Abstract
Purpose
SWOG S0016 was a phase III randomized study that compared the safety and efficacy of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) with CHOP-RIT (CHOP followed by consolidation with iodine-133–tositumomab radioimmunotherapy) for previously untreated patients with follicular lymphoma. Understanding the long-term outcome of patients provides a benchmark for novel treatment regimens for FL.
Patients and Methods
Between 2001 and 2008, 531 previously untreated patients with FL were randomly assigned to receive either six cycles of R-CHOP or six cycles of CHOP-RIT. Patients with advanced-stage disease (bulky stage II, III, or IV) of any pathologic grade (1, 2, or 3) were eligible.
Results
After a median follow-up of 10.3 years, 10-year estimates of progression-free and overall survival were 49% and 78% among all patients, respectively. Patients in the CHOP-RIT arm had significantly better 10-year progression-free survival compared with patients in the R-CHOP arm (56% v 42%; P = .01), but 10-year overall survival was not different between the two arms (75% v 81%; P = .13). There was no significant difference between the CHOP-RIT and R-CHOP arms in regard to incidence of second malignancies (15.1% v 16.1%; P = .81) or myelodysplastic syndrome or acute myeloid leukemia (4.9% v 1.8%; P = .058). The estimated 10-year cumulative incidences of death resulting from second malignancies were not different (7.1% v 3.2%; P = .16), but cumulative incidence of death resulting from myelodysplastic syndrome or acute myeloid leukemia was higher in the CHOP-RIT arm compared with the R-CHOP arm (4% v 0.9%; P = .02).
Conclusion
Given these outstanding outcomes, immunochemotherapy should remain the standard induction approach for patients with high-risk FL until long-term follow-up of alternative approaches demonstrates superiority.
INTRODUCTION
Follicular lymphoma (FL) remains an incurable disease, and the goal of treatment is to achieve durable remissions using treatments with minimal short- and long-term complications. To date, immunochemotherapeutic regimens are the most effective treatment of FL in the first-line setting.1,2 The choice of immunochemotherapy has been the subject of controversy, with limited data regarding the long-term efficacy or toxicity of commonly used regimens.3-6 The SWOG S0016 study compared the efficacy and safety of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) with CHOP-RIT (CHOP followed by consolidation with iodine-131 [131I] –tositumomab radioimmunotherapy) as first-line treatment of patients with FL to determine the superior anti-CD20 compound.7 At the time of initial reporting, S0016 demonstrated excellent outcomes, with 2-year progression-free survival (PFS) of 76% and 80% and 2-year overall survival (OS) of 97% and 93% in the R-CHOP and CHOP-RIT arms, respectively.7
At the time of study design, CHOP was the standard backbone,8 and both rituximab and RIT had previously been shown to increase treatment efficacy with acceptable toxicity.9,10 A randomized phase III study (FOLL05) later showed that R-CHOP had the best efficacy-to-toxicity ratio versus other commonly used regimens.3 More recently, bendamustine with rituximab (BR) has become a preferred regimen among experts based on superior PFS and lower incidence of common toxicities in the randomized StiL (Study Group Indolent Lymphoma) trial after a median follow-up of 9 years.1,2,11 However, there was no OS benefit after a median 45 months of follow-up.5 The BRIGHT trial confirmed noninferiority of BR compared with R-CHOP or R-CVP (rituximab plus cyclophosphamide, vincristine, prednisone) in complete response (CR) and overall response rates, and a trend for improved PFS was recently reported after 5-year follow-up.4,12 More recently, novel agents have been introduced for treatment of lymphoid malignancies.13 These include B-cell receptor inhibitors like idelalisib14 and ibrutinib,15 immunomodulatory drugs (lenalidomide),16,17 and a B-cell lymphoma 2 antagonist (venetoclax).18 A growing number of studies are investigating the role of chemotherapy-free combinations using these agents for FL.13 Given the long expected survival of patients with FL, it is imperative to understand the long-term efficacy and toxicity of these regimens. Here, we report the 10-year follow-up of the S0016 study.
PATIENTS AND METHODS
Eligibility
Patients with previously untreated CD20-positive FL of any histologic grade1-3 were eligible if they had stage III to IV disease or stage II bulky disease (lymphadenopathy > 10 cm or > one third of thoracic diameter). Performance status of 0 to 2, granulocytes ≥ 1,500 cells/µL, and platelets ≥ 100,000/µL were required for enrollment. Patients were excluded if they had CNS involvement, were HIV positive, were pregnant or lactating, or had severe heart disease. Prior malignancies except for nonmelanoma skin cancer or in situ cervical cancer were also excluded. Investigators were encouraged not to enroll patients with asymptomatic or low-burden disease for whom a watch-and-wait strategy was reasonable.
