Abstract
Background
Exposure to a traumatic event leads to posttraumatic stress disorder in 10% to 20% of exposed individuals. Predictors of risk are needed to target early interventions to those who are most vulnerable. The objective of the study was to test whether a noninvasive mobile device that measures a physiological biomarker of autonomic nervous system activation could predict future posttraumatic stress disorder symptoms.
Methods
Skin conductance response was collected during a trauma interview in the emergency department within hours of exposure to trauma in 95 individuals. Trajectories of posttraumatic stress disorder symptoms over 12-month posttrauma were identified using latent growth mixture modeling.
Results
Skin conductance response was significantly correlated with the probability of being in the chronic posttraumatic stress disorder trajectory following trauma exposure in the emergency department (r = 0.489, p < 0.000001). Lasso regression with elastic net was performed with demographic and clinical measures obtained in the emergency department, demonstrating that skin conductance response was the most significant predictor of the chronic posttraumatic stress disorder trajectory (p < 0.00001).
Conclusions
This study is the first prospective study of posttraumatic stress disorder showing skin conductance response in the immediate aftermath of trauma predicts subsequent development of chronic posttraumatic stress disorder. This finding points to an easily obtained, and neurobiologically informative, biomarker in emergency departments that can be disseminated to predict the development of posttraumatic stress disorder.
Keywords: posttraumatic stress disorder, trauma, skin conductance, prediction, emergency department, sympathetic nervous system
Introduction
Posttraumatic stress disorder (PTSD) is a complex and heterogeneous syndrome that can develop in individuals who are exposed to a traumatic event. Approximately 60% of the population will experience at least one traumatic stressor in their lifetime;1 however, the lifetime prevalence for PTSD is only about 6.8%,2 demonstrating the need for tools to identify at-risk individuals at the time of trauma. A number of early interventions that can be deployed in the emergency departments (EDs) may eventually be effective in diminishing the development of PTSD symptoms following such traumas.3 However, limited access to these interventions in hospitals that receive over 100,000 injury-related emergency room visits per day4 necessitates the ability to quickly identify individuals at the highest risk for developing PTSD. Robust predictors of risk in the immediate aftermath of trauma will lead to targeted use of evidence-based treatment and prevention of the disorder. Biomarkers that can quickly ascertain risk, independent of subjective self-report symptoms, demographic and cultural factors, are especially important for early intervention efforts.
One of the hallmarks of PTSD is increased psychophysiological arousal driven by the autonomic nervous system.5,6 Outputs of the sympathetic nervous system (SNS) include changes in heart rate (HR), blood pressure (BP), skin conductance (SC), and respiration rate and are all heightened in individuals with PTSD.5 It has been previously shown that increased HR at the time of trauma is significantly correlated with an increased likelihood to develop PTSD in the months following the trauma,3 suggesting that initial SNS responses to the trauma may reflect a risk mechanism for future symptoms. A limitation of previous studies is the use of large and elaborate psychophysiological data acquisition equipment that requires dedicated space, specialized training, and a substantial financial investment. Furthermore, most biomarkers, including traditional psychophysiological methods, typically require some time for processing and analysis. A novel paradigm that is highly relevant in the ED setting is rapid measurement of psychophysiological reactivity during a trauma interview assessing aspects of the traumatic event that brought the patient to the hospital.
Technological advances now make it possible for SC or HR to be recorded continuously during a trauma interview using mobile applications on smartphones and tablets via very simple and cost-effective devices in clinical settings. We have previously shown that we can differentiate patients with chronic PTSD compared to those without PTSD using this approach during a standardized trauma interview in a cross-sectional study of clinic patients.7
This study investigated SNS response by capturing the skin conductance response (SCR) immediately following the trauma and the subsequent development of PTSD in a prospective longitudinal study conducted in the ED of a large Level 1 trauma center. SCR to a psychological trigger of a previously experienced trauma offers a noninvasive quantitative, biological output that is associated with current PTSD status and symptom severity.5–9 This predictive SNS-based biomarker may prove useful in identifying which individuals are at the highest risk of developing PTSD after a trauma such that early interventions can be most effectively targeted.
In this study, a low-cost, mobile SC recording application was used to quantify differences in SCR during a standard trauma interview (STI) in the immediate aftermath of trauma exposure. Although many individuals will experience some degree of acute stress symptoms in the days and weeks following a trauma, PTSD can be classified as a failure of natural recovery from these symptoms. We hypothesized that higher SNS reactivity at the time of trauma exposure would predict a chronic disease trajectory, in that PTSD symptoms would be high initially as well as remain high over the course of the year following the trauma. On the other hand, we predicted that individuals who had less SNS activation during the trauma interview would be classified in a resilient trajectory, having low PTSD symptoms over the year posttrauma. Furthermore, we hypothesized that higher SNS reactivity would be predictive of future PTSD diagnosis.
