Abstract
GI stromal tumors (GISTs) are neoplasms with a varying malignancy potential ranging from virtually indolent tumors to rapidly progressing cancers. GISTs occur throughout the intestinal tract, and most harbor an activating mutation in either KIT or platelet-derived growth factor A (PDGFRA). Diagnosis is made using immunohistochemistry, but molecular testing with mutation analysis is paramount for selection of appropriate therapy. Most small GISTs are cured with surgery. Tyrosine kinase inhibitor (TKI) therapy has led to substantial improvements in survival, both for patients with localized GIST and those with advanced disease. Adjuvant therapy with imatinib benefits patients with a high risk of recurrence, with studies suggesting most benefit with at least 3 years of therapy. Neoadjuvant imatinib therapy should be considered for patients requiring extensive surgery, aiming at shrinking the tumor to allow organ preservation and less extensive surgery. The following three TKIs have been approved for the management of advanced disease: imatinib, sunitinib, and regorafenib; imatinib is usually the best tolerated of the three and the standard first-line treatment. TKIs benefit the majority of patients with advanced GIST but have no or limited efficacy in patients with the PDGFRA D842V mutation or patients with GIST lacking KIT and PDGFRA mutations. Surgery, the mainstay of primary tumor management, also plays a role in the advanced disease setting for selected patients, as do some other approaches such as palliative radiation therapy. Research continues to identify novel therapies, in particular effective agents to treat TKI-refractory disease.
INTRODUCTION
GI stromal tumors (GIST) compose approximately 20% of soft tissue sarcomas with an annual incidence of approximately 10 per million population.1,2 In addition, small (< 1 cm) gastric micro-GISTs are common (10% to 35%) in the middle-aged and elderly populations.3,4 Micro-GISTs have low or no mitotic activity and have little clinical significance.
GISTs occur throughout the GI tract, most commonly in the stomach or small intestine. GISTs rarely (< 5%) arise within the abdominal cavity without an apparent connection to the GI tract. Such GISTs are known as extra-GI GISTs.
PATIENT PRESENTATION
GISTs occur at any age, with a median age at detection of 65 years, but they rarely occur (< 0.5%) in individuals younger than age 20 years.5 GIST occurs with similar frequency in males and females. The median tumor size at diagnosis is approximately 6 cm, but it may be > 20 cm.
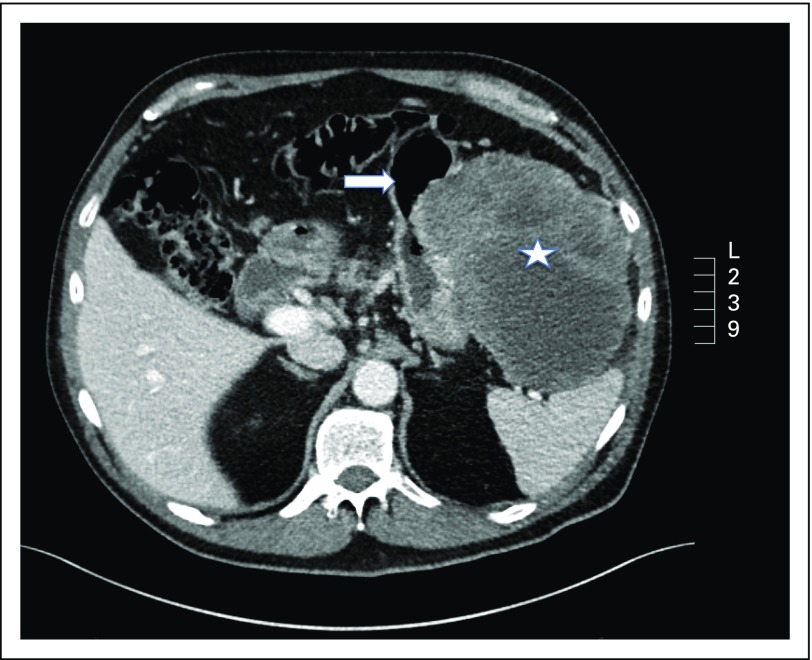
GISTs are often vascular tumors that bulge from the GI tract into spaces between the abdominal organs (Fig 1). Tumor bleeding into the abdominal cavity or bowel is a common presentation. Bleeding may be slow, resulting in anemia, or sudden, causing tachycardia, fainting, stomach pain, melena, or hematemesis. GISTs may cause other symptoms depending on size and location, such as abdominal pain, fullness or pressure, or bowel obstruction. Asymptomatic GISTs may be detected by palpation, during imaging, or at surgery for other conditions.