Study Design and Protocol Treatments
The study design was previously described in detail.7 Patients were randomly assigned to one of two treatment arms: R-CHOP or CHOP-RIT. In the initial design, there was a CHOP-only arm, which was closed after accrual of only 17 patients because of the convincing evidence of benefit with addition of rituximab to chemotherapy in patients with FL. Randomization was performed in a one-to-one fashion, with stratification based on β2 microglobulin (β2M) level. In the R-CHOP arm, patients received treatment per the Czuczman et al19 protocol. CHOP was administered every 21 days at standard doses (cyclophosphamide 750 mg/m2 intravenously, doxorubicin 50 mg/m2 intravenously, and vincristine 1.4 mg/m2 and prednisone 100 mg orally once per day for 5 days), and rituximab was administered on days 1, 6, 48, 90, 134, and 141. Patients in the CHOP-RIT arm received CHOP at the same doses and interval followed by 131I-tositumomab 4 to 8 weeks after the sixth cycle. The therapeutic dose was administered 1 to 2 weeks after the dosimetric dose with 450 mg of tositumomab followed by 35 mg of 131I-tositumomab to deliver a 0.75-Gy whole-body dose in patients with normal platelet count or 0.65-Gy dose in patients with platelet count of 100,000 to 149,000/µL. Patients were removed from the study if they had disease progression or unacceptable toxicity or because of personal preference.
Assessment of Clinical Outcomes
All patients were assessed for response 200 and 365 days after initiation of therapy and whenever there was concern for disease progression. Computed tomography scans were performed every 6 months for the first 2 years and annually for 7 years or until relapse. A bone marrow examination was performed on day 200 if there was involvement before treatment and on day 365 in all patients unless there was disease progression. Development of secondary malignancies, especially myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), was assessed at each follow-up visit, and an expedited (within 30 days) report of MDS or AML to SWOG and the National Cancer Institute was required.
Statistical Considerations
The original clinical trial design of S0016 required approximately 250 eligible patients to be randomly assigned to each treatment arm to have a power of 0.86 to detect an improvement in PFS with CHOP-RIT over R-CHOP, corresponding to a hazard ratio (HR) of 1.50 based on a one-sided P value of .025 using a stratified log-rank test and assuming exponential PFS distribution. PFS and OS were primary end