Supplemental Digital Content is available in the text.
Background.
Membranes surrounding the fetus play a crucial role in providing a physical and immunological barrier between a semiallogeneic fetus and mother during pregnancy. In this study, we tested whether cotransplantation of fetal membranes (FMs) and allogeneic donor cells would improve the retention and function of allografts in mice.
Methods.
Intact and enzyme-digested membranes obtained from E18-E19 pregnant mice were subcutaneously cotransplanted with 10F7MN hybridoma cells that are of BALB/cByJ (Balb) origin and secrete anti-human CD235a antibody. Cells were transplanted into C57BL/6J (B6, allogeneic), Balb (syngeneic), and FVB/NJ (third-party) mice. Serum was collected after 1 and 3 weeks of cell transplantation and tested using flow cytometry for the presence of anti-human CD235a antibody. Immunosuppressive functions of membranes were further investigated by analyzing the cytokine profile of supernatants collected from allo-reactive mixed lymphocyte reactions (MLRs) using a multiplex cytokine assay.
Results.
B6 mice transplanted with 10F7MN cells along with membranes syngeneic to the host had significantly higher levels of CD235a antibody when compared to B6 mice that received cells without membranes, allogenic membranes, or third-party membranes. Syngeneic membranes significantly inhibited T-cell proliferation in the presence of allogeneic stimuli and suppressed the release of Th1-cytokines such as IFNγ, TNFα, and IL-2 in MLRs. Additionally, increases in the levels of Th2-cytokines were found in MLRs containing membrane-derived cells.
Conclusions.
Our study highlights the potential use of syngeneic FMs to act as potent cell-carriers that could improve graft retention as well as graft-specific immunoprotection during allograft transplantation.
An intricate crosstalk between maternal and fetal systems is vital for a successful pregnancy in which a semiallogeneic fetus is protected against rejection by the maternal immune system. The developing conceptus is surrounded by the fetal membranes (FMs), composed of an outer chorion and inner amnion, which act as protective barriers against the immunological, structural, and mechanical provocations of pregnancy.1,2 Additionally, the maternal uterine decidua, which abuts the chorion, plays a critical role in the maintenance of tolerance through secretion of immunosuppressive cytokines and inhibition of cytotoxic T and NK3 cell responses against fetal antigens at the feto-maternal interface.4,5 Overall, the complex interactions across the FMs and maternal decidual cells are crucial for a successful pregnancy.6
In addition to their immunomodulatory and semipermeable barrier functions, the structural composition of membranes encircling the embryo also influences the biomechanical tensile strength needed to protect and support the fetus from the stage of implantation through parturition. Extracellular matrix (ECM) proteins such as collagen, fibronectin, laminin, vitronectin, hyaluronan, decorin, and biglycan form the integral structural units of FMs and decidua, which regulate the biomechanical changes in the membranes at different stages of pregnancy.7,8
Cell-based therapies offer great promise to treat various diseases and malignancies. However, cell transplantation employing allogeneic donor cells faces rejection by the host in the absence of immunosuppression, resulting in loss of the majority of the donor cells within few hours after transplantation.9-11 Administration of immunosuppressants and providing human leukocyte antigen-matched donor cells are some of the routinely used approaches to improve the success of allogeneic cell engraftment. However, morbidity and mortality issues associated with immunosuppression and lack of suitable donors are the major obstacles in the clinical application of allogeneic cell therapies.
Natural biomaterials such as alginate hydrogels have been tested as cell-carriers in therapeutic interventions targeting various disorders.12 These biomaterials provide a suitable microenvironment that allows communication between transplanted grafts and the hosts, facilitating improved graft survival and function. The ECM protein-rich composition and immunosuppressive barrier properties of membranes encircling the fetus point to their role as natural immune barriers. Moreover, the ready availability of membranes that are routinely discarded postpartum has drawn attention to their possible use as cell and tissue sources for developing new therapies.13,14
Taking cues from the natural immune evasion and tolerance toward the semiallogeneic fetus, during both biological and fully allogeneic surrogate pregnancies, we assessed whether envelopment of foreign cells by membranes surrounding the fetus, including both FMs and decidua (for simplicity, hereafter referred to as “membranes”), could lead to protection of allografts from rejection by the host’s immune system. Using a murine transplant model, we have tested the hypothesis that allogeneic donor cells may be protected from the host immune response by cotransplantation with near-term membranes.
MATERIALS AND METHODS
Isolation and Processing of Membranes
This research was performed with the approval of the Institutional Animal Care and Use Committee at Covance Laboratories, Inc. Mice were maintained and used according to the National Institutes of Health and Institutional Animal Care and Use Committee guidelines. Adult C57BL/6J (B6), BALB/cByJ (Balb), and FVB/NJ (FVB) mice were purchased from the Jackson Laboratory and maintained in the pathogen-free facility at Vitalant Research Institute. Intact membranes were isolated from embryonic day (E)18-E19 pregnant dams (Figure S1A–C, SDC, http://links.lww.com/TXD/A213). For experiments involving membrane-derived cells, membranes were digested with collagenase IV (1 mg/mL) (Thermo Fisher Scientific, Life Technologies) for 1 hour followed by DNase I (5 µg/mL) (Sigma-Aldrich) for 15 minutes at 37°C.
Flow Cytometry
Cell isolates from membranes were digested as described above and stained with CD3, CD4, CD8, Gr-1, and B220 antibodies (BioLegend) for 30 minutes at 4°C. After washing, the stained cells were run on an LSR II flow cytometer (BD) and data were analyzed using FlowJo software. Propidium iodide was used to discriminate live and dead cells (Figure S1D and E, SDC, http://links.lww.com/TXD/A213).
Immunohistochemistry
Freshly isolated E18/E19 membranes were fixed and embedded as described previously.15 Membrane cryosections of 10-µM thickness were stained for the expression of proliferin, periostin, and α-fetoprotein (AFP) antibodies (Santa Cruz Biotechnology). Nuclei were counterstained with ProLong Gold antifade reagent with 6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific). Images were captured on Leica Microsystems CTR6500 and further analyzed using NIH-ImageJ software.
