Abstract
Objective
Assessing the epidemiological association between herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) infections in the United States, and characterizing the trends in the standardized HSV-1 and HSV-2 antibody prevalences (seroprevalences), 1999–2016.
Methods
Source of data was the cross-sectional and nationally-representative biennial surveys of the National Health and Nutrition Examination Survey (NHANES). All nine NHANES rounds for 1999–2016 were included in analysis. Datasets of these rounds were combined and analyzed accounting for survey design and applying weighting procedures. Logistic regressions were used to identify associations with seropositivity. Sensitivity analyses were conducted.
Results
Odds of HSV-1 infection declined by 2.84% (95% CI: 1.70%-4.00%) annually among men, and by 2.22% (95% CI: 1.23%-3.21%) among women. Declines were highest at younger ages. Odds of HSV-2 infection declined by 2.23% (95% CI: 0.71%-3.82%) annually among men, and by 2.89% (95% CI: 1.57%-4.28%) among women. Odds ratio of the association between HSV-2 and HSV-1 seropositivity was 0.71 (95% CI: 0.60–0.84) for men and 0.81 (95% CI: 0.72–0.91) for women, after adjustment for age, ethnicity, and year.
Conclusion
HSV-1 and HSV-2 seroprevalences showed a strong declining trend for at least two decades, for both sexes and for the different ethnicities, possibly reflecting improvements in hygiene and living conditions (for HSV-1), and safer sexual behavior (for HSV-2). HSV-1 seroprevalence declines are most pronounced among young individuals. There is evidence for cross protection between the two infections, suggestive of HSV-1 seropositivity being partially protective against HSV-2 infection.
Introduction
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are viruses that establish life-long infections in humans [1]. The major route of transmission for HSV-1 is oral, although (oral) sexual transmission is increasingly common in Western countries and Asia [2–4]. Sexual transmission is the main route of transmission for HSV-2 [5]. Infection by these viruses is often latent and asymptomatic, with frequent reactivations and occasional intermittent symptomatic episodes [6, 7]. Infection can be ascertained by antibody tests (“seroprevalence”) [8]. HSV-1/2 vaccine development is a focus of ongoing international effort [9, 10].
The epidemiologies of HSV-1 and HSV-2 infections are important because of the clinical and psychosocial disease burden and inconvenience these infections can cause [2, 11]. In addition, HSV-2 has been implicated as a cofactor in HIV transmission [12, 13], although recent work has cast doubts on this association [14]. Nevertheless, if true, its control, say through a vaccine, may lead to substantial reductions in HIV transmission [15, 16].
HSV-1 and HSV-2 infections are also indicators of behaviors that facilitate the spread of other infections. Notably, HSV-2 seroprevalence (and seroincidence) reflects sexual risk behavior, and may serve as a marker for potential HIV spread [17–19]. As these infections are viral, they are not influenced by treatment patterns, in contrast to curable bacterial infections. However, as they persist for life, changes in transmission dynamics affect their seroprevalences with variable delays.
In the United States, HSV-2 seroprevalence increased between the 1970s and early 1990s, and was projected to increase to 39% among men and 49% among women aged 15–39 by 2025 [20]. Contrary to these projections, using data from the National Health and Nutrition Examination Survey (NHANES), Xu et al. found a 19% decline in seroprevalence over the period 1999–2004, relative to 1988–1994 [21]. More recently, a data brief, using more recent rounds of NHANES data, suggested persistent declines in seroprevalence [22]. This may suggest that either the earlier high seroprevalence may have been a transient phenomenon, or that the projected increase had been delayed, or avoided, perhaps due to safer sexual behavior following concerns about HIV. With the advent of HIV antiretroviral therapy and pre-exposure prophylaxis [23, 24], however, such concerns might have diminished, leading potentially to a reversion in HSV-2 seroprevalence trajectory. We aimed in this article to statistically investigate and characterize the recent trends in HSV-2 seroprevalence over the period 1999–2016.