Methods
Participants
Study participants (n = 107) were recruited as part of a large prospective study from the ED of Grady Memorial Hospital in Atlanta, GA, the largest Level 1 ED in GA, USA (Figure 1). Participants were enrolled in the emergency room an average of 4.2 h (range 0.5–12 h) after experiencing a Diagnostic and Statistical Manual (DSM)-IV-TR Criterion A trauma. Study participants were English-speaking men and women between the ages of 18 and 65 years of who provided written informed consent. Exclusion criteria were a history of mania, schizophrenia, other psychosis, current suicidal ideation, suicide attempt in the previous three months, and current intoxication. Participants were excluded for respiratory distress or if they were medically unstable or hemodynamically compromised. All participants underwent assessment of trauma exposure, baseline depression, and PTSD by trained research staff in the ED. All study procedures were reviewed and approved by the Emory Institutional Review Board and the Grady Hospital Research Oversight Committee. All data were captured and managed using Health Insurance Portability and Accountability Act-compliant, REDCap electronic data capture tools hosted by Emory University.
Figure 1.
Prospective, Longitudinal Emergency Department Study Design at Grady Memorial Hospital (a Level 1 Trauma Center), in Atlanta, GA. N = 9822 participants were screened for eligibility in the study, with 1755 meeting initial inclusion/exclusion criteria and approached for consent. N = 505 participants consented to participation (28.8%) with 377 returning for at least one follow-up visit being included in the trajectory analyses (74.7%). Skin conductance recording was introduced to the study at a later date with N = 107 participants receiving this assessments (no participants declined this assessment) and N = 95 participants with usable SCR data.
Measures
The STI was administered in the ED. The STI is a 41-item interview covering demographics, detailed characteristics of the trauma, patient-rated severity of the trauma, and social support available to the patient. The STI also allows for an open-ended description of the trauma by the participant. The trauma-related items on the STI are publicly available as part of the PhenX Toolkit protocol (https://www.phenxtoolkit.org/index.php?pageLink=browse.protocoldetails&id=630901). SC data analyzed in this study were collected during the STI at the time of enrollment in the ED.
PTSD diagnosis and symptom severity were assessed at 1-, 3-, 6-, and 12-month posttrauma and were measured using the PTSD Symptom Scale (PSS).10 The PSS is a psychometrically valid 17-item self-report scale assessing PTSD symptoms over the past two weeks.11 A categorical PTSD diagnosis was based on DSM-IV criteria, if participants met at least one reexperiencing symptom, three avoidance and/or numbing symptoms, two hyperarousal symptoms, and if the duration of symptoms was greater than one month.12 For a continuous measure of PTSD severity, we summed the PSS items. Similarly, we computed continuous measures for PTSD symptom clusters, including reexperiencing, avoidance, and hyperarousal symptom clusters. Childhood trauma and preexisting PTSD and depression symptoms were collected as part of the larger study and used as control variables (see Supplement for full description and analysis methods described later in this article).
Trajectory Analysis
To determine how many distinct latent classes best describe the trajectories of PTSD symptom severity based on PSS collected at the follow-up visits, a series of latent growth mixture modeling (LGMM) analyses was applied using MPlus 7. Individuals were assigned to one of the identified trajectories based on their most likely class membership (highest posterior probability). To identify the best fitting number of classes, we started with one, two, three, and so on, up to six classes. We examined linear and quadratic slope to identify the best fitting trajectory shape. A nested model approach was used, testing a progressive number of classes until the model fit indices no longer favor the addition of any more classes. Relevant criteria for determining the number of classes included the reduction in the Bayesian Information Criterion, sample-size adjusted Bayesian Information Criterion, Akaike Information Criterion indices, and significance indicated by the Vuong–Lo–Mendell–Rubin Likelihood ratio test, and the Bootstrap Likelihood ratio test, together with parsimony and interpretability. Entropy was used to determine the clarity of class specification. Trajectories were based on the larger study sample (not all of whom had SC measures) with at least one follow-up visit (N = 377, see Figure 1 for participant flow chart). Participants were assigned a trajectory class membership and a probability of being in each of the three trajectory classes via this analysis.
SC Assessment
SC was assessed in the ED using the mobile eSense system (Mindfield Biosystems, Inc., Berlin, Germany) on an iPad (iOS10) as previously validated in chronic PTSD.7 The eSense application was launched on the study iPad and two finger electrodes were attached to the middle phalanges of the middle and index finger of the nondominant hand with Velcro straps. Isotonic paste was added to the electrodes prior to attaching to the fingers to increase signal quality and ensure contact with skin. The electrodes were connected to the iPad using the audio connection input. eSense acquired data at a sampling rate of 5 Hz and the data were exported for analyses in Excel. Baseline SC level was recorded during a 2-min rest period. The baseline recording was immediately followed by an SC level recording during the administration of the STI. As in our previous study, SC response (SCR) was calculated by subtracting the maximum SC value during the STI from the average SC for the final 30 s at the end of the baseline recording.7 Usable SC data were defined as recordings with no technical errors or faults in the recording system/electrical noise. In all participants, peak SCR occurred prior to 360 s into the trauma interview, and there was no correlation between duration and PTSD outcomes; therefore, all files were trimmed to the same length of 600 s duration.
