Abstract
Background:
Opioids may increase cancer risk and progression through multiple pathways. Our objective was to estimate the association between chronic opioid use and risk of second breast cancer events (SBCEs).
Methods:
Cohort study of women ≥18 years, diagnosed with early stage breast cancer between January 1, 1990 and December 31, 2008, and enrolled in a large health plan for 1+ years before and after (unless died) diagnosis. SBCEs were defined as evidence of recurrence or second primary breast cancer in the medical chart. Chronic opioid use was defined as 75+ days of use in any moving 90-day window after breast cancer diagnosis and varied to 150+ days in a 180-day window in a sensitivity analysis. Using Cox proportional hazards models, we estimated hazard ratios (HR) and 95% confidence intervals (CI) for SBCE and components of SBCE by chronic opioid use.
Results:
Almost 10% met the criteria for chronic use and almost a third of users were taking opioids for > 3 years. Risk of SBCEs (HR=1.20; 95% CI: 0.85–1.70), including second primary breast cancer (HR=1.38; 95% CI: 0.71–2.70), was non-significantly higher among chronic users vs non-chronic/non-users. The HR for recurrence was 1.14 (95% CI, 0.76–2.70). Results of the sensitivity analyses on longer opioid use does support an association with SBCE or recurrence.
Conclusion:
This first US-based study on chronic opioid use and cancer outcomes provides some reassurance on safety. However, the question warrants further exploration in other populations and settings.
Keywords: Breast cancer, opioids, recurrence, survivorship
Introduction
Opioids are one of the most commonly prescribed drug classes in the United States (US).1 The US observed a 50–79% increase in use between 2001 and 2010. In people with cancer, a population where pain is prevalent during and often after treatment, opioid use may be even higher. The long-term effects of opioid use in cancer survivors are unknown, but several biological mechanisms support the hypothesis that opioid use may increase risk of cancer progression and recurrence. 2,3
Opioids bind directly to opioid receptors in cells. μ-Opioid receptor overexpression, which may influence tumor growth and cancer progression, was noted in several cancers4–6 including non-small cell lung, prostate, and breast. However, studies of high morphine doses in mice do not demonstrated tumor progression.5,6 Opioids were shown to stimulate angiogenesis in some, but not all studies of human endothelial cells and mice.7 Contrary to this, opioids enhance apoptosis in breast cancer, small cell lung cancer, and prostate cancer cells, suggesting opioids may have anti-cancer effects.7 Finally, opioids may suppress immune function, in particular natural killer cells, which spontaneously recognize and kill a variety of tumor cells.4–7 Opioids may also increase concentrations of vascular endothelial growth factor, which increases angiogenesis and cell migration. Increases in tumor metastasis with opioids were observed with progression of lung cancer in cell and animal models,8 and with fentanyl in rats.9
The little evidence available in humans is primarily focused on the association between anesthetic techniques with or without opioids during oncologic surgery and cancer survival.10–13 Two studies suggest that patients receiving opioids during surgical removal of breast and prostate cancer tumors have higher recurrence rates, likely through immune suppression, compared to patients receiving paravertebral analgesia.12,13 Two other studies failed to find an association between epidural analgesia with opioids and cancer-free survival.10,11
We are aware of only one study on post-diagnosis opioid use and long-term cancer outcomes.14 The study was conducted in Denmark and found no associations between any use of opioids or chronic use and risk of breast cancer recurrence. The results may not be generalizable to US populations because of differences that include variation in types of commonly prescribed opioids.
Any effect of opioids on cancer outcomes has implications for pain management of cancer or non-cancer pain in cancer survivors. Using data from an existing cohort, we examined the trend in regular use of opioids and the association between chronic use and second breast cancer events (SBCE). Given the opioid epidemic and increasing number of cancer survivors, this is a timely, understudied research question of public health importance.
METHODS
Population and Setting
The parent study, COmmonly Used Medications and Breast Cancer Outcomes (COMBO) is a cohort study within Kaiser Permanente Washington (KPWA) (formerly Group Health Cooperative).15,16 KPWA is a nonprofit integrated delivery system that provides comprehensive health care and insurance to approximately 600,000 individuals in Washington State. KPWA is located within the geographic reporting region of the western Washington Cancer Surveillance System, a population-based cancer registry and member of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program.17,18 The COMBO cohort included women ≥18 years, diagnosed with histologically confirmed unilateral stage I or II breast cancer between January 1, 1990 and December 31, 2008, enrolled at KPWA for at least 1 year before and after (unless died) diagnosis, definitive surgery, alive and recurrence-free for 120 days after surgery, and having a medical record for review.15,16 The final cohort included 4,216 women.
The KPW Institutional Review Board approved the study with a waiver of consent.
Data collection
Data were obtained from medical record review, SEER tumor registry, and electronic health records (EHR) from one year prior to incident breast cancer diagnosis through the end of follow-up defined as the earliest of death, disenrollment from KPWA, or end of study (i.e., chart abstraction date). The EHR includes demographics, enrollment, inpatient and outpatient diagnoses, procedures including breast imaging and results, pharmacy dispensings, laboratory results, vital signs, and death.19 The pharmacy database includes all medications dispensed at KPW’s outpatient pharmacies as well as claims from contracting pharmacies. Pharmacy data are estimated to be 97% complete.19–21 Death data are obtained from Washington State death certificates, internal sources, and SEER.
Opioid exposure
We identified all opioids dispensed in the year before breast cancer diagnosis through the end of follow-up. The date when the dispensing should run out (runout date) was estimated for each dispensing based on the date of the dispensing plus the days supply. Successive dispensings with ≤2-day gap between the runout date of one dispensing and dispensing date of the subsequent were considered a continuous episode of use. For each day in a continuous use episode, women were considered to have possession of opioids.
To evaluate trends in regular opioid use, a woman was categorized as a regular user (yes, no) in each fixed and independent calendar quarter pre- and post-breast cancer diagnosis if she was in possession of opioids for at least 45 days, regardless of whether it was continuous, in the quarter of interest (Figure 1). Classification as a regular user in each fixed quarter was independent of classification in the other quarters but days of opioid use that extended from one quarter into a subsequent quarter were counted as part of the subsequent quarter.
Figure 1.
