to its original state if the plasmodium stops encountering the repellent for 2–3 days (recovery phase). This suggests that the repellent absorbed throughout the habituation phase might be extruded during the recovery phase. To test this hypothesis, we trained 24 plasmodia using the mass habituation protocol described above while 24 plasmodia were used as controls. After 6 days, once the training period ended, all plasmodia were transferred on standard oat gel (without NaCl) for 3 days. We measured the sodium content of each plasmodium at three different time points: day 6 (habituation), day 8 and day 9 (recovery). Here, each plasmodium was weighed immediately to perform sodium assay based on wet weight.
(c). Long-term habituation
Slime moulds have the potential to temporarily interrupt their life cycle by entering a state of dormancy (sclerotium). Taking advantage of this life cycle particularity we tested if slime moulds could recall a habituation acquired before entering the dormant stage. We first trained eight large plasmodia to habituate to salt following the mass habituation protocol described above and reared eight other plasmodia as controls. Then, we initiated the transition from plasmodia to sclerotia. To do so, we cut 12 circular samples from each plasmodium (Ø = 23 mm) and placed each sample on a moist filter paper (Ø = 145 mm) for one week to dry. Control plasmodia were dried on filter papers moistened with water (N = 96 sclerotia) while habituated plasmodia were dried on filter papers moistened with a solution of NaCl 75 mM (N = 96 sclerotia). The sclerotia were then stored for a month before being tested for ‘long-term habituation’.
(i). Test for long-term habituation: bridge crossing test
The bridge crossing test was similar to the one described above except for dimensions (bridge: L = 10 mm, W = 5 mm; patch of oat gel: Ø = 8 mm). On the day of testing, control sclerotia (N = 13) and habituated sclerotia (N = 12) chosen randomly were soaked in water and cut into several square samples (mean ± 95% confidence interval (CI95), 16.14 ± 0.75 mg, N = 400). Each sample was assigned to one of four conditions (CA, CS, HA and HS, N = 100 for each condition). Only four samples out of 400 failed to turn into viable plasmodia (N = 2 CA and N = 2 CS). Here, we recorded both the time to contact the bridge and the time to reach the food for each plasmodium. Plasmodia that never reached the food within the duration of the experiment were assigned the maximum value of 24 h.
(ii). Test for long-term habituation: exploration test
This new test inspired from [26] aims at automatically assessing habituation in the absence of an attractant i.e. the food patch (figure 1d). On the day of testing, control sclerotia (N = 11) and habituated sclerotia (N = 13), chosen randomly, were soaked in water and cut into several square samples (mean ± CI95, 13.98 ± 0.42 mg, N = 460). Each sclerotium sample was introduced into the centre of a circular arena (Ø 55 mm) containing a layer of agar gel (1% agar) to which we added NaCl (100 mM, Arena S) or did not (Arena A). Thus we had again four conditions (CA, CS, HA and HS, N = 115 for each condition). Out of the 460 samples tested, only five failed to turn into plasmodia (1 HS and 4 CS). To measure the exploration rate of each plasmodium, we used custom software adapted from the tracking method used in [26]. Our analysis consisted in monitoring the evolution of the intensity of each pixel within the arena as plasmodial movements translated into variations in pixel intensity. This method allowed us to assess precisely the surface covered by the plasmodium over time. We defined two variables that describe slime mould behaviour while exploring the arena. First the time needed to explore a surface of 10 mm2 in the arena, which corresponds to the appearance of the first pseudopod [22]. Second, since slime moulds always display exponential growth during the first hours of their exploration [26], we estimated the exponential rate of exploration starting from the first pseudopod appearance. High values of time to form the first pseudopod as well as low values of expansion rate indicate that slime moulds were avoiding contact with the arena surface.
(iii). Test for uptake of repellent
We tested if plasmodia accumulated a certain amount of repellent throughout the sclerotization process. Soaked (N = 21 habituated and N = 21 controls) and dry sclerotia (N = 20 habituated and N = 20 controls) that had not been used for habituation tests were used for sodium assays.
(iv). Test for recovery
During sclerotization, plasmodia were allowed to dry while the repellent was still in the environment i.e. we used filter papers moistened with salt water. Here, we tested if plasmodia could recover from the habituation if we removed the repellent during the transition from plasmodia to sclerotia. We trained six large plasmodia to habituate to salt (HW) using the mass habituation protocol while six plasmodia were used as controls (C). Unlike before, circular samples (Ø = 23 mm) of habituated plasmodia (N = 72) were placed on filter papers moistened only with water as the controls (N = 72). Once the sclerotization was over, sclerotia were stored for a month before being tested for recovery using the exploration test. On the day of testing, control sclerotia (N = 11) and habituated sclerotia (N = 15) were soaked in water and cut into several square samples (mean ± CI95, 13.76 ± 0.37 mg, N = 400). Each sample was assigned to one of four conditions (CA, CS, HA and HS, N = 100 for each condition). Only two samples out of 400 failed to turn into viable plasmodia (N = 2 HS).
(v). Test for repellent extrusion
Before entering the sclerotium stage, the plasmodium moved on the paper and left behind a thick mat of non-living, translucent, extracellular slime [28] easily discernable on the paper. Hence, once each plasmodium entered the sclerotium stage, we estimated the sodium content of the explored filter papers (HW N = 39, C N = 53). Regarding the sclerotia, we assessed the sodium content in dry samples (unmodified sclerotia) (HW N = 19, C N = 29) and in wet samples (soaked sclerotia) (HW N = 36, C N = 31).