points. PFS was defined as the time from random assignment to first observation of progression or death. Patients last known to be alive and progression free were censored at the date of last contact. OS was defined as the time from random assignment to date of death or last follow-up. PFS and OS are reported on a modified intent-to-treat basis, in which only patients known to be ineligible were excluded. PFS and OS were estimated according to the Kaplan-Meier method. Treatment differences for PFS and OS were assessed by a stratified log-rank test at a two-sided α level of .05. A two-sided Fisher’s exact test was used to compare the incidence of secondary malignancy development and probability of cause of death between treatment arms. Estimates of the cumulative incidence of death resulting from secondary malignancies or AML or MDS were calculated using the nonparametric Nelson-Aalen estimator. For comparisons between treatment arms, Gray20 test statistics were used. Death resulting from other causes was considered a competing risk. Multivariable Cox proportional hazards models were used to test the prognostic significance of clinical factors regarding OS and PFS.21
RESULTS
Patient Characteristics
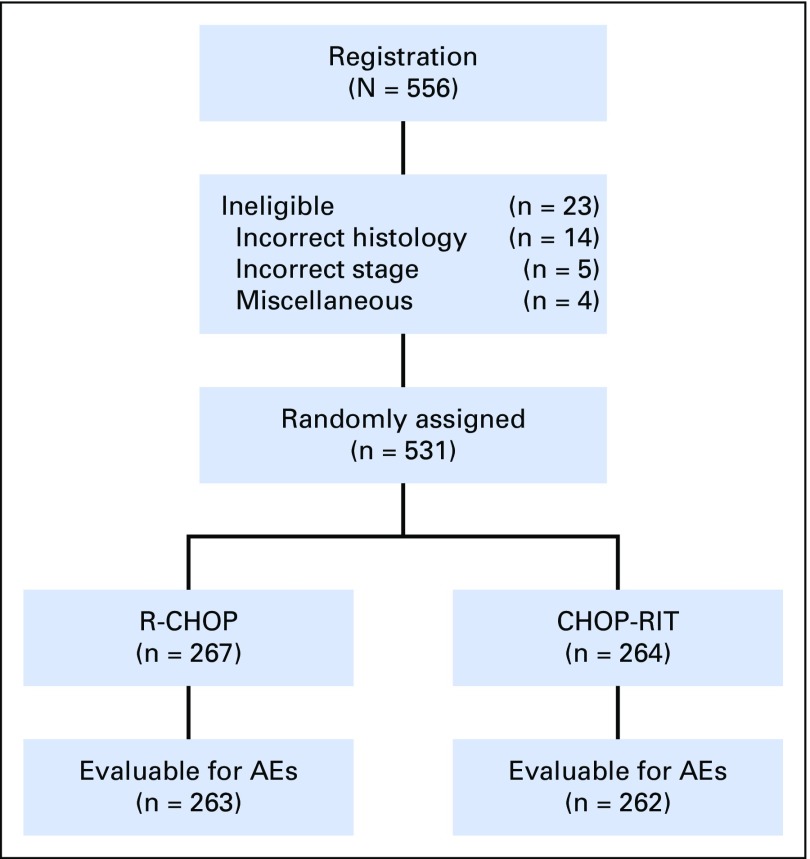
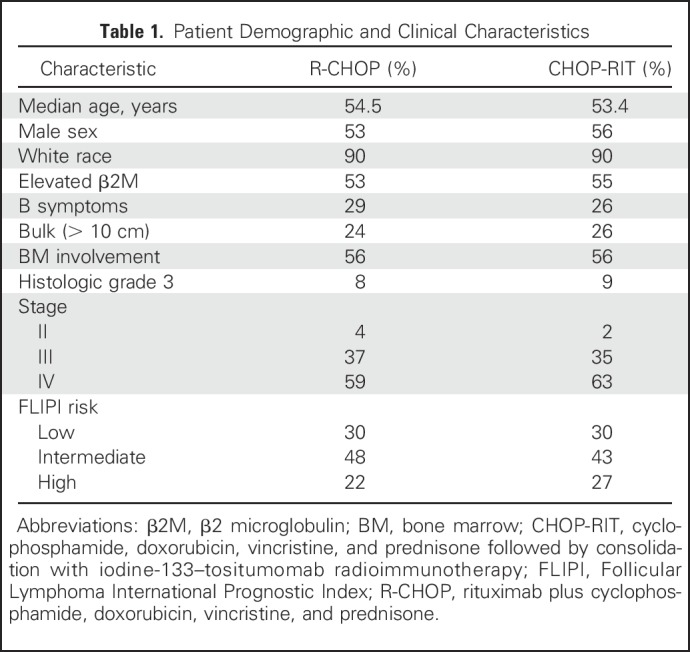
Between 2001 and 2008, 554 patients were randomly assigned to receive either R-CHOP (n = 279 patients) or CHOP- RIT (n = 275 patients). Twenty-three patients were later determined to be ineligible, and therefore, in the final analysis, 264 patients in the R-CHOP arm and 267 patients in the CHOP-RIT arm were included (Fig 1). Baseline characteristics were well balanced between the two arms (Table 1). B symptoms were present in 29% of the patients treated with R-CHOP and 26% of those receiving CHOP-RIT, and 8% and 9% of patients had histologic grade 3 disease in the R-CHOP and CHOP-RIT arms, respectively. Seventy percent of patients in each treatment arm were considered intermediate or high risk according to the Follicular Lymphoma International Index (FLIPI), and one quarter (24% and 26%) of patients had bulky disease. Treatment was completed in 95% of patients in the R-CHOP arm and 92% of patients in the CHOP-RIT arm, for an overall completion rate of 93%.
Fig 1.
CONSORT diagram illustrating patient flow in SWOG protocol S0016. AE, adverse event; CHOP-RIT, cyclophosphamide, doxorubicin, vincristine, and prednisone followed by consolidation with iodine-133–tositumomab radioimmunotherapy; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
Table 1.
