Abstract
Fission yeast Swi6 is a human HP1 homolog that plays important roles in multiple cellular processes. In addition to its role in maintaining heterochromatin silencing, Swi6 is required for cohesin enrichment at the pericentromere. Loss of Swi6 leads to abnormal mitosis, including defects in the establishment of bioriented sister kinetochores and microtubule attachment. Swi6 interacts with Dfp1, a regulatory subunit of DBF4-dependent kinase (DDK), and failure to recruit Dfp1 to the pericentromere results in late DNA replication. Using the dfp1-3A mutant allele, which specifically disrupts Swi6-Dfp1 association, we investigated how interaction between Swi6 and Dfp1 affects chromosome dynamics. We find that disrupting the interaction between Swi6 and Dfp1 delays mitotic progression in a spindle assembly checkpoint-dependent manner. Artificially tethering Dfp1 back to the pericentromere is sufficient to restore normal spindle length and rescue segregation defects in swi6-deleted cells. However, Swi6 is necessary for centromeric localization of Rad21-GFP independent of DDK. Our data indicate that DDK contributes to mitotic chromosome segregation in pathways that partly overlap with, but can be separated from both, Swi6 and the other HP1 homolog, Chp2.
Keywords: fission yeast, kinetochore, Cdc7/Hsk1, heterochromatin, minichromosome, mitosis, Cnp1, Chp1 and SAC
THE repetitive DNA sequences in the pericentromere and their assembled heterochromatin are associated with normal chromosome segregation and structural integrity (Janssen et al. 2011; Forsburg 2013). A key player is Swi6 (HP1), a heterochromatin protein conserved from fission yeast to human that plays multiple roles in chromosome dynamics. Swi6 contains a conserved methyl-histone-binding chromodomain (CD) and an adjacent chromo shadow domain (CSD). The CD is required for Swi6 binding to methylated histone H3K9 in heterochromatin regions, including the pericentromere, while the CSD is necessary for the dimerization and recruitment of cell cycle regulators (Paro 1990; Paro and Hogness 1991; Aasland and Stewart 1995; Brasher et al. 2000; Eissenberg and Elgin 2000; Smothers and Henikoff 2000). Dynamic distribution of Swi6 in the pericentromere is regulated in a cell cycle-dependent manner. During mitosis, Swi6 delocalizes from the pericentromere when methylation of histone H3K9 is reduced, resulting in transient loss of heterochromatic silencing (Chen et al. 2008; Kloc et al. 2008; Li et al. 2013b). In G1 and early S phase, an RNA interference-mediated pathway recruits the RNA-induced transcriptional silencing (RITS) complex containing Chp1, which in turn recruits the methyltransferase Clr4, and restores methylation of H3K9 (Lejeune et al. 2010; Alper et al. 2012). This allows Swi6 binding, restoring pericentromeric heterochromatin silencing and contributing to faithful chromosome segregation (Allshire et al. 1995). Swi6 also contributes to the stability of the pericentromere repeats under conditions of replication stress; in its absence, the pericentromere domain is prone to recombination and chromosome rearrangements (Li et al. 2013b).
Swi6 is also required for the early replication observed in the fission yeast pericentromere (Hayashi et al. 2009). This requires its interaction with Dfp1, the regulatory subunit of the replication-initiating DBF4-dependent kinase (DDK), via the Dfp1 MOD1-interacting region (MIR) domain (Bailis et al. 2003; Hayashi et al. 2009). Mutations in DDK also result in chromosome segregation defects including lagging chromosomes, suggesting that it impacts centromere or kinetochore function (Snaith et al. 2000; Bailis et al. 2003). A specific mutation in the MIR motif, dfp1-3A, disrupts the interaction with Swi6 and leads to late replication in the pericentromere domain, while tethering Dfp1 back to the pericentromere via a CD fusion restores early replication (Hayashi et al. 2009).
In addition to its role in heterochromatin silencing, Swi6 is required for the enrichment of cohesin at the centromere (Bernard et al. 2001; Nonaka et al. 2002). Centromeric cohesin is required to establish bioriented sister kinetochores and proper microtubule attachments; therefore, the increase of lagging chromosome and minichromosome loss in swi6∆ may reflect a loss of centromeric cohesin (Bernard et al. 2001; Nonaka et al. 2002; Li et al. 2013b).
Fission yeast Chp2 is another HP1 homolog that contributes to heterochromatin silencing, although its function is distinct from Swi6 (Motamedi et al. 2008). Biochemical studies of Swi6 and Chp2 suggest that Swi6 interacts with nucleosomal and nonnucleosomal ligands more strongly than Chp2, although they share similar dimerization and oligomerization equilibriums (Isaac et al. 2017). Mass spectrometry analysis of binding partners of Swi6 and Chp2 reveals that Swi6 associates with numerous nuclear proteins while Chp2 mainly binds to Snf2/histone deacetylase-containing repressor complexes (Isaac et al. 2017). These different biophysical properties of Swi6 and Chp2 suggest that they have nonoverlapping biological functions. Chromosome segregation and silencing defects in chp2∆ cells are more modest than those of swi6∆ cells (Isaac et al. 2017).
In this report, we use different alleles of Dfp1 to manipulate DDK association with the pericentromere. This allows us to examine how the interaction between DDK and Swi6 contributes to pericentromeric silencing, cohesion enrichment, and chromosome segregation. Unexpectedly, our data suggest that DDK and Swi6 have overlapping, but nonidentical, requirements in chromosome segregation and stability. Our data indicate that Swi6 plays a major role in ensuring faithful chromosome segregation, but that this can be substituted by artificially recruiting Dfp1 to the pericentromere. However, a Dfp1 mutant that fails to associate with Swi6 causes striking rearrangements without showing substantial defects in segregation. This indicates that interaction between DDK and Swi6 differentially affects numerical and structural chromosome stability.
Materials and Methods
Yeast strains and media
Media and general genetic techniques used for Schizosaccharomyces pombe have been previously described in Forsburg and Rhind (2006) and Sabatinos and Forsburg (2010). Strains used in this study are listed in Table 1. Gene deletion, endogenous green fluorescent protein (GFP) tagging, and mCherry red fluorescent protein (RFP) tagging (chRFP) were achieved using a fusion polymerase chain reaction (PCR), as previously described (Yang et al. 2004). Plasmids pFA6a-GFP(S65T)-KanMX6, pFA6a-mCherry-natMX6, and pFA6a-hphMX6 (Hentges et al. 2005) were used as templates for fusion PCR reactions. Yeast transformations were completed by electroporation (Sabatinos and Forsburg 2010). The GFP- and chRFP-tagged strains showed no growth defects, indicating that they are functional.