(d). Constrained absorption of repellent
In this last experiment, we investigated if habituation could be induced by directly increasing sodium concentration within the plasmodia using topic injections of NaCl. We cut eight large plasmodia reared on oat gel into 20 circular samples (Ø 15 mm). On each sample, we placed either a 50 µl droplet of distilled water (control treatment) or a 50 µl droplet of a 100 mM NaCl solution (habituation treatment) (figure 1e). After two hours, the droplets were totally absorbed by the plasmodia and we tested them for habituation with the exploration test described above (CA, CS, HA and HS, N = 40 for each condition). All plasmodia survived the constrained absorption of salt and distilled water.
(e). Statistical analyses
To present our results in the clearest and simplest way, we synthesized each recorded variable with an aversion index as in [23]:
Using those indexes, we normalized each dependent variable value corresponding to the treatments HS and CS by the mean and the standard deviation of their respective controls HA and CA. Values clearly above 0 indicate an aversion towards the repellent, whereas values close to zero indicate habituation to the repellent i.e. slime moulds react the same way to agar and salt.
All statistical analyses were conducted using R version 3.3.1. To compare HI and CI, we took into account the non-independency of slime moulds originating from the same sclerotium and/or plasmodium by running mixed models. We also added day, experiment number and sclerotium weight as random factors when needed. Statistical analyses were performed both on index values and on raw values (see electronic supplementary material for raw values). Both analyses gave consistent outcomes.
3. Results
(a). Mass habituation
(i). Slime moulds habituate through mass habituation
In the first experiment, we verified that slime moulds have the ability to habituate to a repellent using a ‘mass habituation’ protocol. On day 1, habituated and control plasmodia, facing the repellent for the first time, showed a clear aversive behaviour, crossing the bridge slowly. This translated into high aversion indexes (figure 2a). On day 6, habituated and control plasmodia were tested for habituation and were required to cross an agar gel bridge containing the repellent. Control plasmodia showed a strong aversive behaviour and a high aversion index (figure 2a). In contrast, habituated plasmodia, encountering the repellent for the sixth time, showed no aversive behaviour and an aversion index close to zero (glmm, treatment: Wald χ2 = 8.80, p = 0.003; treatment × day: Wald χ2 = 253.11, p < 0.001).
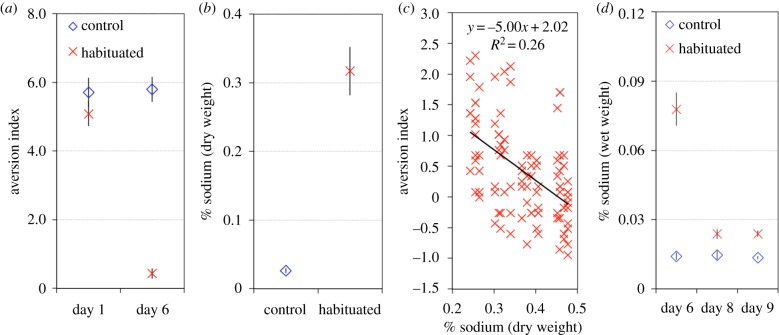
Figure 2.
Mass habituation and repellent uptake. (a) Mass habituation: slime moulds learn to ignore a repellent. Habituated plasmodia were reared on a salt oat gel every day for 6 days while control plasmodia were fed on a standard oat gel. Habituated and control plasmodia were required to cross an agar gel bridge with (N = 200) or without salt (N = 200) to reach a food source on day 1 and on day 6 of the habituation. The aversion index is based on the time to reach the food. An aversion index close to 0 indicates habituation while values clearly above 0 indicate an aversion to the repellent. (b) Percentage of sodium measured on the last day of habituation for the control (N = 20) and the habituated plasmodia (N = 20). % sodium indicates: Na (mg)/plasmodia dry weight (mg) × 100. (c) Habituation performances as a function of plasmodial sodium percentages. (d) Percentages of sodium measured after habituation and during recovery. Habituated (N = 25) and control plasmodia (N = 25) were fed standard oat gel during recovery. % sodium indicates: Na (mg)/plasmodia wet weight (mg) × 100. Error bars indicate CI95. (Online version in colour.)
(ii). Slime moulds absorb the repellent during habituation
Sodium assays indicated that habituated plasmodia contained far more sodium than control plasmodia (glmm, treatment: Wald χ2 = 5049.63, p < 0.001; figure 2b). Interestingly the amount of sodium taken up by habituated plasmodia was negatively correlated with their aversion index i.e. the higher the concentration of sodium within the plasmodia the less the aversive response (F1,98 = 33.93, p < 0.001, figure 2c).
(iii). Slime moulds extrude the repellent during recovery
As expected, during the recovery phase the sodium concentration within the habituated plasmodia decreased greatly but never reached the level of the control plasmodia (glmm, treatment: Wald χ2 = 716.12, p < 0.001; treatment × day: Wald χ2 = 344.99, p < 0.001). This indicates that habituated plasmodia extruded most of the sodium absorbed throughout the habituation training (figure 2d).