Patient Demographic and Clinical Characteristics
Clinical Outcomes
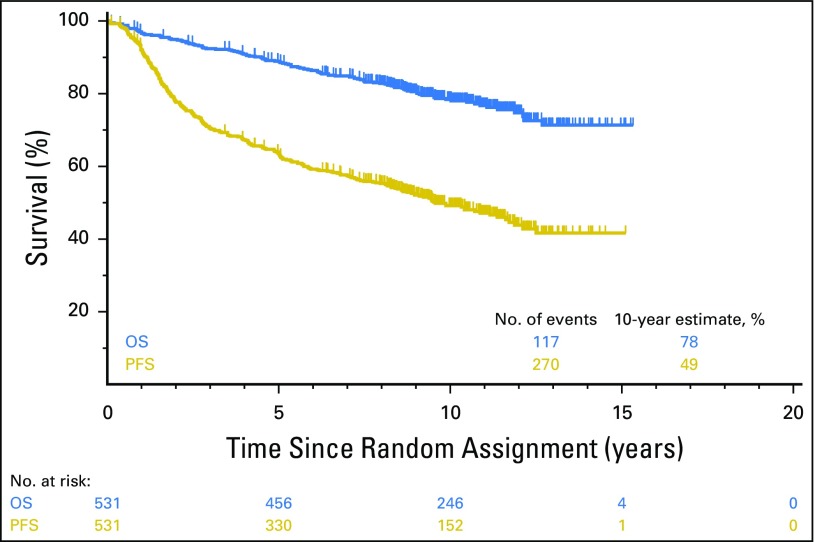
Objective remissions were documented in 263 (99%) of 267 eligible patients treated with R-CHOP and 259 (98%) of 264 patients treated with CHOP-RIT. Twenty-three patients (9%) in the R-CHOP arm and 18 (7%) in the CHOP-RIT arm had inadequate assessments and were assumed to be nonresponders for the purpose of response rate estimation. The CR rates were 37% and 41% in the R-CHOP and CHOP-RIT arms, respectively. Eleven patients were lost to follow-up, and 13 patients withdrew consent. The median follow-up among these patients was 4.5 years (range, 50 days to 11 years). After a median follow-up of 10.3 years (range, 0.1 to 15.3 years), the 10-year PFS estimate was 49% (95% CI, 44.6% to 53.5%) and the 10-year OS estimate was 78% (95% CI, 74.4% to 81.8%) among all patients (Fig 2).
Fig 2.
Overall survival (OS; blue) and progression-free survival (PFS; gold) of all patients (N = 531) in the S0016 study who were randomly assigned to either the R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-RIT (cyclophosphamide, doxorubicin, vincristine, and prednisone followed by consolidation with iodine-133–tositumomab radioimmunotherapy) arm.
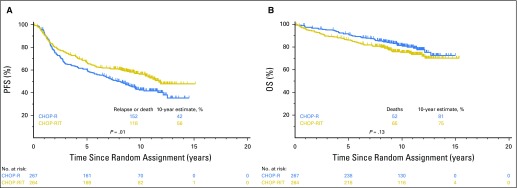
A total of 152 patients in the R-CHOP arm and 118 in the CHOP-RIT arm either experienced progression or died during follow-up, for an estimated 10-year PFS of 42% (95% CI, 36.2% to 48.5%) in the R-CHOP arm and 56% (95% CI, 50.0% to 62.3%) in the CHOP-RIT arm (two-sided stratified log-rank test P = .011). In the R-CHOP arm, 52 patients died, for an estimated 10-year OS of 81% (95% CI, 75.9% to 85.4%); there were 65 deaths in the CHOP-RIT arm, for an estimated 10-year OS of 75% (95% CI, 69.3% to 80.2%; two-sided stratified log-rank test P = .12; Figs 3A and 3B). Patients with grade 1 to 2 FL and those with grade 3 FL did not have different PFS (49% v 47%; P = .82) or OS (79% v 67%; P = .07). Similarly, comparing patients with bulky stage II disease versus patients with stage III to IV disease, there was no significant difference in PFS (HR, 0.69; 95% CI, 0.37 to 1.30; P = .25) or OS (HR, 0.48; 95% CI, 021 to 1.09; P = .08).
Fig 3.
Comparison of (A) progression-free survival (PFS) and (B) overall survival (OS) of patients with advanced-stage follicular lymphoma who received either six cycles of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) or six cycles of CHOP-RIT (cyclophosphamide, doxorubicin, vincristine, and prednisone followed by consolidation with iodine-133–tositumomab radioimmunotherapy).