Data Analyses
The data were analyzed using SPSS (v.24) and were summarized as mean ± standard error of the mean (SEM). The alpha level was set at p < 0.05 for statistical significance. Pearson’s correlations were used to assess the relationship between SCR and the probability of assignment to each of the trajectory classes. In addition, to test whether the trajectory membership results would generalize to a more direct clinical outcome, PTSD symptom severity, as well as PTSD symptom sub-clusters, measured at the six-month follow-up visit was also analyzed. The PSS data from this visit were used because it had the highest sample size of the chronic timepoints (>3 months posttrauma).
To assess the predictive ability of SCR for PTSD trajectory membership, we used a lasso regression with elastic net.13 Elastic net regularization can be used to define the most optimal model among a number of independent variables and is particularly well suited to models wherein the predictors are highly correlated.14 It combines the penalty functions of lasso and ridge methods and was implemented using SPSS default settings. For the predictors in the model, elastic net regression minimizes overfitting by penalizing coefficient estimates (reducing them toward zero), thereby reducing the variance of estimates so that they are more stable and more generalizable to the larger population. The .632 bootstrap method was used to estimate the expected prediction error for each model.15 Using the one-standard-error rule, the most parsimonious model within 1SE of the model with minimum expected prediction error was determined. Trajectory class assignment was used as the outcome measure in this model. Finally, a receiver operating characteristic (ROC) curve was used to evaluate the sensitivity and specificity of classification in the trajectory membership using ED measurement of SCR as the predictor. The area under the curve (AUC) and 95% confidence intervals were determined to test the accuracy of SCR for predicting PTSD outcome.
To assess the variance accounted for by the SCR after accounting for other putative predictors of chronic PTSD, we used a stepwise linear regression with SCR added in the last step. The probability of assignment to the chronic PTSD trajectory was used as the outcome measure in this model. The models were built with SCR as well as common predictors of PTSD including demographic variables (age, gender, race, education level, income level, and social support), trauma-related variables (trauma type, report of intimate partner violence for the study index trauma, patient-rated severity of the trauma, and the number of previous similar traumas), and baseline clinical variables (childhood trauma load, depression symptoms at the time of the trauma, and existing PTSD symptoms from prior traumas at the time of the study index trauma). These variables are defined further in the Supplemental Text.
Results
Sociodemographics
Participant flow chart is shown in Figure 1. Sociodemographic characteristics of the final sample are summarized in Table 1. The sample with SC data was predominately African American, survivors of a motor vehicle collision, with an average monthly income of > $1000. Approximately half (44%) of the participants were female. After removing unusable data due to noise and artifact (N = 9) and three outliers (>3 SD), usable SC data were available for N = 95. There were no demographic (age, sex, or race) differences between those with usable SC data (N = 95) and unusable SC data (N = 12).