Table 1. Strain lists.
| Stain | Genotype | Source |
|---|---|---|
| FY4003 | h- ChL[ubcp4::LEU2::chk1 kan:spccB3.18 spcc1322.09::ura4+ ade6+] ade6Æ ura4-D18 leu1-32 | Takuro Nakagawa |
| FY4255 | h- cdc25-22 leu1-32::hENT-leu1 his7-366::hsv-tk-his7 ura4? ade6-M210 | Li et al. (2013a) |
| FY4293 | h- otr1L(HindIII)::ura4+ ura4D-18 leu1-32 his3-D1 ade6-M210 | This study |
| FY4295 | h- swi6Æ::kanMX6 otr1L(HindIII)::ura4+ ura4-D18 leu1-32 his3-D1 ade6-M210 | This study |
| FY4574 | h+ cdc25-22 swi6Æ::kanMX leu1-32::hENT1-leu1 his7-366::hsv-tk-his7 ura4-D18 ade6-M210 his3-D1 | Li et al. (2013a) |
| FY4942 | h- ura4-D18::ura4+-nmt1-TK Ælys1::(dfp1+-CFP-2CD-hphMX6) | Hisao Masukata |
| FY4944 | h- ura4-D18::ura4+-nmt1-TK dfp1-3A::kanMX6 | Hisao Masukata |
| FY5109 | h- swi6Æ::kanMX6 ChL[ubcp4::LEU2::chk1 hph:spcc1322.09::ura4+ ade6+] ade6Æ-D ura4-D18 leu1-32 his1-102 | Li et al. (2013b) |
| FY5911 | h+ chp1-eGFP::kanMX sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210/216 can1-1? | Li et al. (2013a) |
| FY7554 | h+ CFP-cnp1::kanMX sad1+::DsRed-leu2 leu1-32 ura4-D18 ade6-M216 can1-1? | This study |
| FY7583 | h+ cdc25-22 swi6Æ::kanMX Ælys1::(dfp1+-CFP-2CD-hphMX6) leu1-32::hENT1-leu1 his7-366::hsv-tk-his7 ura4-D18 ade6-M216 his3-D1? | This study |
| FY7585 | h- cdc25-22 Ælys1::(dfp1+-CFP-2CD-hphMX6) leu1-32::hENT-leu1 his7-366::hsv-tk-his7 ura4-D18 ade6-M210 | This study |
| FY7631 | h+ cdc25-22 dfp1-3A::kanMX6 leu1-32::(hENT- leu1+) his7-366::hsv-tk-his7 ura4-D18 ade6-M216 | This study |
| FY7640 | h+ CFP-cnp1::kanMX sad1+::DsRed-leu2 leu1-32 ura4-D18 dfp1-3A::kanMX6 | This study |
| FY7687 | h+ cdc25-22 Æswi6::kanMX dfp1-3A::kanMX6 leu1-32::hENT-leu1 his7-366::hsv-tk-his7 ura4-D18 | This study |
| FY7719 | h- Ælys1::(dfp1+-CFP-2CD-hphMX6) ChL[ubcp4::LEU2::chk1 kan:spcc1322.09::ura4+ ade6+) ade6Æ ura4-D18 leu1-32 | This study |
| FY7814 | h- swi6-GFP::kanMX6 sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210 can1-1 | This study |
| FY7824 | h- dfp1-3A::kanMX6 ChL[ubcp4::Leu2::chk1 hph::spccB3.18 spcc1322.09::ura4+ ade6+] ade6Æ ura4-D18 leu1-32 his1-102? | This study |
| FY7973 | h90 chp1-eGFP::kanMX swi6-mCherry::natMX6 ura4-D18 leu1-32 ade6-M216 | This study |
| FY7978 | h- chp1Æ::ura4+ swi6-GFP::kanMX6 sad1+::DsRed-leu2+ leu1-32 ade6-M210/M216? his7-366? ura4-D18 can1-1? | This study |
| FY7979 | h90 swi6Æ::ura4+ chp1-eGFP-kanMX sad1+::DsRed-leu2+ leu1-32 ura4-(DS/ED18?) ade6-M210/M216? can1-1? | This study |
| FY7987 | h+ swi6Æ::kanMX6 Ælys1::(dfp1+-CFP-2CD-hphMX6) ChL[ubcp4::LEU2::chk1 kan:spccB3.18 spcc1322.09::ura4+ ade6+] ade6Æ ura4-D18 leu1-32 his3-D1? | This study |
| FY8023 | h+ arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 ura4-D18 leu1-32? can1-1? his5D? | This study |
| FY8027 | h- swi6Æ::kanMX6 dfp1-3A::kanMX6 ChL[ubcp4::LEU2::chk1 hph:spccB3.18 spcc1322.09::ura4+ ade6+] ade6Æ ura4-D18 leu1-32 (his1-102 or his3-D1?) | This study |
| FY8051 | h- dfp1-3A::kanMX6 rad21-GFP[leu2] sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210/216 ? his3-D1? | This study |
| FY8113 | h+ dfp1-3A::kanMX6 arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 ura4-D18 leu1-32 his5D can1-1? | This study |
| FY8115 | h- rad21-GFP[leu2] sad1+::DsRed-leu2+ leu1-32 ura4-D18 his3-D1? | This study |
| FY8144 | h- dfp1-3A::kanMX6 otr1L(HindIII)::ura4+ ade6-M210 leu1-32 ura4-D18 his3-D1 | This study |
| FY8146 | h- dfp1-3A::kanMX6 swi6Æ::kanMX6 otr1L(HindIII)::ura4+ ade6-M210 leu1-32 ura4-D18 his3-D1 | This study |
| FY8191 | h+ swi6Æ::kanMX6 Ælys1::(dfp1+-CFP-2CD-hphMX6) otr1L(HindIII)::ura4+ ade6-M210 leu1-32 his3-D1 ura4-D18 | This study |
| FY8213 | h- swi6Æ::ura4+ dfp1-3A::kanMX6 arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 leu1-32 ura4-(DS/E orD18?) can1-1? his5D? | This study |
| FY8214 | h90 swi6Æ::ura4+ arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 leu1-32 ura4(DS/E or D18?) his5D? can1-1? | This study |
| FY8221 | h+ Ælys1::(dfp1+-CFP-2CD-hphMX6) otr1L(HindIII)::ura4+ ura4-D18 leu1-32 ade6-M210 | This study |
| FY8222 | h- tos4-GFP::kanMX6 sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210 can1-1 | This study |