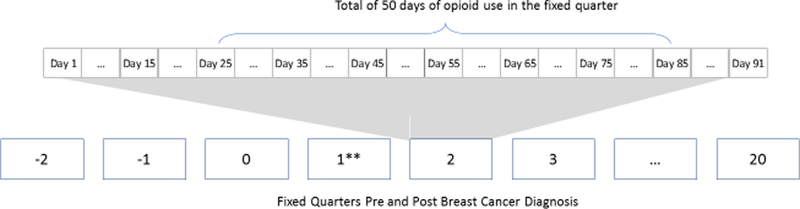
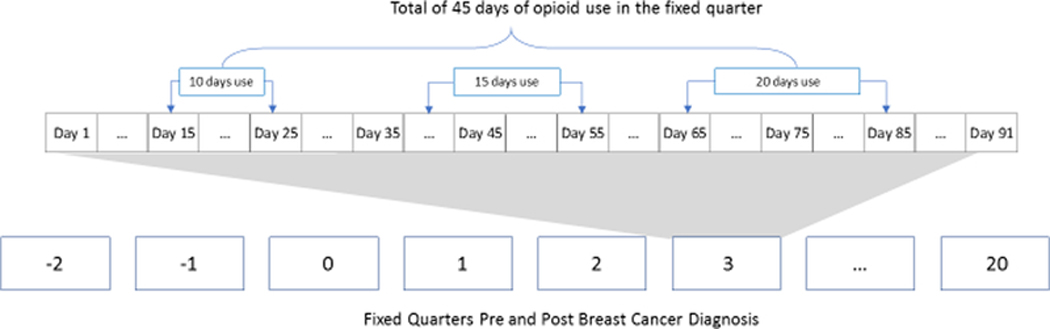
Two examples of regular opioid use per fixed quarters pre- and post-incident breast cancer diagnosis. Regular use defined as 45+ days of opioid use in fixed and independent quarters of interest. Not to scale.
*Regular user in a fixed quarter if 45+ days of opioid use in the fixed quarter of interest. Count starts over at the beginning of each quarter.
**Q1 = quarter of incident brest cancer diagnosis
Chronic opioid therapy after breast cancer diagnosis was our exposure of interest in relation to risk of SBCE. Women were defined, in a time-varying manner, as a chronic user if they had 75+ days possession of opioids within any rolling 90-day window starting at diagnosis through end of follow-up (Figure 2). This is a commonly used definition of chronic opioid therapy 22–25 A moving 90-day window was scanned over the follow-up period, and days with possession of opioids were summed up within each rolling 90-day time window. We lagged exposure by 6-months to reduce protopathic bias. Therefore, women were considered chronic users from 6 months after the day when they accumulated 75 days of opioid possession in a 90-day window and remained as exposed through the end of follow-up. Chronic opioid use in the year prior to breast cancer diagnosis was also estimated by applying the definition above to the year prior to incident breast cancer diagnosis.
Figure 2.
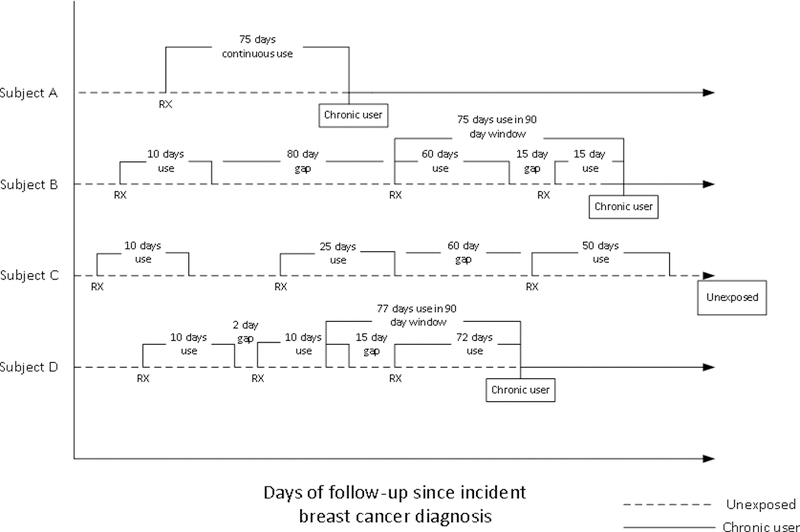
Examples of chronic opioid use post incident breast cancer diagnosis through end of follow-up. Chronic use defined as 75+ days of use in any rolling 90-day window following diagnosis. Not to scale.
Daly dose in morphine equivalent milligrams (MME) was calculated for each dispensing by first multiplying strength, quantity dispensed and a drug-specific conversion factor and then dividing by days supply.26 Based on guidelines,26 we defined high dose as an average daily dose of at least 90 MME/day, mid -dose as 20–90 MME/day and low dose as under 20 MME/day.
Our referent group was non-chronic opioid users and non-users. Ninety-five percent of the referent group filled at least one opioid and we therefore refer to the referent group as non-chronic users.
Outcomes
SBCE were defined as the first of a ductal carcinoma in situ or invasive cancer of the ipsilateral (recurrence) or contralateral (second primary) breast or in any regional or distant sites.27 A woman was at risk for a SBCE starting 120 days after completing definitive surgery for the incident breast cancer.28
Statistical Analysis
Prevalence of regular opioid use (45+ days of use per quarter) was plotted on a histogram for the three quarters prior to breast cancer diagnosis through the last quarter with a full 91 days of follow-up and until 6 months prior to the censoring date. The date of censoring was the earliest of 5 years post diagnosis, first SBCE, death, disenrollment from the health plan, or end of study. Women without at least 6 months of follow-up were excluded from this analysis (n=47). Trends in the number of regular opioid users were estimated from the three fixed quarters prior to incident breast cancer diagnosis (i.e., Quarter 0 was 91 days prior to diagnosis to the day before diagnosis) through the subsequent 20 fixed quarters (5-years) post breast cancer diagnosis (i.e., Quarter 1 was from cancer diagnosis day to the 90th day after cancer diagnosis).
We compared patient, tumor, and treatment characteristics by outcome and by exposure. We estimated the adjusted hazard ratios (HR) and 95% confidence intervals (CI) using the cause-specific Cox proportional hazards model to assess whether chronic use was associated with risk of SBCEs while accounting for competing risks.29 We modeled time from the incident breast cancer with a delayed entry at 120 days post-surgery28 to SBCE as a function of a time-varying chronic opioid exposure while adjusting for potential confounders. Women were followed until the earliest of SBCE, death, disenrollment from the health plan, or end of study. We also modeled recurrences and second primaries separately to obtain a comprehensive assessment of outcomes.29 In analysis of individual events (e.g., recurrence) women were censored at the earliest of disenrollment, end of follow-up, and other competing events (e.g., death and second primary). Chronic use was modeled as time-varying and women were only allowed unidirectional transition (i.e., non-chronic user to chronic user).