Fig 1.
A 15-cm gastric GI stromal tumor (GIST; star) in a 64-year-old man. The GIST harbored a KIT exon 11 deletion mutation of codons 557 and 558. The arrow points at the stomach.
Up to 20% of patients have overt metastases at diagnosis.6 Metastases typically occur in the abdominal cavity or the liver, whereas metastases in the lungs, bones, or brain are rare. Lymph node metastases are found in 20% to 60% of pediatric GISTs, pediatric-type GISTs in young adults, and syndromic GISTs.7,8 An abdominal tumor with lung metastases is likely not GIST.
SYNDROMIC GIST
Most GISTs (97%) are sporadic.9 No risk factors have been recognized apart from rare tumor syndromes, including neurofibromatosis type 1, Carney-Stratakis syndrome, and Carney triad. Neurofibromatosis type 1 presents with multiple intestinal GISTs that harbor mutated NF1. The Carney-Stratakis syndrome is a rare heritable condition with a germline mutation in the succinate dehydrogenase (SDH) complex genes, SDHA (C. Stratakis, personal communication, June 2017), SDHB, SDHC, or SDHD.9 Patients have a high risk for gastric GIST at a young age and paraganglioma. The Carney triad is a rare nonheritable condition. The triad consists of multiple gastric GISTs in young females, paraganglioma, and pulmonary chondroma, but may present without all three components or with adrenal cortical adenoma or esophageal leiomyoma. The molecular pathogenesis depends on epigenetic SDHC inactivation through SDHC hypermethylation.10 Collectively, tumors with SDH gene mutations or hypermethylation are referred to as SDH-deficient GISTs. Rarely, GIST can be familial, with a germline mutation in either KIT or platelet-derived growth factor receptor A (PDGFRA).9
STAGING
Standard imaging consists of computed tomography of the abdomen and pelvis. Imaging of the chest may be considered to exclude rare metastases above the diaphragm. Magnetic resonance imaging may provide additional information, particularly for GIST of the rectum or duodenum. Other examinations, such as computed tomography–positron emission tomography or ultrasound of the liver, may also be considered.
BIOPSY
Histopathologic evaluation of the excised tumor allows best estimation of the risk of recurrence when considering adjuvant therapy. A core needle biopsy can be taken safely endoscopically. Mitotic counts from a needle biopsy may underestimate the risk of recurrence. The safety of a transabdominal wall biopsy is controversial as a result of concern for tumor cell seeding to the abdominal cavity when GIST is necrotic. One study found that performing a diagnostic transabdominal wall biopsy does not adversely influence prognosis in patients who received adjuvant imatinib after surgery.11 Some data suggest that small tumor capsule lesions have little adverse impact on patient outcome, unlike large tumor ruptures.12
TISSUE DIAGNOSIS AND MOLECULAR BIOLOGY
GIST morphology is variable, and immunohistochemical staining usually establishes the diagnosis. Most GISTs stain for KIT (95%)13 and anoctamin-1 (DOG-1; 98%),14 whereas most do not stain with smooth muscle biomarkers such as desmin.
Activating mutations in KIT and PDGFRA (encoding KIT and platelet-derived growth factor receptor tyrosine kinases, respectively) are considered the main oncogenic drivers of GIST.15 Similar mutations occurring in clinical GISTs are found in micro-GISTs,4,16 suggesting that further genetic aberrations are required for tumor progression. Mutations occur occasionally in several other genes in GISTs, including SETD2, SDH, BRAF, TP53, MEN1, MAX, and Rb1, and translocations involving FGFR and NTRK.17-21
Most GISTs (75%) harbor a mutation in KIT,22 occurring in exon 11 (90%) or exon 9 (8%) and, less often, in exon 13 (1%) or exon 17 (1%).23 PDGFRA mutations occur in 10% to 20% of GISTs, most commonly in exons 12, 14, and 18. GISTs that do not harbor a KIT or PDGFRA mutation (5% to 10% of GISTs) were called wild-type GISTs in the past, but such GISTs are now known to have other mutations, frequently in NF1 or genes of the SDH complex.9 GISTs in children typically have SDH mutations or epigenetic silencing of the SDHC promoter.24
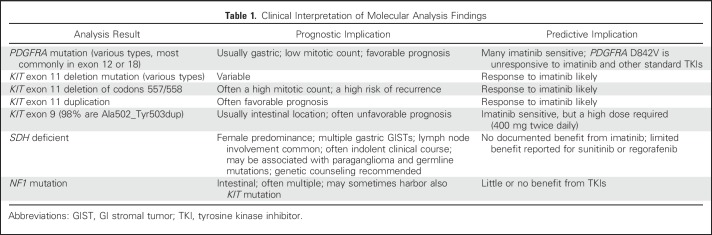
Mutation analysis of KIT and PDGFRA is mandatory for optimal care of GIST. GISTs harboring the PDGFRA mutation D842V (approximately 8% of GISTs)6,23 do not respond to imatinib or other approved tyrosine kinase inhibitors (TKIs),25 but most may respond to BLU-285.26 GISTs that do not contain KIT or PDGFRA mutations are unlikely to benefit from imatinib treatment (Table 1). Tumor immunostaining for SDHB is recommended when no KIT or PDGFRA mutation is present, as absence of SDHB staining indicates SDH deficiency and potentially an SDH mutation,11 in which case genetic counseling is appropriate.