| FY8254 | h- dfp1-3A::kanMX6 tos4-GFP::kanMX6 sad1+::DsRed-leu2+ ade6-M210 leu1-32 ura4-D18 his3-D1? can1-1? | This study |
| FY8269 | h- rad21-GFP[leu2] sad1+::DsRed-leu2+ swi6Æ::kanMX Ælys1::(dfp1+-CFP-2CD-hphMX6) leu1-32 ura4-D18 his3-D1? (ade6-M210 or -M216?) | This study |
| FY8275 | h+ hht1-mRFP::natMX6 arg3+::ccr1N-GFP(D187 aa1-275)::his5+ Ælys1::(dfp1+-CFP-2CD-hphMX6) ura4-D18 leu1-32? his3-D1? his5D? | This study |
| FY8285 | h- swi6Æ::kanMX rad21-GFP[Leu2] sad1+::DsRed-leu2+ leu1-32 his3-D1? ura4-D18 ade6-M210/M216? | This study |
| FY8325 | h- swi6Æ::natMX6 tos4-GFP::kanMX6 sad1-::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210 can1-1 | This study |
| FY8327 | h- swi6Æ::natMX6 dfp1-3A::KanMX6 tos4-GFP::KanMX6 Sad1-DsRed::Leu2 ade6-M210 leu1-32 ura4-D18 his3-D1? can1-1? | This study |
| FY8329 | h+ ark1-GFP::kanR hht1-mRFP::kanMX6 leu1-32 his3-D1 | This study |
| FY8340 | h+ swi6Æ::natMX6 ark1-GFP::kanR hht1-mRFP::kanMX6 leu1-32 his3-D1 | This study |
| FY8375 | h+ swi6Æ::kanMX Ælys1::(dfp1-CFP-2CD-hphMX6) arg3+::ccr1N-GFP(D187 aa1-275)::his5+ hht1-mRFP::natMX6 leu1-32? ade6-M210? his3-D1? his5D1? ura4-D18 | This study |
| FY8376 | h- swi6Æ::natMX6 dfp1-3A::hphMX6 ark1-GFP::kanR hht1-mRFP::kanMX6 leu1-32 his3-D1 ura4-D18? | This study |
| FY8390 | h- mad2Æ::hphMX6 swi6Æ::natMX6 tos4-GFP::kanMX6 sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210 can1-1 | This study |
| FY8393 | h- mad2Æ::hphMX6 tos4-GFP::kanMX6 sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210 can1-1 | This study |
| FY8394 | h- mad2Æ::hphMX6 dfp1-3A::kanMX6 tos4-GFP::kanMX6 sad1+::DsRed-leu2+ ade6-M210 leu1-32 ura4-D18 his3-D1? can1-1? | This study |
| FY8396 | h- mad2Æ::hphMX6 swi6Æ::natMX6 dfp1-3A::KanMX6 tos4-GFP::KanMX6 Sad1-DsRed::Leu2 ade6-M210 leu1-32 ura4-D18 his3-D1? can1-1? | This study |
| FY8410 | h+ chp2Æ::natMX6 arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 ura4-D18 leu1-32? can1-1? his5D? | This study |
| FY8413 | h- chp2Æ::natMX6 dfp1-3A::kanMX6 arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 ura4-D18 leu1-32 can1-1? his5D | This study |
| FY8444 | h+ cdc25-22 swi6-sm1::hygr leu1-32::hENT-Leu1+ his7-366::hsv-tk-his7 ura4-D18 ade6-M210/216?) | This study |
| FY8513 | h- swi6-sm1::hygr hht1-mRFP::natMX6 arg3+::ccr1N-GFP(D187 aa1-275)::his5+ ura4-D18 leu1-32? his5D? (wt ade6-M210 or M-216?) | This study |
| FY8633 | h+ swi6Æ::ura4+ dfp1-3A::kanMX6 chp2Æ::natMX6 leu1-32 ura4-(DS/E orD18?) arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 can1-1? his5D? | This study |
| FY8634 | h- swi6Æ::ura4+ chp2Æ::natMX6 leu1-32 ura4-(DS/E orD18?) arg+3::ccr1N-GFP(D817 aa1-275)::his5+ hht1-mRFP-hphMX6 can1-1? his5D? | This study |
| FY8688 | h+ swi6-sm1::hygr otr1L(HindIII)::ura4+ ura4-D18 leu1-32 ade6-M210/216? his3D1? his7-366? | This study |
| FY8726 | h90 swi6Æ::ura4+ chp2Æ::natMX6 chp1-eGFP-kanMX sad1+::DsRed-leu2+ leu1-32 ura4-(DS/ED18?) ade6-M210/M216 can1-1? | This study |
| FY8795 | h+ chp2Æ::natMX6 chp1-eGFP::kanMX sad1+::DsRed-leu2+ leu1-32 ura4-D18 ade6-M210/216 can1-1? | This study |
| FY8958 | h- swi6-sm1::hygr rad21-GFP[leu2] sad1-DsRed-Leu2 leu1-32 ura4-D18 his3-D1? his7-366? ade6-M210/216? | This study |
| FY9117 | h- rad21-GFP[leu2] Sad+::DsRed-leu2 Ælys1::(dfp1+-CFP-2CD-hphMX6) leu1-32 ura4-D18 his3-D1 ade6-M210 | This study |
A question mark indicates that the marker may or may not be present in the strain.
Live-cell imaging and data analysis
Cells were grown in EMM with appropriate supplements. After cells were grown to the midlog phase, they were harvested and then placed on agarose pads for imaging (Tran et al. 2004; Green et al. 2015). Time-lapse imaging was performed at 25° in a DeltaVision Core (Applied Precision, Issaquah, WA) microscope using a 60× N.A. 1.4 PlanApo objective lens and 12-bit Photo metrics Cool Snap HQII charge-coupled device (CCD). Nine slices with 0.5 µm space distance were captured at each time point. All images were processed by the deconvolution of softWoRx software and are presented as maximum-intensity projections. ImageJ software (National Institutes of Health, Bethesda, MD) was used for image analysis.
