Abstract
The 1918 influenza pandemic is one of the most devastating infectious disease epidemics on record, having caused approximately 50 million deaths worldwide. Control measures, including prohibiting non-essential gatherings as well as closing cinemas and music halls, were applied with varying success and limited knowledge of transmission dynamics. One hundred years later, following developments in the field of mathematical epidemiology, models are increasingly used to guide decision-making and devise appropriate interventions that mitigate the impacts of epidemics. Epidemiological models have been used as decision-making tools during outbreaks in human, animal and plant populations. However, as the subject has developed, human, animal and plant disease modelling have diverged. Approaches have been developed independently for pathogens of each host type, often despite similarities between the models used in these complementary fields. With the increased importance of a One Health approach that unifies human, animal and plant health, we argue that more inter-disciplinary collaboration would enhance each of the related disciplines. This pair of theme issues presents research articles written by human, animal and plant disease modellers. In this introductory article, we compare the questions pertinent to, and approaches used by, epidemiological modellers of human, animal and plant pathogens, and summarize the articles in these theme issues. We encourage future collaboration that transcends disciplinary boundaries and links the closely related areas of human, animal and plant disease epidemic modelling.
This article is part of the theme issue ‘Modelling infectious disease outbreaks in humans, animals and plants: approaches and important themes’. This issue is linked with the subsequent theme issue ‘Modelling infectious disease outbreaks in humans, animals and plants: epidemic forecasting and control’.
Keywords: mathematical modelling, human disease, animal disease, plant disease, public health, one health
1. Introduction
The field of epidemiological modelling is centuries old [1–10]. However, in the past 20 years, modelling has increasingly been used to advise policy during outbreaks [11–16]. Models can be used to forecast the total number of cases (e.g. [13]), as well as to inform intervention strategies (e.g. [11,17]). Recent examples of real-time modelling during outbreaks can be drawn from diseases of humans (e.g. the outbreak of Ebola virus disease in West Africa from 2013 to 2016 [13,18–21]), animals (e.g. the 2001 and 2007 Foot-and-Mouth Disease epidemics in the UK [11,12,22,23]) and plants (e.g. the invasion of the UK by Chalara fraxinea, which causes dieback of ash trees, in 2012 [24]).
Although there are scenarios in which a specific modelling approach is required for pathogens of a particular host type (see §2c), there are also many similarities between human, animal and plant disease systems that suggest that common modelling frameworks can be extremely useful. There are important questions that are of interest in all three fields, such as: how can models be parameterized using data collected during an epidemic? How can uncertainty in the data, or in the estimated values of model parameters, be represented in model outputs and then communicated to decision makers? And how can interventions be introduced to fulfil a particular objective—such as to minimize the number of hosts ever infected or reduce the ecological or economic impacts of an epidemic?
As a result of these shared questions of interest, we contend that increased collaboration between modellers across human, animal and plant disease epidemiology will be beneficial and allow modelling approaches to be optimized. In this pair of theme issues, we therefore present articles written by scientists working at the forefront of epidemiological research for human, animal and plant systems. There is a focus on the links between these topics, thereby encouraging future collaboration and cross-fertilization between these areas.
In this introductory article, we summarize the shape of the epidemiological modelling landscape (§2a) and note that epidemiological modellers most frequently focus on pathogens of humans. We describe some of the types of data available for quantitative analysis of infectious disease epidemics (§2b), as well as the methods used to analyse those data and to represent the dynamics of outbreaks (§2c). We discuss the use of infectious disease modelling to guide forecasting and control (§2d). Throughout, we highlight articles in this pair of theme issues and more widely in the literature. Finally, we provide an overview of these theme issues (§3), as well as propose a unified approach involving mathematical epidemiologists focussed on pathogens of humans, animals and plants to be used going forwards (§4).
2. Modelling epidemics in humans, animals and plants
(a). Focal systems for epidemic modellers
The field of epidemic modelling is dominated by studies of human diseases, with commercially important livestock disease modelling second. Models of plant disease outbreak dynamics are less numerous, despite the economic impacts of plant pests and diseases [25] as well as their effects on biodiversity [26] and ecosystem services [27].
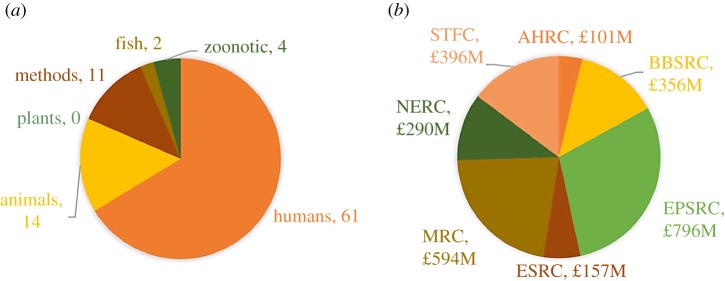
In 2015, the journal Epidemics published a range of articles describing outstanding challenges in modelling infectious disease dynamics [28], including challenges in modelling for public health policy [15], modelling livestock diseases [29] and plant disease modelling [30]. As an illustration that epidemic modelling is dominated by studies of pathogens of humans, however, in the 92 articles published in Epidemics since 2015, we find that two-thirds (61) of articles were on human diseases (figure 1a). Of the other articles, 16 were on animal (mainly livestock) or fish diseases, 11 on general methods that can be applied to a range of systems and four on zoonotic infections. In the past two years, there were no papers at all in that journal on plant disease modelling.
Figure 1.
Most epidemic models represent outbreaks of pathogens of humans, rather than plants or animals. (a) The topics of published studies in the epidemiological modelling journal Epidemics between 2015 and 2019. (b) The funding provided to research councils by the UK government in 2017/18. More funding is directed towards funders that support modelling studies of pathogens of humans rather than plants or animals. AHRC, Arts and Humanities Research Council; BBSRC, Biotechnology and Biological Sciences Research Council; EPSRC, Engineering and Physical Sciences Research Council; ESRC, Economic and Social Research Council; MRC, Medical Research Council; NERC, Natural Environment Research Council; STFC, Science and Technology Facilities Council. (Online version in colour.)
Similarly, there have been theme issues of Philosophical Transactions of the Royal Society B since 2016 on topics such as the 2013–2016 Ebola epidemic [31], marine diseases [32] and the concept of One Health [33]. Yet, despite some theme issues [34] containing studies about plant diseases [35,36], no theme issues exclusively about this topic—and about plant disease modelling in particular—have been compiled.