HSV-1 is commonly acquired in childhood [1], but changes in hygiene and socio-economic conditions reduced exposure in childhood in Western and Asian countries [1, 3, 21]. Young individuals are increasingly entering the sexually active phase without previous HSV-1 exposure, thus being at risk of HSV-1 genital acquisition and genital herpes [2, 4, 25]. HSV-1 could be overtaking HSV-2 as the main cause of first episode of genital herpes in the United States and elsewhere [2, 4, 25]. The extent to which HSV-1 seroprevalence is declining in childhood in the United States, exposing adults to increasing HSV-1 genital herpes, remains not well-understood. Thus, we further aimed here to statistically investigate and characterize the recent trends in HSV-1 seroprevalence over the period 1999–2016.
HSV-1 and HSV-2 are closely-related antigenically raising a question about the potential for an epidemiological interaction between the two infections. Specifically, since HSV-1 is normally acquired in childhood [1, 3], could prior HSV-1 infection be protective against subsequent HSV-2 infection, which normally occurs after sexual debut [1, 5]? Of notice that the two viruses typically infect two different sites, oral versus genital [2, 26]—two different biological niches, with a variation in immune response [27], that may reduce any immunological cross protection [28]. The two viruses could also have evolved to escape any cross protection to sustain circulation in human populations, particularly for HSV-2, which is normally acquired after HSV-1 [28]. Therefore, we further aimed to assess the evidence for an epidemiological interaction between these two infections using the NHANES database—the world’s foremost nationally-representative database of population-based repeated surveys for both, HSV-1 and HSV-2 infections.
Methods
NHANES are nationally-representative (for the non-institutionalized United States population) probability-based surveys. For sampling, the country is divided into geographic areas (mostly single counties), called "primary sampling units (PSUs)". Strata within PSUs are divided into neighborhoods from which households are randomly selected. Eligible inhabitants of those households are interviewed and a subsample tested for the presence of glycoprotein specific HSV-1 (designated gG-1) and HSV-2 (designated gG-2) in sera using highly sensitive and specific solid-phase enzymatic type-specific immunodot assays [29, 30]. As some demographic groups are oversampled, observations are weighted to obtain truly representative samples.
We used the publicly-available continuous NHANES data from a total of nine biennial surveys (“waves” or “rounds”) extending over the period 1999–2016 [31]. All surveys conducted during this period followed a standardized methodology, both analytically and in laboratory procedures. HSV-1 serological test results were available for ages 14–49, and HSV-2 tests for ages 18–49. Rounds, here, will be denoted by their first (calendar) year (e.g. 1999–2000 = “1999”).
Datasets were combined and analyzed using STATA 13.0 [32], taking into account surveys’ design and applying recommended weighting procedures (using PSUs, strata, and sampling weights). NHANES uses separate strata numbers for different rounds so that the combined data is effectively a survey from a superpopulation that consists of nine “replicas” of the United States population, one from each round (S1 Dataset). Graphical illustrations were made in R version 3.5.1 [33].
Logistic regression was used to identify associations with seropositivity. All regressions were stratified by sex. Age (years) was treated as a categorical variable, and calendar year as a continuous variable, after verifying that there were no major non-linear trends. Thus, annual declines in odds of infection (seroprevalence of infection/seroprevalence of non-infection) were obtained from logistic analysis with calendar year (NHANES round) being a continuous variable, and age being a categorical variable. This method of analysis (as opposed to prevalence ratios), was deemed optimal for statistical [34] and scientific reasons relating to the specific research questions of this study, and for better reflection of incidence ratios (or forces of infection).
Further analyses were also carried out, mostly to scrutinize results through sensitivity analyses. First, we explored whether there were heterogeneities in trend among ethnic groups (distinguishing Mexican, Other Hispanic, White, Black, and Other ethnicities) within the United States population. We further examined ethnicity’s interaction with calendar year. An additional interaction that was explored was that between age (here as a continuous variable) and calendar year. This interaction was examined to explore differences in trend among different age groups. Last but not least, in order to explore the association between HSV-1 and HSV-2 infections, we carried out a logistic regression for HSV-2 infection in which HSV-1 infection was included as an additional covariable.
To obtain standardized seroprevalences by year and sex from age-sex-year specific population seroprevalences, we applied direct standardization, using the United States 2010 population as a reference [35] (its 14–49 and 18–49 populations for HSV-1 and HSV-2, respectively). To estimate these age-sex-year specific population seroprevalences, we carried out (survey) logistic regression with all interactions between age and year as independent variables (all as categorical variables), for each sex, thereby adjusting the survey seroprevalences for the NHANES weights and other design aspects. We used loess (locally weighted polynomial regression) to calculate interpolating curves.