Chromatin immunoprecipitation and real-time quantitative PCR analysis
Chromatin immunoprecipitation (ChIP) experiments were carried out according to methods described in Rougemaille et al. (2008). Rabbit anti-GFP antibody (Ab290; Abcam) was used to bring down Rad21-GFP-associated DNA fragments. Immunoprecipitated DNA was analyzed via real-time quantitative PCR using SYBR Green (Bio-Rad, Hercules, CA) as a marker for DNA amplification on a CFX Connect Real-Time System (Bio-Rad). The pericentromere dh locus was amplified using primers including 5′-GTAAGTATGAGCAACTGGCG-3′ and 5′-GGAACAAATCAGGAAACCGAG-3′ (Gomez et al. 2005; Li et al. 2013a). A graph was plotted according to the percentage of immunoprecipitated DNA with respect to the total input DNA used in each immunoprecipitation.
Rate of minichromosome loss and rearrangement
A single colony of cells containing a minichromosome was picked up from selective EMM plates, and then inoculated in 2 ml rich medium [yeast extract with supplements (YES)] and grown overnight at 25° to allow spontaneous recombination. A total of 1 × 104 cells were spread on pombe minimal medium with glutamate (PMG)+5-FOA+All plates and cells were allowed to grow at 32° for 3 days. A total of 250 cells were plated on the YES plates for determination of the plating efficiency. The minichromosome loss and recombination rates were calculated according to the Lea–Coulson method. Bean and box plots were generated with the R program using easyGgplot2.
Silencing assay
Cells were grown in YES medium to saturation and then diluted to OD595 = 1. Fivefold serial dilution was carried out in a 96-well microtiter plate. Cells were then spotted on YES, 5-FOA, and supplemented EMM −Ura plates. Cells were allowed to grow at 32° for 2–4 days.
Data availability
Strains are available upon request. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8009408.
Results
Loss of Swi6-Dfp1 interaction causes mitotic delay
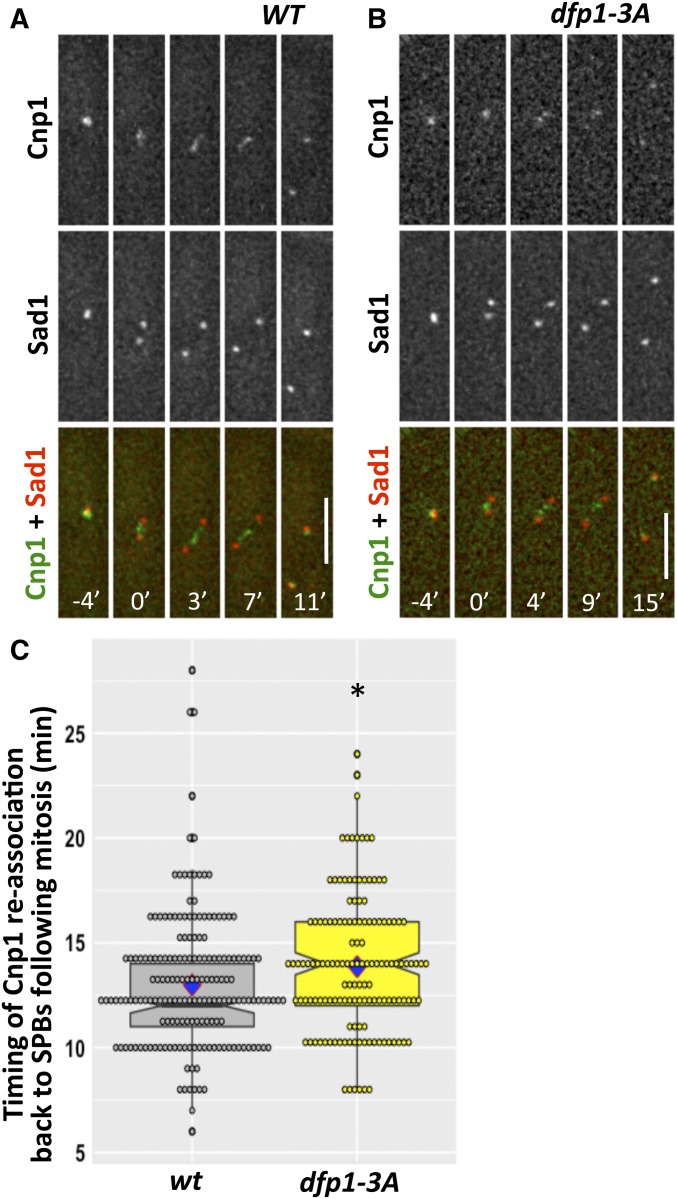
The fission yeast centromere associates with spindle pole bodies (SPBs) before and after mitosis, with a transient dissociation during metaphase and anaphase; relocalization occurs within ∼10 min of SPB duplication (Li et al. 2013a). We asked whether recruitment of DDK to the pericentromere via Swi6-Dfp1 interaction affects the timing of this association in live cells by monitoring the distribution of CFP-Cnp1, a centromere marker, in relation to Sad1-DsRed, an SPB marker (Figure 1, A and B). The return of CFP-Cnp1 to the SPBs following mitosis was modestly delayed in dfp1-3A mutants (14.11 ± 2.74 min) compared to wild-type (12.98 ± 3.23 min) (Figure 1C), suggesting the interaction of Swi6 and Dfp1 contributes to timely mitosis.
Figure 1.
Disruption of Swi6 and Dfp1 interaction affects timing of reassociation of centromere to SPBs. We examined CFP-Cnp1 to mark the clustered centromeres, and Sad1-DsRed to mark the SPB, in live cells during mitosis in (A) wt (FY7554) and (B) dfp1-3A (FY7640). Time 0’ corresponds to the timepoint that precedes the first observed separation of the SPB and defines the initiation of mitosis. (C) Quantification of timing when CFP-Cnp1 locates back to the SPBs following mitosis. Significance was calculated with a two-tailed Student’s t-test with * P < 0.05. Bar, 5 µm. CFP, cyan fluorescent protein; SPB, spindle pole bodies; WT, wild-type.