Despite the overall focus on public health and modelling pathogens of humans (and animals, to a lesser extent), there are a number of research groups working in plant disease epidemic modelling. As described below, these groups have tended to publish their research in journals specifically focused on plant systems such as Phytopathology [37–39], Plant Pathology [40–42] and New Phytologist [43,44], rather than more general (and typically higher-impact) journals, with a few high profile exceptions (e.g. [45,46]).
The dominance of human disease modelling is driven by several factors. First, the availability of funding (figure 1b). In the UK in 2017/8, the Medical Research Council (MRC) received more than one and half times more funding (£594 million) than either the Biotechnology and Biological Sciences Research Council (BBSRC) or the Natural Environment Research Council (NERC), both of which fund animal and plant disease modelling. Human disease modelling is also funded by a number of charitable foundations including the Wellcome Trust. Charities such as the Bill and Melinda Gates Foundation provide funding for animal and plant disease modelling (e.g. the project on West African Virus Epidemiology for Root and Tuber Crops), however they fund epidemiological studies of human pathogens to a higher extent.
Second, there is a wide range of journals that publish human disease modelling studies, and they tend to have higher impact factors. While top-tier general interest journals do accept impactful modelling studies, more specialist journals of veterinary epidemiology or plant disease, for example, tend to be lower impact than human epidemiological journals. As a result, modellers might choose to focus on human diseases. This is despite the huge impact of animal and plant diseases. For example, the annual cost of grain diseases alone in the USA has been estimated at five billion US dollars [47]. To counter this, new plant-focussed journals from high profile publishing organizations, such as the journal Nature Plants that was released in 2015, have begun to redress this balance by providing a high-impact destination for publishing a limited number of plant disease modelling studies (e.g. [48]). However, it is still the general trend that journals that publish analyses of epidemics in human populations tend to have higher impacts.
(b). The data revolution in epidemic modelling
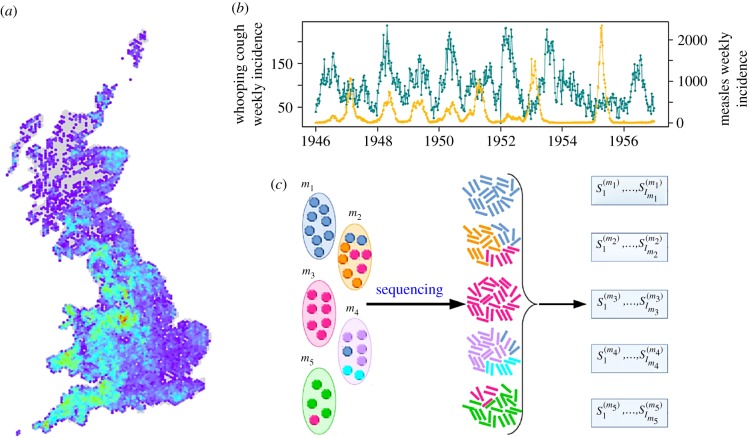
The availability of diverse and detailed data has transformed epidemic modelling over the past few decades. Early models developed to capture the epidemiological dynamics of pathogens of humans, such as the susceptible–infected–removed (SIR) model and its approximation applied to plague in India [8], were parameterized using data on the numbers of cases or deaths observed in each time period, and relied on simplifying assumptions about features such as host mixing within populations. Other studies were informed by epidemiological data such as seroprevalence surveys [49]. While simple models are powerful, more complex models require detailed data regarding, for example, the distribution of hosts in the landscape (figure 2a) or data on numbers of incident cases of multiple pathogens over long timescales (figure 2b). Multi-annual data for measles provided an early example of the richness contained in many real datasets [52], and these data are still being used to understand transmission dynamics: for example, in this theme issue, Noori and Rohani investigate the interaction between childhood diseases using data dating back to 1904 [51].
Figure 2.
Different types of data can be used to parameterize epidemiological models. (a) Data on the locations of hosts in the landscape (here the density of cattle in the UK, adapted from [50]—see that paper for further details). (b) Inference of the values of core parameters governing epidemic dynamics, such as the basic reproduction number, requires temporal data—here, time series of the numbers of new cases in each time period (adapted from [51], see that paper for further details). (c) Schematic showing how pathogen sequence data are recorded from hosts (adapted from [54], see that paper for further details). (Online version in colour.)
Other types of data, such as genomic data (figure 2c), are now used with models for outbreak investigation [53] or to infer epidemiological links (figure 3d) [54]. Although genomic data are most commonly available for human diseases (e.g. [55–57]), in this issue we also have examples of using genomic data to understand the spread of avian influenza in wild bird populations [58,59].
Figure 3.
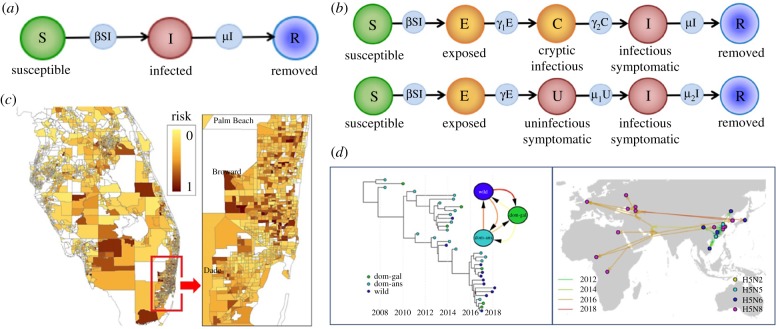
Different types of epidemiological modelling approach can be used to analyse epidemic data. (a) Schematic of the classic susceptible–infected–removed (SIR) compartmental model, in which individuals are classified according to their infection status. (b) Adapted compartmental models to include infectiousness prior to symptoms (SECIR model, e.g. [70,71]) or infectiousness after symptoms (SEUIR model, e.g. [72,73]). (c) A spatial model for predicting the risk of introduction of citrus greening (huanglongbing disease of citrus) in Florida in 2010 (adapted from [62]—see that paper for further details). (d) Phylodynamic and phylogeographic methods can be used to infer transmission routes (adapted from [59]—see that paper for further details). (Online version in colour.)