Potential confounders were determined a priori. The minimally adjusted model included age at incident breast cancer diagnosis and American Joint Committee on Cancer (AJCC) stage. Similar to other studies and the COMBO cohort, 15,16,30–34 fully adjusted models included age, AJCC stage, calendar year,35 hormone receptor, primary breast cancer treatment, body mass index (BMI), smoking status, and menopausal status -- all of which were defined at the time of the incident breast cancer diagnosis -- as well as the time-varying covariates including endocrine therapy, Charlson co-morbidity score,36 diabetes,37–39 non-steroidal anti-inflammatory medication use, and receipt of surveillance mammogram in the prior 12 months. Chronic use in the year prior to breast cancer diagnosis was highly correlated with chronic use post diagnosis (i.e., 68 of the 87 chronic users) pre-diagnosis were chronic users post diagnosis) and therefore we did not adjust for prior chronic opioid use.
Using methods similar to the main analysis described above, we conducted three sensitivity analysis. We modeled chronic use as 150+ days in any 180-day window, lagged exposure by 12-months, and excluded surveillance mammography as a covariate in the model.
Proportional hazards assumptions were evaluated by testing the interaction between the exposure variable and the logarithm of follow-up time. There was no evidence suggesting a violation of the proportional hazards assumption. All analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Median age of the cohort at incident breast cancer diagnosis was 63 years. The majority of women were post-menopausal, Caucasian, non-Hispanic, never smokers, and had at least some college education and a Charlson co-morbidity score of zero (Table 1). The majority were AJCC stage I, lymph node negative, estrogen receptor (ER)+/progesterone receptor (PR)+, ≤2 cm in size, HER-2 negative (if tested), treated with breast conserving surgery +/− radiation, not treated with chemotherapy, and treated with endocrine therapy. Median follow-up was 6.3 years (Interquartile range 3.7–9.7 years).
Table 1.
Characteristics of women included in the COMBO study, overall and by second breast cancer event status
| Characteristics | All | SBCE* | ||||
|---|---|---|---|---|---|---|
| n=4216 | No (n=3658) | Yes (n=558) | ||||
| n | (column %) | n | (column %) | n | (column %) | |
| At incident breast cancer diagnosis | ||||||
| Year of diagnosis | ||||||
| 1990–1994 | 950 | (22.5) | 755 | (20.6) | 195 | (34.9) |
| 1995–1999 | 1191 | (28.2) | 1020 | (27.9) | 171 | (30.6) |
| 2000–2004 | 1201 | (28.5) | 1073 | (29.3) | 128 | (22.9) |
| 2005–2008 | 874 | (20.7) | 810 | (22.1) | 64 | (11.5) |
| Age (years) | ||||||
| Median (Interquartile range) | 63 | (52–73) | 63 | (52–73) | 62 | (50–72) |
| 18–39 | 139 | (3.3) | 112 | (3.1) | 27 | (4.8) |
| 40–49 | 646 | (15.3) | 544 | (14.9) | 102 | (18.3) |
| 50–59 | 995 | (23.6) | 866 | (23.7) | 129 | (23.1) |
| 60–69 | 1018 | (24.1) | 889 | (24.3) | 129 | (23.1) |
| 70–79 | 940 | (22.3) | 824 | (22.5) | 116 | (20.8) |
| 80+ | 478 | (11.3) | 423 | (11.6) | 55 | (9.9) |
| Menopausal status | ||||||
| Peri- or Pre-menopausal | 1145 | (27.2) | 956 | (26.1) | 189 | (33.9) |
| Post-menopausal | 3071 | (72.8) | 2702 | (73.9) | 369 | (66.1) |
| Race | ||||||
| White | 3719 | (88.5) | 3232 | (88.7) | 487 | (87.3) |
| African American | 136 | (3.2) | 104 | (2.9) | 32 | (5.7) |
| American Indian/Alaska Native | 113 | (2.7) | 104 | (2.9) | 9 | (1.6) |
| Asian/Pacific Islander | 233 | (5.5) | 203 | (5.6) | 30 | (5.4) |
| Unknown | 15 | 15 | 0 | |||
| Hispanic ethnicity | ||||||
| Non-Hispanic | 3976 | (94.6) | 3438 | (94.3) | 538 | (96.4) |
| Hispanic | 229 | (5.4) | 209 | (5.7) | 20 | (3.6) |
| Unknown | 11 | 11 | 0 | |||
| Education | ||||||
| High school or less | 418 | (23.4) | 393 | (23.5) | 25 | (21.4) |
| Some college | 634 | (35.4) | 594 | (35.5) | 40 | (34.2) |
| College or post graduates | 737 | (41.2) | 685 | (41) | 52 | (44.4) |
| Unknown | 2427 | 1986 | 441 | |||
| Body mass index (kg/m2) | ||||||
| <18.5 | 69 | (1.6) | 55 | (1.5) | 14 | (2.5) |
| 18.5–24.9 | 1453 | (34.6) | 1269 | (34.8) | 184 | (33.3) |
| 25–29.9 | 1362 | (32.5) | 1186 | (32.6) | 176 | (31.8) |
| 30–34.9 | 766 | (18.3) | 666 | (18.3) | 100 | (18.1) |