Table 1.
Participant demographic and clinical data.
| N = 95 | |
|---|---|
| Age, mean (SD) | 35.6 (13.0) |
| Gender (% female) | 42 (44%) |
| Race (%) | |
| Black | 78 (82%) |
| White | 9 (10%) |
| Mixed | 1 (1%) |
| Other | 7 (7%) |
| Monthly income level (%) | |
| $0–$249 | 7 (7%) |
| $250–$499 | 5 (5%) |
| $500–$999 | 13 (14%) |
| $1000–$1999 | 23 (24%) |
| $2000 or more | 46 (48%) |
| Education level (%) | |
| Master's degree | 10 (10%) |
| Some graduate school | 2 (2%) |
| Bachelor's degree | 8 (8%) |
| Associate's, some college | 32 (34%) |
| High school degree | 28 (30%) |
| Some high school | 15 (17%) |
| Type of trauma (%) | |
| Nonsexual assault | 6 (6%) |
| Motor vehicle collision | 46 (43%) |
| Motor cycle collision | 5 (5%) |
| Pedestrian vs. auto | 12 (11%) |
| Stabbing | 10 (9%) |
| Gunshot wound | 4 (4%) |
| Industrial/home accident | 4 (4%) |
| Fall | 3 (4%) |
| Animal attack/bite | 4 (4%) |
| Bicycle accident | 4 (4%) |
| Sexual assault | 6 (6%) |
| Intimate partner violence (%) | 4 (4%) |
| Pain after trauma (0–10), mean (SD) | 6.59 (2.8) |
| Patient-rated trauma severity (0–5), mean (SD) | 3.89 (1.26) |
| Number of Similar Prior Traumas, mean (SD) | 2.07 (1.7) |
| Social support, mean (SD) | 2.33 (1.09) |
| Childhood trauma (CTQ), mean (SD) | 43.3 (20.4) |
| Depression symptoms (BDI) in the ED, mean (SD) | 14.34 (11.3) |
| PTSD symptoms (PDS) in the ED, mean (SD) | 11.4 (10.7) |
| PTSD symptoms (PSS) at six months (mean, SD) | 11.8 (11.6) |
SCR and PTSD Trajectories
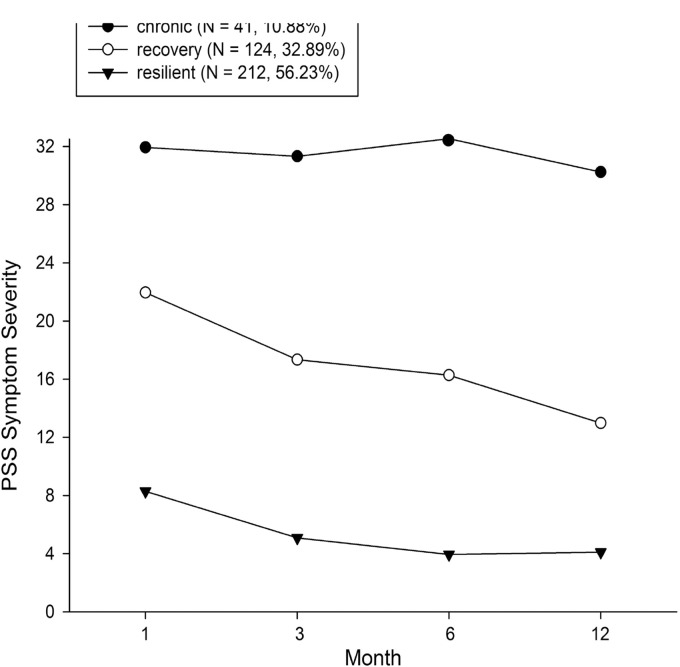
When determining trajectory outcomes, a three-class solution with fixed variance for intercept and slope was the best-fitted model (see Supplement Table 1). The LGMM analysis included a larger sample of 377 participants recruited in the ED, of which 41 participants were assigned to the chronic trajectory (10.88%), 124 participants to the recovery trajectory (32.89%) and 212 participants to the resilient trajectory (56.23%) (Figure 2). Of this larger sample, 95 participants with SC measures in the ED were assigned into one of the trajectories: N = 12 participants were assigned to the chronic trajectory (12.6%), N = 28 participants were assigned to the recovery trajectory (29.5%), and N = 55 participants to the resilient trajectory (57.9%).
Figure 2.
Unconditional LGMM—Identification of three heterogeneous trajectories of PTSD symptom severity (chronic, recovery, or resilient) based on PSS scores. Trajectory assignment was based on the larger study sample of n = 377 with PSS score at any follow-up timepoint. PSS: posttraumatic stress disorder Symptom Scale.
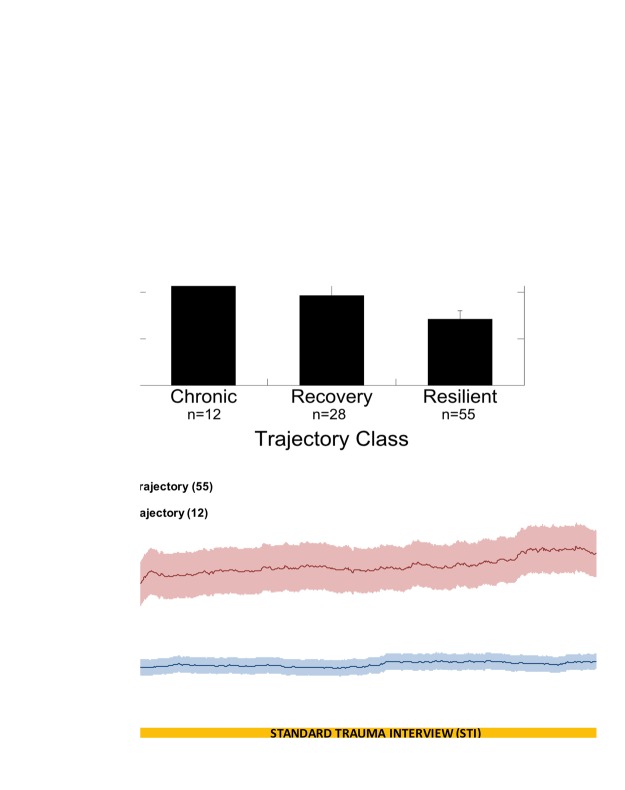
SCR of participants in the chronic trajectory was significantly higher than that of participants in both the resilient and recovery trajectories (Figure 3(a) and (b)) t12.026 = 4.260, p < 0.0000001 and t15.026 = 3.313, p = 0.005, respectively. There was a significant positive correlation between SCR in the ED and the probability of assignment to the chronic PTSD trajectory (r = 0.489, p < 0.000001) and a significant negative correlation between SCR and probability of assignment to the resilient trajectory (r = −0.377, p < 0.000001). There was no significant correlation between SCR and probability of assignment to the recovery trajectory. Further SCR analysis was completed using only the chronic and resilient trajectories due to the recovery trajectory not being correlated with SC in the ED.