Equally important in models are the assumptions that define the demographic processes of a population. A diverse range of large-scale datasets are being repurposed to understand the interactions that lead to transmission. For example, mobile phone data have recently been used to identify travel patterns to inform epidemiological models [60,61]. In this issue, we have examples of a range of data types being used to direct model development, such as the use of census data to predict emergence sites of imported pathogens [62], data on global travel being used to consider the likelihood of a large-scale pandemic in the modern day [63], and animal movement data being used to predict pathogen spread [50]. There are challenges to repurposing data: Chaters et al. [50] describe methods for using sparse routinely collected data to define high-dimensional network models.
Perhaps most pertinent for plant diseases given the central role of environmental conditions in determining when new infections are possible, high-resolution climate data are becoming increasingly essential for capturing the impact of environmental changes on outbreak dynamics [64]. Bebber [65] uses hourly microclimate data to describe the invasion of black sigatoka disease in banana plants. Chaloner et al. [66] discuss the challenges of resolving the spatio-temporal scales of climate data with host data for septoria leaf blotch disease of wheat. We hope that these illuminating examples will lead to a more widespread interest in plant disease modelling among other epidemiological modellers.
(c). Types of modelling approach
The most commonly used epidemiological modelling approach is compartmental modelling [67]. Compartmental models can be either deterministic or stochastic, and individuals are categorized according to their infection [68] or symptom [69] status. At the beginning of this theme issue, Kleczkowski et al. [68] provide an overview of compartmental modelling, predominantly focusing on the commonly used SIR model (figure 3a). This framework has been extended to include a range of epidemiological features, including transmission between hosts via vectors—which is a route of transmission for a number of pathogens of humans, plants and animals. In this theme issue, Alonso et al. [74] consider a complex compartmental model of malaria that accounts for features such as superinfection of human hosts, and Chowell et al. [75] use a model including pathogen transmission via mosquitoes to consider the impacts of hurricanes on epidemic dynamics.
As well as being able to address questions that are common to diseases of humans, plants and animals, compartmental models can account for differences between systems comprising these different host types. As an example, cryptic infectiousness has been included in plant disease models to represent the fact that some hosts become infectious before the onset of symptoms (see [70,71] and figure 3b top), whereas simple models of Ebola virus disease in humans have incorporated the onset of infectiousness following a non-infectious but symptomatic period (see [72,73] and figure 3b bottom).
There are a number of other differences between human, animal and plant disease systems that require different epidemiological modelling approaches. Perhaps the most obvious of these is host movement. Plant populations are typically stationary and livestock are contained within strict boundaries, whereas humans have significantly more freedom of movement that permits long-distance pathogen transmission. However, plant hosts can be motile—for example in plant trade networks—and animal movement underlies the spread of a number of pathogens. Transmission of plant pathogens is also possible via the dispersal of airborne spores over long distances [76,77]. Accordingly, epidemiological models of pathogens of humans, animals and plants often include a spatial component. In this issue, there are examples of spatial livestock disease models based on cattle movements [50], a spatial model of wildlife rabies [78] and spatial models of huanglongbing disease of citrus plants [16,62] (e.g. figure 3c). There are also models of epidemics in human populations that illustrate the importance of spatial processes for reproducing real-world observations [63,79,80].
Aside from compartmental modelling, other approaches are commonly used to analyse epidemic data. Renewal process models are often used to characterize the numbers of cases of disease (e.g. [81]), and statistical models are used for forecasting numbers of cases [82] or representing processes such as disease surveillance [83]. In this issue, Bourhis et al. [84] link a statistical approach for estimating infection prevalence to the susceptible–infected compartmental model to account for temporal variations in the number of infected hosts during an outbreak.
The optimal type of model to use depends on the questions of interest in the particular host–pathogen system under consideration, and the wide range of available models reflects the increasing number of data sources described in the previous section. For example, analyses of pathogen sequencing data have been linked with models of epidemic dynamics leading to the field of phylodynamics [85,86]. This issue includes a review paper by Lycett et al. [59] in which the history of avian influenza is summarized, including the inference of transmission routes using phylodynamic methods (figure 3d).
Such a wide range of modelling approaches provides a suite of techniques for forecasting and planning control when epidemics are ongoing.
(d). Forecasting and control
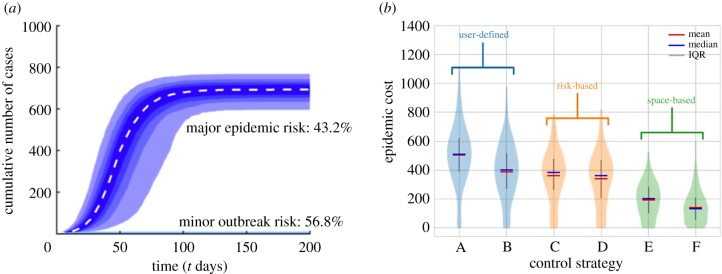
As described in §1, mathematical modelling is increasingly used in real-time when epidemics are ongoing for forecasting (figure 4a) and testing interventions (figure 4b). This has led to the field named ‘outbreak analytics’ in the context of pathogens of humans [53,88]. Whilst this term has been introduced for human diseases, there are key challenges common to any infectious disease epidemic.
Figure 4.
Forecasting and control. Epidemiological models can be used during an outbreak to: (a) forecast whether or not a major epidemic is going to occur and predict the total number of cases (adapted from [63]; see that paper for further details); (b) assess the likely impacts of an epidemic under different possible control interventions (adapted from [87]—see that paper for further details). IQR, interquartile range. (Online version in colour.)
Disease modelling often starts before a pathogen has entered a new population. Risk maps of potential introduction sites can be produced (see the submission by Gottwald et al. [62]), and these can, in turn, be used to guide where to target surveillance. Having decided where surveillance should be focused, it is also necessary to choose which diagnostic method to use. Detection is key in the early stages of an outbreak. Mastin et al. [89] present a framework for comparing different approaches for pathogen detection early in an outbreak and show that visual detection might be the optimal approach for sudden oak death in the UK.
During the initial phase of an outbreak, once the pathogen has been detected in the host population, a challenge is forecasting whether initial cases will fade out or whether they will go on to spark a major epidemic [72,90]. This question is common to pathogens in a range of systems. Using pandemic influenza as an example, Thompson et al. [63] consider the probability of a major epidemic when a pathogen arrives in a population following a prior epidemic of a related pathogen, but also note examples such as the plant pathogen Podosphaera plantaginis [45] to which their conclusions might apply.