(b). Long-term habituation
(i). Slime moulds retain the habituation through their dormant stage
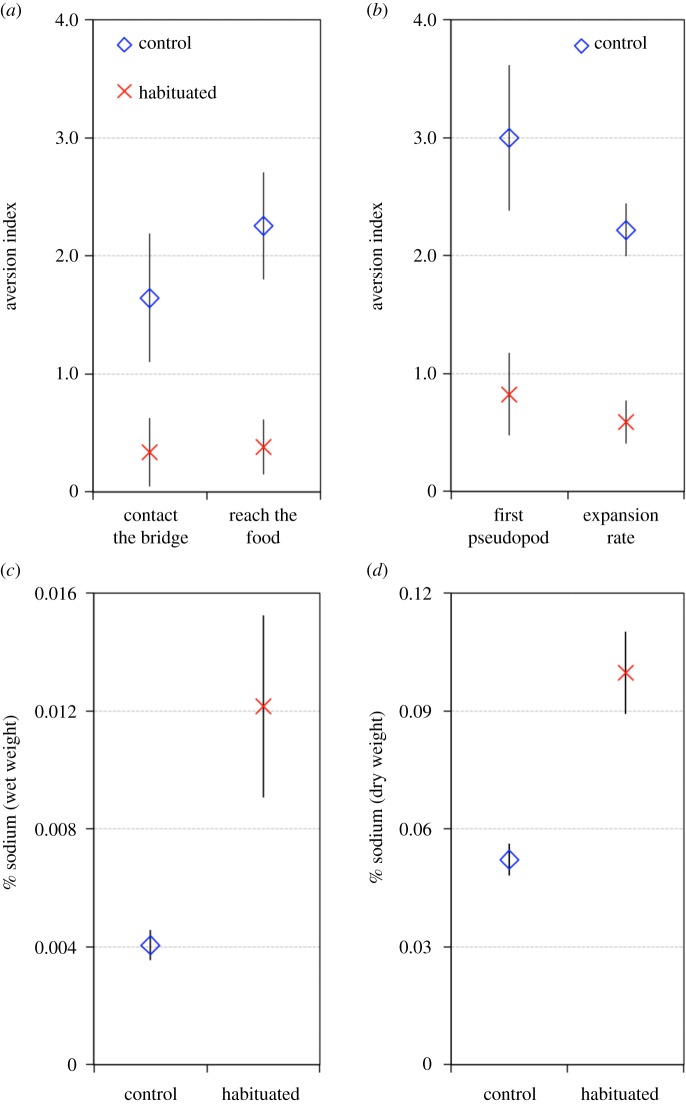
Almost all plasmodia offered a plain agar gel bridge crossed the bridge and reached the food patch within 24 h (95/98 and 98/100 for CA and HA respectively). In contrast, only half of the control plasmodia (54/98 for CS) were able to cross the salt bridge whereas habituated plasmodia did not experience any difficulty (94/100 crossed the salt bridge for HS). This indicates already that habituated plasmodia were less troubled by the repellent than control plasmodia. Moreover, as shown in figure 3a, habituated plasmodia, encountering the repellent again after the dormant phase, showed a much lower aversion index than control plasmodia facing the repellent for the first time (glmm, time to contact the bridge: Wald χ2 = 7.76, p = 0.005; time to contact the food: Wald χ2 = 33.39, p < 0.001). We confirmed these results using the exploration test. Habituated plasmodia emerging from a dormant stage in a salt arena were quicker to explore and expanded faster than control plasmodia (figure 3b; glmm, first pseudopod: Wald χ2 = 14.71, p = 0.005; expansion rate: Wald χ2 = 42.19, p < 0.001). These results indicate that habituation persisted throughout the dormant stage.
Figure 3.
Long-term habituation and repellent uptake. Plasmodia were trained following a mass habituation protocol and turned into sclerotia. (a) Bridge crossing test for long-term habituation. Plasmodia reinitiated from habituated and control sclerotia were required to cross an agar gel bridge with (N = 200) or without salt (N = 200) to reach a food source. The aversion indexes are based on the time to contact the bridge and the time to reach the food. Aversion indexes close to 0 indicate habituation while values clearly above 0 indicate an aversion to the repellent. (b) Exploration test for long-term habituation. Plasmodia reinitiated from habituated and control sclerotia were required to explore an arena with (N = 230) or without salt (N = 230). The aversion indexes are based on the time to form the first pseudopod and the expansion rate. (c) Percentages of sodium measured in habituated (N = 21) and control soaked sclerotia (N = 21). % sodium indicates: Na (mg)/sclerotia wet weight (mg) × 100. (d) Percentages of sodium measured in habituated (N = 20) and control dry sclerotia (N = 20). % sodium indicates: Na (mg)/sclerotia dry weight (mg) × 100. (Online version in colour.)
(ii). Slime moulds kept the repellent during the dormant stage
Habituated sclerotia contained more sodium than control sclerotia (glmm, treatment: Wald χ2 = 8.25, p = 0.004 and Wald χ2 = 41.92, p < 0.001 for wet and dry sclerotia respectively; figure 3c), indicating that habituated plasmodia kept a certain amount of sodium throughout the sclerotization process.