10F7MN Cell Culture and In Vivo Transplantation
10F7MN cells are murine hybridoma cells that secrete anti-human glycophorin A (CD235a) antibody, recognizing human erythrocytes. Details of 10F7MN cells are described in the Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213).
For transplantation of 10F7MN cells, B6 (allogeneic) and Balb (syngeneic) mice were used as hosts. To create a viscous gel suspension, 1 × 106 10F7MN cells were mixed with Matrigel matrix high concentration (Corning) and transplanted subcutaneously. Membranes were obtained from syngeneic, allogeneic, and third-party pregnant dams, and transplantations were carried out for following conditions: (a) 10F7MN cells only, (b) intact membranes only, (c) 10F7MN cells + intact membranes, (d) 10F7MN cells + digested membranes, and (e) Matrigel only. The progress of the tumors was monitored for 3 weeks after 10F7MN cell transplantation. Serum samples were collected from the transplanted mice at 1 and 3 weeks’ time points to determine the presence of anti-human CD235a antibody.
Detection for Anti-Human CD235a Antibody Levels in the Serum of the Mice After Transplantation
Human venous blood was collected under a protocol approved by the University of California at San Francisco Institutional Review Board (approval number: 11-06262), and all methods were performed following relevant guidelines and in accordance with the principles of the Declarations of Helsinki.
Erythrocytes were isolated from the blood of a healthy donor, as described in the Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213). Human erythrocytes (2 × 105 cells/well) were incubated for 30 minutes at 4°C with 2 μL of mouse serum and collected at 1 and 3 weeks after transplantation of 10F7MN cells. Erythrocytes were then washed and stained with goat anti-mouse IgG1-PE antibody (Thermo Fisher Scientific) for 30 minutes at 4°C. Median fluorescence intensities (MFIs) of stained cells were calculated using FlowJo software. A standard curve was produced from erythrocytes stained with known concentrations of anti-human CD235a antibody, which was used to extrapolate the anti-human CD235a titers in the serum of the mice transplanted with 10F7MN cells.
Mouse Alloantibody Screening Assay
Alloantibodies were screened as previously described16 and detailed in Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213). Briefly, Balb splenocytes were incubated with serum collected from syngeneic (Balb) and allogeneic (B6) mice transplanted with 10F7MN cells. Serum samples were collected 3 weeks after 10F7MN cell transplantation. Cells were washed and stained with anti-Igk antibody (BD Pharmingen) to detect alloantibody binding. Cells were additionally stained with B220 and TCRβ antibodies (BD Pharmingen), and samples were analyzed by flow cytometry. MFIs of Igκ staining on the TCRβ + B220-population were calculated.
In Vitro Mixed Lymphocyte Reaction Assay
B6 and Balb splenocytes were used as responder and stimulator cells, respectively. Responder B6 splenocytes were labeled with 15 μM carboxyfluorescein succinimidyl ester (CFSE) (Affymetrix) as described previously.17 As a positive control to determine the T-cell proliferative response, Dynabeads Mouse T-Activator CD3/CD28 (Thermo Fisher Scientific) was used. To determine the immunosuppressive activity of membranes, allogen and mitogen-induced T-cell responses were tested both with and without membrane-derived cells. The mixed lymphocyte reaction (MLR) was performed at 37°C in 5% CO2 for 4 days. Details of the MLR assay are described in Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213). Cells were stained with CD4 and CD8 antibodies (BioLegend) and analyzed on a flow cytometer.
Cytokine Detection Assay
B6 and Balb splenocytes were cultured in the presence and absence of syngeneic B6 membrane cells at 37°C in 5% CO2. After 96 hours, culture supernatants were assayed for targets: IFNγ, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-5, IL-6, TNFα, GM-CSF, IL-18, IL-10, IL-17A, IL-22, IL-23, IL-27, and IL-9 cytokines following the manufacturer’s (Thermo Fisher Scientific) protocol. Cytokines were analyzed using Luminex 200 platform (Luminex) with BioManager Software (BioRad). The details of assay are described in Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213).
Statistics
Data are presented as mean ± SEM. The nonparametric Mann–Whitney t-test was used for unpaired comparisons. Means of multiple groups were compared by one- and two-way analyses of variance (ANOVA) using Tukey’s multiple comparison test. Details of the statistical analyses used are described in Supplemental Material and Methods (SDC, http://links.lww.com/TXD/A213). Analyses were performed using GraphPad Prism version 6.00 and P ≤ 0.05 was considered significant.
RESULTS
Membranes Contained Immunologically Naïve Cells
Analysis of cells derived from E18-E19 membranes showed that the membranes expressed significantly fewer T- (CD4 and CD8) and B- (B220) lymphocytes as compared to adult spleens, and there was also a general paucity of Gr-1+ myeloid cells in membranes (Figure 1A and B) (Figure S1D and E, SDC http://links.lww.com/TXD/A213). Additionally, intact membranes from B6 mice expressed the mesenchymal-protein, periostin, and epithelial proteins, proliferin (Figure 1C), indicating the layered composition of different cell types comprising the membranes.
FIGURE 1.