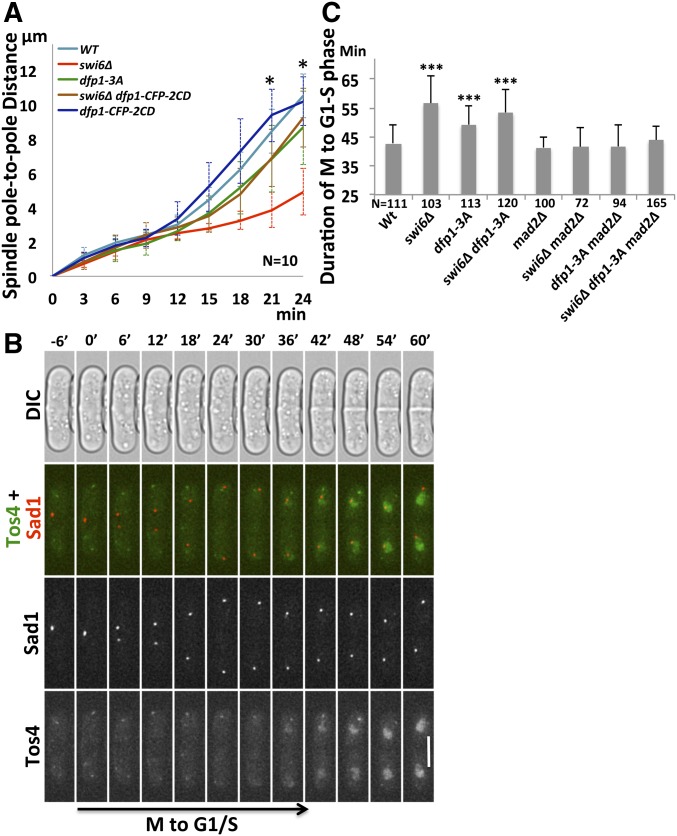
Establishment of sister-kinetochore biorientation and proper attachment of microtubules is important to generate microtubule-pulling force for chromosome segregation (Tanaka 2002; Gregan et al. 2011). Failure to establish proper attachment results in defects in kinetochore tension during the transition from metaphase to anaphase. Previous work has suggested that loss of Swi6 leads to increased length of metaphase spindles (Choi and McCollum 2012). We examined the spindle pole-to-pole distance in swi6∆ and dfp1-3A mutants as cells proceeded through anaphase. In contrast to the results in metaphase, we observed that spindle pole-to-pole distance during anaphase was considerably shorter in swi6∆ (21 min: 3.85 ± 1.02 µm and 24 min: 4.92 ± 1.35 µm) compared to wild-type (21 min: 8.42 ± 1.27 µm and 24 min: 10.51 ± 1.28 µm) (Figure 2A), consistent with a kinetochore tension defect (Dumont and Mitchison 2009). The dfp1-3A mutant that disrupts the Swi6 interaction has only a slightly reduced pole-to-pole distance. However, when Dfp1 recruitment is independent of Swi6, by fusing it to a CD in the Dfp1-CFP-2CD construct (Hayashi et al. 2009), the severe spindle pole-to-pole shortening in swi6∆ is largely restored, suggesting that enriching Dfp1 at the pericentromere is sufficient to restore much of the kinetochore tension in swi6∆ mutants in anaphase.
Figure 2.
Artificially tethering Dfp1 to the pericentromere via 2CD rescues mitotic spindle tension. (A) Spindle pole-to-pole distance was used as a marker for mitotic spindle tension, and was determined by the distance between Sad1-DsRed foci through mitosis in wt (FY8115), swi6∆ (FY8285), dfp1-3A (FY8051), dfp1-CFP-2CD (FY9117), and swi6∆ dfp1-CFP-2CD (FY8269). (B) Duration of M-to-G1/S phase transition was determined by the timing between separation of duplicated SPBs (labeled by Sad1-DsRed) and the appearance of nuclear Tos4-GFP, an S phase marker, which coincides with the appearance of the septum, visualized by DIC. Time 0’ corresponds to the timepoint that precedes the first observed separation of the SPB and defines the initiation of mitosis. Representative image from wt (FY8222). (C) Quantification of timing from the assay in (B) for wt (FY8222), swi6∆ (FY8325), dfp1-3A (FY8254), mad2∆ (FY8393), mad2∆ dfp1-3A (FY8394), and mad2∆ swi6∆ (FY8390). The delay of M-to-G1/S phase transition in swi6∆ and dfp1-3A was abolished after deleting mad2. A two-tailed Student’s t-test was used to determine significance: * P < 0.05, *** P < 0.001. Error bars represent Standard Deviation (SD). Bar, 5 µm. CD, chromodomain; CFP, cyan fluorescent protein; SPB, spindle pole bodies; WT, wild-type.
Failure of kinetochore–microtubule attachment causes spindle assembly checkpoint (SAC) activation and mitotic delay (Musacchio and Salmon 2007; Lara-Gonzalez et al. 2012). We investigated the timing of the M-to-G1/S phase transition in swi6∆ and dfp1-3A mutants using a live-cell imaging approach. We used separation of duplicated SPBs, revealed by Sad1-DsRed, to mark the beginning of mitosis, and the appearance of Tos4-GFP, which shows S phase-specific nuclear localization (Kiang et al. 2009), to mark the beginning of S phase. We measured the time between these events to estimate the duration of the M-to-G1/S phase transition of the cell cycle. Appearance of nuclear Tos4-GFP signals was coincident with the appearance of septation, as revealed by DIC images (Figure 2B). The time from SPB duplication to the appearance of Tos4 was extended in swi6∆ (56.64 ± 9.43 min) swi6∆ dfp1-3A (53.53 ± 7.8 min), and, to a lesser extent, dfp1-3A (49.22 ± 6.5 min), compared to wild-type cells (42.76 ± 6.29 min) (Figure 2C).
We reasoned that this delay in the M-to-G1/S phase transition is likely a consequence of SAC activation. We deleted mad2, a component of the SAC effector complex (Fang et al. 1998), in wild-type, swi6∆, and dfp1-3A mutants. We observed that the duration of the M-to-G1/S phase transition was similar to wild-type in mad2∆ cells (41.28 ± 3.66 min) (Figure 2C). The time from SPB duplication to the appearance of Tos4 was dramatically reduced in swi6∆ mad2∆ (41.38 ± 6.99 min), dfp1-3A mad2∆ (41.68 ± 7.26 min), and swi6∆ dfp1-3A mad2∆ (43.89 ± 4.82 min) mutants, compared with swi6∆ (56.64 ± 9.43 min), dfp1-3A (49.22 ± 6.5 min), and swi6∆ dfp1-3A (53.53 ± 7.8 min) (Figure 2C). Thus, the mitotic delay in both swi6∆ and dfp1-3A is consistent with activation of the SAC.