Table 1.
Clinical Interpretation of Molecular Analysis Findings
RISK STRATIFICATION
The malignant potential of GISTs varies greatly from virtually benign tumors to rapidly progressing cancers. The estimation of the risk of recurrence is particularly important for localized tumors when considering adjuvant treatment.
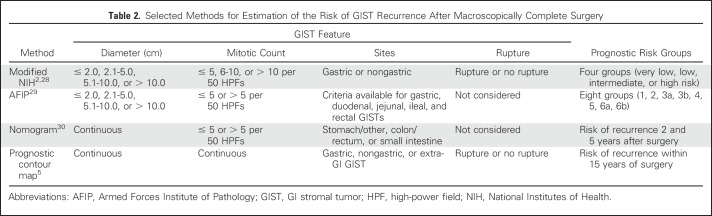
Several validated stratification schemes to estimate the risk of recurrence after macroscopically complete surgery are available (Table 2).2,6,27-30 These methods are largely equivalent,31 although factors included and the cutoff values vary between methods. It may be best to consult another scheme when either the tumor diameter or the mitotic count is close to the cutoff value or to use a method where size and mitotic count are expressed as continuous variables,5,30 because the true risk of recurrence does not change abruptly at any variable cutoff. Some novel prognostic approaches, such as multigene panels and circulating tumor DNA, seem promising.
Table 2.
Selected Methods for Estimation of the Risk of GIST Recurrence After Macroscopically Complete Surgery
TREATMENT OF LOCALIZED GIST
Surgery
Approximately 60% of patients with localized GIST are cured with surgery.5 The aim is to completely excise GIST macroscopically and microscopically, without rupturing the tumor capsule. This can be done at open surgery or, for smaller tumors, by laparoscopic surgery.32,33 A 1- to 2-cm macroscopic margin may be sufficient to achieve microscopically negative margins.2 Lymph node dissection is usually not indicated in adults.
Preoperative Imatinib
Preoperative imatinib, an inhibitor of KIT and PDGFRA, should be considered to shrink the tumor and allow organ sparing when up-front surgery might lead to extensive organ resections.34 Indications for preoperative imatinib include rectal GISTs in an attempt to avoid abdominoperineal resection, some duodenal and esophageal GISTs, and large gastric GISTs that might require total gastrectomy. A tissue diagnosis using a core needle biopsy should be taken before starting imatinib to confirm the diagnosis, and a mutation analysis should be obtained whenever feasible. The optimal duration of preoperative imatinib is unknown, but it is often administered for 6 to 12 months to allow maximal tumor shrinkage. Response to treatment should be monitored because some GISTs do not respond and a few may develop imatinib resistance. Imatinib is continued until surgery and should be resumed after surgery to complete 3 years of treatment when the risk of recurrence is substantial.
A limitation of preoperative imatinib is that tumor mitotic activity often cannot be estimated reliably from the diagnostic biopsy or from the residual resected GIST after imatinib therapy. Because standard risk estimation often cannot be done, the benefits of adjuvant imatinib may become challenging to assess, particularly when the tumor diameter is < 10 cm.