(iii). Slime moulds do not recover during the sclerotization process
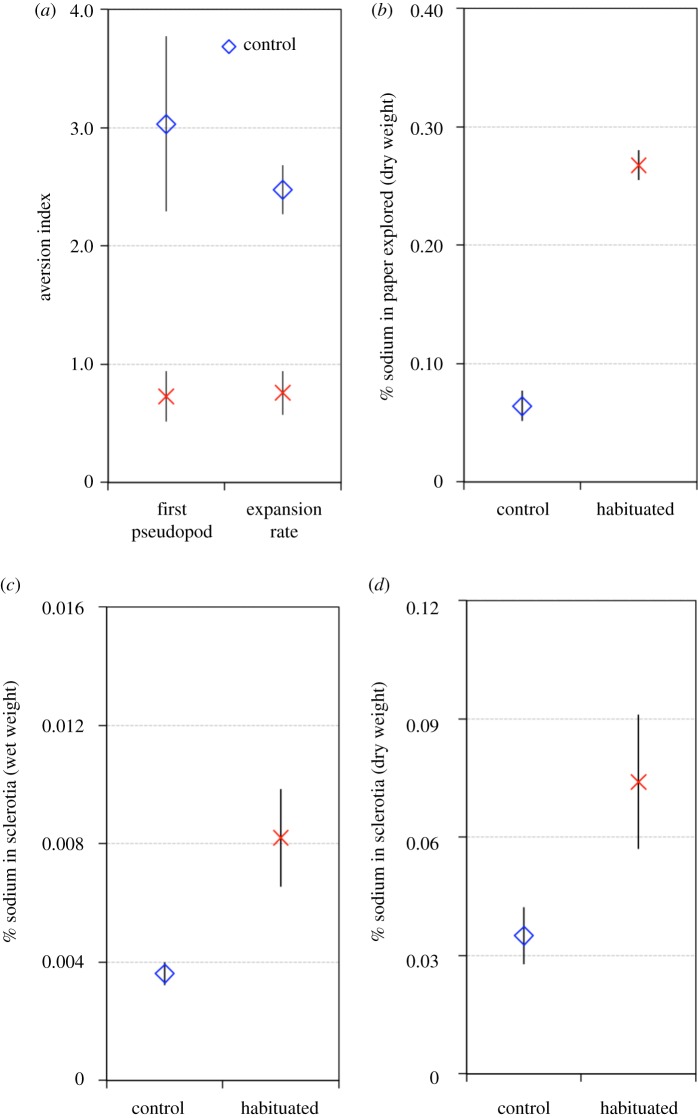
Habituated plasmodia that entered the dormant stage in an environment without repellent (HW) still showed habituated behaviour when they were revived and introduced into a salt arena (figure 4a; glmm, first pseudopod: Wald χ2 = 5.50, p = 0.019; expansion rate: Wald χ2 = 23.96, p < 0.001). During the sclerotization process, habituated plasmodia (HW) extruded part of the repellent absorbed during the habituation, as the filter papers explored by habituated plasmodia contained more sodium than the ones explored by controls (glmm, treatment: Wald χ2 = 130.91, p < 0.001; figure 4b). However, habituated sclerotia (HW) formed in an environment deprived of salt still contained more sodium than control sclerotia (glmm, treatment: Wald χ2 = 11.01, p < 0.001 and Wald χ2 = 21.54, p < 0.001 for wet and dry samples respectively; figure 4c,d), indicating that they retained part of the sodium accumulated throughout habituation.
Figure 4.
Recovery and repellent extrusion. Plasmodia were trained following a mass habituation protocol and turned into sclerotia in an environment without salt. (a) Exploration test for recovery. Plasmodia reinitiated from habituated and control sclerotia were required to explore an arena with (N = 200) or without salt (N = 200). The aversion indexes are based on the time to form the first pseudopod and the expansion rate. Aversion indexes close to 0 indicate habituation while values clearly above 0 indicate an aversion to the repellent. (b) Percentage of sodium measured in paper explored by habituated (N = 39) and control plasmodia (N = 53) while they were transiting from plasmodia to sclerotia. % sodium indicates: Na (mg)/paper dry weight (mg) × 100. (c) Percentages of sodium measured in habituated (N = 19) and control soaked sclerotia (N = 20). % sodium indicates: Na (mg)/sclerotia dry weight (mg) × 100. (d) Percentages of sodium measured in habituated (N = 36) and control dried sclerotia (N = 31). % sodium indicates: Na (mg)/sclerotia (mg wet weight) × 100. (Online version in colour.)
The sodium content was slightly lower in sclerotia formed in an environment deprived of salt (HW) than in sclerotia generated in a salt environment (H) (glmm, treatment: Wald χ2 = 1.92, p = 0.164 and Wald χ2 = 6.47, p = 0.011 for wet and dry weight respectively; figure 3c,d and figure 4c,d). These results indicate that habituated slime moulds did not recover their initial state during the transition from plasmodia to sclerotia, even when this process occurred in a non-aversive environment.
(c). Constrained absorption of repellent
A topic application of salt was able to mimic training to some extent. All plasmodia placed in a salt arena were reluctant to form the first pseudopod (figure 5; glmm, first pseudopod: Wald χ2 = 1.46, p = 0.226). However, once the exploration started, plasmodia that were constrained to absorb salt water before the test expanded quicker than plasmodia that had absorbed only water (figure 4; glmm, expansion rate Wald χ2 = 12.79, p < 0.001)
Figure 5.