Second Malignancies
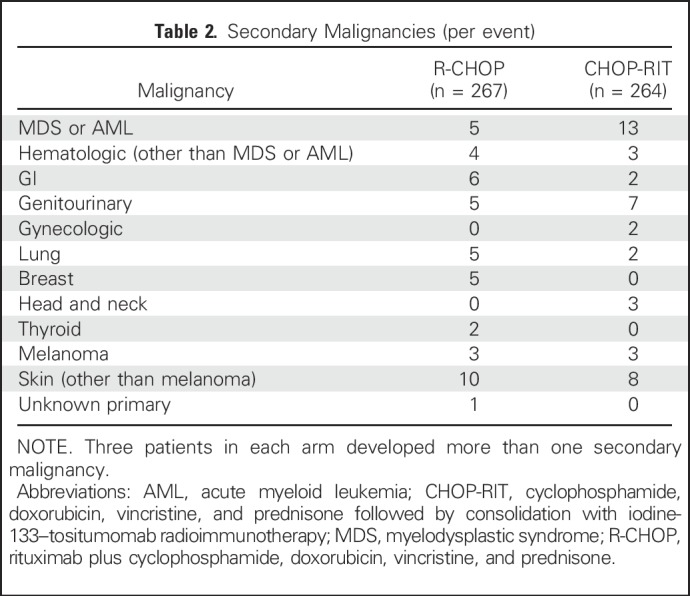
Incidence of second malignancies was not different between the two arms. Forty-three patients (16.1%) in the R-CHOP arm and 40 (15.1%) in the CHOP-RIT arm developed secondary malignancies (P =.81). Three patients in each arm developed more than one secondary malignancy. Nonmelanoma skin cancers were the most common type of nonhematologic malignancy, occurring in 10 (3.7%) and seven patients (2.6%; one patient had two skin cancer events) in the R-CHOP and CHOP-RIT arms, respectively (Table 2). Five patients (1.8%) who were treated with R-CHOP and 13 (4.9%) who received CHOP-RIT developed myeloid malignancies (AML or MDS; P = .058). In the R-CHOP arm, two patients developed AML and three patients developed MDS. Detailed follow-up data were available for two of the three patients with MDS. Using the International Prognostic Scoring System score for MDS,22 one patient had intermediate-risk and one had high-risk disease. In the CHOP-RIT arm, six and seven patients developed AML and MDS, respectively. MDS details were available for two patients who had high-risk or very high–risk disease.
Table 2.
Secondary Malignancies (per event)
Causes of Death
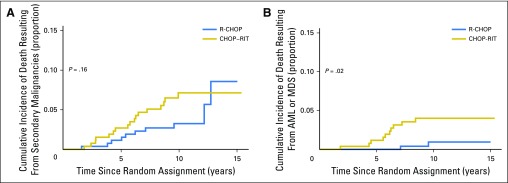
There were 117 deaths (R-CHOP arm, n = 52; CHOP-RIT arm, n = 65) during follow-up. Lymphoma or complications from lymphoma treatment (including stem-cell transplantTION) caused most of the deaths in both arms, with 29 events in the R-CHOP arm (57.7% of deaths) and 35 in the CHOP-RIT arm (53.8% of deaths). Second malignancies were responsible for 10 deaths (19.2% of deaths) in the R-CHOP arm and 17 deaths (26.1% of deaths) in the CHOP-RIT arm, followed by medical etiologies other than cancer, which caused death in nine patients (17.3% of deaths) in the R-CHOP arm and eight (12.3% of deaths) in the CHOP-RIT arm. Cause of death was unknown in four and five patients in the R-CHOP and CHOP-RIT arms, respectively (7.6% of deaths in each arm). There was no overall difference in causes of death between the two arms (two-sided Fisher’s exact P = .33). To examine the contribution of secondary malignancies, particularly MDS and AML, to the death rate in each arm, we used a cumulative incidence function. Ten patients (3.7%) in the R-CHOP arm and 17 (6.4%) in the CHOP-RIT arm died as a result of secondary malignancies during follow-up. This translated to an estimated 10-year cumulative incidence of death of 3.2% (95% CI, 1.5% to 6.1%) in the R-CHOP arm and 7.1% (95% CI, 4.3% to 10.9%) in the CHOP-RIT arm; there was no statistical difference in incidence of death resulting from secondary malignancies (P = .16). AML or MDS caused death in two (0.7%) and 10 patients (3.7%) in the R-CHOP and CHOP-RIT arms, respectively. The estimated 10-year cumulative incidence of death was 0.9% (95% CI, 0.2% to 3.2%) in R-CHOP arm and 4.0% (95% CI, 2.0% to 7.0%) in the CHOP-RIT arm (P = .02; Figs 4A and 4B).
Fig 4.
Cumulative incidences of death resulting from (A) secondary malignancies and (B) acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Estimates of 10-year cumulative incidence were (A) 3.2% and 7.1% (P = .16) and (B) 0.9% and 4.0% (P = .02) in the R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOP-RIT (cyclophosphamide, doxorubicin, vincristine, and prednisone followed by consolidation with iodine-133–tositumomab radioimmunotherapy) groups, respectively.