Adjuvant Imatinib
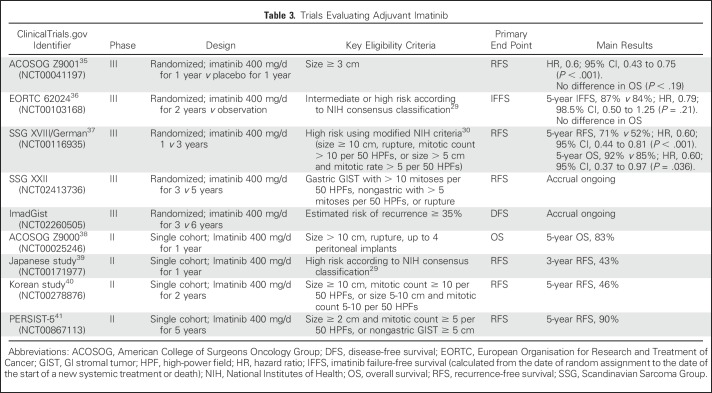
Imatinib is the only TKI that has been studied as adjuvant treatment of operable GIST, at a dose of 400 mg/d (Table 3).35-42 In two of three randomized trials with results, adjuvant imatinib administered for 1 year35 or 2 years36 improved recurrence-free survival compared with placebo or observation; overall survival (OS) benefit was not observed in either study. In the third trial, patients with GIST with a high risk for recurrence received adjuvant imatinib for 1 or 3 years after surgery. With a median follow-up of 7.5 years, patients who received 3 years of imatinib had longer recurrence-free survival and OS times compared with those who were on imatinib for 1 year.37 In these studies, patients with KIT exon 11 deletion mutations benefitted most from imatinib.35,42
Table 3.
Trials Evaluating Adjuvant Imatinib
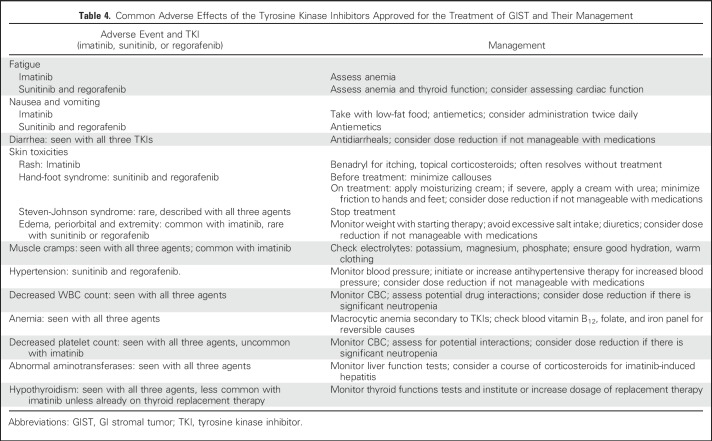
Imatinib is relatively well tolerated. However, 26% of patients receiving 3 years of adjuvant imatinib discontinued treatment for reasons other than GIST recurrence,42 and 49% of patients in the PERSIST trial discontinued imatinib before 5 years.41 Supportive measures (Table 4) can alleviate adverse effects and help in maintaining compliance.
Table 4.
Common Adverse Effects of the Tyrosine Kinase Inhibitors Approved for the Treatment of GIST and Their Management
Patients who have a substantial risk for recurrence should be treated with at least 3 years of imatinib.43,44 The optimal duration remains unknown. Two randomized trials are comparing 5 or 6 years to 3 years of adjuvant imatinib (Table 3). GISTs with PDGFRA D842V mutation or lacking a mutation in KIT or PDGFRA are unlikely to benefit from adjuvant imatinib. Patients with KIT exon 9 mutation are usually treated with higher imatinib doses (up to 800 mg/d, if tolerated) based on data from patients with advanced GIST,45 without prospective data in the adjuvant setting.
Follow-Up
The optimal patient follow-up strategies after surgery or during and after adjuvant imatinib are unknown. Periodic imaging of the abdomen is likely beneficial because detecting GIST recurrences when tumors are small may result in a longer time to imatinib resistance compared with bulky recurrences. A reasonable strategy may be to image the abdomen at 6- to 12-month intervals for the first 10 years after surgery. More frequent imaging at 3- or 4-month intervals may be considered for 2 years after stopping adjuvant imatinib because many GISTs recur during this time period.44
ADVANCED GIST
TKIs
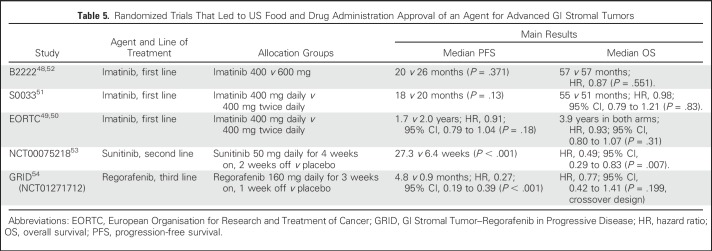
Before the availability of TKIs, chemotherapy was the primary treatment modality for advanced GIST, but responses were few and the median survival was only approximately 1 year.46,47 In phase I evaluation of imatinib administered at 400 mg daily to 500 mg twice daily, a dose of 400 mg twice daily was established as the maximum-tolerated dose (MTD).48 The complete and partial response rates in the phase I and II studies with the 400-mg daily dose ranged from 40% to 74%, which was similar to the rates of 45% to 52% with the MTD but with less toxicity.48,49 The pivotal phase III European Organisation for Research and Treatment of Cancer 6200550,51 and S0033 trials52 compared imatinib 400 mg once daily with 400 mg twice daily (Table 5). Both trials confirmed the meaningful clinical benefit of imatinib 400 mg once daily, first reported in the B2222 study,49,55 with complete response rates of 3% to 6%, partial response rates of 45% to 48%, and stable disease (SD) rates of 26% to 32%. No difference was noted in OS between the 400-mg and 800-mg doses, establishing 400 mg once daily as the standard dose. OS ranged from 47 to 55 months, a substantial improvement compared with chemotherapy.