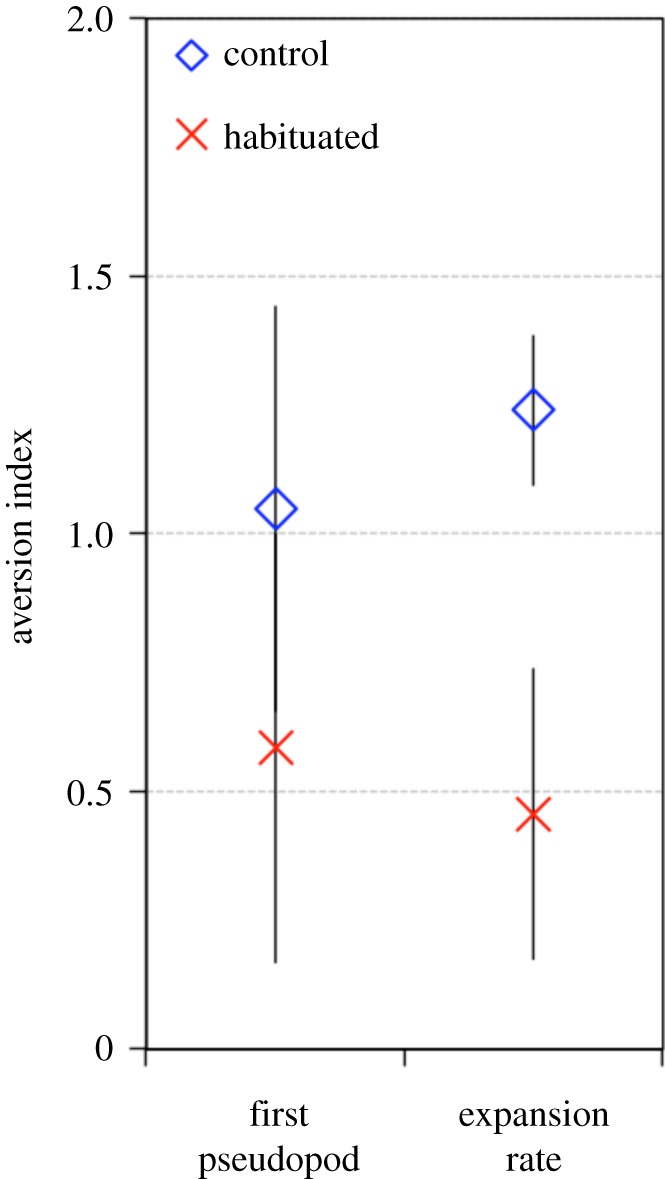
Constrained absorption of repellent. Exploration test for habituation. Plasmodia were constrained to absorb salt (habituated) or distilled water (control) and required to explore an arena with (N = 80) or without salt (N = 80). The aversion indexes are based on the time to form the first pseudopod and the expansion rate. Aversion indexes close to 0 indicate habituation while values clearly above 0 indicate an aversion to the repellent.
4. Discussion
Here, we found that habituation to a repellent persisted through the dormant stage in slime moulds. To our knowledge, this is the first study to show long-term habituation in a non-neural organism. We also found that repellent uptake could be one of the mechanisms underlying short- and long-term habituation in slime moulds. Four main results support this hypothesis. First, training slime moulds to habituate to a repellent led to an increase in intracellular repellent concentration. Second, the repellent absorbed during habituation was extruded during recovery. Third, a certain amount of repellent absorbed during habituation was stored throughout the dormant stage. Lastly, we succeeded in inducing habituation in naive slime moulds by constraining them to absorb a repellent.
(a). Short-term habituation and repellent uptake
Using a different protocol, named ‘mass habituation’, we corroborated previous studies and showed short-term habituation in slime moulds [22,23]. Slime moulds reared with a salt diet for 6 days stopped showing any aversive behaviour toward the salt. In previous training protocols, slime moulds were in contact with the repellent intermittently while in this new protocol slime moulds were continuously in contact with the repellent. This last feature renders the training more ecologically relevant as slime moulds are more likely to experience an aversive substance uniformly scattered while feeding. This protocol enabled high throughput training and we succeeded in achieving similar performances to a previous protocol [23]. In addition, we did not observe any detrimental effect of salt on slime mould survival. The other advantage of this protocol is that we were able to train slime moulds while running chemical assays since a single cell can be cut into multiple viable cells. This allowed us to demonstrate that slime moulds absorbed the salt throughout the training and extruded it during recovery. The level of sodium measured in control slime moulds was relatively low and in agreement with previous measurements (0.008%—1.4 mM kg−1 wet weight, 0.05%—9.3 mM kg−1 dry weight [38], as water content of plasmodia is 85% [39]). In contrast, the level of sodium measured in habituated slime moulds was 10 times the level of the controls, confirming that slime moulds can pick up sodium from the environment [36]. Thus, slime moulds tolerated large variation in intracellular sodium concentration without being impaired. To our surprise, intracellular sodium concentration was positively correlated with slime mould performance, supporting the role of sodium uptake in habituation.
(b). Long-term habituation and repellent storage
We showed that information acquired during the training could also be preserved through the dormant stage even after one month. We also reinitiated sclerotia after 1 year but did not include the data in this paper as most of the control slime moulds died (224 out of 267) while half of the habituated ones survived (149 out of 288). This suggests a link between sclerotium age and resistance to osmotic stress [35]. On exploring a salt environment, habituated slime moulds revived after one month showed some degree of aversive behaviour but they were far less repelled by salt than control slime moulds. Interestingly, the level of aversion toward the salt in controls was lower for long-term habituation than for short-term habituation (figure 2a and figure 3a). This might have been due to differences in their physiological states. For short-term habituation tests, control slime moulds were well fed, i.e. they were literally sitting on a patch of food before having to cross the salt bridge. In contrast, for long-term habituation tests, slime moulds were turn into sclerotia, a process that took one week under starved condition, and then, they were revived after a one month, placed on agar gel and faced with a salt bridge. Hence, in these two experiments, the level of motivation might have been different and might have affected the slime moulds' willingness to cross a salt bridge.