Swi6 and Dfp1 independently contribute to faithful chromosome segregation
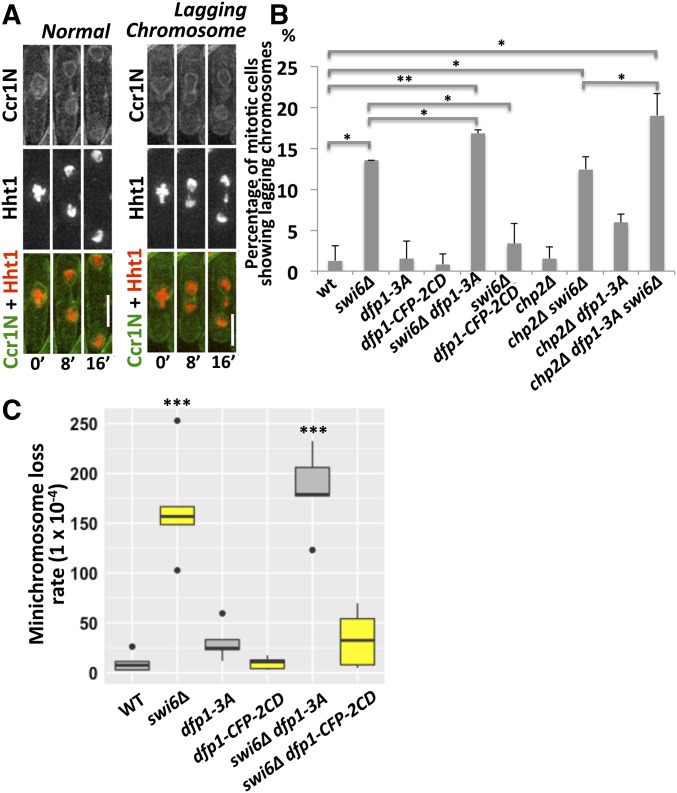
Cells lacking Swi6 show increased lagging chromosomes, a hallmark of merotelic attachment (Ekwall et al. 1995). Lagging chromosomes have also been observed in DDK mutants (Snaith et al. 2000; Bailis et al. 2003). We examined whether recruitment of DDK to the pericentromere is required to prevent lagging chromosomes. We generated strains expressing tagged histone hht1-mRFP and the membrane marker ccr1N-GFP, and used time-lapse imaging to analyze mitotic progression and the formation of lagging chromosomes (Figure 3A).
Figure 3.
DBF4-dependent kinase (DDK) contributes to proper chromosome segregation in the absence of Swi6. (A) We monitored mitosis in strains expressing ccr1N-GFP (D187 amino acids 1–275) (a membrane marker) and hht1-mRFP (a histone marker). Representative normal mitosis and lagging chromosome images are shown for wt (FY8023), swi6∆ (FY8214), dfp1-3A (FY8113), dfp1-CFP-2CD (FY8275), swi6∆ dfp1-3A (FY8213), swi6∆ dfp1-CFP-2CD (FY8375), chp2∆ (FY8410), chp2∆ swi6∆ (FY8634), chp2∆ dfp1-3A (FY8413), and chp2∆ swi6∆ dfp1-3A (FY8633). Time 0’ corresponds to the timepoint that precedes the first observed separation of the histone mass and defines the initiation of mitosis. (B) Quantification of mitotic cells showing lagging chromosomes in (A). (C) The frequency of chromosome loss was determined using a minichromosome with multiple genetic markers. Cells that survived on EMM +5-FOA +histidine +uracil +leucine +adenine +lysine plates have lost the right arm of the minichromosome, and were screened for leucine auxotrophy to determine whether the left arm was also missing. wt (FY4003), swi6∆ (FY5109), dfp1-3A (FY7824), dfp1-CFP-2CD (FY7719), swi6∆ dfp1-3A (FY8027), swi6∆ dfp1-CFP-2CD (FY7987), chp2∆ (FY8690), chp2∆ dfp1-3A (FY8692), chp2∆ swi6∆ (FY8700), and chp2∆ swi6∆ dfp1-3A (FY8702). Frequencies of minichromosome loss were calculated according to the Lea–Coulson method. A two-tailed Student’s t-test was used to determinate significance. P-values are reported as follows: * P < 0.05, ** P < 0.01, *** P < 0.001. Bar, 5 µm. WT, wild-type.
Representative images of normal mitosis and lagging chromosomes are shown in Figure 3A. In dfp1-3A (1.5%) and dfp1-CFP-2CD mutants (0.8%), we did not observe an increase in lagging chromosomes compared to wild-type cells (1%) (Figure 3B). As expected, lagging chromosomes were frequent in swi6Δ cells (13.5%). This suggests that Dfp1 recruitment by Swi6, which is abolished by dfp1-3A, is not required to prevent lagging chromosomes. However, swi6∆ dfp1-CFP-2CD mutants show a low proportion of lagging chromosomes (3.4%) compared to swi6∆ alone (13.5%). Thus, recruiting Dfp1 back to the pericentromere via 2CD is sufficient to rescue lagging chromosomes in the absence of Swi6. Unexpectedly, the frequency of lagging chromosomes in the swi6∆ dfp1-3A double mutant (16.8%) is significantly higher than in the swi6∆ mutant (13.5%). Thus, the dfp1-3A allele contributes to lagging chromosome formation but only when Swi6 is missing. This suggests that DDK has a redundant, Swi6-independent function in preventing lagging chromosome formation, which requires the MIR motif, and is particularly important in the absence of Swi6.
As an independent assay for chromosome stability, we monitored loss of a minichromosome in swi6∆, dfp1-3A, dfp1-CFP-2CD, swi6∆ dfp1-3A, and swi6 dfp1-CFP-2CD mutants. Chromosome loss in swi6∆ and swi6∆ dfp1-3A strains occurred to a similar extent, but was not observed in dfp1-3A single mutants, which resembled wild-type cells. However, both dfp1-CFP-2CD and swi6∆ dfp1-CFP-2CD mutants had low rates of minichromosome loss, similar to wild-type (Figure 3C). The frequency of lagging chromosomes observed in swi6∆ dfp1-CFP-2CD correlates with minichromosome loss.