Table 5.
Randomized Trials That Led to US Food and Drug Administration Approval of an Agent for Advanced GI Stromal Tumors
In the joint analysis of the S0033 and European Organisation for Research and Treatment of Cancer 62005 trials, progression-free survival (PFS) was longer in the imatinib 400 mg twice daily arm.56 The improvement was a result of KIT exon 9 mutated tumors treated at the higher dose.45 Therefore, patients with exon 9 mutations should receive imatinib 400 mg twice daily if tolerated.57 Starting imatinib at 400 mg once daily and increasing the dose up to 400 mg twice daily decreases adverse effects of higher-dose therapy.57
The impact of stopping therapy after 1, 3, or 5 years in patients whose tumors had responded or stabilized on imatinib was tested in the BFR14 trial.58,59 Patients randomly assigned to stop therapy had shorter PFS compared with those who remained on treatment, with a suggestion that the time to progression increases with longer time on imatinib before discontinuation. Fortunately, the majority of patients achieved disease control again after restarting imatinib, and OS was no different between the groups. These data support uninterrupted treatment with imatinib.
Sunitinib is a multitargeted TKI with activity against KIT, PDGFR, VEGFR, and FLT-1/KDR. Phase I studies identified 50 mg daily for 28 days with 14 days of rest as the MTD.53 Response data in an early trial53 and the phase III placebo-controlled trial60 demonstrated no complete responses and partial response rates of 7% to 13%. In the phase III trial, both the median PFS and OS were longer in the sunitinib group than in the placebo group despite the study crossover design60 (Table 5). Disease symptoms and metabolic activity by positron emission tomography may return on the 2-week treatment break.53 To avoid recurrent tumor symptoms during the 2-week break and to manage adverse effects, a phase II trial of continuous daily dosing starting at 37.5 mg was conducted.61 Given that the safety, tolerability, and response rates were similar to those with the 4 weeks on/2 weeks off regimen, continuous administration is a valid option.
In a study of imatinib-resistant or -intolerant GIST, response to sunitinib was obtained in seven (37%) of 19 patients with KIT exon 9 mutations and in two (5%) of 44 patients with exon 11 mutation.62 One of the 10 patients with KIT exon 11 mutation and a second mutation in exon 13 or 14 responded, and six patients had SD for ≥ 6 months, whereas none of the 10 patients with a second mutation in KIT exon 17 benefitted, consistent with in vitro findings. Treatment with sunitinib in five pediatric patients with KIT/PDGFRA mutant-negative tumors resulted in one patient with partial response and four patients with SD.63
Regorafenib, a multitargeted TKI targeting VEGFR1-3, TEK, KIT, RET, RAF1, BRAF, PDGFR, and FGFR, was approved for treatment of patients with GIST previously treated with imatinib and sunitinib.54,64 Regorafenib is dosed at 160 mg daily for 21 days with 7 days off, repeated every 28 days. The phase III GIST–Regorafenib in Progressive Disease (GRID) trial was a randomized placebo-controlled study with crossover to regorafenib at the time of disease progression on placebo (Table 5).64 The median PFS was 4 months longer with regorafenib, but no difference in OS was observed, probably because 85% of the patients on placebo crossed over to active therapy. The response rates were low (4.5% for regorafenib and 1.5% for placebo). As with sunitinib, regorafenib benefited patients with primary tumor KIT exon 11 or exon 9 mutations, with in vitro data showing activity for some secondary mutations in exon 17; two patients with SDH-deficient GIST demonstrated partial response, with four others achieving SD.54
Mutational Testing in Advanced Disease
Tumor tissue mutation analysis is indicated when it is not available from the primary tumor. In refractory disease, a single biopsy under-represents the potential tumor heterogeneity. Circulating plasma DNA may be more representative of the spectrum of clones with variable secondary mutations, but to date, it is rarely available for routine management of advanced disease.