Again, we were able to correlate habituation with intracellular sodium concentration. Habituated sclerotia had higher sodium content than control ones. Interestingly, during sclerotization habituated sclerotia extruded a large amount of sodium accumulated throughout habituation. This is not surprising as differentiation of plasmodium to dormant sclerotium is characterized by a large change in metabolism [32]. This result might indicate either that a low level of repellent is sufficient to trigger and preserve the memory of habituation or that the repellent is not the only information supporting habituation. Internalizing a chemical repellent to retain habituation might be an acceptable gambit for the future since the chemical substances encountered before sclerotization might still be there when the slime mould is reinitiated.
(c). A simple mechanism
In a previous study [23], it was shown that slime moulds habituated to salt could transfer this adaptive response by cell fusion to naive individuals in less than 3 h. Here, we showed that habituation can be induced in slime moulds in only 2 h by constrained absorption of the repellent. Slime moulds forced to absorb salt for 2 h showed only a mild aversion toward the salt while controls presented a pronounced aversive behaviour. Taken together, all these results suggest a key role of sodium uptake in the emergence and maintenance of habituation. Sodium is known to decrease migration rate in slime moulds [37] via a depolarization of the membrane potential [40]. Sodium entry might counteract this process by restoring membrane potential and allowing migration. Using radioactive markers, past studies have shown that slime moulds can pick up and retain sodium within the cytoplasm [36]. Yet, we do not know if the entry of sodium is an active or a passive process. In plants and yeast, sodium is driven into the cell passively by the negative electrical potential difference across the membrane resulting from the difference between the concentrations of sodium inside and outside the cell [41]. Sodium enters through transport protein channels used to acquire potassium, as those transporters do not discriminate between the two ions. Similar transporters have been described in the cellular slime mould Dictyostelium discoideum [42] and might certainly exist in P. polycephalum.
Sodium entry might allow habituation but migrating in a salt environment could impose two major stresses on slime moulds. First, water is drawn out of the cells through osmosis (osmotic stress), which might lead to desiccation. Second, in plants and yeast, sodium competes with potassium for binding sites, leading to a decrease of the K+/Na+ ratio. This ionic stress impairs cell functions as protein synthesis depends strongly on potassium concentration and sodium might damage DNA [41]. Plants and yeast have found active ways to overcome this damage: cell volume regulation, sodium extrusion, compartmentalization of sodium into vacuoles and synthesis of osmoprotectants [41,43]. Our results have shown that slime moulds were capable of releasing sodium to recover and of storing sodium throughout the dormant stage. We did not observe any cell volume changes and further analysis is needed to investigate synthesis of osmoprotectants.
(d). Beyond habituation to NaCl
In a previous study, slime moulds were trained to habituate to different chemical substances: quinine or caffeine [22]. Thus, we might wonder if the repellent were also absorbed and kept within the cell. Salt can be passively absorbed by transport proteins but what about quinine and caffeine? In plants, alkaloids such as quinine and caffeine are easily taken up and released but they can also be accumulated in large amounts and stored inside vacuoles [44]. Alkaloid transport is done via ATP-binding cassette (ABC) transporters, which constitute a large, diverse and ubiquitous superfamily of proteins found in a large range of organisms including slime moulds [45]. Hence, it might be possible that repellent uptake plays a key role in alkaloid habituation as well. Quinine, caffeine and NaCl are all chemical stimuli but slime moulds also respond to other types of stimuli such as light, temperature gradients, humidity and gravity [32,34]. If habituation extends to all sensory modalities, it ought to rely on different mechanisms. In animals, it has been shown that behind the concept of habituation is a large range of cellular mechanisms that are differentially activated by different stimulus paradigms [31]. Similarly, habituation in slime moulds could be encoded via different physiological, transcriptional, morphological and/or biophysical processes, depending on the type of stimulus used. We aim to address this question in subsequent rounds of experimentation.
5. Conclusion
Habituation is critical for survival, in that it allows organisms to ignore irrelevant stimuli so that they can pay attention to more important ones [31]. Thus, it is not surprising that habituation is observed in a large range of living beings such as single celled organisms [17–23], plants [46] and animals [30,31]. Although the mechanisms underpinning habituation are still unknown for most non-neural organisms, habituation might have evolved independently in those phylogenetically distant groups [47]. In non-neural organisms various candidate mechanisms for habituation have been suggested: epigenetic reprogramming in unicellulars [11] and in plants [45], ion flows propagated by cell–cell junctions in plants [45] and chemical signalling networks in both unicellulars [15] and plants [45]. Here in this paper, we have presented the first evidence of long-term habituation in non-neural organisms and provided the first glimpse of the underlying mechanism. We are convinced that habituation is just one example of cognitive abilities that might be shared by most living organisms. As Godfrey-Smith [14, p. 236] would say ‘There are lots of ways to process information and control behaviour; a central nervous system is one way, but not the only way’.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Edgar Dusacre, Maxence Mougon, Morgan Pezet and Pauline Vinet for technical assistance, François-Xavier Dechaume-Moncharmont and Vincent Fourcassié for statistical advice, and Deborah Federico and Jean-Louis Deneubourg for fruitful discussions.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
A.D. conceived the study. A.B. and A.D. designed the study. A.B. and J.D. performed the experiments. A.B. and A.P.-E. carried out data acquisition and data analysis. A.D. and J.D. performed the chemical assays. A.D., A.B. and A.P.-E. wrote the manuscript. A.D. secured funding. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The research was supported by a grant from the ‘Agence Nationale de la Recherche’, reference no. ANR-17-CE02-0019-01 - SMARTCELL.