Once an outbreak has started, it is necessary to estimate its current size from the often limited available data. Bourhis et al. [84] demonstrate how the current outbreak size can be estimated from periodically sampled data. Such data might most commonly be derived from animals and plants that are regularly checked for infection; however, these data might also be obtained when surveillance takes place at regular intervals during an epidemic in humans (e.g. village visitations to identify Ebola infections in rural areas where access to healthcare is limited [91]). A key parameter for assessing the current outbreak size early in an epidemic is the sensitivity of surveillance, which is also important at the opposite end of the epidemic for predicting whether or not the epidemic has finished once the final symptomatic infectious cases appear to have been safely removed, as demonstrated in this pair of theme issues in the context of Ebola epidemics [91].
Once a major epidemic is ongoing, modelling is used to forecast the total number of cases and to plan how and when to intervene [17]. Gaydos et al. [92] show how participatory modelling can be used for forecasting and control, using any of a number of possible types of model from conceptual models to spatial stochastic simulation models. Using stochastic simulations to assess the impact of existing interventions on epidemiological dynamics often involves exploring epidemiological dynamics with and without a proposed control strategy—a problem considered by Lessler et al. [93]. Potential interventions include prophylactic controls such as vaccination, considered in the context of wildlife rabies by Baker et al. [78] and Ebola in humans by Getz et al. [79] and movement bans, an intervention assessed in the context of livestock diseases by Chaters et al. [50]. Probert et al. [94] and Bussell et al. [87] also focus on using models to guide interventions—using reinforcement learning and optimal control theory, respectively—and these approaches could also potentially apply to pathogens irrespective of the type of host.
In any single study, the models used for forecasting and guiding control described here have tended to focus on a specific plant, animal or human disease system. However, there has been recent interest in considering entire systems holistically [33], rather than focussing solely on a limited number of interactions, which might improve forecasts and assessments of the impacts of interventions.
3. Summary of these theme issues
To encourage collaboration between modellers of infectious disease epidemics in human, animal and plant populations, in this pair of theme issues we present articles from researchers from across these fields.
In the first theme issue, papers have been contributed that include commonly used methodological approaches and explore important themes in mathematical epidemiology. Methods include compartmental epidemiological models (e.g. [68,79]) and phylodynamic/phylogenetic analyses (e.g. [58,59]). Important themes include interactions between different pathogens [51] or different strains of the same pathogen [63], pathogen evolution to escape interventions [95], and the impact of weather or climate on the dynamics of epidemics in human and animal populations [75,96] as well as plant populations [64–66].
In the second theme issue, the main focus is on how epidemiological modelling can be used in real-time during an outbreak. This includes the use of modelling to guide surveillance [62] or assess the vulnerability of a population to disease [97] just before an outbreak has started or early in an outbreak [84,89]. The use of modelling for forecasting or control [16,78,87,92,94] once a major epidemic is ongoing is also considered. There is a contribution about using models to determine when an epidemic has finished [91], as well as articles by public health decision makers about the role of quantitative approaches to guide outbreak responses [53,88].
4. Outlook
In the modern world, over a century after the devastating 1918 influenza pandemic, mathematical models are frequently used as a tool for understanding infectious disease outbreak dynamics and guiding responses to epidemics in human, animal and plant populations. A number of recent advances, such as the availability of increasingly detailed datasets (e.g. whole-genome sequences available in real-time during epidemics) and increases in computational power, are generating opportunities for understanding epidemics in more detail than ever before.
This pair of theme issues brings together research in the fields of mathematical epidemiology for human, animal and plant systems. Given the large number of questions of interest in common to modellers of epidemics in these different host types, we contend that increased collaboration will aid efficient development of methods applicable across these topics, even if certain aspects of specific models must be conditioned to the particular system being considered.
Increased modelling capabilities have been recognized by decision makers, who have recently begun to turn to modellers when epidemics are ongoing. One of the first uses of epidemic modelling to guide interventions in real-time occurred during the 2001 Foot-and-Mouth disease epidemic in the UK [11,12,22]. More recently, initiatives such as the Centers for Disease Control and Prevention ‘Predict the influenza season challenge’ [98] and the RAPIDD ‘Ebola forecasting challenge’ [99] have sought to bring together decision makers and mathematical modellers, particularly during epidemics in human populations. There has recently also been significant attention directed towards epidemic modelling, with the television documentary Contagion! The BBC Four Pandemic [61] raising public awareness in the UK, as well as events such as the 30th anniversary of World AIDS Day. There were also a number of journal highlights and special issues marking the centenary of the 1918 Spanish influenza pandemic, including a special issue of the journal Annals of Epidemiology [100] and a web focus of the journal Nature [101].
Not only might increased collaboration lead to novel modelling approaches that can be used to represent the dynamics of outbreaks in human, animal and plant populations, but it might also facilitate a ‘One Health’ approach to infectious disease management [33]. Such an integrated approach, in which multiple disciplines are considered and included in a single framework, has been widely advocated as an opportunity for improving outbreak control, with a particular focus on epidemics of zoonotic diseases [102]. Collaborative research therefore has the potential to further improve understanding of epidemic dynamics, and lead to enhanced surveillance, forecasting and disease management in future epidemics. We contend that the need for a unified approach involving modellers from the complementary fields of human, animal and plant disease epidemiology is central to this.
Acknowledgements
This pair of theme issues is dedicated to Michael Thompson, who died at the age of 59 on 15 October 2018. He had the original idea to compile a special issue of a journal to coincide (approximately!) with the centenary of the 1918 ‘Spanish flu’ pandemic, and so these theme issues would not exist without his encouragement. Thanks also to Helen Eaton for commissioning these theme issues and being available to answer our (many!) questions, Sunetra Gupta for discussions about the theme issues while they were compiled, and Nik Cunniffe for discussions about this introductory article.
Biographies
Authors' profile

Dr Robin Thompson is a Junior Research Fellow at the University of Oxford, UK, and is the lead guest editor of this pair of theme issues. His research involves using mathematical models to represent the epidemiological or evolutionary dynamics of infectious disease outbreaks in human, animal and plant populations. This includes using statistical methods to estimate parameters associated with pathogen transmission and developing stochastic or deterministic models for generating outbreak forecasts. These forward projections can be used to predict the effects of proposed control interventions. Robin has constructed models for a range of infectious diseases in human and plant populations, including Ebola virus disease, HIV, sudden oak death and citrus greening. He also recently developed a method for determining the optimal time to introduce control of an invading pathogen, with applications to diseases of livestock.