Results
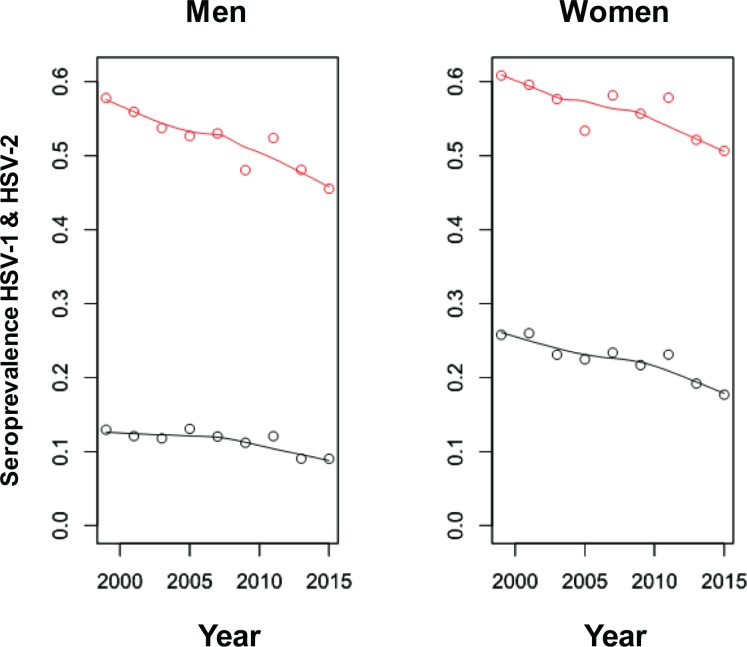
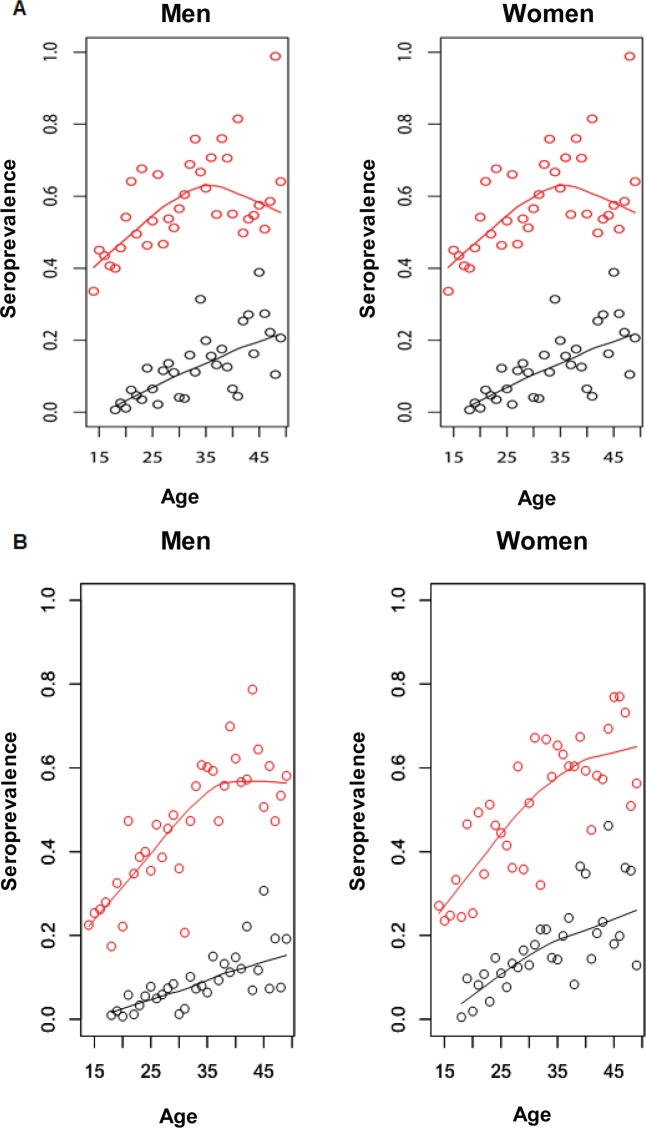
Fig 1 shows the trends for the standardized seroprevalences of HSV-1 and HSV-2 infections, as well as the loess smoothed estimates. HSV-1 and HSV-2 seroprevalences, stratified by age and sex, over the period 1999–2016, can be found in S1 Table. Fig 2A and 2B show the estimated age-specific seroprevalences of HSV-1 and HSV-2 infections, for men and women, in the years 1999–2000 and 2015–2016, respectively, as well as their loess smoothed estimates.