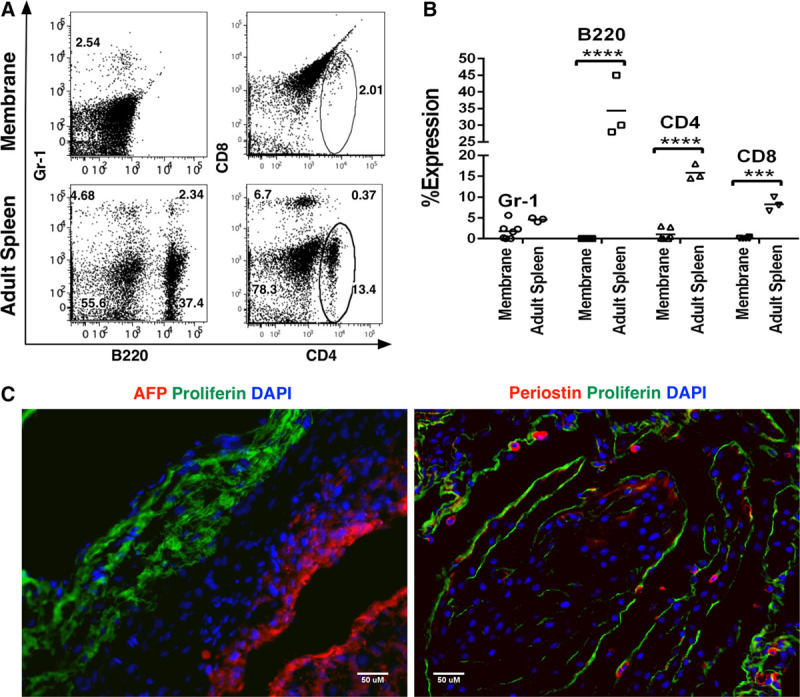
Flow cytometric and immunohistochemical characterization of membranes derived from E18-19 pregnant mice. A and B, Dot plots and bar graphs representing the frequencies of Gr-1 (myeloid), B-220 (B-cells), and CD4 and CD8 (T cells) cells in membranes (n = 7) and adult spleen, respectively (n = 3). C, Demonstration of expression of α-fetoprotein (AFP) (red) and proliferin (green, left panel) and periostin (red) and proliferin (green, right panel) on intact B6 membranes. Nuclei were stained with 6-diamidino-2-phenylindole (DAPI) (blue). Scale bars represent 50 µM.
Membranes, Syngeneic to the Host Genetic Background, Facilitated Allogeneic Donor Cell Engraftment
The potential for membranes to serve as immunosuppressive cell-carriers for allogeneic transplantations was investigated using 10F7MN hybridoma cells as donor cell grafts. For syngeneic transplantations, 10F7MN cells were injected into Balb host, with and without membranes obtained from syngeneic Balb and allogeneic B6 dams. For allogeneic transplants, B6 mice were injected subcutaneously with allogeneic 10F7MN cells, with and without membranes derived from B6, Balb, and FVB (third-party) mice. Assessment of engraftment was based on tumor formation by the 10F7MN cells. It was observed that 10F7MN cells formed tumors only in syngeneic Balb recipients, either in the presence or absence of membranes but not in the allogeneic B6 hosts (Figure S2, SDC, http://links.lww.com/TXD/A213).
Retention of 10F7MN cells was assessed by analyzing the presence of anti-human CD235a antibody produced by this cell line and found in the serum of the transplanted mice. It was found that levels of anti-human CD235a antibody increased over 3 weeks following transplantation in syngeneic Balb hosts, when cells were transplanted either with or without membranes (Figure 2A). Interestingly, even at 1 week post-transplantation, 10F7MN cells were retained in allogeneic B6 recipients when they received these cells along with the intact membranes derived from B6 dams in comparison to those who received cells with Balb (allogeneic) (P = 0.005) and FVB/NJ (third-party) (P = 0.002) membranes (Figure 2B). Moreover, despite the absence of gross tumor formation by the allogeneic hybridoma cells in B6 hosts, after 3 weeks following transplantation the levels of anti-human CD235a antibody were significantly higher in B6 hosts that received 10F7MN cells with the B6 membranes compared to hosts that received cells without membranes (P = 0.01) or with membranes from allogeneic Balb or third party FVBN mice (P < 0.0001 and P = 0.0005, respectively) (Figure 2B).
FIGURE 2.
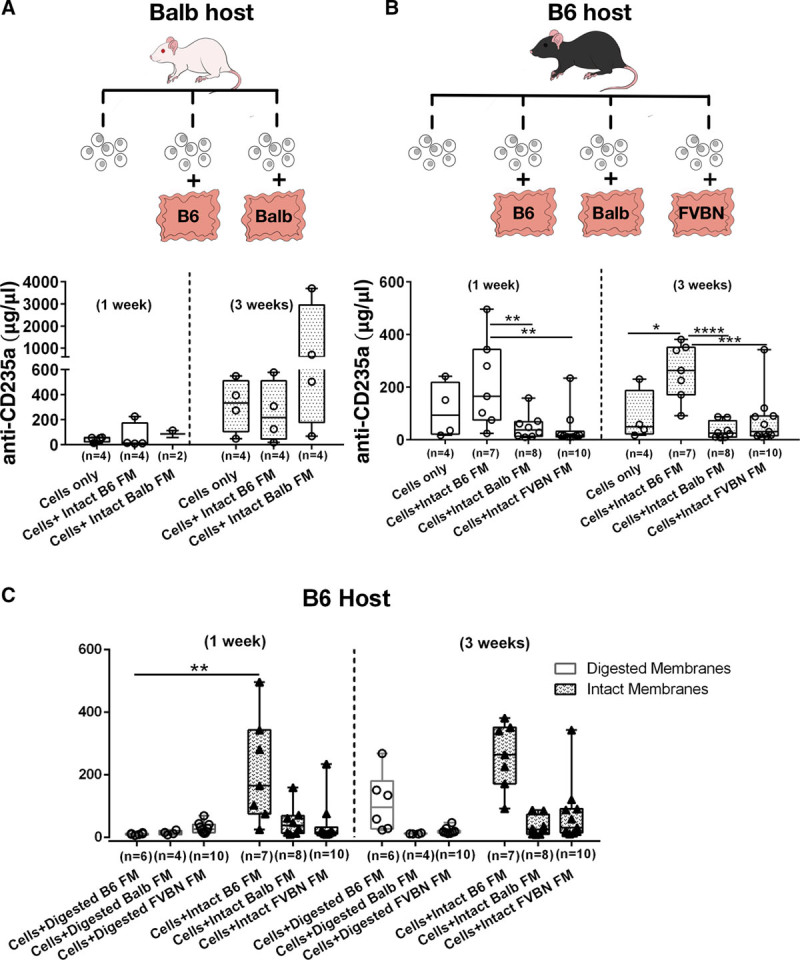
Assessment of 10F7MN cell function following transplantation in mice. A and B, Experimental scheme of transplantation of 10F7MN cells and membranes in Balb and B6 recipients; analyses of serum anti-human CD235a antibody levels secreted by the injected 10F7MN cells following 1 and 3 wk after transplantation are shown using box and whisker plots. Balb mice were syngeneic hosts, B6 mice were allogeneic hosts, and transplants of FVB membranes were employed as third party allogeneic tissues (Balb; cells injected without and with membranes of B6 and Balb mice) and allogeneic (B6, cells injected without and with membranes of B6, Balb, and FVB mice) recipients, respectively. C, Graphs showing comparison of anti-human CD235a antibody levels in B6 recipients that received 10F7MN cells with intact and digested membranes obtained from B6 (syngenic), Balb (allogeneic), and FVB (third-party) pregnant mice. Number of mice (n) transplanted for each experimental condition has been mentioned in the bar graphs. FM, fetal membrane.