Figure 3.
SCR posttrauma correlates to PTSD development and differs significantly based on outcome. (a) Mean ± SEM skin conductance response (difference between SC to trauma interview and baseline SC) in the Emergency Department (SCR, microSiemens, µS) by PTSD trajectory **p < 0.00001. (b) Average skin conductance levels with ± SEM (shaded) for participants in the chronic class (n = 12, top trace) and the resilient class (n = 55, bottom trace).
Lasso regression with elastic net was used to determine the optimal model to predict trajectory class assignment using demographic and clinical measures and SCR. Table 2 displays the regression coefficients for only the variables included in the most parsimonious model found to predict trajectory class. For the selected model, the apparent proportion of explained variance was 53% (F(14, 80) = 5.048, p = 0.000001). SCR was the strongest predictor of trajectory class in the model (p = 0.001) with all three trajectory classes included (n = 95). Baseline PTSD (p = 0.024) was also a significant factor in the final model. Negative Beta values in this model indicate risk due to the chronic trajectory class assignment being assigned a value of 1, the recovery trajectory class is assigned a value of 2, while the resilient trajectory class was assigned a value of 3.
Table 2.
Lasso regression coefficients for predicting trajectory class: Predictive variables model using lasso regression with elastic net.
| Trajectory class | Beta | SE | df | F | p |
|---|---|---|---|---|---|
| Trauma type | 0.056 | 0.092 | 10 | 0.364 | 0.958 |
| Patient-rated severity | −0.009 | 0.028 | 1 | 0.105 | 0.747 |
| Depression symptoms in ED (BDI) | −0.131 | 0.082 | 1 | 2.558 | 0.114 |
| PTSD symptoms in ED (PDS) | −0.207 | 0.090 | 1 | 5.308 | 0.024* |
| SCR | −0.294 | 0.085 | 1 | 11.972 | 0.001*** |
Note: Lasso regression with elastic net was performed in the original sample (n = 95; all three trajectory classes included). Regression coefficients only for variables included in the most optimal models to predict trajectory class are presented. BDI: Beck Depression Inventory; PDS: Posttraumatic Stress Diagnostic Scale; SCR: Skin conductance response; ED: emergency department.
p < 0.05; ***p < 0.001.
To identify the unique variance accounted for by SCR, we ran a stepwise linear regression with probability of assignment to the chronic trajectory as the dependent variable. After controlling for demographic variables, trauma history, baseline PTSD, and depression, SCR was added in the final step. Table 3 shows the variables added in each step and the statistics for each model. SC alone accounted for approximately 28% of the variance in the chronic trajectory membership, R2 change = 0.283, F change = 37.720, and p = 0.000001 with all 95 individuals included. Finally, we tested the sensitivity and specificity of the SCR predicting trajectory membership; the AUC for the ROC curve analysis for SCR on chronic trajectory class assignment was 0.90 (p < 0.00001) with 95% confidence intervals of 0.80 and 0.99 (Figure 4).
Table 3.
Stepwise linear regression for probability of chronic trajectory membership.
| R2 | SE | df | F | p | |
|---|---|---|---|---|---|
| Model 1—Demographic variables | 0.216 | 0.258 | 6, 53 | 2.431 | 0.038* |
| Model 2—Trauma-related variables | 0.325 | 0.249 | 10, 49 | 2.361 | 0.023* |
| Model 3—Baseline psychiatric variables | 0.379 | 0.247 | 13, 46 | 2.160 | 0.028* |
| Model 4—SCR | 0.662 | 0.184 | 14, 45 | 6.302 | 0.000001*** |
Note: Stepwise linear regression was performed with probability of chronic trajectory membership (all individuals with SCR included in analysis (n = 95)). In the first model, only demographic variables of age, gender, race, education, income and social support were included. In Model 2, trauma-related variables were added including trauma type, if the index trauma was intimate partner violence, patient-rated severity of the trauma, and the number of similar traumas previously experienced. In Model 3, baseline psychiatric variables of childhood trauma load, baseline PTSD, and baseline depression were added. In Model 4, the SCR in the ED was added; R2 change = 0.283, F change = 37.72. PTSD: posttraumatic stress disorder; SCR: skin conductance response; ED: emergency department.
p < 0.05; ***p < 0.001.
Figure 4.
The ROC curve for SCR to trauma interview as the predictor for chronic PTSD trajectory assignment–—the AUC for the ROC curve was 0.90 (p < 0.00001) with 95% confidence intervals of 0.80 and 0.99. PTSD: posttraumatic stress disorder; ROC: receiver operating characteristic; SCR: skin conductance response.