Fig 1. Trends in seroprevalence of HSV-1 (red) and HSV-2 (black) infections in the United States, 1999–2015.
Seroprevalences were standardized with respect to the United States 2010 population; ages 14–49 for HSV-1 and 18–49 for HSV-2. Interpolating curves were estimated using loess.
Fig 2. Estimated age-specific HSV-1 (red) and HSV-2 (black) seroprevalence in the United States.
A) Estimates using 1999–2000 data. B) Estimates using 2015–2016 data. Estimates were derived from survey logistic regression (points), as well as loess smoothed estimates (curves).
HSV-1 seroprevalence
There were an (unweighted) total of 7144 and 6713 HSV-1 negative tests, and 8761 and 10330 positive tests for men and women, aged 14–49, respectively. Using (survey) logistic regression, the odds of HSV-1 infection declined by 2.84% (95% CI: 1.70%-4.00%) and 2.22% (95% CI: 1.23%-3.21%) per year among men and women, respectively. While there were large differences in HSV-1 seroprevalence by ethnicity, the relative declines in seroprevalence were rather similar among the different ethnicities (S1 Fig).
Through sensitivity analysis, there was, however, a highly significant interaction between age (as a continuous variable) and year, with the strongest declines in HSV-1 seroprevalence in the younger age groups for both men and women—the declines decreased in strength (odds) by 0.0013, i.e. 0.13% (95% CI: 0.05–0.21%) per year of age among men, and by 0.18% (95% CI: 0.10–0.25%) per year of age among women.
HSV-2 seroprevalence
There were a total of 10657 and 10316 HSV-2 negative tests, and 1631 and 3311 positive tests for men and women, respectively—much lower overall seroprevalence than for HSV-1. The odds of HSV-2 infection declined by 2.23% (95% CI: 0.71%-3.82%) and 2.89% (95% CI: 1.57%-4.28%) per year among men and women, respectively. While there were large differences in HSV-2 seroprevalence by ethnicity, the relative declines in seroprevalence were overall similar among the different ethnicities (S2 Fig). Through sensitivity analysis, there was no significant interaction between age and year.
Association between HSV-1 and HSV-2 infections
In the additional logistic regression for HSV-2 infection, in which HSV-1 infection was included as an independent covariable, the odds ratio of HSV-2 infection with HSV-1 seropositivity was 0.71 (95% CI: 0.60–0.84; p≤0.001) for men, and 0.81 (95% CI: 0.72–0.91; p≤0.001) for women, after adjustment for age, ethnicity, and year.
Discussion
Recent trends in HSV-1 and HSV-2 seroprevalences in the United States were assessed using the NHANES database. Findings showed continuing declines in both HSV-1 and HSV-2 seroprevalences up to the present (Fig 1), consistent with earlier observed declines [21, 36, 37], and confirming a recent summary report on these data [22]. These declines were strong regardless of sex or ethnicity (Fig 1 and S1 and S2 Figs), but for HSV-1, were more pronounced for the younger age cohorts. Women showed consistently higher HSV-2 seroprevalence than men, possibly due to differences in biological susceptibility [1, 38–40]. Importantly, there was evidence for a negative association between HSV-1 and HSV-2 infections, suggestive of some immunity cross-protection.
The continuing declines in HSV-1 seroprevalence, notably in childhood, may reflect progressive improvements in hygiene and living conditions, and demonstrate how HSV-1 seroprevalence has deviated from its historical pattern of nearly universal infection, still observed in other parts of the globe, where no declines have been documented [3, 41, 42]. While a decline in infection seroprevalence is a positive development, implying decreases in oral herpes and more serious sequelae [2], it is of concern, as young individuals are increasingly unprotected against HSV-1 genital acquisition (with their steadily declining seroprevalence before sexual debut). The role of HSV-1 in genital herpes will probably increase, as HSV-1 seroprevalence declines further in the younger age cohorts.