Another intriguing finding was that the allogeneic cells were retained significantly better at 1 week after cell transplantation (P = 0.001), as scored by the levels of anti-human CD235a antibodies in B6 recipients, when intact syngeneic membranes were used for cotransplantations compared to cells derived from digested syngeneic membranes (Figure 2C). Effective retention and function of the injected allogeneic 10F7MN cells was observed at 3 weeks post-transplantation only when intact syngeneic membranes were used and not when digested syngeneic, allogeneic (intact or digested), or third-party membranes (intact or digested) were used (Figure 2C).
Mice Transplanted With 10F7MN Cells Along With Membranes Showed Weak Alloantibody Response
To investigate the alloantibody response, serum collected from B6 recipients after 3 weeks of allogeneic 10F7MN cell transplantations, performed with and without membranes, was tested against allogeneic Balb splenocytes (Figure 3A); serum collected from syngeneic transplants, that is, from Balb mice transplanted with 10F7MN cells, with and without membranes served as controls for the assay. The antibody response was estimated from the MFI values of the Igκ+ TCRβ+ B220− Balb splenocytes (Figure S3A, SDC, http://links.lww.com/TXD/A213).
FIGURE 3.
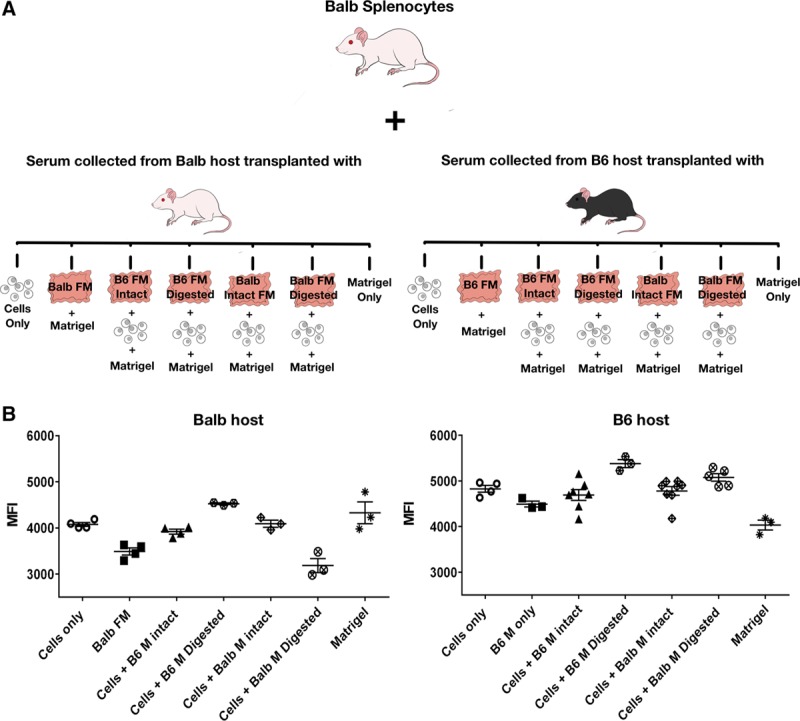
Analysis of alloantibody response in recipient mice transplanted with 10F7MN cells with and without membranes. A, Experimental scheme depicting the analysis of antibody responses against Balb splenocytes in the serum of mice transplanted with 10F7MN cells and different combinations of membranes; (B) dot plots showing antibody responses against Balb splenocytes in the serum of the syngeneic (Balb) and allogeneic (B6) hosts, respectively, that received 10F7MN cells in the presence and absence of intact and digested membranes obtained from Balb and B6 mice. The antibody responses are depicted as median fluorescent intensities (MFI) of Igκ+ TCRβ+ B220− cell population of Balb splenocytes. Number of mice (n) transplanted for each experimental condition has been mentioned in the bar graphs. FM, fetal membrane.
It was found that even though the antibody response to allogeneic 10F7MN cells was significantly higher in B6 recipients compared to their Balb counterparts (P = 0.02) (Figure S3B, SDC, http://links.lww.com/TXD/A213), the levels were not remarkably different in B6 mice that received allogeneic cells either with or without membranes (Figure 3B). In fact the genetic strain of the membranes did not significantly impact the antibody responses against the allogeneic cells in B6 mice (Figure 3B). However, it was found that alloantibody responses were elevated in mice that received cells with digested membranes (Figure 3B), in line with our findings of CD235a antibody levels in Figure 2C. Although 10F7MN cells are syngeneic to Balb mice, similar to B6 recipients, the antibody responses were higher in Balb mice that were cotransplanted with cells, along with digested B6 membranes (Figure 3B). Antibody levels were lowest in the Balb mice that received cells with digested Balb membranes.