References
- 1.Baluška F, Mancuso S, Volkmann D, Barlow P. 2009. The ‘root-brain’ hypothesis of Charles and Francis Darwin. Plant Signal. Behav. 4, 1121–1127. ( 10.4161/psb.4.12.10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo GP, Keijzer F. 2011. Plants: adaptive behaviour, root-brains, and minimal cognition. Adapt. Behav. 19, 155–171. ( 10.1177/1059712311409446) [DOI] [Google Scholar]
- 3.Trewavas A. 2017. The foundations of plant intelligence. Interface Focus 7, 20160098 ( 10.1098/rsfs.2016.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyon P. 2015. The cognitive cell: bacterial behaviour reconsidered. Front. Microbiol. 6, 264 ( 10.3389/fmicb.2015.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garnier S, Gautrais J, Theraulaz G. 2007. The biological principles of swarm intelligence. Swarm Intell. 1, 3–31. ( 10.1007/s11721-007-0004-y) [DOI] [Google Scholar]
- 6.Bonabeau E, Dorigo M, Théraulaz G. 1999. Swarm intelligence: from natural to artificial systems. New York, NY: Oxford University Press. [Google Scholar]
- 7.DasGupta D. 1993. An overview of artificial immune systems and their applications. In Artificial immune systems and their applications (ed. DasGupta D.), pp. 3–21. Berlin, Germany: Springer. [Google Scholar]
- 8.Farmer JD, Packard NH, Perelson AS. 1986. The immune system, adaptation, and machine learning. Physica D 22, 187–204. ( 10.1016/0167-2789(86)90240-X) [DOI] [Google Scholar]
- 9.Lyon P. 2006. The biogenic approach to cognition. Cogn. Process. 7, 11–29. ( 10.1007/s10339-005-0016-8) [DOI] [PubMed] [Google Scholar]
- 10.Baluška F, Levin M. 2016. On having no head: cognition throughout biological systems. Front. Psychol. 7, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsburg S, Jablonka E. 2009. Epigenetic learning in non-neural organisms. J. Biosci. 34, 633–646. ( 10.1007/s12038-009-0081-8) [DOI] [PubMed] [Google Scholar]
- 12.van Duijn M. 2017. Phylogenetic origins of biological cognition: convergent patterns in the early evolution of learning. Interface Focus 7, 20160158 ( 10.1098/rsfs.2016.0158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallverdú J, et al. 2018. Slime mould: the fundamental mechanisms of biological cognition. Biosystems 165, 57–70. ( 10.1016/j.biosystems.2017.12.011) [DOI] [PubMed] [Google Scholar]
- 14.Godfrey-Smith P. 2002. Environmental complexity and the evolution of cognition. In The evolution of intelligence (ed. Sternberg RJ, Kaufman J), pp. 233–249. Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- 15.Dukas R. 2009. Learning: mechanisms, ecology and evolution. In Cognitive ecology II (ed. Dukas R, Ratcliffe JM), pp. 7–26. Chicago, IL: University of Chicago Press. [Google Scholar]
- 16.McGregor S, Vasas V, Husbands P, Fernando C. 2012. Evolution of associative learning in chemical networks. PLoS Comput. Biol. 8, e1002739 ( 10.1371/journal.pcbi.1002739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn D, Blair HJ, Thomas J, Eisenstein EM. 1973. The effects of vibratory and electrical stimulation on habituation in the ciliated protozoan, Spirostomum ambiguum. Behav. Biol. 8, 655–664. ( 10.1016/S0091-6773(73)80150-6) [DOI] [PubMed] [Google Scholar]
- 18.Hamilton TC, Thompson JM, Eisenstein EM. 1974. Quantitative analysis of ciliary and contractile responses during habituation training in Spirostomum ambiguum. Behav. Biol. 12, 393–407. ( 10.1016/S0091-6773(74)91601-0) [DOI] [PubMed] [Google Scholar]
- 19.Hennessey TM, Rucker WB, McDiarmid CG. 1979. Classical conditioning in paramecia. Anim. Learn. Behav. 7, 417–423. ( 10.3758/BF03209695) [DOI] [Google Scholar]
- 20.Wood DC. 1988. Habituation in Stentor: a response-dependent process. J. Neurosci. 8, 2248–2253. ( 10.1523/JNEUROSCI.08-07-02248.1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunita I, Yamaguchi T, Tero A, Akiyama M, Kuroda S, Nakagaki T. 2016. A ciliate memorizes the geometry of a swimming arena. J. R. Soc. Interface 13, 20160155 ( 10.1098/rsif.2016.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisseau RP, Vogel D, Dussutour A. 2016. Habituation in non-neural organisms: evidence from slime moulds. Proc. R. Soc. B 283, 20160446 ( 10.1098/rspb.2016.0446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel D, Dussutour A. 2016. Direct transfer of learned behaviour via cell fusion in non-neural organisms. Proc. R. Soc. B 283, 20162382 ( 10.1098/rspb.2016.2382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagaki T, Yamada H, Tóth Á. 2000. Intelligence: maze-solving by an amoeboid organism. Nature 407, 470 ( 10.1038/35035159) [DOI] [PubMed] [Google Scholar]