Dr Ellen Brooks-Pollock is a Lecturer at the University of Bristol, UK. She is interested in applying mathematical modelling and data science to applied questions in the control of infectious diseases. She has spent a lot of time thinking about tuberculosis (TB) in humans, bovine TB in cattle and zoonotic TB transmission from cattle to humans, but is also branching out into hepatitis A, influenza and vaccination strategies. Ellen has spoken about bovine TB on BBC 1's Countryfile and BBC Radio 4's Farming Today and sits on the Editorial board for Mathematics Today.
Data accessibility
This article does not contain any additional data.
Competing interests
We declare we have no competing interests.
Funding
R.N.T. was funded by a Junior Research Fellowship from Christ Church, Oxford. E.B.-P. was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The NIHR had no role in writing the manuscript or the decision to publish.
References
- 1.Graunt J.1662. Natural and political observations made upon the bills of mortality. London, UK: John Martyn.
- 2.Bernoulli D. 1760. Essai d'une nouvelle analyse de la mortalité causée par la petite vérole et des avantages de l'inoculation pour la prévenir. Mem. Math. Phys. Acad. R. 1, 1–45. [Google Scholar]
- 3.Farr W. 1866. On the cattle plague. J. Soc. Sci. 1, 349–351. [Google Scholar]
- 4.Snow J. 1853. On continuous molecular changes, more particularly in their relation to epidemic diseases. London, UK: John Churchill. [PubMed] [Google Scholar]
- 5.Snow J. 1855. On the mode of communication of cholera, 2nd edn London, UK: John Churchill. [Google Scholar]
- 6.Hamer WH. 1906. The Milroy lectures on epidemic disease in England: the evidence of variability and of persistency of type. London, UK: Bedford Press. [Google Scholar]
- 7.McKendrick AG. 1914. Studies on the theory of continuous probabilities, with special reference to its bearing on natural phenomena of a progressive nature. Proc. Lond. Math. Soc. 2, 401–416. ( 10.1112/plms/s2-13.1.401) [DOI] [Google Scholar]
- 8.Kermack WO, McKendrick AG. 1927. A contribution to the mathematical theory of epidemics. I. Proc. R. Soc. Lond. A 115, 700–721. ( 10.1098/rspa.1927.0118) [DOI] [Google Scholar]
- 9.Kermack WO, McKendrick AG. 1932. A contribution to the mathematical theory of epidemics. II. The problem of endemicity. Proc. R. Soc. Lond. A 138, 55–83. ( 10.1098/rspa.1932.0171) [DOI] [Google Scholar]
- 10.Kermack WO, McKendrick AG. 1933. A contribution to the mathematical theory of epidemics. III. Further studies of the problem of endemicity. Proc. R. Soc. Lond. A 141, 94–122. ( 10.1098/rspa.1933.0106) [DOI] [Google Scholar]
- 11.Ferguson NM, Donnelly CA, Anderson RM. 2001. The foot-and-mouth epidemic in Great Britain: pattern of spread and impact of interventions. Science 292, 1155–1160. ( 10.1126/science.1061020) [DOI] [PubMed] [Google Scholar]
- 12.Keeling MJ, et al. 2001. Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science 294, 813–818. ( 10.1126/science.1065973) [DOI] [PubMed] [Google Scholar]
- 13.WHO Ebola Response Team. 2014. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl. J. Med. 371, 1481–1495. ( 10.15678/EBER.2017.050110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funk S, Camacho A, Kucharski AJ, Eggo RM, Edmunds WJ. 2018. Real-time forecasting of infectious disease dynamics with a stochastic semi-mechanistic model. Epidemics 22, 56–61. ( 10.1016/j.epidem.2016.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalf CJE, Edmunds WJ, Lessler J. 2014. Six challenges in modelling for public health policy. Epidemics 10, 93–96. ( 10.1016/j.epidem.2014.08.008) [DOI] [PubMed] [Google Scholar]
- 16.McRoberts N, Figuera SG, Olkowski S, McGuire B, Luo W, Posny D, Gottwald T. 2019. Using models to provide rapid programme support for California's efforts to suppress Huanglongbing disease of citrus. Phil. Trans. R. Soc. B 374, 20180281 ( 10.1098/rstb.2018.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson RN, Gilligan CA, Cunniffe NJ. 2018. Control fast or control smart: when should invading pathogens be controlled? PLoS Comput. Biol. 14, e1006014 ( 10.1371/journal.pcbi.1006014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camacho A, et al. 2015. Temporal changes in Ebola transmission in Sierra Leone and implications for control requirements: a real-time modelling study. PLoS Curr. 7, 1–12. ( 10.1371/currents.outbreaks.406ae55e83ec0b5193e30856b9235ed2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey A, Atkins KE, Medlock J, Wenzel N, Townsend JP, Childs JE, Nyenswah TG, Ndeffo-Mbah ML, Galvani AP. 2014. Strategies for containing Ebola in West Africa. Science 346, 991–995. ( 10.1126/science.1260612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merler S, et al. 2014. Spatio-temporal spread of the Ebola 2014 outbreak in Liberia and the effectiveness of nonpharmaceutical interventions: a computational modelling analysis. Lancet Infect. Dis. 15, 204–211. ( 10.1016/S1473-3099(14)71074-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes MFC, Piontti AP, Rossi L, Chao D, Longini I, Halloran ME, Vespignani A. 2014. Assessing the international spreading risk associated with the 2014 West African Ebola outbreak. PLoS Curr. 6 ( 10.1371/currents.outbreaks.cd818f63d40e24aef769dda7df9e0da5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson NM, Donnelly CA, Anderson RM. 2001. Transmission intensity and impact of control policies on the foot and mouth epidemic in Great Britain. Nature 413, 542–548. ( 10.1038/35097116) [DOI] [PubMed] [Google Scholar]
- 23.Anderson I. 2008. Foot and Mouth disease 2007: a review and lessons learned London, UK: Stationary Office. [Google Scholar]
- 24.DEFRA. 2013. Chalara management plan. See https://www.gov.uk/government/publications/chalara-management-plan (accessed 15 April 2019).