Chp2 is a second HP1 homolog in fission yeast. We reasoned that Dfp1 might also be recruited via interaction of its MIR motif with the Chp2 CSD and that this might explain the additive phenotype of the swi6∆ dfp1-3A allele. Therefore, we tested the contributions of chp2∆ to chromosome segregation. First, we observed that chp2∆ single mutants have a low frequency of lagging chromosomes (1.6%), which is similar to that of wild-type (1.3%) and dfp1-3A (1.5%) cells. We found that the frequency of lagging chromosomes in chp2∆ dfp1-3A (5.8%) increases about fourfold compared to that in the single mutants (Figure 3B). The frequency of lagging chromosomes in chp2∆ swi6∆ double mutants (12.5%) resembles that of single swi6∆ mutants (13.5%). However, the frequency in the chp2∆ swi6∆ dfp1-3A triple mutant rises to 18.6% (Figure 3B). These data suggest that Swi6 plays a major role in preventing the formation of lagging chromosomes, but that Chp2 and DDK also prevent lagging chromosome formation independently of Swi6 function.
We examined additional phenotypes linked to chromosome mis-segregation. Cells lacking Swi6 show higher sensitivity to the microtubule depolymerization drug Thiabendazole (TBZ). We observed slight increases in TBZ sensitivity in dfp1-3A, dfp1-CFP-2CD, and chp2∆ single mutants (Supplemental Material, Figure S1). chp2∆ dfp1-3A double mutants are much more sensitive to TBZ than chp2∆ and dfp1-3A single mutants (Figure S1). Surprisingly, swi6∆ dfp1-CFP-2CD mutants are also sensitive to TBZ, which we did not expect as these cells show less-frequent lagging chromosomes and restored the long spindle pole-to-pole distance.
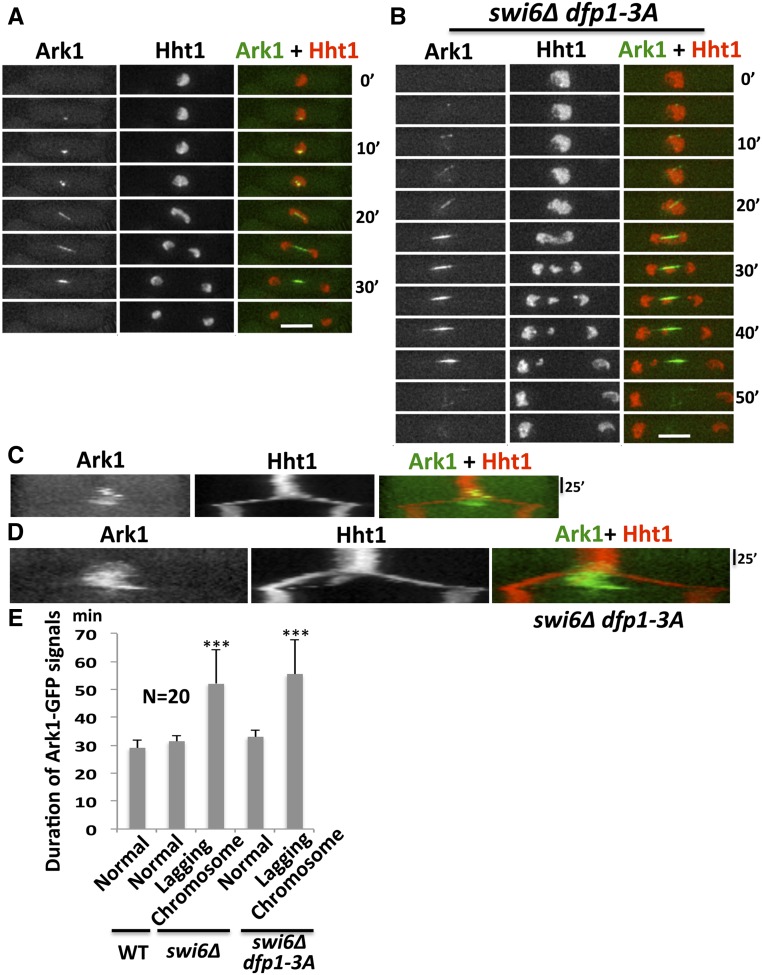
Ark1, the fission yeast Aurora B kinase, plays a key role in controlling the activation of the spindle checkpoint (Petersen and Hagan 2003). We examined ark1-GFP and hht1-mRFP localization throughout mitosis using time-lapse imaging in wild-type, swi6∆, and swi6∆ dfp1-3A strains. Ark1-GFP first appears as a dot at the kinetochore and then moves to the mitotic spindles, ultimately localizing to the central region of the spindle before mitotic exit. We observed a prolonged duration of Ark1-GFP signal in swi6∆ and swi6∆ dfp1-3A mutants that had increased lagging chromosomes compared to cells with normal mitosis (Figure 4), consistent with the delayed mitosis in these strains.
Figure 4.
Prolonged nuclear Ark1-GFP correlates with increased lagging chromosomes. Localization of Ark1-GFP in relation to Hht1-mRFP (a histone marker) was observed in wt (FY8329), swi6∆ (FY8340), and swi6∆ dfp1-3A (FY8376) during (A) representative normal mitosis and (B) lagging chromosome mitosis. (C and D) Kymograph analysis of (A and B), respectively shows prolonged duration of Ark1-GFP in strains with lagging chromosomes. (E) Duration of Ark1-GFP signals in (A and B). The difference in duration of Ark1-GFP signals in normal mitotic cells compared to lagging chromosome cells was significant using a two-tailed Student’s t-test: *** P < 0.001. Bar, 5 µm. RFP, red fluorescent protein; WT, wild-type.