| 35+ | 546 | (13) | 467 | (12.8) | 79 | (14.3) |
| Unknown | 20 | 15 | 5 | |||
| Smoking status | ||||||
| Current | 253 | (6.0) | 230 | (6.3) | 23 | (4.1) |
| Past | 352 | (8.3) | 318 | (8.7) | 34 | (6.1) |
| Never/Unknown | 3611 | (85.6) | 3110 | (85.0) | 501 | (89.8) |
| AJCC stage | ||||||
| I | 2648 | (62.8) | 2384 | (65.2) | 264 | (47.3) |
| IIA | 1078 | (25.6) | 906 | (24.8) | 172 | (30.8) |
| IIB | 490 | (11.6) | 368 | (10.1) | 122 | (21.9) |
| Lymph node | ||||||
| Negative | 2847 | (75.6) | 2525 | (77.4) | 322 | (64.3) |
| Positive | 918 | (24.4) | 739 | (22.6) | 179 | (35.7) |
| Unknown | 451 | 394 | 57 | |||
| ER/PR status** | ||||||
| ER-/PR- | 667 | (16.7) | 531 | (15.3) | 136 | (25.7) |
| ER+/PR- | 383 | (9.6) | 319 | (9.2) | 64 | (12.1) |
| ER-/PR+ | 61 | (1.5) | 47 | (1.4) | 14 | (2.6) |
| ER+/PR+ | 2888 | (72.2) | 2572 | (74.1) | 316 | (59.6) |
| ER and/or PR unknown | 217 | 189 | 28 | |||
| Tumor size | ||||||
| ≤2cm | 3110 | (73.8) | 2785 | (76.1) | 325 | (58.5) |
| >2cm | 1104 | (26.2) | 873 | (23.9) | 231 | (41.5) |
| Unknown | 2 | 0 | 2 | |||
| HER2 status | ||||||
| Test done | 2074 | (49.2) | 1874 | (51.2) | 200 | (35.8) |
| Positive/borderline | 353 | (17.0) | 311 | (16.6) | 42 | (21.0) |
| Negative | 1714 | (82.6) | 1556 | (83.0) | 158 | (79.0) |
| No result | 7 | (0.3) | 7 | (0.4) | 0 | (0) |
| Surgical treatment | ||||||
| Mastectomy including radical ± radiation | 1521 | (36.1) | 1289 | (35.2) | 232 | (41.6) |
| Breast conserving, + radiation | 2172 | (51.5) | 1927 | (52.7) | 245 | (43.9) |
| Breast conserving, no radiation | 523 | (12.4) | 442 | (12.1) | 81 | (14.5) |
| Other treatment | ||||||
| Any chemotherapy | 1376 | (32.6) | 1142 | (31.2) | 234 | (41.9) |
| Any endocrine therapy | 2363 | (56.0) | 2101 | (57.4) | 262 | (47.0) |
| Tamoxifen only | 1394 | (59.0) | 1297 | (61.7) | 97 | (37.0) |
| Aromatase inhibitors only | 288 | (12.2) | 275 | (13.1) | 13 | (5.0) |
| Both tamoxifen and aromatase inhibitors | 673 | (28.5) | 522 | (24.9) | 151 | (57.6) |
| Unknown | 8 | (0.3) | 7 | (0.3) | 1 | (0.4) |
| Charlson co-morbidity score | ||||||
| 0 | 3229 | (76.6) | 2784 | (76.1) | 445 | (79.7) |
| 1 | 704 | (16.7) | 625 | (17.1) | 79 | (14.2) |
| 2+ | 283 | (6.7) | 249 | (6.8) | 34 | (6.1) |
| Throughout study follow-up*** | ||||||
| Years of follow-up, Median (interquartile range) | 6.3 | (3.7–9.7) | 6.7 | (4.2–10.2) | 3.3 | (1.8–5.9) |
| Diabetes | 610 | (14.5) | 539 | (14.7) | 71 | (12.7) |
| % Follow-up years with yearly surveillance mammography | ||||||
| <50% | 939 | (22.3) | 793 | (21.7) | 146 | (26.2) |
| 50–79% | 1439 | (34.1) | 1284 | (35.1) | 155 | (27.8) |
| 80%+ | 1838 | (43.6) | 1581 | (43.2) | 257 | (46.1) |
| Pain conditions | ||||||
| Abdominal | 1875 | (44.5) | 1690 | (46.2) | 185 | (33.2) |
| Arthritis/gout | 2993 | (71.0) | 2700 | (73.8) | 293 | (52.5) |
| Back | 1074 | (25.5) | 987 | (27.0) | 87 | (15.6) |
| Chest | 2077 | (49.3) | 1876 | (51.3) | 201 | (36.0) |
| Fibromyalgia | 706 | (16.8) | 633 | (17.3) | 73 | (13.1) |
| General chronic | 163 | (3.9) | 157 | (4.3) | 6 | (1.1) |
| Limb/extremity | 1916 | (45.4) | 1745 | (47.7) | 171 | (30.6) |
| Neck | 369 | (8.8) | 327 | (8.9) | 42 | (7.5) |
| Neuropathic | 278 | (6.6) | 254 | (6.9) | 24 | (4.3) |
| Pelvic | 52 | (1.2) | 42 | (1.2) | 10 | (1.8) |
| Migraine/TMJ | 1213 | (28.8) | 1099 | (30.0) | 114 | (20.4) |
SBCE=second breast cancer event includes recurrence or second primaries, in-situ and invasive
ER/PR=Estrogen receptor/progesterone receptor
Earliest of SBCE, death, disenrollment from health plan, or end of study period.
In Figure 3, we display the number of women regularly using opioids in each quarter before and after incident breast cancer diagnosis. The proportion varied from a low of 2.0% in two quarters prior to incident breast cancer diagnosis to a high of 5.0% in the quarter which breast cancer was diagnosed. It consistently hovered around the median of 3.2% during the years following diagnosis and treatment.
Figure 3.
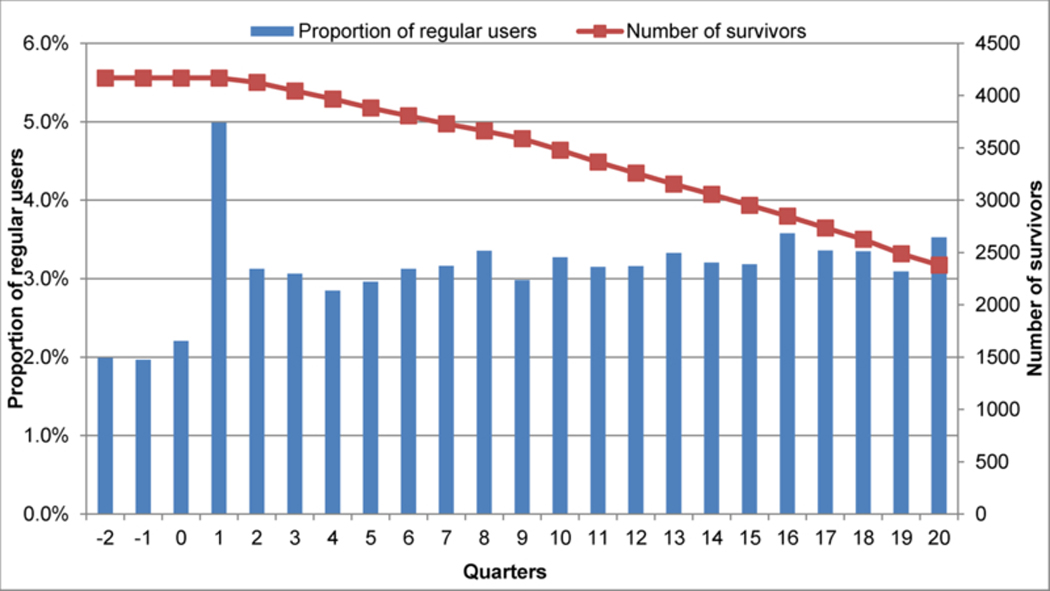
The proportion of regular opioid users, defined as 45+ days of use in each fixed 91-day quarter, during the quarters pre (0, −1, and −2) and post (quarters 1–20) incident breast cancer diagnosis. The red line denotes the number of breast cancer survivors at each quarter.