Surgical Management
The role of metastasectomy in patients whose disease is controlled with imatinib is controversial.65 The extent of tumor bulk was identified as a factor impacting PFS on imatinib.66 This raises the possibility that debulking of metastatic disease after an initial stabilization or response to imatinib may help in prolonging disease control by preventing emergence of resistant clones. Prospective randomized trials studying the benefit of debulking surgery unfortunately failed to accrue enough patients, but the results of one small randomized study suggest that resection of the residual disease on imatinib treatment improves OS.67 A retrospective report demonstrated longer OS with debulking that achieved R0 or R1 sections compared with those where surgery left gross tumor behind.68 Resection of a focal progressing lesion may allow the use of imatinib to be prolonged, whereas surgery for diffuse progression does not.65
The role of metastasis surgery in patients on sunitinib or regorafenib is unclear, except for patients who require emergency surgery.66 These agents target VEGFR and require a longer period off-therapy to undergo surgery safely and achieve wound closure; this contrasts with imatinib, which may be taken the day before surgery and resumed when a patient is able to eat. Patients with progressive disease on sunitinib and regorafenib also likely have multiple resistant clones and greater tumor bulk, and therefore, the potential benefits versus risks of surgery need to be carefully considered.
Other Palliative Approaches
Although not used in the primary management of GIST, radiotherapy plays a role in palliation and may stabilize single progressing liver or intra-abdominal lesions for several months.69 In addition, local interventional modalities, such as embolization or radiofrequency ablation, are considerations for liver metastases. For patients with progressive GIST after all standard therapies, discontinuing TKI therapy is not recommended given the rapid rate of progression documented after discontinuation.60,64 Restarting imatinib after progression on at least prior imatinib and sunitinib increased PFS slightly from 0.9 to 1.8 months compared with best supportive care (P = .005).70 In a small randomized trial of patients whose GIST was resistant to imatinib and sunitinib, pazopanib plus best supportive care improved PFS compared with best supportive care alone (median, 3.4 v 2.3 months, respectively; P = .03).71
In conclusion, the management of GIST changed radically with the understanding of its molecular drivers. Although first described as having oncogenic mutations in KIT, we now know that PDGFRA mutations and SDH deficiency by mutation and methylation each account for a subset of GISTs, with additional drivers. TKI therapy has greatly impacted survival in patients with GIST, increasing the life expectancy from about 1 year to 5 years in the advanced setting; adjuvant imatinib for 3 years also improves survival. These advances underscore the need for molecular testing of GIST, recognizing that some patients (eg, those with PDGFRA D842V mutation) do not respond to the present standard therapies. Patients with SDH mutation or hypermethylation require specialized surgical considerations but may benefit from VEGFR-targeted agents.
Patients with advanced disease that has progressed on standard therapies often are clinically well and candidates for additional therapy. Trials continue testing novel TKIs and combination therapies that exploit resistance pathways. The role of immunotherapy is yet to be defined in GIST but includes use of antibodies targeting tumor or immune pathways and use of chimeric antigen receptor T cells. Finally, the role of serum surveillance is developing and will potentially better define disease progression with molecular information to target emerging resistance clones.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gastrointestinal Stromal Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Margaret von Mehren
Consulting or Advisory Role: CytRx Corporation, Blueprint Medicines, Janssen Oncology, Deciphera
Research Funding: ArQule
Travel, Accommodations, Expenses: Janssen Oncology, Blueprint Medicines, Arog
Other Relationship: National Comprehensive Cancer Network
Heikki Joensuu
Stock or Other Ownership: Orion Pharma, Sartar Therapeutics, Faron Pharmaceuticals
Honoraria: Orion Pharma
Consulting or Advisory Role: Blueprint Medicines, Neutron Therapeutics
REFERENCES
- 1.Ducimetière F, Lurkin A, Ranchère-Vince D, et al. : Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 6:e20294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joensuu H, Hohenberger P, Corless CL: Gastrointestinal stromal tumour. Lancet 382:973-983, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Kawanowa K, Sakuma Y, Sakurai S, et al. : High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 37:1527-1535, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A, Wünsch PH, Hofstaedter F, et al. : Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 31:113-120, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Joensuu H, Vehtari A, Riihimäki J, et al. : Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol 13:265-274, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Emile JF, Brahimi S, Coindre JM, et al. : Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs. Med Oncol 29:1765-1772, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Agaimy A, Wünsch PH: Lymph node metastasis in gastrointestinal stromal tumours (GIST) occurs preferentially in young patients < or = 40 years: An overview based on our case material and the literature. Langenbecks Arch Surg 394:375-381, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Smyrk TC, Young WF, Jr, et al. : Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: Findings in 104 cases. Am J Surg Pathol 34:53-64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricci R: Syndromic gastrointestinal stromal tumors. Hered Cancer Clin Pract 14:15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller F, Moskalev EA, Faucz FR, et al. : Aberrant DNA hypermethylation of SDHC: A novel mechanism of tumor development in Carney triad. Endocr Relat Cancer 21:567-577, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson M, Reichardt P, Sundby Hall K, et al. : Needle biopsy through the abdominal wall for the diagnosis of gastrointestinal stromal tumour: Does it increase the risk for tumour cell seeding and recurrence? Eur J Cancer 59:128-133, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Hølmebakk T, Bjerkehagen B, Boye K, et al. : Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg 103:684-691, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Medeiros F, Corless CL, Duensing A, et al. : KIT-negative gastrointestinal stromal tumors: Proof of concept and therapeutic implications. Am J Surg Pathol 28:889-894, 2004 [DOI] [PubMed] [Google Scholar]