The continuing declines in HSV-2 seroprevalence affirm that the earlier observed declines [21] were not just a transient phenomenon, but may reflect consistently lower sexual risk behavior, including changes in sexual networking, or use of barrier contraceptive methods. The high observed seroprevalence of late 1970s to early 1990s [20] may have been exceptional, and the consequence of riskier sexual behavior during the sexual revolution of the preceding two decades. Trends in HSV-2 seroprevalence in other parts of the world are yet to be ascertained with confidence [39].
Remarkably, rates of human papillomavirus infection, another viral STI, seem also to have declined in recent decades in the United States [43]. Based on suggestive evidence, these declines may not have been solely due to HPV vaccination [44, 45], thus supporting declines in sexual risk behavior as an explanation. Self-reported behavior data further indicates lower levels of sexual behavior, particularly among young individuals. In the National Survey of Family Growth [46] of 2006–2008 compared to that of 2002, the percentage of men and women aged 15–24 who never had a sexual contact increased from 22% (men and women) to 27% (men) and 29% (women). The declines could be further due to changing sexual-mixing patterns, which can significantly affect the spread of STIs [47, 48]. Whether age-mixing has changed over the past decades, say due to rising college education with approximately (currently) equal men and women participation, remains to be explored.
Current evidence on a possible epidemiological interaction between HSV-1 and HSV-2 infections is conflicting [28]. Pooled findings of prospective studies suggest a lower risk of HSV-2 infection with prior HSV-1 infection [28]; but pooled findings of cross-sectional studies suggest a higher risk of HSV-2 infection with HSV-1 infection in low sexual risk populations and in Europe, but no association in high sexual risk populations and in the United States [28]. Our results suggest some cross-protection. Most likely, assuming that HSV-1 infection precedes HSV-2 infection [3, 17, 41], a lower risk of HSV-2 infection after HSV-1 infection [49], just as the pooled finding of prospective studies. Of notice, a recent study on a merged multi-national database of seroprevalence studies among men, also suggested that HSV-1 infection provided protection against HSV-2 acquisition, with an adjusted odds ratio of 0.51 (95% CI 0.30–0.87; p-value = 0.013) (Nasrallah G.K., Personal communication), similar to the present study result. It would be useful to explore further this negative association and its underlying causes within the complex and delicate web of innate signaling pathways and adaptive immune responses against HSV infections [49].
Our study was concerned with seroprevalence, a measure of lifetime exposure to the infection, and thus may not necessarily reflect actual current trends in transmission dynamics. The analyzed data are based on repeated rounds of surveys, and therefore, no seroconversions were identifiable, allowing only indirect conclusions about incidence. NHANES rounds did not include the institutionalized populations of the United States, such as the incarcerated population, and thus, our results may not apply to the institutionalized populations. The association between HSV-1 and HSV-2 infections was assessed using cross-sectional data, and thus we cannot strictly infer the temporal sequence of cause and effect.
In conclusion, HSV-1 and HSV-2 epidemiologies continue to evolve in the United States, with the seroprevalence of both infections progressively declining for both sexes, and for the different ethnicities. The declines in HSV-1 seroprevalence are more pronounced for the younger age cohorts. Younger cohorts are increasingly acquiring the infection for the first time genitally after sexual debut, as opposed to orally during childhood, thus pointing towards an increasing role for HSV-1 in genital herpes. Importantly, there was evidence for an HSV-1/2 epidemiological interaction, suggestive of HSV-1 infection being partially protective against HSV-2 infection.
Supporting information
(XLS)
(DOCX)
Seroprevalence was standardized with respect to the United States 2010 population (ages 14–49). Interpolating curves were estimated using loess.
(TIF)
Seroprevalence was standardized with respect to the United States 2010 population (ages 18–49). Interpolating curves were estimated using loess.
(TIF)
Acknowledgments
The authors are grateful to the NHANES program of the Centers of Disease Control and Prevention, United States, for making surveys’ data publicly available. The authors are also grateful for the administrative support of Ms. Adona Canlas.
Data Availability
The database used for this study is publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.