Prognostic Factors for Clinical Outcomes
In a multivariable analysis, we examined the prognostic significance of clinical factors regarding OS and PFS. In the initial report of this study previously published, elevated serum β2M, elevated lactate dehydrogenase (LDH), and high FLIPI score were associated with lower PFS and OS.7 For this analysis presented here, we included those factors and added bulky disease to assess the possible differential efficacy of RIT. Similar to the previous analysis, high FLIPI score (HR, 2.02; 95% CI, 1.47 to 2.79), elevated LDH (HR, 1.33; 95% CI, 1.02 to 1.75), elevated serum β2M (HR, 1.59; 95% CI, 1.24 to 2.03), and presence of bulky disease (HR, 1.59; 95% CI, 1.22 to 2.06) were associated with inferior PFS. Also, high FLIPI score (HR, 2.65; 95% CI, 1.62 to 4.34), elevated LDH (HR, 1.54; 95% CI, 1.03 to 2.29), and elevated serum β2M (HR, 2.33; 95% CI, 1.56 to 3.47), but not bulky disease (HR, 1.26; 95% CI, 0.84 to 1.89), were associated with inferior OS. In a retrospective subgroup analysis, patients with bulky disease (R-CHOP arm, n = 63; CHOP-RIT arm, n = 64) were assessed, and no significant difference in response rate (85.7% v 79.6%) was observed (P = .37). Among patients who had bulky disease, there was also no significant difference in PFS or OS between the two treatment arms (two-sided log-rank P = .33 and .19, respectively).
DISCUSSION
With almost 10 years of follow-up, patients with FL treated in the SWOG 0016 study showed outstanding survival in both R-CHOP and CHOP-RIT arms and represent the best-published long-term outcomes to date to our knowledge in this disease. At 10 years, almost 80% of the entire cohort was alive and 50% were disease free. Patients treated with CHOP-RIT had significantly longer PFS, but OS was not different between the two arms. This study provides the longest follow-up data on first-line treatment of FL using immunochemotherapy to our knowledge, and the clinical outcomes can serve as a benchmark in this setting.
These results emphasize the importance of long-term follow-up and call into question the validity of PFS as a surrogate end point for OS in this patient population. Poor correlation of PFS with OS in FL has previously been shown,23 and a number of alternative surrogate end points like CR at 30 months,24 PFS at 24 months,25 and event-free survival at 12 months26 have been suggested for clinical trials and practice, given their strong correlation with OS. Assessment of these novel end points may not be feasible in previously conducted studies because of the differences in clinical assessment time points and evolving surveillance strategies. Therefore, long-term follow-up for OS rates remains critical. In recent years, bendamustine-based regimens have become the treatment of choice based on promising results from prospective clinical trials. Rummel et al5 reported significantly better PFS in the BR arm compared with R-CHOP in a subgroup analysis of patients with FL (median PFS not reached v 40.9 months). A recent 9-year follow-up of that study showed that patients treated with BR had superior PFS and time to next treatment compared with those who received R-CHOP.11 The poor performance of R-CHOP in that study compared with survival in the S0016 or FOLL053 study could at least in part be explained by the different criteria used for treatment initiation, but it also raises questions about the true superiority of BR. In the BRIGHT study, BR was reported to be noninferior to R-CHOP and R-CVP based on initial response rates. A recently reported 5-year follow-up of the BRIGHT study showed a trend toward better PFS for BR (HR, 0.70; P = .058) but no OS benefit among patients with indolent non-Hodgkin lymphoma.4,12 Details of those follow-up studies are critically important from the efficacy standpoint and in regard to safety, especially in light of the GALLIUM study, which demonstrated the PFS advantage of obinutuzumab over rituximab when added to chemotherapy for FL but also showed that fatal adverse events were more common in patients who received bendamustine in both the rituximab and obinutuzumab arms.6
In our study, CHOP-RIT was superior to R-CHOP from a PFS standpoint, but this advantage did not translate to an OS benefit. Given the potential risk of second malignancies, especially MDS and AML, with the RIT approach,27 we examined the incidence and possible contribution of second malignancies to death in both arms. Patients who received CHOP-RIT had a numerically higher rate of MDS or AML (4.9% v 1.8%) that almost reached the level of statistical significance (P = .05). These rates are consistent with previously reported MDS or AML rates of 3.5% after RIT28 and 2.1% after immunochemotherapy for indolent non-Hodgkin lymphoma.29 Detailed information on the characteristics of MDS was available for only 40% of the patients. With this limitation in mind, 60% of patients with MDS or AML in each arm had either AML or high- or very high–risk MDS per IPSS-R.22 Overall, we did not find differences in causes of death between the two arms. However, although there was no difference in the cumulative incidence of death resulting from second malignancies between the two arms, cumulative incidence of death resulting from MDS or AML was higher in the CHOP-RIT arm. It should be noted that only 12 patients died as a result of MDS or AML during follow-up in the study, and therefore, these results should be interpreted with caution given the small number of events. Incidence of histologic transformation (HT) could not be assessed in this study, because biopsy was not mandatory at the time of relapse. Considering a majority of HTs occur early after induction treatment, along with the comparable OS rates between the two arms and among other studies, a major difference in the HT rate in our study seems unlikely.30,31
Although the introduction of novel and noncytotoxic therapeutic agents may change future treatment options,32 chemoimmunotherapy offers outstanding long-term disease control and survival, with almost half of patients in S0016 still progression free after a single line of treatment with 10 years of follow-up. Given these outstanding outcomes, immunochemotherapy should remain the standard induction approach for patients with high-risk FL, with CHOP-based regimens as viable alternatives to bendamustine-based regimens. Our results offer a benchmark for comparison of future regimens.