SCR and PTSD Symptom Development
In order to examine whether the trajectory analyses mirrored clinical data, we analyzed PSS severity in N = 75 participants with six-month follow-up data. This timepoint was chosen given that it had the largest sample size. SCR to the trauma interview during the ED evaluation immediately posttrauma was significantly correlated with overall PTSD symptom severity (r = 0.41, p < 0.0001) (Figure 5). SCR in the ED was also significantly associated with all symptom cluster subscales at six months: intrusive (r = 0.30, p = 0.01), avoidance/numbing (r = 0.49, p < 0.0001), and hyperarousal symptoms (r = 0.29, p = 0.011). Table 4 shows a stepwise linear regression matching that used for the analysis with trajectory outcomes and the variables added in each step and the statistics for each model. SC alone accounted for approximately 16% of the variance in PTSD symptom severity, R2 change = 0.159, F change = 27.399 and p < 0.000001 with 68 individuals included.
Figure 5.
The SCR recorded in the ED at the time of trauma was significantly correlated to the severity of PTSD symptoms at the six-month follow-up visit (r = 0.41, p < 0.001). PSS: PSS: posttraumatic stress disorder Symptom Scale; PTSD: posttraumatic stress disorder.
Table 4.
Stepwise linear regression for six-month PTSD symptoms.
| R2 | SE | df | F | p | |
|---|---|---|---|---|---|
| Model 1—Demographic variables | 0.246 | 0.258 | 6, 62 | 3.366 | 0.006* |
| Model 2—Trauma-related variables | 0.402 | 0.249 | 10, 58 | 3.895 | 0.00044* |
| Model 3—Baseline psychiatric variables | 0.528 | 0.247 | 13, 55 | 4.739 | 0.00002* |
| Model 4—SCR | 0.687 | 0.184 | 14, 54 | 8.470 | <0.000001*** |
Note: Stepwise linear regression was performed with six-month PTSD symptom severity (n = 68). In the first model, only demographic variables of age, gender, race, education, income, and social support were included. In Model 2, trauma related-variables were added including trauma type, if the index trauma was intimate partner violence, patient-rated severity of the trauma, and the number of similar traumas previously experienced. In Model 3, baseline psychiatric variables of childhood trauma load, baseline PTSD, and baseline depression were added. In Model 4, the SCR in the ED was added; R2 change = 0.159, F change = 27.399. PTSD: posttraumatic stress disorder; SCR: skin conductance response; ED: emergency department.
p < 0.05; ***p < 0.000001.
Discussion
This study is the first to show that SCR to trauma-relevant stimuli collected using a mobile device in the ED, in the immediate aftermath of trauma exposure, is predictive of future development of PTSD symptoms during the year following the trauma. The predictive utility of SCR was independent of psychiatric status at the time of the trauma, demographic characteristics, as well as the type and severity of traumatic event, revealing a robust noninvasive biomarker of risk for developing PTSD symptoms. This is a rapid and simple assessment that can be easily completed by a first responder or ED technician/nurse/provider for early detection of individuals at high risk for chronic symptoms. Early interventions by mental health providers could then be targeted specifically at those high-risk individuals.
Previous work has largely used HR or BP in exploring a biological indicator of risk for PTSD development after a trauma, but with many caveats. Shalev et al. showed that mean HR levels at the time of trauma were significantly higher in subjects who subsequently developed PTSD at four months than those who did not.20 Studies of HR in the acute period posttrauma have largely assessed resting HR using the vital signs collected as part of hospital medical records20,21 rather than HR changes in response to a trauma challenge, as has been done within this study. Bryant et al.21 reported strong sensitivity and specificity in predicting PTSD using resting HR at discharge from the hospital, that is, 2 to 26 days posttrauma exposure. Although these studies showed early promise in using SNS measures to predict PTSD, the reliance on hospital records and inherent variability in postinjury HR hampered its widespread use.
Using a more experimental approach, Blanchard et al.22 utilized a stressful challenge (mental arithmetic and audio and videotapes of motor vehicle accidents (MVA)) while measuring HR and BP in MVA survivors. This study found that increased HR to trauma-specific stimuli in the months after MVA predicted PTSD one-year posttrauma4 and showed that the initial physiological reactivity of the first presentation of audio recording was the most salient predictor of future PTSD. The main limitation of this study is that assessment was conducted one to four months after the trauma in a controlled, laboratory setting. In this study, we used one of the very first descriptions of the trauma in the ED situation by the participant as the stimulus for SCR recording, thereby providing one of the earliest timepoints posttrauma for assessment and a feasible timepoint when ED staff could collect these data.
The SCR measured in this study likely reflects the underlying biology of the stress response, specifically related to autonomic function, since SC primarily measures SNS activity.23 The SNS has also been implicated in the etiology of PTSD. Pitman et al. hypothesized that PTSD results from the traumatic experience leading to exaggerated catecholamine response.8 The increased levels of NE modulate postencoding memory stabilization processes, potentially leading to a phenomenon dubbed “overconsolidation” of the trauma memory in individuals at risk for later PTSD. This hypothesis was supported by early findings that propranolol, a β-adrenergic receptor antagonist, could prevent PTSD if administered acutely posttrauma.24 However, a recent meta-analysis of propranolol studies did not find consistent results25—suggesting that targeting such interventions only to those at highest risk may be more effective. Our results support the idea that some aspect of the development of PTSD—potentially including the “overconsolidation” of distressing memories of the trauma–—occurs while the SNS is in a hyperactive state. The increased SNS activity would disrupt normal memory processing and, particularly, could sustain distress associated with reexperiencing and reprocessing of the traumatic event in the initial posttrauma stages. This disruption would lead to a cascade of events that would result in an escalation of intrusive symptomology and a resultant disruption in the fear circuitry.