- 25.Pimentel D, et al. 2001. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 84, 1–20. ( 10.1016/S0167-8809(00)00178-X) [DOI] [Google Scholar]
- 26.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. ( 10.1016/j.tree.2004.07.021) [DOI] [PubMed] [Google Scholar]
- 27.Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ. 2013. The consequence of tree pests and diseases for ecosystem services. Science 342, 1235773 ( 10.1126/science.1235773) [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Smith JO, Mollison D, Metcalf CJE, Klepac P, Heesterbeek JAP. 2015. Challenges in modelling infectious disease dynamics: preface. Epidemics 10, iii–iv. ( 10.1016/j.epidem.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 29.Brooks-Pollock E, de Jong MCM, Keeling MJ, Klinkenberg D, Wood JLN. 2015. Eight challenges in modelling infectious livestock diseases. Epidemics 10, 1–5. ( 10.1016/j.epidem.2014.08.005) [DOI] [PubMed] [Google Scholar]
- 30.Cunniffe NJ, Koskella B, Metcalf CJE, Parnell S, Gottwald TR, Gilligan CA. 2015. Thirteen challenges in modelling plant diseases. Epidemics 10, 6–10. ( 10.1016/j.epidem.2014.06.002) [DOI] [PubMed] [Google Scholar]
- 31.Piot P, Coltart CEM, Atkins KE. 2017. Preface: ‘The 2013–2016 West African Ebola epidemic: data, decision-making and disease control.’ Phil. Trans. R. Soc. B 372, 20170020 ( 10.1098/rstb.2017.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lafferty KD, Hofmann EE.. 2016. Marine disease impacts, diagnosis, forecasting, management and policy. Phil. Trans. R. Soc. B 371, 20150200 ( 10.1098/rstb.2015.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham AA, Scoones I, Wood JLN, Cunningham AA.. 2017. One Health for a changing world: new perspectives from Africa. Phil. Trans. R. Soc. B 372, 20160162 ( 10.1098/rstb.2016.0162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher MC, Gow NAR, Gurr SJ.. 2016. Tackling emerging fungal threats to animal health, food security and ecosystem resilience. Phil. Trans. R. Soc. B 371, 20160332 ( 10.1098/rstb.2016.0332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo D, Gurr SJ, Bebber DP.. 2016. Modelling coffee leaf rust risk in Colombia with climate reanalysis data. Phil. Trans. R. Soc. B 371, 20150458 ( 10.1098/rstb.2015.0458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godfray HCJ, D'Croz DM, Robinson S. 2016. Food system consequences of a fungal disease epidemic in a major crop. Phil. Trans. R. Soc. B 371, 20150467 ( 10.1098/rstb.2015.0467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elderfield JAD, Lopez-Ruiz FJ, van den Bosch F, Cunniffe NJ. 2018. Using epidemiological principles to explain fungicide resistance management tactics: why do mixtures outperform alternations? Phytopathology 108, 803–817. ( 10.1094/PHYTO-08-17-0277-R) [DOI] [PubMed] [Google Scholar]
- 38.Andersen K, Buddenhagen C, Rachkara P, Gibson R, Kalule S, Phillips D, Garrett KA. 2019. Modeling epidemics in seed systems and landscapes to guide management strategies: the case of sweetpotato in Northern Uganda. Phytopathology. ( 10.1094/PHYTO-03-18-0072-R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilker FM, et al. 2017. Modeling virus coinfection to inform management of maize lethal necrosis in Kenya. Phytopathology 107, 1095–1108. ( 10.1094/PHYTO-03-17-0080-FI) [DOI] [PubMed] [Google Scholar]
- 40.Mikaberidze A, Mcdonald BA, Bonhoeffer S. 2015. Developing smarter host mixtures to control plant disease. Plant Pathol. 64, 996–1004. ( 10.1111/ppa.12321) [DOI] [Google Scholar]
- 41.van den Bosch F, Lopez-Ruiz F, Oliver R, Paveley N, Helps J, van den Berg F. 2018. Identifying when it is financially beneficial to increase or decrease fungicide dose as resistance develops. Plant Pathol. 67, 549–560. ( 10.1111/ppa.12787) [DOI] [Google Scholar]
- 42.Suffert F, Thompson RN. 2018. Some reasons why the latent period should not always be considered constant over the course of a plant disease epidemic. Plant Pathol. 67, 1831–1840. ( 10.1111/ppa.12894) [DOI] [Google Scholar]
- 43.Hyatt-Twynam SR, Parnell S, Stutt ROJH, Gottwald TR, Gilligan CA, Cunniffe NJ. 2017. Risk-based management of invading plant disease. New Phytol. 214, 1317–1329. ( 10.1111/nph.14488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Djidjou-Demasse R, Moury B, Fabre F. 2017. Mosaics often outperform pyramids: insights from a model comparing strategies for the deployment of plant resistance genes against viruses in agricultural landscapes. New Phytol. 216, 239–253. ( 10.1111/nph.14701) [DOI] [PubMed] [Google Scholar]
- 45.Jousimo J, Tack AJM, Ovaskainen O, Mononen T, Susi H, Tollenaere C, Laine AL. 2014. Ecological and evolutionary effects of fragmentation on infectious disease dynamics. Science 344, 1289–1293. ( 10.1126/science.1253621) [DOI] [PubMed] [Google Scholar]
- 46.Cunniffe NJ, Cobb RC, Meentemeyer RK, Rizzo DM, Gilligan CA. 2016. Modeling when, where, and how to manage a forest epidemic, motivated by sudden oak death in California. Proc. Natl Acad. Sci. USA 113, 5640–5645. ( 10.1073/pnas.1602153113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savary S, Ficke A, Aubertot J, Hollier C. 2012. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 4, 519–537. ( 10.1007/s12571-012-0200-5) [DOI] [Google Scholar]
- 48.Meyer M, Cox JA, Hitchings MDT, Burgin L, Hort MC, Hodson DP, Gilligan CA. 2017. Quantifying airborne dispersal routes of pathogens over continents to safeguard global wheat supply. Nat. Plants 3, 780 ( 10.1038/s41477-017-0017-5) [DOI] [PubMed] [Google Scholar]
- 49.Farrington CP. 1990. Modelling forces of infection for measles, mumps and rubella. Stat. Med. 9, 953–967. ( 10.1002/sim.4780090811) [DOI] [PubMed] [Google Scholar]
- 50.Chaters GL, et al. 2019. Analysing livestock network data for infectious disease control: an argument for routine data collection in emerging economies. Phil. Trans. R. Soc. B 374, 20180264 ( 10.1098/rstb.2018.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noori N, Rohani P. 2019. Quantifying the consequences of measles-induced immune modulation for whooping cough epidemiology. Phil. Trans. R. Soc. B 374, 20180270 ( 10.1098/rstb.2018.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grenfell BT, Bjùrnstad ON, Kappey J. 2001. Travelling waves and spatial hierarchies in measles epidemics. Nature 414, 716–723. ( 10.1038/414716a) [DOI] [PubMed] [Google Scholar]