Funding Statement
This publication was made possible by NPRP grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors. The authors are also grateful for pilot funding provided by the Biomedical Research Program and infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine in Qatar. JST, PRESTO provided research funding support in the form of salary for RO, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of authors are articulated in the ‘Authors’ Contributions’ section.
References
- 1.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186 Suppl 1:S3-28. 10.1086/343739 . [DOI] [PubMed] [Google Scholar]
- 2.Bernstein DI, Bellamy AR, Hook EW, 3rd, Levin MJ, Wald A, Ewell MG, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–51. 10.1093/cid/cis891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khadr L, Harfouche M, Omori R, Schwarzer G, Chemaitelly H, Abu-Raddad LJ. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2018. Epub 2018/07/19. 10.1093/cid/ciy562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: model-based predictions. BMC Med. 2019;17(1):57 10.1186/s12916-019-1285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11 Suppl 1:24A–35A. . [PubMed] [Google Scholar]
- 6.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198(8):1141–9. Epub 2008/09/12. 10.1086/591913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffer JT, Abu-Raddad LJ, Mark KE, Zhu J, Selke S, Magaret A, et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Science Translational Medicine. 2009;1(7):7ra16 Epub 2010/02/18. 10.1126/scitranslmed.3000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald A, Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis. 2002;35(Suppl 2):S173–82. 10.1086/342104 . [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb SL, Giersing B, Boily MC, Chesson H, Looker KJ, Schiffer J, et al. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: Key findings from the World Health Organization Consultation on HSV Vaccine Impact Modelling. Vaccine. 2017. Epub 2017/06/26. 10.1016/j.vaccine.2017.03.074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016;34(26):2939–47. 10.1016/j.vaccine.2016.03.111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnann JW Jr., Whitley RJ. Genital Herpes. New England Journal of Medicine. 2016;375(7):666–74. 10.1056/NEJMcp1603178 . [DOI] [PubMed] [Google Scholar]
- 12.Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM Jr., Self SG, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One. 2008;3(5):e2230 10.1371/journal.pone.0002230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17(12):1303–16. 10.1016/S1473-3099(17)30405-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omori R, Nagelkerke N, Abu-Raddad LJ. HIV and herpes simplex virus type 2 epidemiological synergy: misguided observational evidence? A modelling study. Sex Transm Infect. 2018;94(5):372–6. Epub 2017/12/06. 10.1136/sextrans-2017-053336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddow LJ, Mindel A. Genital herpes vaccines—cause for cautious optimism. Sex Health. 2006;3(1):1–4. Epub 2006/04/13. . [DOI] [PubMed] [Google Scholar]
- 16.Alsallaq RA, Schiffer JT, Longini IM Jr., Wald A, Corey L, Abu-Raddad LJ. Population level impact of an imperfect prophylactic vaccine for herpes simplex virus-2. Sex Transm Dis. 2010;37(5):290–7. 10.1097/OLQ.0b013e3181d3d023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Raddad LJ, Schiffer JT, Ashley R, Mumtaz G, Alsallaq RA, Akala FA, et al. HSV-2 serology can be predictive of HIV epidemic potential and hidden sexual risk behavior in the Middle East and North Africa. Epidemics. 2010;2(4):173–82. 10.1016/j.epidem.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omori R, Abu-Raddad LJ. Sexual network drivers of HIV and herpes simplex virus type 2 (HSV-2) transmission: a comparative mathematical modeling analysis. AIDS. 2017. 10.1097/QAD.0000000000001542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouyoumjian SP, Heijnen M, Chaabna K, Mumtaz GR, Omori R, Vickerman P, et al. Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS. 2018;32(10):1343–52. Epub 2018/05/26. 10.1097/QAD.0000000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisman DN, Lipsitch M, Hook EW 3rd, Goldie SJ. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex Transm Dis. 2002;29(10):608–22. Epub 2002/10/09. . [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. 10.1001/jama.296.8.964 . [DOI] [PubMed] [Google Scholar]
- 22.McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016. NCHS Data Brief. 2018;(304):1–8. . [PubMed] [Google Scholar]
- 23.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30(10):797–800. 10.1097/01.OLQ.0000092387.58746.C7 . [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127–37. 10.1016/S0140-6736(07)61908-4 . [DOI] [PubMed] [Google Scholar]
- 27.Stanberry LR, Cunningham AL, Mindel A, Scott LL, Spruance SL, Aoki FY, et al. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30(3):549–66. Epub 2000/03/18. 10.1086/313687 . [DOI] [PubMed] [Google Scholar]
- 28.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81(2):103–7. Epub 2005/04/01. 81/2/103 [pii] 10.1136/sti.2004.012039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee FK, Coleman RM, Pereira L, Bailey PD, Tatsuno M, Nahmias AJ. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol. 1985;22(4):641–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee FK, Pereira L, Griffin C, Reid E, Nahmias A. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J Virol Methods. 1986;14(2):111–8. . [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey 1999–2016. 2017.