Footnotes
Supported by Grants No. CA180888, CA180819, CA180821, CA189953, CA180801, CA189808, CA189957, CA189952, CA189822, CA189872, CA189853, CA189804, CA180818, CA189860, CA189848, CA189972, CA180828, CA189997, CA180846, CA189858, CA180835, and CA189809 from the National Cancer Institute, National Institutes of Health, and in part by GlaxoSmithKline.
Clinical trial information: NCT00006721.
AUTHOR CONTRIBUTIONS
Conception and design: Ajay K. Gopal, David G. Maloney, Michael LeBlanc, Richard I. Fisher, Jonathan W. Friedberg, Oliver W. Press
Provision of study materials or patients: Ajay K. Gopal, Bruce D. Cheson
Collection and assembly of data: Mazyar Shadman, Hongli Li, Lisa Rimsza, John P. Leonard, Rita M. Braziel, Ajay K. Gopal, Jonathan W. Friedberg, Oliver W. Press
Data analysis and interpretation: Mazyar Shadman, Hongli Li, Lisa Rimsza, John P. Leonard, Mark S. Kaminski, Catherine M. Spier, Ajay K. Gopal, David G. Maloney, Bruce D. Cheson, Shaker Dakhil, Sonali M. Smith, Jonathan W. Friedberg, Oliver W. Press
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Continued Excellent Outcomes in Previously Untreated Patients With Follicular Lymphoma After Treatment With CHOP Plus Rituximab or CHOP Plus 131I-Tositumomab: Long-Term Follow-Up of Phase III Randomized Study SWOG-S0016
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Mazyar Shadman
Consulting or Advisory Role: AbbVie
Research Funding: Pharmacyclics (Inst), Gilead Sciences (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), Merck (Inst), Celgene (Inst), Genentech (Inst)
Hongli Li
No relationship to disclose
Lisa Rimsza
Consulting or Advisory Role: Celgene
Research Funding: ProNAi
Patents, Royalties, Other Intellectual Property: Coinventor on patent undergoing commercialization by NanoString
John P. Leonard
Consulting or Advisory Role: Roche/Genentech
Mark S. Kaminski
Consulting or Advisory Role: Pharmacyclics, Genentech, Nordic Nanovector
Research Funding: Merck
Rita M. Braziel
Patents, Royalties, Other Intellectual Property: Named inventor on a patent: “Evaluation of mantle cell lymphoma and methods related thereof”
Catherine M. Spier
No relationship to disclose
Ajay K. Gopal
Stock or Other Ownership: Compliment
Honoraria: Janssen Pharmaceuticals, Compliment, Gilead Sciences, Sanofi, Pfizer, Seattle Genetics, Brim, Incyte
Consulting or Advisory Role: Pfizer, Sanofi, Seattle Genetics, Janssen Pharmaceuticals, Gilead Sciences, Compliment, Brim
Research Funding: Merck, Janssen Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, Spectrum Pharmaceuticals, Seattle Genetics, eFFECTOR Therapeutics, TEVA Pharmaceuticals Industries, TG Therapeutics, Takeda Pharmaceuticals
David G. Maloney
Honoraria: Celgene, Gilead Sciences, Kite Pharma, Roche
Research Funding: Juno Therapeutics (Inst)
Bruce D. Cheson
No relationship to disclose
Shaker Dakhil
No relationship to disclose
Michael LeBlanc
No relationship to disclose
Sonali M. Smith
No relationship to disclose
Richard I. Fisher
Consulting or Advisory Role: Pharmacyclics, Roche, Kite Pharma, Seattle Genetics, Sandoz, Celgene, Genentech, Bayer HealthCare Pharmaceuticals
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals
Research Funding: Seattle Genetics (Inst), Kite Pharma (Inst)
Travel, Accommodations, Expenses: Roche
Oliver W. Press
Stock or Other Ownership: PhaseRx, Emergent Biosolutions
Consulting or Advisory Role: Roche, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Genentech (Inst), Presage Biosciences (Inst)
Travel, Accommodations, Expenses: Bayer HealthCare Pharmaceuticals
REFERENCES
- 1.Horwitz SM, Zelenetz AD, Gordon LI, et al. : NCCN guidelines insights: Non-Hodgkin’s lymphomas, version 3.2016. J Natl Compr Canc Netw 14:1067-1079, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Kahl BS, Yang DT: Follicular lymphoma: Evolving therapeutic strategies. Blood 127:2055-2063, 2016 [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.1200/JCO.2012.45.0866. Federico M, Luminari S, Dondi A, et al: R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: Results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. J Clin Oncol 31:1506-1513, 2013 [Erratum: J Clin Oncol 32:1095, 2014] [DOI] [PubMed] [Google Scholar]