- 53.Polonsky JA, et al. 2019. Outbreak analytics: a developing data science for informing the response to emerging pathogens. Phil. Trans. R. Soc. B 374, 20180276 ( 10.1098/rstb.2018.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alamil M, Hughes J, Berthier K, Desbiez C, Thébaud G, Soubeyrand S. 2019. Inferring epidemiological links from deep sequencing data: a statistical learning approach for human, animal and plant diseases. Phil. Trans. R. Soc. B 374, 20180258 ( 10.1098/rstb.2018.0258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanini F, Brodin J, Thebo L, Lanz C, Bratt G, Albert J, Neher RA. 2015. Population genomics of intrapatient HIV-1 evolution. Elife. 4, e11282 ( 10.7554/eLife.11282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson RN, Wymant C, Spriggs RA, Raghwani J, Fraser C, Lythgoe KA. 2019. Link between the numbers of particles and variants founding new HIV-1 infections depends on the timing of transmission. Virus Evol. 5, vey038 ( 10.1093/ve/vey038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Obolski U, Gori A, Lourenço J, Thompson C, Thompson R, French N, Heyderman RS, Gupta S. 2019. Identifying genes associated with invasive disease in S. pneumoniae by applying a machine learning approach to whole genome sequence typing data. Sci. Rep. 9, 4049 ( 10.1038/s41598-019-40346-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill SC, et al. 2019. Comparative micro-epidemiology of pathogenic avian influenza virus outbreaks in a wild bird population. Phil. Trans. R. Soc. B 374, 20180259 ( 10.1098/rstb.2018.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lycett SJ, Duchatel F, Digard P. 2019. A brief history of bird flu. Phil. Trans. R. Soc. B 374, 20180257 ( 10.1098/rstb.2018.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesolowski A, et al. 2014. Quantifying travel behavior for infectious disease research: a comparison of data from surveys and mobile phones. Sci. Rep. 4, 5678 ( 10.1038/srep05678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klepac P, Kissler S, Gog J. 2018. Contagion! The BBC four pandemic—the model behind the documentary. Epidemics. Elsevier 24, 49–59. ( 10.1016/j.epidem.2018.03.003) [DOI] [PubMed] [Google Scholar]
- 62.Gottwald T, Luo W, Posny D, Riley T, Louws F. 2019. A probabilistic census-travel model to predict introduction sites of exotic plant, animal and human pathogens. Phil. Trans. R. Soc. B 374, 20180260 ( 10.1098/rstb.2018.0260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson RN, Thompson CP, Pelerman O, Gupta S, Obolski U. 2019. Increased frequency of travel in the presence of cross-immunity may act to decrease the chance of a global pandemic. Phil. Trans. R. Soc. B 374, 20180274 ( 10.1098/rstb.2018.0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah DA, Paul PA, De Wolf ED, Madden LV. 2019. Predicting plant disease epidemics from functionally represented weather series. Phil. Trans. R. Soc. B 374, 20180273 ( 10.1098/rstb.2018.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bebber DP. 2019. Climate change effects on Black Sigatoka disease of banana. Phil. Trans. R. Soc. B 374, 20180269 ( 10.1098/rstb.2018.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaloner TM, Fones HN, Varma V, Bebber DP, Gurr SJ. 2019. A new mechanistic model of weather-dependent Septoria tritici blotch disease risk. Phil. Trans. R. Soc. B 374, 20180266 ( 10.1098/rstb.2018.0266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keeling MJ, Rohani P. 2011. Modeling infectious diseases in humans and animals. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Kleczkowski A, Hoyle A, McMenemy P. 2019. One model to rule them all? Modelling approaches across OneHealth for human, animal and plant epidemics. Phil. Trans. R. Soc. B 374, 20180255 ( 10.1098/rstb.2018.0255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart WS, Hochfilzer LFR, Cunniffe NJ, Lee H, Nishiura H, Thompson RN. 2019. Accurate forecasts of the effectiveness of interventions against Ebola may require models that account for variations in symptoms during infection. bioRxiv ( 10.1101/592030) [DOI] [PubMed]
- 70.Gilligan CA. 2008. Sustainable agriculture and plant diseases: an epidemiological perspective. Phil. Trans. R. Soc. B 363, 741–759. ( 10.1098/rstb.2007.2181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunniffe NJ, Stutt ROJH, DeSimone RE, Gottwald TR, Gilligan CA. 2015. Optimising and communicating options for the control of invasive plant disease when there is epidemiological uncertainty. PLoS Comput. Biol. 11, e1004211 ( 10.1371/journal.pcbi.1004211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson RN, Gilligan CA, Cunniffe NJ. 2016. Detecting presymptomatic infection is necessary to forecast major epidemics in the earliest stages of infectious disease outbreaks. PLoS Comput. Biol. 12, e1004836 ( 10.1371/journal.pcbi.1004836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson RN, Hart WS. 2018. Effect of confusing symptoms and infectiousness on forecasting and control of Ebola outbreaks. Clin. Infect. Dis. 67, 248 ( 10.1093/cid/ciy248) [DOI] [PubMed] [Google Scholar]
- 74.Alonso D, Dobson A, Pascual M. 2019. Critical transitions in malaria transmission models are consistently generated by superinfection. Phil. Trans. R. Soc. B 374, 20180275 ( 10.1098/rstb.2018.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chowell G, Mizumoto K, Banda JM, Poccia S, Perrings C. 2019. Assessing the potential impact of vector-borne disease transmission following heavy rainfall events: a mathematical framework. Phil. Trans. R. Soc. B 374, 20180272 ( 10.1098/rstb.2018.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown JKM, Hovmøller MS. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297, 537–541. ( 10.1126/science.1072678) [DOI] [PubMed] [Google Scholar]