*Regular opioid use defined as having 45+ days of use in each 91-days quarter.Quarter #1 is the quarter in which the incident brest cancer was diagnosed.
Among the 4,216 eligible women, 558 (13.2%) experienced a SBCE (first of: 415 recurrences and 143 second primary breast cancers). Median time to the first SBCE was 3 years. Among recurrences, 67% were distant, 32% local or regional, and 1% DCIS. Among second primary breast cancers, 21% were DCIS, 49% stage I, 21% stage II, 4% stage III/IV, and 5% unknown stage.
Women with a SBCE were more likely to be peri- or premenopausal, AJCC stage II, lymph node positive, ER and/or PR negative, tumor size > 2 cm, HER-2 positive, treated with mastectomy, treated with chemotherapy, and not treated with endocrine therapy than women without a SBCE (Table 1). Women with SBCEs had few pain diagnoses than disease free women during follow-up.
Approximately 9.7% of women (n=410) met the definition of chronic opioid use during follow-up (Table 2). The most commonly dispensed opioids among the chronic users were oxycodone (33%), hydrocodone (31%), codeine (14%), and morphine (9%). The median duration of use was 23.5 months (interquartile range (IQR): 12.9–42.1). Approximately 45% of chronic users had 1–3 years of use and 32% >3 years of use. The median duration of use in the non-chronic user referent group was 0.8 months (IQR: 0.3–1.9). We did not examine SBCE by duration of opioid use because of large differences in follow up time (i.e., censoring at SBCEs) and small numbers. Compared to non-chronic users, chronic users were older, less educated, and more likely to be menopausal and obese. Chronic users also had more extensive breast surgery and more comorbidities including diabetes than non-users. Chronic users on endocrine therapy were less likely to use aromatase inhibitors than non-chronic users. Adherence to screening surveillance was lower among chronic users than non-chronic users. Chronic users had more diagnoses of pain conditions than non-chronic users.
Table 2.
Characteristics of women included in the COMBO study, overall and by chronic opioid use after breast cancer diagnosis
| Characteristics | Chronic opioid use* | |||
|---|---|---|---|---|
| No (n=3,806) | Yes (n=410) | |||
| n | (column %) | n | (column %) | |
| At incident breast cancer diagnosis | ||||
| Year of diagnosis | ||||
| 1990–1994 | 858 | (22.5) | 92 | (22.4) |
| 1995–1999 | 1062 | (27.9) | 129 | (31.5) |
| 2000–2004 | 1065 | (28) | 136 | (33.2) |
| 2005–2008 | 821 | (21.6) | 53 | (12.9) |
| Age (years) | ||||
| Median (Interquartile range) | 62 | (51–72) | 68 | (58–76) |
| 18–39 | 131 | (3.4) | 8 | (2) |
| 40–49 | 616 | (16.2) | 30 | (7.3) |
| 50–59 | 917 | (24.1) | 78 | (19) |
| 60–69 | 905 | (23.8) | 113 | (27.6) |
| 70–79 | 820 | (21.5) | 120 | (29.3) |
| 80+ | 417 | (11) | 61 | (14.9) |
| Menopausal status | ||||
| Peri- or Pre-menopausal | 1073 | (28.2) | 72 | (17.6) |
| Post-menopausal | 2733 | (71.8) | 338 | (82.4) |
| Race | ||||
| White | 3344 | (88.2) | 375 | (91.7) |
| African American | 120 | (3.2) | 16 | (3.9) |
| American Indian/Alaska Native | 101 | (2.7) | 12 | (2.9) |
| Asian/Pacific Islander | 227 | (6) | 6 | (1.5) |
| Unknown | 14 | 1 | ||
| Hispanic ethnicity | ||||
| Non-Hispanic | 3579 | (94.3) | 397 | (96.8) |
| Hispanic | 216 | (5.7) | 13 | (3.2) |
| Unknown | 11 | 0 | (0) | |
| Education | ||||
| High school or less | 369 | (22.4) | 49 | (34.3) |
| Some college | 586 | (35.6) | 48 | (33.6) |
| College or post graduates | 691 | (42) | 46 | (32.2) |
| Unknown | 2160 | 267 | ||
| Body mass index (kg/m2) | ||||
| <18.5 | 62 | (1.6) | 7 | (1.7) |
| 18.5–24.9 | 1349 | (35.6) | 104 | (25.4) |
| 25–29.9 | 1236 | (32.6) | 126 | (30.7) |
| 30–34.9 | 673 | (17.8) | 93 | (22.7) |
| 35+ | 466 | (12.3) | 80 | (19.5) |
| Unknown | 20 | 0 | (0) | |
| Smoking status | ||||
| Current | 221 | (5.8) | 32 | (7.8) |
| Past | 321 | (8.4) | 31 | (7.6) |
| Never/Unknown | 3264 | (85.8) | 347 | (84.6) |
| AJCC stage | ||||
| I | 2395 | (62.9) | 253 | (61.7) |
| IIA | 977 | (25.7) | 101 | (24.6) |
| IIB | 434 | (11.4) | 56 | (13.7) |
| Lymph node | ||||
| Negative | 2586 | (75.9) | 261 | (73.3) |
| Positive | 823 | (24.1) | 95 | (26.7) |
| Unknown | 397 | 54 | ||
| ER/PR status** | ||||
| ER-/PR- | 612 | (16.9) | 55 | (14.3) |
| ER+/PR- | 342 | (9.5) | 41 | (10.7) |
| ER-/PR+ | 54 | (1.5) | 7 | (1.8) |
| ER+/PR+ | 2607 | (72.1) | 281 | (73.2) |
| ER and/or PR unknown | 191 | 26 | ||
| Tumor size | ||||
| ≤2cm | 2812 | (73.9) | 298 | (72.7) |
| >2cm | 992 | (26.1) | 112 | (27.3) |
| Unknown | 2 | 0 | (0) | |
| HER2 status | ||||
| Test done | 1888 | (49.6) | 186 | (45.4) |
| Positive/borderline | 328 | (17.4) | 25 | (13.4) |
| Negative | 1553 | (82.3) | 161 | (86.6) |
| No result | 7 | (0.4) | 0 | (0) |
| Surgical treatment | ||||
| Mastectomy including radical ± radiation | 1339 | (35.2) | 182 | (44.4) |
| Breast conserving, + radiation | 1989 | (52.3) | 183 | (44.6) |
| Breast conserving, no radiation | 478 | (12.6) | 45 | (11) |
| Other treatment | ||||
| Any chemotherapy | 1259 | (33.1) | 117 | (28.5) |
| Any endocrine therapy*** | 2123 | (55.8) | 240 | (58.5) |
| Tamoxifen only | 1240 | (58.4) | 154 | (64.2) |
| Aromatase inhibitors only | 272 | (12.8) | 16 | (6.7) |