- 14.West RB, Corless CL, Chen X, et al. : The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 165:107-113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corless CL, Barnett CM, Heinrich MC: Gastrointestinal stromal tumours: Origin and molecular oncology. Nat Rev Cancer 11:865-878, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Rossi S, Gasparotto D, Toffolatti L, et al. : Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am J Surg Pathol 34:1480-1491, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Huang KK, McPherson JR, Tay ST, et al. : SETD2 histone modifier loss in aggressive GI stromal tumours. Gut 65:1960-1972, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Huss S, Pasternack H, Ihle MA, et al. : Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol 62:206-214, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Pantaleo MA, Urbini M, Indio V, et al. : Genome-wide analysis identifies MEN1 and MAX mutations and a neuroendocrine-like molecular heterogeneity in quadruple WT GIST. Mol Cancer Res 15:553-562, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Merten L, Agaimy A, Moskalev EA, et al. : Inactivating mutations of RB1 and TP53 correlate with sarcomatous histomorphology and metastasis/recurrence in gastrointestinal stromal tumors. Am J Clin Pathol 146:718-726, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Shi E, Chmielecki J, Tang C-M, et al. : FGFR1 and NTRK3 actionable alterations in “wild-type” gastrointestinal stromal tumors. J Transl Med 14:339, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota S, Isozaki K, Moriyama Y, et al. : Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577-580, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Joensuu H, Rutkowski P, Nishida T, et al. : KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 33:634-642, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Boikos SA, Pappo AS, Killian JK, et al. : Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: A report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2:922-928, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassier PA, Fumagalli E, Rutkowski P, et al. : Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 18:4458-4464, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Rose S: BLU-285, DCC-2618 show activity against GIST. Cancer Discov 7:121-122, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher CD, Berman JJ, Corless C, et al. : Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 33:459-465, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Joensuu H: Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39:1411-1419, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Miettinen M, Lasota J: Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol 23:70-83, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Gold JS, Gönen M, Gutiérrez A, et al. : Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol 10:1045-1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmieder M, Henne-Bruns, Mayer B, et al: Comparison of different risk classification systems in 558 patients with gastrointestinal stromal tumors after R0-resecrion. Front Pharmacol 7:504, 2016. [DOI] [PMC free article] [PubMed]
- 32.Chi JL, Xu M, Zhang MR, et al. : Laparoscopic versus open resection for gastric gastrointestinal stromal tumors (GISTs): A size-location-matched case-control study. World J Surg 41:2345-2352, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Nishida T, Goto O, Raut CP, et al. : Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 122:3110-3118, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Zhang Q, Blanke CD, et al. : Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: Long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 19:1074-1080, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corless CL, Ballman KV, Antonescu CR, et al. : Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: The ACOSOG Z9001 trial. J Clin Oncol 32:1563-1570, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casali PG, Le Cesne A, Poveda Velasco A, et al. : Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup randomized trial in collaboration with the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 33:4276-4283, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Joensuu H, Eriksson M, Sundby Hall K, et al. : Adjuvant imatinib for high-risk GI stromal tumor: Analysis of a randomized trial. J Clin Oncol 34:244-250, 2016 [DOI] [PubMed] [Google Scholar]
- 38.DeMatteo RP, Ballman KV, Antonescu CR, et al. : Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 258:422-429, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanda T, Nishida T, Wada N, et al. : Adjuvant therapy with imatinib mesylate after resection of primary high-risk gastrointestinal stromal tumors in Japanese patients. Int J Clin Oncol 18:38-45, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Kang YK, Kang BW, Im SA, et al. : Two-year adjuvant imatinib mesylate after complete resection of localized, high-risk GIST with KIT exon 11 mutation. Cancer Chemother Pharmacol 71:43-51, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Raut CP, Espat J, Maki RG, et al: Extended treatment with adjuvant imatinib for patients with high-risk primary gastrointestinal stromal tumor (GIST): The PERCIST-5 study. J Clin Oncol 35:556s, 2017 (suppl 15S; abstr 11009)
- 42. doi: 10.1001/jama.2012.347. Joensuu H, Eriksson M, Sundby Hall K, et al: One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 307:1265-1272, 2012. [DOI] [PubMed] [Google Scholar]
- 43. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology. Soft tissue sarcoma. V. 2.2017-February 8, 2017. https://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf.