- 77.Thompson RN, Cobb RC, Gilligan CA, Cunniffe NJ. 2016. Management of invading pathogens should be informed by epidemiology rather than administrative boundaries. Ecol. Modell. 324, 28–32. ( 10.1016/j.ecolmodel.2015.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker L, Matthiopoulos J, Müller T, Freuling C, Hampson K. 2019. Optimizing spatial and seasonal deployment of vaccination campaigns to eliminate wildlife rabies. Phil. Trans. R. Soc. B 374, 20180280 ( 10.1098/rstb.2018.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Getz WM, Salter R, Mgbara W. 2019. Adequacy of SEIR models when epidemics have spatial structure: Ebola in Sierra Leone. Phil. Trans. R. Soc. B 374, 20180282 ( 10.1098/rstb.2018.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rushton SP, Sanderson RA, Reid WDK, Shirley MDF, Harris JP, Hunter PR, O'Brien SJ. 2019. Transmission routes of rare seasonal diseases: the case of norovirus infections. Phil. Trans. R. Soc. B 374, 20180267 ( 10.1098/rstb.2018.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nouvellet P, et al. 2018. A simple approach to measure transmissibility and forecast incidence. Epidemics 22, 29–35. ( 10.1016/j.epidem.2017.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly JD, et al. 2018. Projections of Ebola outbreak size and duration with and without vaccine use in Equateur, Democratic Republic of Congo, as of May 27, 2018. PLoS ONE 14, e0213190 ( 10.1371/journal.pone.0213190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cameron AR, Baldock FC. 1998. A new probability formula for surveys to substantiate freedom from disease. Prev. Vet. Med. 34, 1–17. ( 10.1016/S0167-5877(97)00081-0) [DOI] [PubMed] [Google Scholar]
- 84.Bourhis Y, Gottwald T, van den Bosch F. 2019. Translating surveillance data into incidence estimates. Phil. Trans. R. Soc. B 374, 20180262 ( 10.1098/rstb.2018.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grenfell BT, Pybus OG, Gog JR, Wood JLN, Daly JM, Mumford JA, Holmes EC. 2004. Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303, 327–333. ( 10.1126/science.1090727) [DOI] [PubMed] [Google Scholar]
- 86.Frost SDW, Pybus OG, Gog JR, Viboud C, Bonhoeffer S, Bedford T. 2015. Eight challenges in phylodynamic inference. Epidemics 10, 88–92. ( 10.1016/j.epidem.2014.09.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bussell EH, Dangerfield CE, Gilligan CA, Cunniffe NJ. 2019. Applying optimal control theory to complex epidemiological models to inform real-world disease management. Phil. Trans. R. Soc. B 374, 20180284 ( 10.1098/rstb.2018.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgan O. 2019. How decision makers can use quantitative approaches to guide outbreak responses. Phil. Trans. R. Soc. B 374, 20180365 ( 10.1098/rstb.2018.0365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mastin AJ, van den Bosch F, van den Berg F, Parnell SR. 2019. Quantifying the hidden costs of imperfect detection for early detection surveillance. Phil. Trans. R. Soc. B 374, 20180261 ( 10.1098/rstb.2018.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Craft ME, Beyer HL, Haydon DT. 2013. Estimating the probability of a major outbreak from the timing of early cases: an indeterminate problem? PLoS ONE 8, e57878 ( 10.1371/journal.pone.0057878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson RN, Morgan OW, Jalava K. 2019. Rigorous surveillance is necessary for high confidence in end-of-outbreak declarations for Ebola and other infectious diseases. Phil. Trans. R. Soc. B 374, 20180431 ( 10.1098/rstb.2018.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaydos DA, Petrasova A, Cobb RC, Meentemeyer RK. 2019. Forecasting and control of emerging infectious forest disease through participatory modelling. Phil. Trans. R. Soc. B 374, 20180283 ( 10.1098/rstb.2018.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaminsky J, Keegan LT, Metcalf CJE, Lessler J. 2019. Perfect counterfactuals for epidemic simulations. Phil. Trans. R. Soc. B 374, 20180279 ( 10.1098/rstb.2018.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Probert WJM, Lakkur S, Fonnesbeck CJ, Shea K, Runge MC, Tildesley MJ, Ferrari MJ. 2019. Context matters: using reinforcement learning to develop human-readable, state-dependent outbreak response policies. Phil. Trans. R. Soc. B 374, 20180277 ( 10.1098/rstb.2018.0277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rousseau E, Bonneault M, Fabre F, Moury B, Mailleret L, Grognard F. 2019. Virus epidemics, plant-controlled population bottlenecks and the durability of plant resistance. Phil. Trans. R. Soc. B 374, 20180263 ( 10.1098/rstb.2018.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akhmetzhanov AR, Asai Y, Nishiura H. 2019. Quantifying the seasonal drivers of transmission for Lassa fever in Nigeria. Phil. Trans. R. Soc. B 374, 20180268 ( 10.1098/rstb.2018.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kajero O, Del Rio Vilas V, Wood JLN, Lo Iacono G. 2019. New methodologies for the estimation of population vulnerability to diseases: a case study of Lassa fever and Ebola in Nigeria and Sierra Leone. Phil. Trans. R. Soc. B 374, 20180265 ( 10.1098/rstb.2018.0265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biggerstaff M, et al. 2016. Results from the centers for disease control and prevention's predict the 2013–2014 influenza season challenge. BMC Infect. Dis. 16, 357 ( 10.1186/s12879-016-1669-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viboud C, Sun K, Gaffey R, Ajelli M, Fumanelli L. 2018. The RAPIDD Ebola forecasting challenge: synthesis and lessons learnt. Epidemics 22, 13–21. ( 10.1016/j.epidem.2017.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Influenza Pandemic Symposium. 2018. Introduction to symposium: a century after the 1918 influenza pandemic. Ann. Epidemiol. 28, 265–266. ( 10.1016/j.annepidem.2018.03.010) [DOI] [PubMed] [Google Scholar]
- 101.Web focus: 1918 influenza pandemic [Internet]. See https://www.nature.com/collections/dpjcqwsqts#rese.
- 102.World Bank. 2010. People, pathogens, and Our planet, vol. 1: towards a One health approach for controlling zoonotic diseases. Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.