- 4.Flinn IW, van der Jagt R, Kahl BS, et al. : Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 123:2944-2952, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rummel MJ, Niederle N, Maschmeyer G, et al. : Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 381:1203-1210, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Marcus R, Davies A, Ando K, et al. : Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med 377:1331-1344, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Press OW, Unger JM, Rimsza LM, et al. : Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol 31:314-320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czuczman MS, Weaver R, Alkuzweny B, et al. : Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin’s lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol 22:4711-4716, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Press OW, Unger JM, Braziel RM, et al. : Phase II trial of CHOP chemotherapy followed by tositumomab/iodine I-131 tositumomab for previously untreated follicular non-Hodgkin’s lymphoma: Five-year follow-up of Southwest Oncology Group Protocol S9911. J Clin Oncol 24:4143-4149, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Press OW, Unger JM, Braziel RM, et al. : A phase 2 trial of CHOP chemotherapy followed by tositumomab/iodine I 131 tositumomab for previously untreated follicular non-Hodgkin lymphoma: Southwest Oncology Group Protocol S9911. Blood 102:1606-1612, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Rummel MJ, Maschmeyer G, Ganser A, et al: Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: Nine-year updated results from the StiL NHL1 study. J Clin Oncol 35, 2017 (suppl; abstr 7501)
- 12. Flinn I, van der Jagt R, Chang JE, et al: First-line treatment of iNHL or MCL patients with BR or R-CHOP/R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol 35, 2017 (suppl; abstr 7500) [DOI] [PMC free article] [PubMed]
- 13.Reagan PM, Friedberg JW: Follicular lymphoma: First-line treatment without chemotherapy for follicular lymphoma. Curr Treat Options Oncol 16:32, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Gopal AK, Kahl BS, de Vos S, et al. : PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370:1008-1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gopal AK, Schuster SJ, Fowler N, et al: Ibrutinib as treatment for chemoimmunotherapy-resistant patients with follicular lymphoma: First results from the open-label, multicenter, phase 2 DAWN study. Blood 128, 2016 (abstr 1217) [Google Scholar]
- 16.Smith SM, Pitcher BN, Jung SH, et al. : Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: The Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 4:e176-e182, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard JP, Jung SH, Johnson J, et al. : Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol 33:3635-3640, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davids MS, Roberts AW, Seymour JF, et al. : Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 35:826-833, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czuczman MS, Grillo-López AJ, White CA, et al. : Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 17:268-276, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988. [Google Scholar]
- 21. Cox DR: Regression models and life-tables. J R Stat Soc B 34:187-220, 1972. [Google Scholar]
- 22.Greenberg PL, Tuechler H, Schanz J, et al. : Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120:2454-2465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee L, Wang L, Crump M: Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin’s lymphoma: Correlation of complete response, time-to-event and overall survival end points. Ann Oncol 22:1392-1403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. doi: 10.1200/JCO.2016.70.8651. Shi Q, Flowers CR, Hiddemann W, et al: Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: An individual patient-level analysis of multiple randomized trials. J Clin Oncol 35:552-560, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Casulo C, Byrtek M, Dawson KL, et al. : Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare Study. J Clin Oncol 33:2516-2522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer MJ, Bachy E, Ghesquières H, et al. : Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol 91:1096-1101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roboz GJ, Bennett JM, Coleman M, et al. : Therapy-related myelodysplastic syndrome and acute myeloid leukemia following initial treatment with chemotherapy plus radioimmunotherapy for indolent non-Hodgkin lymphoma. Leuk Res 31:1141-1144, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Bennett JM, Kaminski MS, Leonard JP, et al. : Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. Blood 105:4576-4582, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Sacchi S, Marcheselli L, Bari A, et al. : Secondary malignancies after treatment for indolent non-Hodgkin’s lymphoma: A 16-year follow-up study. Haematologica 93:398-404, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Salles G, Seymour JF, Offner F, et al. : Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 377:42-51, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Sarkozy C, Trneny M, Xerri L, et al. : Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol 34:2575-2582, 2016 [DOI] [PubMed] [Google Scholar]
- 32. ClinicalTrials.gov: Combined rituximab and lenalidomide treatment for untreated patients with follicular lymphoma (RELEVANCE). https://clinicaltrials.gov/ct2/show/NCT01476787.