Importantly, SCR alone was found to be a stronger predictor than other canonical factors thought to predict PTSD, such as type of trauma or past PTSD symptoms. As Yehuda noted, “Variables that have emerged as salient predictors of PTSD in retrospective studies have very little predictive value in determining the development of this disorder, when gauged from a prospective vantage point.”26 This is evident in our elastic net regression analysis, which resulted in other predictors being excluded from, or nonsignificant in the most parsimonious model. These included even very well-accepted risk factors for PTSD including childhood trauma exposure, dissociation, pain level, and type of trauma. It is possible that SNS reactivity, as one of the putative biological bases of the disorder, incorporates the effects of other trauma-related factors, providing further support for the use of biomarkers in risk prediction.
In previous studies that have prospectively studied the development of PTSD in participants who were seen in the ED after trauma exposure,22,27 it was observed that PTSD risk is predictable from data collected in the ED, including neuroendocrine, physiological, demographic, or early clinical symptoms, and that chronic stress trajectory membership is influenced by pretrauma regulation of cortisol.28 Based on these findings, it is possible with the trajectory approach to identify and validate risk factors and to target potential treatments strategies related to different clinically relevant phenotypes. The LGMM computational approach has empirically shown that heterogeneous responses in populations exposed to trauma are to be expected and distinct trajectories for resilience, recovery, and chronic posttraumatic stress response have been identified.29,30 This illustrates how a data-driven approach can characterize clinically relevant populations and provide an avenue to test putative risk factors and to target potential treatments leading to promising venues for early prevention of chronic posttraumatic stress responses soon after a potential trauma.
As SCR has been found to be elevated in response to trauma-related stimuli in both chronic PTSD patients7 as well as in the immediate aftermath of trauma, it provides a unique opportunity to identify risk for PTSD in the population as traumas occur. Traditional methods of recording SCR have been cumbersome, cost-prohibitive, and have required special training to ensure reliable data acquisition and analysis. The mobile SCR recording method utilized in this study, however, provides for a low-cost and easy-to-use method for paramedics in the field, or medical staff in the ED, to quickly assess the patients’ physiological response immediately following the trauma. Furthermore, it can be deployed in other challenging settings, such as combat zones after trauma exposure. Therefore, this method can help to specifically target early delivery of resource-intensive or SNS-targeting pharmacological interventions to these patients.
One of the limitations of this study is the relatively small sample size, which has shown to produce idiosyncratic results in other small, prospective studies of physiological predictors of PTSD.24 Yet, despite the limited sample size, rigorous statistical analyses showed that this measure is a strong predictor for PTSD. To address the reproducibility and generalizability of our results, future studies should replicate this finding and generalize to other samples and populations. This mobile SCR assessment method is currently employed in a large ongoing multisite prospective study of PTSD (AURORA); while it is still too early to see results of the study, it has already proven to be scale-able to large sample sizes. An additional limitation of this study is the high prevalence of traditionally “low severity” observed trauma exposures, with nearly 50% of participants experiencing a motor vehicle collision. We believe that the current results provide a more conservative test of our hypothesis given this prevalence of low severity trauma, for example, the increased psychological risk of interpersonal trauma would potentially suggest a higher association of SCR and PTSD in such trauma cohorts.31,32 However, the current sample is representative of the types of traumas commonly seen in EDs and potentially leads to more generalizable findings.
Conclusion
This study was the first prospective study of PTSD that used SCR paired with an STI to predict subsequent development of PTSD. This finding is unique due to the strength and specificity by which this biomarker (an easy to obtain and compute value) can be disseminated to predict the trajectory of the severity of PTSD symptoms after exposure to a traumatic event.
Supplemental Material
Supplemental Material for Increased Skin Conductance Response in the Immediate Aftermath of Trauma Predicts PTSD Risk by Rebecca Hinrichs, Sanne J. H. van Rooij, Vasiliki Michopoulos, Katharina Schultebraucks, Sterling Winters, Jessica Maples-Keller, Alex O. Rothbaum, Jennifer S. Stevens, Isaac Galatzer-Levy, Barbara O. Rothbaum, Kerry J. Ressler and Tanja Jovanovic in Chronic Stress
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by fuding from the National Institutes of Mental Health R01MH094757 (KJR) and Brain and Behavior Research Foundation NARSAD Indepentent Investigator Award (BOR).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52(12): 1048–1060. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demier O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence of age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 3.Kearns MC, Ressler KJ, Zatzick D, Rothbaum B. Early interventions for PTSD: a review. Depress Anxiety 2012; 29(10): 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rui P, Kang K, Albert M. National Hospital Ambulatory Medical Care Survey: 2013 Emergency Department Summary Tables. In: Branch AaHCS, ed. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf. Accessed April 4, 2019.