Membrane-Derived Cells Inhibited T-Cell Proliferation and Suppressed the Proinflammatory Cytokine Response
Higher anti-human CD235a antibody levels in the B6 recipients that received allogeneic 10F7MN cells cotransplanted with syngeneic membranes prompted us to further investigate the effects of membranes on the host T cells. MLR assays were performed using B6 and Balb splenocytes as responder and stimulator cells, respectively. To better understand the immune-regulatory function of membranes on alloantigen-induced T-cell proliferation, Balb splenocytes were used as strong allogeneic stimulators, instead of 10F7MN hybridoma cells, for B6 splenocytes responders.
It was found that membrane-derived cells from B6 dams were able to suppress the proliferation of responder B6 T-cells when exposed to allogeneic Balb splenocytes, though the suppressive effect of membranes was statistically significant only for CD8+ T cells (P = 0.019) (Figure 4A and C). As a negative control for the experiment, the background T-cell proliferation of B6 splenocytes alone was measured (Figure S4, SDC, http://links.lww.com/TXD/A213). The inhibitory effect of B6 membrane cells on T-cell proliferation was also tested using CD3/CD28 beads as a direct TCR stimulant for the B6 responders. Similar to the observation with Balb splenocytes as stimulators, we found that in the presence of B6 membrane-derived cells, proliferation of B6 T cells was decreased when exposed to CD3/CD28 beads and proliferation was significantly lowered in the CD4+ T-cell population (P = 0.0001) (Figure 4B and C), implying that syngeneic FMs globally suppressed T-cell responses.
FIGURE 4.
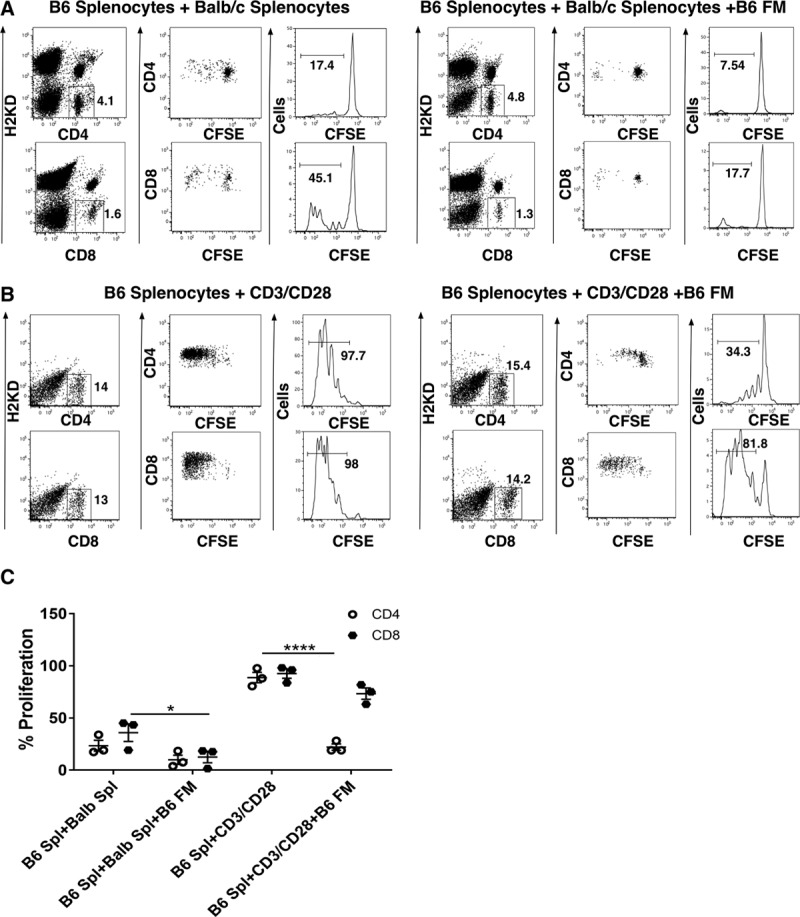
Analysis of the effect of membranes on T-cell (CD4 and CD8) proliferation in mixed lymphocyte reactions (MLRs): inhibition of B6 T-cell proliferation in the presence of (A) left panel, B6 and Balb mixed-splenocyte cultures without membranes; right panel, B6 and Balb mixed splenocyte cultures with B6 (syngeneic) membrane-derived cells; (B) left panel, B6 and CD3/CD28 bead mixed-cultures without membranes; right panel, B6 and CD3/CD28 bead mixed-cultures with B6 (syngeneic) membrane-derived cells; and (C) summarized data showing significant inhibition in % B6 T-cell proliferation in allo-reactive mixed-cell cultures grown in the presence of B6 (syngeneic) membrane-derived cells. The MLR assay was performed 3 times and each experimental condition was run in duplicate. CFSE, carboxyfluorescein succinimidyl ester; FM, fetal membrane.