| Both tamoxifen and aromatase inhibitors | 603 | (28.4) | 70 | (29.2) |
| Unknown | 8 | (0.4) | 0 | 0 |
| Charlson co-morbidity score | ||||
| 0 | 2964 | (77.9) | 265 | (64.6) |
| 1 | 613 | (16.1) | 91 | (22.2) |
| 2+ | 229 | (6) | 54 | (13.2) |
| Throughout study follow-up*** | ||||
| Years of follow-up, Median (interquartile range) | 6.1 | (3.6–9.6) | 7.5 | (4.5–10.6) |
| Diabetes | 519 | (13.6) | 91 | (22.2) |
| % Follow-up years with yearly surveillance mammography | ||||
| <50% | 826 | (21.7) | 113 | (27.6) |
| 50–79% | 1265 | (33.2) | 174 | (42.4) |
| 80%+ | 1715 | (45.1) | 123 | (30) |
| Pain conditions | ||||
| Abdominal | 1603 | (42.2) | 272 | (66.3) |
| Arthritis/gout | 2632 | (69.2) | 361 | (88.1) |
| Back | 859 | (22.6) | 215 | (52.4) |
| Chest | 1787 | (47.0) | 290 | (70.7) |
| Fibromyalgia | 573 | (15.1) | 133 | (32.4) |
| General chronic | 78 | (2.0) | 85 | (20.7) |
| Limb/extremity | 1686 | (44.3) | 230 | (56.1) |
| Neck | 304 | (8.0) | 65 | (15.9) |
| Neuropathic | 219 | (5.8) | 59 | (14.4) |
| Pelvic | 44 | (1.2) | 8 | (2.0) |
| Migraine/TMJ | 1050 | (27.6) | 163 | (39.8) |
Chronic use defined as 75+ days of use in any 90-day window
ER/PR=Estrogen receptor/progesterone receptor
Throughout study follow up - earliest of SBCE, death, disenrollment from health plan, or end of study period
Among chronic users, the mean and median daily dose of opioids was 31 MME and 20 MME (IQR: 10–35), respectively. Few of the women on chronic therapy (6.1%) were using high doses of ≥ 90 mg MME/day and 50% were using low doses of <20 mg MME/day.
Chronic use was associated with a non-significant increased risk of SBCEs (HR=1.20; 95% CI: 0.85–1.70), including second primary breast cancer (HR=1.38; 95% CI: 0.71–2.70), compared to non-chronic users/non-users (Table 3). The HR for recurrence was 1.14 (95% CI, 0.76–2.70). However, all confidence intervals included 1.0 and were overlapping for recurrence and second primary.
Table 3.
Risk of second breast cancer events (SBCE) by chronic opioid use after breast cancer diagnosis.
| Outcomes | Exposure status* |
Number of events ** |
Unadjusted Incidence rate (per 1000- person year) |
Minimally adjusted Hazard Ratio *** |
95% Confidence Interval |
Multivariate adjusted Hazard Ratio† |
95% Confidence Interval |
|---|---|---|---|---|---|---|---|
| SBCE | Non | 521 | 18.4 | Reference | Reference | ||
| Chronic | 37 | 23.6 | 1.23 | 0.88–1.72 | 1.20 | 0.85–1.70 | |
| Recurrence | Non | 388 | 13.7 | Reference | Reference | ||
| Chronic | 27 | 17.2 | 1.21 | 0.81–1.79 | 1.14 | 0.76–1.70 | |
| Second primary | Non | 133 | 4.7 | Reference | Reference | ||
| Chronic | 10 | 6.4 | 1.28 | 0.67–2.47 | 1.38 | 0.71–2.70 | |
Chronic opioid use was defined as 75+ days of use in any 90-day window and modeled as a time-varying covariate.
Second breast cancer event includes recurrence or second primaries, in-situ and invasive. Women were censored at the earliest of disenrollment, death, any SBCE event, or end of study period.
Adjusted for age at diagnosis (18–49,50–59, 60–69, 70–79, 80+ years) and AJCC stage (I, IIA, IIB).
Adjusting for age at diagnosis (18–49,50–59, 60–69, 70–79, 80+ years); diagnosis year (1990–1994,1995–1999, 2000–2004, 2005–2008); AJCC stage (I, IIA, IIB); hormone receptor status (estrogen receptor [ER]-/progesterone receptor [PR]-, ER+/PR-, ER-/PR+, ER+/PR+, and ER and/or PR unknown); primary treatment for initial breast cancer (mastectomy, breast conserving surgery with radiation, breast conserving surgery without radiation); endocrine therapy for the incident breast cancer (yes/no, time-varying); body mass index (BMI) at diagnosis (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35+kg/m2); smoking status at diagnosis (current, past, never/unknown); menopausal status at diagnosis (peri- or pre-menopausal, post-menopausal); Charlson comorbidity score (0, 1, 2+, time-varying); diabetes (yes/no, time-varying); use of NSAIDs (yes/no, time-varying) and receipt of screening mammogram in the 12 months prior to events (yes/no, time-varying).
Results from the sensitivity analysis of chronic use as defined as 150+ days in a 180-day window was attenuated toward the null. The adjusted HRs and respective 95% CI were 0.97 (0.62–1.54) for SBCE, 0.87 (0.50–1.49) for recurrence and 1.29 (0.55–3.00) for second primaries. Lagging exposure by 12-months in a sensitivity analysis yielded point estimates closer to the null with HR=1.05 (95% CI; 0.71–1.54) for SBCE, HR=0.98 (95% CI: 0.63–1.55) for recurrence and HR=1.24 (95% CI: 0.64–2.42) for second primaries. Results changed minimally when we took surveillance mammography out of adjusted models.
DISCUSSION
This was the first US based observational study on the association between chronic opioid use and risk of cancer recurrence and second primary breast cancer. Our study may provide some reassurance to women with breast cancer that chronic use post breast cancer diagnosis was not associated with a statistically significant increased risk of SBCEs in this study. However, an elevated risk of SBCEs with chronic opioid therapy cannot be ruled out, in part, due to a limited sample size to detect small differences in risk and a cohort of chronic users with relatively low dose use of non-immunosuppressive opioids.