- 5.Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25(2): 271–293. [DOI] [PubMed] [Google Scholar]
- 6.Keane TM, Wolfe J, Taylor KL. Post-traumatic stress disorder: Evidence for diagnostic validity and method for psychological assessment. J Clin Psychol 1987; 43: 32–43. [DOI] [PubMed] [Google Scholar]
- 7.Hinrichs R, Michopoulos V, Winters S, et al. Mobile assessment of heightened skin conductance in posttraumatic stress disorder. Depress Anxiety 2017; 34: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry 1999; 4(4): 234–241. [DOI] [PubMed] [Google Scholar]
- 9.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry 2010; 67(4): 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder 6. J Trauma Stress 1993, pp. 459–473. [Google Scholar]
- 11.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv 2005; 56(2): 212–215. [DOI] [PubMed] [Google Scholar]
- 12.Falsetti SA, Resnick HS, Resick PA, Kilpatrick DG. The Modified PTSD Symptom Scale: a brief self-report measure of posttraumatic stress disorder. Behav Ther 1993; 16: 161–162. [Google Scholar]
- 13.Van der Kooij AJ, Meulman JJ. Regression with optimal scaling. In: JJ Meulman WJH and SPSS Inc., eds. SPSS Categories 10.0. Chicago, IL: SPSS Inc.; 1999: pp. 1–8, 77–101, 239–246.
- 14.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol 2005; 67(2): 301–320. [Google Scholar]
- 15.Van der Kooij AJ. Prediction Accuracy and Stability of Regression With Optimal Scaling Transformations [doctoral thesis]. Leiden, the Netherlands: Leiden University, Faculteit der Sociale Wetenschappen; 2007.
- 16.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994; 151(8): 1132–1136. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 1988; 8(1): 77–100. [Google Scholar]
- 18.Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol Assess 1997; 9(4): 445. [Google Scholar]
- 19.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version and the Clinician-Administered PTSD scale. J Trauma Stress 2000; 13(2): 181–191. [DOI] [PubMed] [Google Scholar]
- 20.Shalev AY, Sahar T, Freedman S, et al. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Arch Gen Psychiatry 1998; 55(6): 553–559. [DOI] [PubMed] [Google Scholar]
- 21.Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. J Abnorm Psychol 2000; 109: 341–344. [PubMed] [Google Scholar]
- 22.Blanchard EB, Hamilton HK, Galovski T, Veazey C. Emergency room vital signs and PTSD in a treatment seeking sample of motor vehicle accident survivors. J Trauma Stress 2002; 15: 199–204. [DOI] [PubMed]
- 23.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry 1998; 44: 1248–1263. [DOI] [PubMed] [Google Scholar]
- 24.Pitman RK, Sanders KM, Zusman RM, et al. Effects of propanolol on PTSD symptoms and psychophysiological responding in acute trauma victims. Biol Psychiatry 2002; 51(2): 189–192. [DOI] [PubMed] [Google Scholar]
- 25.Steenen SA, van Wijk AJ, van dr Heijden GJMG, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J Psychopharmacol 2016; 30(2): 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 1998; 44: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 27.Galatzer-Levy IR, Ma S, Statnikov A, Yehuda R, Shalev AY. Utilization of machine learning for prediction of post-traumatic stress: a re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Transl Pyschiatry. 2017; 7(3): e1070. [DOI] [PMC free article] [PubMed]
- 28.Galatzer-Levy IR, Steenkamp MM, Brown AD, et al. Cortisol response to an experimental stress paradigm prospectively predicts long-term distress and resilience trajectories in response to active police service. J Psychiatr Res 2014; 56: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galatzer-Levy IR, Ankri Y, Freedman S, et al. Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS One 2013; 8(8): e70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter B, Bonanno GA, Frasco MA, Dursa EK, Boyko EJ. Prospective post-traumatic stress disorder symptom trajectories in active duty and separated military personnel. J Psychiatr Res 2017; 89: 55–64. [DOI] [PubMed] [Google Scholar]
- 31.Golding JM. Intimate partner violence as a risk factor for mental disorders: a meta-analysis. J Family Violence 1999; 14(2): 99–132. [Google Scholar]
- 32.Woods SJ. Intimate partner violence and post-traumatic stress disorder symptoms in women: what we know and need to know. J Interpersonal Violence 2005; 20(4): 394–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Increased Skin Conductance Response in the Immediate Aftermath of Trauma Predicts PTSD Risk by Rebecca Hinrichs, Sanne J. H. van Rooij, Vasiliki Michopoulos, Katharina Schultebraucks, Sterling Winters, Jessica Maples-Keller, Alex O. Rothbaum, Jennifer S. Stevens, Isaac Galatzer-Levy, Barbara O. Rothbaum, Kerry J. Ressler and Tanja Jovanovic in Chronic Stress