Further, investigation on the immunosuppressive response of the T-cell proliferation in the presence of membranes showed that the levels of proinflammatory cytokines such as IFNγ and TNFα were significantly decreased in the cultures containing B6 splenocytes (responder), CD3/CD28 beads (stimulator), and B6 membrane cells when compared to the cultures containing B6 splenocytes and CD3/CD28 beads only (P < 0.05) (Figure 5). Although not statistically significant, a similar trend of decreased IFNγ and TNFα levels was observed in the cultures containing B6 splenocytes (responder), Balb (stimulator), and B6 membrane cells (Figure 5). Moreover, the levels of IL-2 and IL-4 were distinctly decreased when B6 responder cells were cultured in the presence of B6 membrane cells with Balb splenocytes and CD3/CD28 stimulators (Figure 5). The presence of B6 membrane cells in the cultures increased the production of anti-inflammatory Th2 cytokine IL-10 production (Figure 5). Interestingly, the pleotropic cytokine IL-6 was seen to be highly produced by the membrane cells. IL-6 levels were significantly lower in cultures containing B6 splenocytes (responder) and Balb splenocytes or CD3/CD28 beads compared to the aforementioned cells cultured in the presence of B6 membrane cells (Figure 5). Overall, a suppression of the Th1 cytokine profile was found in the MLRs in the presence of membrane-derived cells. Further, the increase of the membrane cells caused an additive suppressive effect on the Th1 cytokine response, that is, lowering IFNγ, TNFα, IL-2, and IL-4 levels (Figure 5). In the presence of Balb splenocytes, B6 membranes stimulated increased IL-17 and IL-22 production. However, doubling the ratio of B6 membrane cells decreased the IL-17 and IL-22 responses (Figure S5A, SDC, http://links.lww.com/TXD/A213). Although differences were not statistically significant, a trend of suppression in the levels of IL-13 was found in the cultures containing B6 and Balb splenocytes (alloantigen) and B6 splenocytes and CD3/CD28 beads (mitogen) grown in the presence of increased doses of membrane-derived cells (Figure S5B, SDC, http://links.lww.com/TXD/A213). Interestingly, IL-5 was found to be higher in the presence of membrane cells in cultures containing B6 splenocytes and CD3/CD28 beads, but the levels decreased by doubling the number of membrane cells corresponding to the number of B6 splenocytes and CD3/CD28 beads (Figure S5B, SDC, http://links.lww.com/TXD/A213). Noticeable differences in the levels of IL-1β, IL-9, IL-12p70, IL-18, and GM-CSF cytokines were not found among different experimental groups (Figure S5B, SDC, http://links.lww.com/TXD/A213). The levels of IL-23 and IL-27 were below the range of detection.
FIGURE 5.
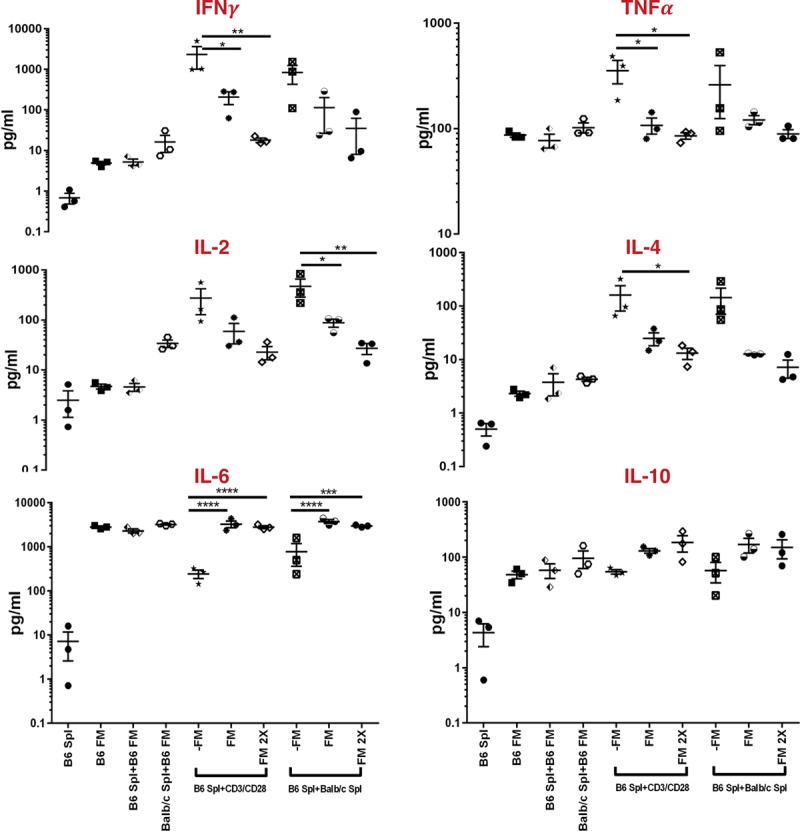
Luminex-based multiplex analysis of the levels of pro and anti-inflammatory cytokines in the supernatants collected from alloantigen and mitogen stimulated mixed lymphocyte reaction (MLR) cultures: levels of IFNγ, TNFα, IL-2, IL-4, IL-6, and IL-10 expressed as pg/mL units, in the supernatants obtained from the cultures containing following experimental groups: B6 splenocytes (B6 Spl), B6 membranes (B6 FM), B6 splenocytes + B6 membranes (B6 Spl + B6FM), Balb splenocytes + B6 membranes (Balb Spl + B6 FM), Balb splenocytes + B6 splenocytes (-FM), Balb splenocytes + B6 splenocytes + B6 membranes (FM), Balb splenocytes + B6 splenocytes + B6 membranes (2X, B6 membranes used at double the cell concentrations corresponding to the responders and stimulators), B6 splenocytes+CD3/CD28 beads (-FM), B6 splenocytes+CD3/CD28 beads+B6 membranes (FM), and B6 splenocytes + CD3/28 beads + B6 membranes (2X). FM, fetal membrane.
DISCUSSION
The present study shows that membranes surrounding the fetus can act as immunosuppressive barriers as well as carriers to significantly improve retention of allogeneic donor cells in transplant recipients without immunosuppressive conditioning. Employing a Balb to B6 allogeneic transplantation model, our results show that 10F7MN hybridoma cells of Balb origin could be retained in a B6 host when transplanted along with B6 membranes. Hybridoma survival was evaluated from the serum antibody levels secreted by the retained donor cells. Function of the donor hybridoma, measured by anti-human CD235a antibody level in the recipient serum, was significantly increased when the genetic background of the membranes was matched to the recipient mice.
Owing to their immunosuppressive properties, intact FMs and the decidua have been studied for their potential application for tissue engineering and cell therapies. FMs consist of a number of cell types,18-20 and our data showed the presence of mesenchymal and epithelial cells. Some hematopoietic cells are also observed in the FMs, primarily consisting of macrophages (Hofbauer cells).21 We found few immune cells in our membrane preparations, suggesting a low likelihood that membrane transplantation will result in significant allogeneic T-cell transfer.