- 44.ESMO/European Sarcoma Network Working Group : Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii21-iii26, 2014. (suppl 3): [DOI] [PubMed] [Google Scholar]
- 45. doi: 10.1016/j.ejca.2006.01.030. Debiec-Rychter M, Sciot R, Le Cesne A, et al: KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42:1093-1103, 2006. [DOI] [PubMed] [Google Scholar]
- 46.DeMatteo RP, Lewis JJ, Leung D, et al. : Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg 231:51-58, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edmonson JH, Marks RS, Buckner JC, et al. : Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest 20:605-612, 2002 [DOI] [PubMed] [Google Scholar]
- 48.van Oosterom AT, Judson I, Verweij J, et al. : Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: A phase I study. Lancet 358:1421-1423, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Demetri GD, von Mehren M, Blanke CD, et al. : Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472-480, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Verweij J, Casali PG, Zalcberg J, et al. : Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet 364:1127-1134, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Casali PG, Zalcberg J, Le Cesne A, et al. : Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol 35:1713-1720, 2017 [DOI] [PubMed] [Google Scholar]
- 52.Blanke CD, Rankin C, Demetri GD, et al. : Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626-632, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Blanke CD, Demetri GD, von Mehren M, et al. : Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26:620-625, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Demetri GD, Heinrich MC, Fletcher JA, et al. : Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res 15:5902-5909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Ami E, Barysauskas CM, von Mehren M, et al. : Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol 27:1794-1799, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) : Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol 28:1247-1253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel S, Zalcberg JR: Optimizing the dose of imatinib for treatment of gastrointestinal stromal tumours: Lessons from the phase 3 trials. Eur J Cancer 44:501-509, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Le Cesne A, Ray-Coquard I, Bui BN, et al. : Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: An open-label multicentre randomised phase 3 trial. Lancet Oncol 11:942-949, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Patrikidou A, Chabaud S, Ray-Coquard I, et al. : Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol 24:1087-1093, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Demetri GD, van Oosterom AT, Garrett CR, et al. : Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368:1329-1338, 2006 [DOI] [PubMed] [Google Scholar]
- 61.George S, Blay JY, Casali PG, et al. : Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45:1959-1968, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Janeway KA, Albritton KH, Van Den Abbeele AD, et al. : Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer 52:767-771, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Heinrich MC, Maki RG, Corless CL, et al. : Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 26:5352-5359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demetri GD, Reichardt P, Kang Y-K, et al. : Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:295-302, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raut CP, Posner M, Desai J, et al. : Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 24:2325-2331, 2006 [DOI] [PubMed] [Google Scholar]
- 66. von Mehren M, Heinrich MC, Joensuu H, et al: Follow-up results after 9 years (yrs) of the ongoing, phase II B2222 trial of imatinib mesylate (IM) in patients (pts) with metastatic or unresectable KIT + gastrointestinal stromal tumors (GIST). J Clin Oncol 29, 2011 (suppl; abstr 10016)
- 67.Du CY, Zhou Y, Song C, et al. : Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: A prospective randomised trial in China. Eur J Cancer 50:1772-1778, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Bauer S, Rutkowski P, Hohenberger P, et al. : Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib: Analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 40:412-419, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Joensuu H, Eriksson M, Collan J, et al. : Radiotherapy for GIST progressing during or after tyrosine kinase inhibitor therapy: A prospective study. Radiother Oncol 116:233-238, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Kang YK, Ryu MH, Yoo C, et al. : Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): A randomised, placebo-controlled, phase 3 trial. Lancet Oncol 14:1175-1182, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mir O, Cropet C, Toulmonde M, et al. : Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): A randomised, multicentre, open-label phase 2 trial. Lancet Oncol 17:632-641, 2016 [DOI] [PubMed] [Google Scholar]