The only other published study of chronic opioid use and breast cancer recurrence was conducted in Denmark.14 Cronin-Fenton et al. studied 34,188 women with stage I-III incident breast cancer between 1996 and 2008. The average follow-up was 7 years and 15.6% developed a recurrence. Chronic opioid use was defined as having 1+ opioid dispensing per month for at least six months and approximately 2% met this criteria during follow-up. The study found no association between chronic opioid users (HR=1.1; 95% CI: 0.93–1.4) and risk of recurrence compared to non-chronic users. Risk did not differ by cumulative dose. Contrary to hypotheses, the study results suggest strongly immunosuppressive opioids reduce risk of recurrence (HR=0.75; 95% CI: 0.57–0.99) in comparison to non-users. The authors note that channeling bias among those with high competing risks such as mortality may explain why recurrence rates were lower among users of strong but not weakly immunosuppressive opioids. Tramadol (36%) and codeine (23%) were the most frequently prescribed opioids.
Because of other safety concerns with opioids and lack of evidence on the long-term benefits of opioids,25 it may be concerning that almost 10% of the women in our cohort met the criteria for chronic use at some point during follow-up and approximately a third of them took opioids for more than 3 years. It is somewhat reassuring that relatively few were high dose users. However, regular use of opioids in the years following breast cancer diagnosis and treatment (~3.2% in any given quarter) was on the high end of estimates reported in the general U.S. population (2–3.5%).40–43 A large US claims-based study of cancer patients undergoing curative-intent surgery found that 10% of opioid-naïve patients developed persistent opioid use after surgery (defined as 1+ opioid dispensings attributed to surgery plus 1+ opioid dispensings 90–180 days after surgery).44 This is higher than the 6% to 8% reported for noncancer surgery.45–47 These patients continued using opioids at modestly high doses (25 MMEs) even one year after cancer surgery. This data taken together should prompt discussions on whether there is a need to reduce excess opioid prescribing during the years post cancer diagnosis and treatment among cancer survivors.
COMBO is one of only a few population-based US cohorts of breast cancer survivors that contains comprehensive and high quality data on incident breast cancer characteristics and treatment through both a registry and medical charts, demographics, unbiased health care utilization including medication use and breast services, breast cancer outcomes, and death. Complete information on death and disenrollment allows the application of robust methods to address potential competing risks and informative censoring. Detailed information on breast cancer screening and relatively long follow-up are other strengths of the study.
However, our study is not without limitations. COMBO uses data from a single health plan and includes an insured, educated, and primarily Caucasian population. This may limit generalizability to some populations but the results are generalizable to a large majority of women and we do not hypothesize a difference in association by race. Loss to follow-up is a possible source of bias with 18% censored due to disenrollment from the health plan. Residual confounding is possible in any observational study. We considered numerous potential confounders, but lacked information on lifestyle factors, over-the-counter medications, and alcohol intake. We lacked data on use of non-prescribed opioids but expect illicit use to be relatively rare in older women. We had no information on opioid use in the inpatient setting including perioperative use, which was shown to influence recurrence.12 While not the objective of the study, we are unable to describe lifetime opioid use prior to breast cancer diagnosis. Protopathic bias where opioids are prescribed to treat symptoms of undiagnosed SBCEs is possible but we used standard methods of lagging the exposure to minimize this bias.48 Due to limited statistical power, we were unable to evaluate associations with SBCEs by cumulative dose, duration of use, individual opioids, and immunosuppressive vs non-immunosuppressive opioids. The majority of chronic users in this study were on low doses and non-immunosuppressive opioids49–51 (i.e., oxycodone and hydrocodone) which may partly explain the overall null results.
The gap in evidence on the safety of opioids with respect to cancer risk and cancer outcomes is of concern. Even a small increase in risk is of importance given the high prevalence of opioid use. Chronic pain is one of the most common symptoms in cancer survivors, especially in the first few years after treatment.52–54 For example, half of breast cancer survivors report pain55,56 and pain remains common even among long-term breast cancer survivors.57,58 As many as 30% of breast cancer survivors report above-average pain 10 years after treatment.59 Another example is pelvic pain syndrome arising in patients who undergo radiation therapy for cancers of the rectum, prostate, bladder, and uterus.60 Neuropathic pain is also commo in cancer survivors who have undergone surgery.61,62 As cancer survivors live longer, pain from other conditions becomes common.63,64 A study out of Canada found opioid prescribing to be 1.2 times higher among cancer survivors than matched controls.61 This coupled with evidence that opioids influence multiple well-established cancer pathways2,3 such as immune suppression, cell proliferation, cell invasion and angiogenesis, points to a need for clinical studies of the effects of chronic use on cancer outcomes in diverse population and across different cancers. Our results can be used in planning future studies in this area.
Take home messages:
The long-term effects of opioid use in cancer survivors are unknown, but several biological mechanisms support the hypothesis that opioid use may increase risk of cancer progression and recurrence.
Regular use of opioids among breast cancer survivors is common. Regular use varied from 2.0% in quarters prior to incident breast cancer diagnosis to a high of 5.0% in the quarter which breast cancer was diagnosed. It consistently hovered around 3.2% during the years following diagnosis and treatment.
Chronic use of opioids is common with 10% of breast cancer survivors meeting the definition of chronic opioid use during follow-up. Median duration of chronic use was 23.5 months: 45% had 1–3 years of use and 32% >3 years of use.
This first US based study of women with early stage breast cancer provides some reassurance that chronic opioid use does not increase the risk of second breast cancer events (SBCEs). While statistically non-significant, the observed higher risk estimates for SBCEs warrants further study in larger and different populations.