We hypothesized that immune barrier function of membranes might be achieved using membranes readily available from various genetic backgrounds. However, significant improvement in the retention and function of cells could only be achieved when the allogeneic cells were cotransplanted with syngeneic membranes. Indeed, transplantation of intact membranes has been shown to induce an adverse graft-specific immune response by the host.22,23 Moreover, intradonor variability, including mother’s age, race, and health, and even gestational age and sex of the fetus have been reported to contribute to the differences in the functional abilities of FMs.24-26 Our findings suggest that autologous membranes help alleviate adverse immune reactions in the recipients and thus might serve as graft carriers in allogeneic transplantations.
Cotransplantation of allogeneic 10F7MN cells with dissociated membrane cells did not have the same protective and functional effects as intact membranes. We hypothesize that the enzyme-mediated digestion of membranes might inactivate or infer irreversible alterations in the key regulators of cell-to-cell communications, which in turn might impact their functional role as protective carriers in cell transplantations. This finding suggests that intact membranes can act as potent cell-cargos to support the survival allogeneic cells in the host environment. Indeed, it has been observed that without a carrier, <5% of the injected cells persist due to the loss the number and viability of the cells during injection process and exposure to the adverse host factors.10,11,27 Overall, our data suggest that intact syngeneic membranes might provide an immunoprivileged microenvironment facilitating sustained graft function.
An immunomodulatory function of membranes was observed as they showed significant inhibition of alloantigen and mitogen-induced T-cell proliferation in MLR assays. Unraveling the mechanism of suppression of responder T-cell proliferation showed that the alloantigen and mitogen stimulated responder-cultures containing syngeneic membrane cells yielded reduced proinflammatory IFNγ, TNFα, IL-2, and IL-4 as well as increased IL-6 and IL-10 cytokine levels. Interestingly, we observed that even in the absence of cell-cell contact, high amounts IL-6 and IL-10 cytokines were secreted by membranes, suggesting that membranes are rich producers of anti-inflammatory cytokines.
Similar to our findings, previous studies have shown that cellular components of membranes surrounding the fetus produce high levels of IL-6 and IL-10 and suppress IFNγ secretion in MLRs.18,27-31 These cytokine effects are believed to support the immunosuppressive and tolerogenic environment at the feto-maternal interface. Interestingly, in the alloantigen and mitogen stimulated T-cell cultures, we observed an association between the increase in the number of membrane cells to the decrease in the production of IFNγ, TNFα, IL-2, and IL-4. These data are consistent with earlier studies highlighting the dose-dependent immunomodulatory functions of amnion and placenta-derived cells.19,28 In this context, even though our results showed that cultures containing B6 membranes exposed to Balb splenocytes had an increase in the levels of IL-17 and IL-22 production, a reduction in amounts of these cytokines was found as the number of B6 membrane cells were increased. Overall, these data indicated that cells derived from the membranes syngeneic to the responders have the potential to strongly suppress allo-reactive T-cell proliferation and Th1 cytokine production. Interestingly, as we did not observe reduction in alloantibody responses, we hypothesize that membranes might be exerting immunosuppressive effects primarily through T cells rather than B cells.
Various types of natural and synthetic biomaterials have been widely used as cell-carriers in diseases such as hemophilia and diabetes mellitus.29-31 Natural biopolymers are biocompatible and less immunogenic, leading to improved cell survival and function.32 ECM rich, immunoprotective FMs might serve as natural cell-carriers for improving graft survival and function and limiting chances of cytotoxicity in allogeneic transplantations. Research is in progress to engineer biomaterials with integrated anti-inflammatory cytokines to overcome the need of immunosuppressants for long-term donor cell survival and function.33,34 Our findings show that membranes can produce anti-inflammatory cytokines, highlighting their role as promising natural scaffolds in adoptive cell transplantation. Moreover, our findings of improved allogeneic graft function after subcutaneous transplantation is supported by the recent studies that have illustrated the advantage of subcutaneous route of cell transplantation in improving the engraftment of allogeneic pancreatic islet cells.35-37 However, our findings show significant survival of allogeneic cells only when cotransplanted with membranes syngeneic to that of the host. Further research is needed to understand the functional benefit of membranes in improving graft engraftment and function across various diverse genetic backgrounds, to be applicable to clinical practices.
In conclusion, our study highlights the significance of membranes surrounding the fetus that are clinical waste after child birth as potential cell-carriers for allogeneic grafts. Further, these data indicate that membranes might be used as cell-delivery vehicles to deliver therapeutic proteins from injected cells for diseases such as diabetes mellitus and hemophilia. Moreover, the noninvasive procedures involved in membrane collection minimize the ethical considerations involved in their usage, making them a promising substitute as natural, biocompatible, and immunomodulatory cell-carriers over commercially available biomaterials in adoptive cellular transplantations.
ACKNOWLEDGMENTS
The authors are indebted to the administrative staff at Vitalant Research Institute for their tremendous support.
Supplementary Material
Footnotes
Published online 29 May, 2019.
This work was supported by the RIVA Foundation and the National Institutes of Health—grant numbers R01 HL133024 and P01 DK088760. N.D. and E.Y. were supported by a Bridges to Stem Cell Training grant TB1-01188 from the California Institute of Regenerative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases; the National Heart, Lung and Blood Institute; the National Institutes of Health; the California Institute for Regenerative Medicine; or any other agency of the state of California.
The authors declare no conflicts of interest.