- 32.StataCorp, inventorStata Statistical Software: Release 13. College Station, TX: StataCorp LP2013.
- 33.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. Vienna, Austria: ISBN 3-900051-07-0, http://www.R-project.org; 2018.
- 34.Tamhane AR, Westfall AO, Burkholder GA, Cutter GR. Prevalence odds ratio versus prevalence ratio: choice comes with consequences. Stat Med. 2016;35(30):5730–5. Epub 2016/07/28. 10.1002/sim.7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Age and Sex Composition in the United States: 2010 2010. Available from: https://www.census.gov/data/tables/2010/demo/age-and-sex/2010-age-sex-composition.html.
- 36.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis. 2014;209(3):325–33. 10.1093/infdis/jit458 . [DOI] [PubMed] [Google Scholar]
- 37.Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years—United States, 1988 to 2010. Sex Transm Dis. 2013;40(11):860–4. 10.1097/OLQ.0000000000000043 . [DOI] [PubMed] [Google Scholar]
- 38.MacDonald EM, Savoy A, Gillgrass A, Fernandez S, Smieja M, Rosenthal KL, et al. Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol Reprod. 2007;77(6):1049–59. 10.1095/biolreprod.107.063933 . [DOI] [PubMed] [Google Scholar]
- 39.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10(1):e114989 10.1371/journal.pone.0114989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. . [DOI] [PubMed] [Google Scholar]
- 41.Chaabane S, Harfouche M, Chemaitelly H, Schwarzer G, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci Rep. 2019;9(1):1136 10.1038/s41598-018-37833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sukik L, Alyafei M, Harfouche M, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in Latin America and the Caribbean: Systematic review and meta-analytics. PLoS One. 2019;14(4):e0215487 10.1371/journal.pone.0215487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickson EL, Vogel RI, Luo X, Downs LS. Recent trends in type-specific HPV infection rates in the United States. Epidemiol Infect. 2015;143(5):1042–7. Epub 2015/03/07. 10.1017/S0950268814001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis RM, Markowitz LE. Human papillomavirus vaccination coverage among females and males, National Health and Nutrition Examination Survey, United States, 2007–2016. Vaccine. 2018;36(19):2567–73. 10.1016/j.vaccine.2018.03.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–63. 10.1016/S2214-109X(16)30099-7 . [DOI] [PubMed] [Google Scholar]
- 46.Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. Natl Health Stat Report. 2011;(36):1–36. Epub 2011/05/13. . [PubMed] [Google Scholar]
- 47.Catania JA, Binson D, Stone V. Relationship of sexual mixing across age and ethnic groups to herpes simplex virus-2 among unmarried heterosexual adults with multiple sexual partners. Health Psychol. 1996;15(5):362–70. Epub 1996/09/01. . [DOI] [PubMed] [Google Scholar]
- 48.Kraut-Becher JR, Aral SO. Patterns of age mixing and sexually transmitted infections. Int J STD AIDS. 2006;17(6):378–83. Epub 2006/06/01. 10.1258/095646206777323481 . [DOI] [PubMed] [Google Scholar]
- 49.Chew T, Taylor KE, Mossman KL. Innate and adaptive immune responses to herpes simplex virus. Viruses. 2009;1(3):979–1002. 10.3390/v1030979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
Seroprevalence was standardized with respect to the United States 2010 population (ages 14–49). Interpolating curves were estimated using loess.
(TIF)
Seroprevalence was standardized with respect to the United States 2010 population (ages 18–49). Interpolating curves were estimated using loess.
(TIF)
Data Availability Statement
The database used for this study is publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.