Acknowledgements:
This manuscript was supported by grant numbers CA120562 (Boudreau), CRTG-03–024-01 (Buist), and CA093772 (Silliman). The collection of cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center (Contract No. N01-CN-67009 and N01-PC-35142) from the Surveillance, Epidemiology and End Results Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington. Dr. Chen’s time was supported by a Group Health Foundation Fellowship. Ms. Bowles’ time was supported by the National Cancer Institute of the National Institutes of Health under Award Number R50CA211115. The contents are solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Kuehn BM: Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA 297:249–51, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Afsharimani B, Cabot P, Parat MO: Morphine and tumor growth and metastasis. Cancer Metastasis Rev 30:225–38, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Lennon FE, Moss J, Singleton PA: The mu-opioid receptor in cancer progression: is there a direct effect? Anesthesiology 116:940–5, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Tedore T: Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth 115 Suppl 2:ii34–45, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Le-Wendling L, Nin O, Capdevila X: Cancer Recurrence and Regional Anesthesia: The Theories, the Data, and the Future in Outcomes. Pain Med 17:756–75, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Ash SA, Buggy DJ: Does regional anaesthesia and analgesia or opioid analgesia influence recurrence after primary cancer surgery? An update of available evidence. Best Pract Res Clin Anaesthesiol 27:441–56, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Wigmore T, Farquhar-Smith P: Opioids and cancer: friend or foe? Curr Opin Support Palliat Care 10:109–18, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Mathew B, Lennon FE, Siegler J, et al. : The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 112:558–67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shavit Y, Ben-Eliyahu S, Zeidel A, et al. : Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation 11:255–60, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Myles PS, Peyton P, Silbert B, et al. : Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ 342:d1491, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Tsui BC, Rashiq S, Schopflocher D, et al. : Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth 57:107–12, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Exadaktylos AK, Buggy DJ, Moriarty DC, et al. : Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 105:660–4, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biki B, Mascha E, Moriarty DC, et al. : Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology 109:180–7, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Cronin-Fenton DP, Heide-Jorgensen U, Ahern TP, et al. : Opioids and breast cancer recurrence: A Danish population-based cohort study. Cancer 121:3507–14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirtz HS, Buist DS, Gralow JR, et al. : Frequent antibiotic use and second breast cancer events. Cancer Epidemiol Biomarkers Prev 22:1588–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudreau DM, Yu O, Chubak J, et al. : Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat 144:405–16, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fred Hutchinson Cancer Research Center: Cancer Surveillance System. Seattle, WA, 2018 [Google Scholar]
- 18.National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program, 2018 [Google Scholar]
- 19.Saunders KW, Davis RL, Stergachis A: Group Health Cooperative, in Strom BL (ed): Pharmacoepidemiology (ed 4th). West Sussex, England, John Wiley and Sons, 2005, pp 223–239 [Google Scholar]
- 20.Boudreau DM, Doescher MP, Jackson JE, et al. : Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother 38:1317–8, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Buist DS, LaCroix AZ, Brenneman SK, et al. : A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc 52:1130–7, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Von Korff M, Dublin S, Walker RL, et al. : The Impact of Opioid Risk Reduction Initiatives on High-Dose Opioid Prescribing for Patients on Chronic Opioid Therapy. J Pain 17:101–10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders K, Shortreed S, Thielke S, et al. : Evaluation of Health Plan Interventions to Influence Chronic Opioid Therapy Prescribing. Clin J Pain 31:820–829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakral M, Walker RL, Saunders K, et al. : Impact of Opioid Dose Reduction and Risk Mitigation Initiatives on Chronic Opioid Therapy Patients at Higher Risk for Opioid-Related Adverse Outcomes. Pain Med, 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou R, Turner JA, Devine EB, et al. : The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 162:276–86, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Dowell D, Haegerich TM, Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 65:1–49, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Hudis CA, Barlow WE, Costantino JP, et al. : Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25:2127–32, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Geiger AM, Thwin SS, Lash TL, et al. : Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer 109:966–74, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Varadhan R, Weiss CO, Segal JB, et al. : Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care 48:S96–105, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Ahern TP, Pedersen L, Tarp M, et al. : Statin prescriptions and breast cancer recurrence risk: a danish nationwide prospective cohort study. J Natl Cancer Inst 103:1461–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganz PA, Habel LA, Weltzien EK, et al. : Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat 129:549–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chae YK, Valsecchi ME, Kim J, et al. : Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest 29:585–93, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Chae YK, Brown EN, Lei X, et al. : Use of ACE Inhibitors and Angiotensin Receptor Blockers and Primary Breast Cancer Outcomes. J Cancer 4:549–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwan ML, Habel LA, Flick ED, et al. : Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat 109:573–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene FL: The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg 87:13–5, 2002 [PubMed] [Google Scholar]
- 36.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–9, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Newton KM, Wagner EH, Ramsey SD, et al. : The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol 52:199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Boudreau DM, Malone DC, Raebel MA, et al. : Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 7:305–14, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Malone DC, Boudreau DM, Nichols GA, et al. : Association of cardiometabolic risk factors and prevalent cardiovascular events. Metab Syndr Relat Disord 7:585–93, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Parsells Kelly J, Cook SF, Kaufman DW, et al. : Prevalence and characteristics of opioid use in the US adult population. Pain 138:507–13, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Paulose-Ram R, Hirsch R, Dillon C, et al. : Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III). Pharmacoepidemiol Drug Saf 12:315–26, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MD, Edlund MJ, Steffick D, et al. : Regular use of prescribed opioids: association with common psychiatric disorders. Pain 119:95–103, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Sullivan MD, Edlund MJ, Zhang L, et al. : Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med 166:2087–93, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lee JS, Hu HM, Edelman AL, et al. : New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 35:4042–4049, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke H, Soneji N, Ko DT, et al. : Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 348:g1251, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soneji N, Clarke HA, Ko DT, et al. : Risks of Developing Persistent Opioid Use After Major Surgery. JAMA Surg 151:1083–1084, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Brummett CM, Waljee JF, Goesling J, et al. : New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg 152:e170504, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chubak J, Boudreau DM, Wirtz HS, et al. : Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst 105:1456–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacerdote P, Manfredi B, Mantegazza P, et al. : Antinociceptive and immunosuppressive effects of opiate drugs: a structure-related activity study. Br J Pharmacol 121:834–40, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budd K: Pain management: is opioid immunosuppression a clinical problem? Biomed Pharmacother 60:310–7, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Sacerdote P: Opioids and the immune system. Palliat Med 20 Suppl 1:s9–15, 2006 [PubMed] [Google Scholar]
- 52.Patrick DL, Ferketich SL, Frame PS, et al. : National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst Monogr:9–16, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Sun V, Borneman T, Piper B, et al. : Barriers to pain assessment and management in cancer survivorship. J Cancer Surviv 2:65–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glare PA, Davies PS, Finlay E, et al. : Pain in cancer survivors. J Clin Oncol 32:1739–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung BF, Ahrendt GM, Oaklander AL, et al. : Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain 104:1–13, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Saxena AK, Kumar S: Management strategies for pain in breast carcinoma patients: current opinions and future perspectives. Pain Pract 7:163–77, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Macdonald L, Bruce J, Scott NW, et al. : Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 92:225–30, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peuckmann V, Ekholm O, Rasmussen NK, et al. : Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain 13:478–85, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Forsythe LP, Alfano CM, George SM, et al. : Pain in long-term breast cancer survivors: the role of body mass index, physical activity, and sedentary behavior. Breast Cancer Res Treat 137:617–30, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levy MH, Chwistek M, Mehta RS: Management of chronic pain in cancer survivors. Cancer J 14:401–9, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Rogers ML, Duffy JP: Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 18:711–6, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Tasmuth T, von Smitten K, Hietanen P, et al. : Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol 6:453–9, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Ritchie CS, Kvale E, Fisch MJ: Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract 7:371–4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganz PA: Survivorship: adult cancer survivors. Prim Care 36:721–41, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: A population based matched cohort study among individuals with and without a history of cancer. Cancer 123:4286–4293, 2017 [DOI] [PubMed] [Google Scholar]