- 25.Tero A, Takagi S, Saigusa T, Ito K, Bebber DP, Fricker MD, Nakagaki T. 2010. Rules for biologically inspired adaptive network design. Science 327, 439–442. ( 10.1126/science.1177894) [DOI] [PubMed] [Google Scholar]
- 26.Vogel D, Nicolis SC, Perez-Escudero APE, Nanjundiah V, Sumpter DJT, Dussutour A. 2015. Phenotypic variability in unicellular organisms: from calcium signalling to social behaviour. Proc. R. Soc. B 282, 20152322 ( 10.1098/rspb.2015.2322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saigusa T, Tero A, Nakagaki T, Kuramoto Y. 2008. Amoebae anticipate periodic events. Phys. Rev. Lett. 100, 018101 ( 10.1103/PhysRevLett.100.018101) [DOI] [PubMed] [Google Scholar]
- 28.Reid CR, Latty T, Dussutour A, Beekman M. 2012. Slime mould uses an externalized spatial ‘memory’ to navigate in complex environments. Proc. Natl Acad. Sci. USA 109, 17 490–17 494. ( 10.1073/pnas.1215037109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RF. 2009. Habituation: a history. Neurobiol. Learn. Mem. 92, 127–134. ( 10.1016/j.nlm.2008.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rankin CH, et al. 2009. Habituation revisited: an updated and revised description of the behavioural characteristics of habituation. Neurobiol. Learn. Mem. 92, 135–138. ( 10.1016/j.nlm.2008.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldrich H. 2012. Cell biology of Physarum and Didymium V1: organisms, nucleus, and cell cycle. New York, NY: Elsevier. [Google Scholar]
- 33.Oettmeier C, Brix K, Döbereiner HG. 2017. Physarum polycephalum—a new take on a classic model system. J. Phys. D Appl. Phys. 50, 413001 ( 10.1088/1361-6463/aa8699) [DOI] [Google Scholar]
- 34.Alvarado CR, Stephenson SL (eds). 2017. Myxomycetes: biology, systematics, biogeography and ecology. San Diego, CA: Elsevier. [Google Scholar]
- 35.Gehenio PM. 1944. Longevity of the sclerotia of Mycetozoa. Biodynamica 4, 359–368. [Google Scholar]
- 36.Miller DM, Anderson JD, Abbot BC. 1968. Potentials and ionic exchange in slime mould plasmodia. Comp. Biochem. Physiol. 27, 633–646. ( 10.1016/0010-406X(68)90603-8) [DOI] [PubMed] [Google Scholar]
- 37.Denbo JR, Miller DM. 1976. Factors affecting the movement of slime mould plasmodia. Comp. Biochem. Physiol. 55, 5–12. ( 10.1016/0300-9629(76)90114-6) [DOI] [PubMed] [Google Scholar]
- 38.Kuroda R, Kuroda H. 1980. Calcium accumulation in vacuoles of Physarum polycephalum following starvation. J. Cell Sci. 44, 75–85. [DOI] [PubMed] [Google Scholar]
- 39.Tran H, Stephenson S, Pollock E. 2015. Evaluation of Physarum polycephalum plasmodial growth and lipid production using rice bran as a carbon source. BMC Biotechnol. 15, 67 ( 10.1186/s12896-015-0188-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueda T, Terayama K, Kurihara K, Kobatake Y. 1975. Threshold phenomena in chemoreception and taxis in slime mould Physarum polycephalum. J. Gen. Physiol. 65, 223–234. ( 10.1085/jgp.65.2.223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumwald E. 2000. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 12, 431–434. ( 10.1016/S0955-0674(00)00112-5) [DOI] [PubMed] [Google Scholar]
- 42.Müller U, Hartung K. 1990. Properties of three different ion channels in the plasma membrane of the slime mould Dictyostelium discoideum. Biochim. Biophys. Acta 1026, 204–212. ( 10.1016/0005-2736(90)90065-V) [DOI] [PubMed] [Google Scholar]
- 43.Tester M, Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91, 503–527. ( 10.1093/aob/mcg058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shitan N, Yazaki K. 2007. Accumulation and membrane transport of plant alkaloids. Curr. Pharm. Biotechnol. 8, 244–252. ( 10.2174/138920107781387429) [DOI] [PubMed] [Google Scholar]
- 45.Verrier PJ, et al. 2008. Plant ABC proteins—a unified nomenclature and updated inventory. Trends Plant Sci. 13, 151–159. ( 10.1016/j.tplants.2008.02.001) [DOI] [PubMed] [Google Scholar]
- 46.Gagliano M, Renton M, Depczynski M, Mancuso S. 2014. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175, 63–72. ( 10.1007/s00442-013-2873-7) [DOI] [PubMed] [Google Scholar]
- 47.Moore BR. 2004. The evolution of learning. Biol. Rev. 79, 301–335. ( 10.1017/S1464793103006225) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.