P.P.T. participated in research design, performed in vivo transplantations, in vitro MLR assays and luminex experiments, conducted the data analysis, prepared figures, and drafted the article. N.D. participated in the in vivo transplantation experiments and figure preparation. E.Y. participated in the in vivo transplantation experiments and performed flow cytometry and data analysis on the serum of transplanted mice. J.W.H. performed the luminex experiments. R.P.J. participated in data analysis and edited the article. M.G. participated in research design. P.J.N. participated in data analysis and edited the article. A.B. participated in research design and edited the article. M.O.M. participated in research design and data analysis, secured funding for the research, and edited the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Manabe Y, Himeno N, Fukumoto M. Tensile strength and collagen content of amniotic membrane do not change after the second trimester or during delivery. Obstet Gynecol. 1991;78:24. [PubMed] [Google Scholar]
- 2.Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195:510–515.. [DOI] [PubMed] [Google Scholar]
- 3.Ponder KL, Bárcena A, Bos FL, et al. Preeclampsia and inflammatory preterm labor alter the human placental hematopoietic niche. Reprod Sci. 2016;23:1179–1192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badet MT, Bell SC, Billington WD. Immunoregulatory activity of supernatants from short-term cultures of mouse decidual tissue. J Reprod Fertil. 1983;68:351–358.. [DOI] [PubMed] [Google Scholar]
- 5.Mori M, Bogdan A, Balassa T, et al. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol. 2016;38:635–649.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328–334.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huppertz B, Kertschanska S, Frank HG, et al. Extracellular matrix components of the placental extravillous trophoblast: immunocytochemistry and ultrastructural distribution. Histochem Cell Biol. 1996;106:291–301.. [DOI] [PubMed] [Google Scholar]
- 8.Kisalus LL, Herr JC, Little CD. Immunolocalization of extracellular matrix proteins and collagen synthesis in first-trimester human decidua. Anat Rec. 1987;218:402–415.. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449.. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202.. [DOI] [PubMed] [Google Scholar]
- 11.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–213.. [DOI] [PubMed] [Google Scholar]
- 12.Qi C, Yan X, Huang C, et al. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell. 2015;6:638–653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niknejad H, Peirovi H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99.. [DOI] [PubMed] [Google Scholar]
- 14.Riau AK, Beuerman RW, Lim LS, et al. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31:216–225.. [DOI] [PubMed] [Google Scholar]
- 15.Togarrati PP, Sasaki RT, Abdel-Mohsen M, et al. Identification and characterization of a rich population of CD34+ mesenchymal stem/stromal cells in human parotid, sublingual and submandibular glands. Sci Rep. 2017;7:3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muench MO, Heitman JW, Inglis H, et al. Reduced alloimmunization in mice following repeated transfusion with pathogen-reduced platelets. Transfusion. 2016;56:1419–1429.. [DOI] [PubMed] [Google Scholar]
- 17.Chen JC, Chang ML, Muench MO. A kinetic study of the murine mixed lymphocyte reaction by 5,6-carboxyfluorescein diacetate succinimidyl ester labeling. J Immunol Methods. 2003;279:123–133.. [DOI] [PubMed] [Google Scholar]
- 18.Lim R. Concise review: fetal membranes in regenerative medicine: new tricks from an old dog? Stem Cells Transl Med. 2017;6:1767–1776.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talwadekar MD, Kale VP, Limaye LS. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci Rep. 2015;5:15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kmiecik G, Niklińska W, Kuć P, et al. Fetal membranes as a source of stem cells. Adv Med Sci. 2013;58:185–195.. [DOI] [PubMed] [Google Scholar]
- 21.Reyes L, Wolfe B, Golos T. Hofbauer cells: placental macrophages of fetal origin. Results Probl Cell Differ. 2017;62:45–60.. [DOI] [PubMed] [Google Scholar]
- 22.Wilshaw SP, Kearney J, Fisher J, et al. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A. 2008;14:463–472.. [DOI] [PubMed] [Google Scholar]
- 23.Díaz-Prado S, Rendal-Vázquez ME, Muiños-López E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11:183–195.. [DOI] [PubMed] [Google Scholar]
- 24.López-Valladares MJ, Teresa Rodríguez-Ares M, Touriño R, et al. Donor age and gestational age influence on growth factor levels in human amniotic membrane. Acta Ophthalmol. 2010;88:e211–e216.. [DOI] [PubMed] [Google Scholar]
- 25.Litwiniuk M, Radowicka M, Krejner A, et al. The influence of amniotic membrane extracts on cell growth depends on the part of membrane and childbirth mode selected: a proof-of-concept study. J Wound Care. 2017;26:498–503.. [DOI] [PubMed] [Google Scholar]
- 26.Litwiniuk M, Grzela T. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen. 2014;22:451–456.. [DOI] [PubMed] [Google Scholar]
- 27.Burdick JA, Mauck RL, Gerecht S. To serve and protect: hydrogels to improve stem cell-based therapies. Cell Stem Cell. 2016;18:13–15.. [DOI] [PubMed] [Google Scholar]
- 28.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–1183.. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, He H, Yen C, et al. A biodegradable, immunoprotective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29:4253–4259.. [DOI] [PubMed] [Google Scholar]
- 30.Desai TA, Chu WH, Rasi G, et al. Microfabricated biocapsules provide short-term immunoisolation of insulinoma xenografts. Biomed Microdevices. 1999;1:131–138.. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan ES, Vegas A, Anderson DG, et al. Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews JA, Wnek GE, Simpson DG, et al. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238.. [DOI] [PubMed] [Google Scholar]
- 33.Hume PS, He J, Haskins K, et al. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials. 2012;33:3615–3625.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarado-Velez M, Pai SB, Bellamkonda RV. Hydrogels as carriers for stem cell transplantation. IEEE Trans Biomed Eng. 2014;61:1474–1481.. [DOI] [PubMed] [Google Scholar]
- 35.Kuwabara R, Hamaguchi M, Fukuda T, et al. Long-term functioning of allogeneic islets in subcutaneous tissue pretreated with a novel cyclic peptide without immunosuppressive medication. Transplantation. 2018;102:417–425.. [DOI] [PubMed] [Google Scholar]
- 36.Luan NM, Iwata H. Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant. 2014;14:1533–1542.. [DOI] [PubMed] [Google Scholar]
- 37.Bertuzzi F, De Carlis LG. Subcutaneous islet allotransplantation without immunosuppression therapy: the dream of the diabetologists and of their patients. Transplantation. 2018;102:351–352.. [DOI] [PubMed] [Google Scholar]