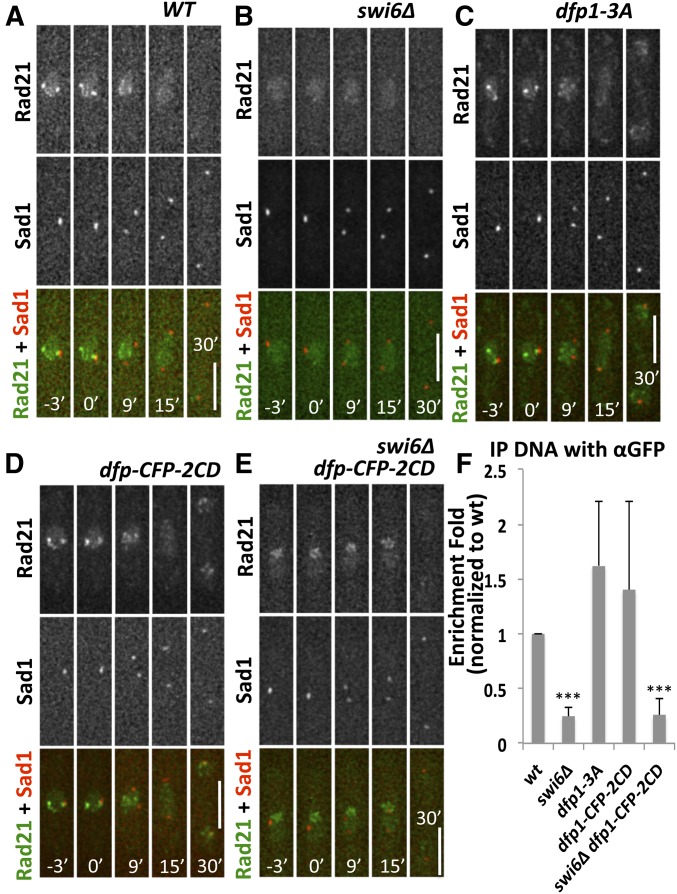
Swi6 is required for centromeric enrichment of Rad21 in interphase independent of DDK
Next, we investigated whether the segregation defects observed in swi6 and dfp1 mutants are related to cohesion recruitment. Centromeric cohesin is required for the establishment of sister-kinetochore biorientation and the attachment of microtubules (Tanaka et al. 2013; Forsburg and Shen 2017). DDK interacts with cohesin via Swi6 (Bailis et al. 2003). We asked whether disrupting the interaction between Swi6 and Dfp1 affects the distribution of Rad21, a subunit of cohesin. We tagged Rad21 and Sad1 with GFP and DsRed, respectively, in wild-type, swi6∆, and dfp1-3A cells. Rad21-GFP distribution in relation to Sad1-DsRed was observed throughout the cell cycle.
In wild-type cells, Rad21-GFP had a diffused nuclear GFP signal and also produced several bright foci inside the nucleus. One focus colocalized with Sad1-DsRed, suggesting that it represents the centromeric enrichment of Rad21-GFP (Figure 5A; Tomonaga et al. 2000). During mitosis, the Rad21-GFP signal was dramatically reduced in the nucleus, consistent with the degradation of the protein after anaphase onset. In the absence of swi6, we no longer observed a Rad21-GFP focus colocalizing with Sad1-DsRed (Figure 5B), agreeing with previous studies showing that Swi6 recruits pericentromere cohesion (Bernard and Allshire 2002; Nonaka et al. 2002). In dfp1-3A cells, Rad21-GFP still colocalized with Sad1-DsRed before mitosis (Figure 5C). To test if artificially targeting Dfp1 to the pericentromere via 2CD was sufficient to restore the enrichment of centromeric Rad21-GFP in swi6-deleted cells, we followed the distribution of Rad21-GFP and Sad1-DsRed throughout mitosis in dfp1-CFP-2CD and swi6∆ dfp1-CFP-2CD mutants (Figure 5, D and E). We observed that dfp1-CFP-2CD swi6∆ did not restore Rad21-GFP enrichment at the centromere (Figure 5E). We confirmed these observations using ChIP followed by real-time quantitative PCR analysis to detect Rad21-GFP at the pericentromere dh locus, with consistent results (Figure 5F). These data suggest that recruitment of Dfp1 to the pericentromere is neither necessary nor sufficient for enrichment of Rad21, but that Swi6 is strictly required for this.
Figure 5.
Centromeric localization of Rad21 depends on Swi6. (A–E) The position of Rad21-GFP in relation to the centromere defined by an SPB marker, Sad1-DsRed, was observed in live cells. Time 0’ corresponds to the timepoint that precedes the first observed separation of the SPB and defines the initiation of mitosis. (A) WT (FY8115), (B) swi6∆ (FY8285), (C) dfp1-3A (FY8051), (D) dfp1-CFP-2CD (FY9117), and (E) swi6∆ dfp1-CFP-2CD (FY8269). (F) Association of Rad21-GFP at the pericentromeric dh locus in strains FY8051, FY8115, FY8269, FY8285, and FY9117 was examined using chromatin IP-quantitative PCR, and is consistent with the visualization in (A–E). Significance was determined using a two-tailed Student’s t-test; *** P < 0.001. Bar, 5 µm. CD, chromodomain; CFP, cyan fluorescent protein; IP immunoprecipitation; SPB, spindle pole bodies; WT, wild-type.
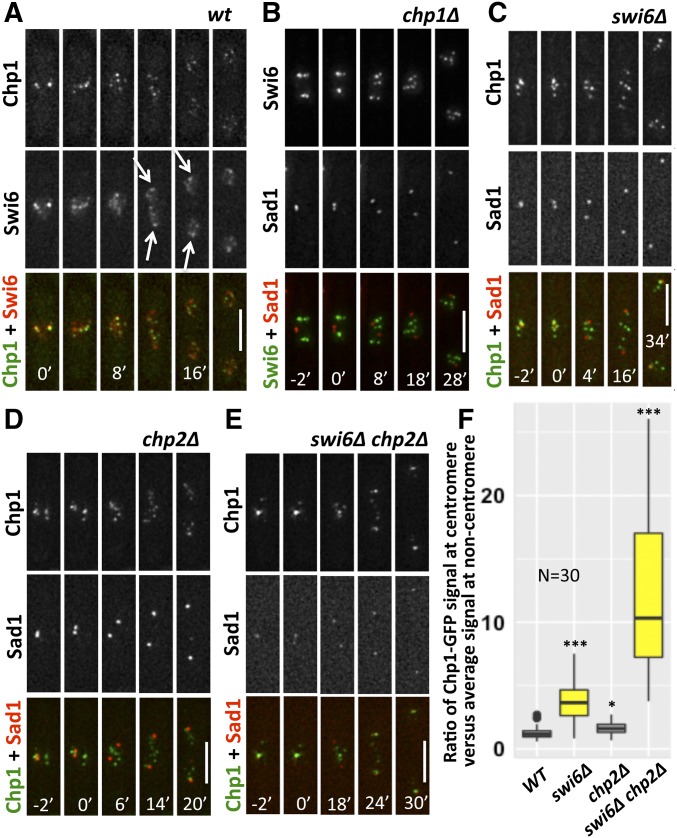
Swi6 and Chp2 are required for noncentromere localization of Chp1
We previously observed that GFP-Swi6 (which expresses an N-terminal GFP-tagged version of Swi6 under the control of the nmt1 promoter) relocalizes to the SPB more quickly than Chp1-GFP following mitosis (Li et al. 2013a). Similar results were observed in this study using an endogenously expressed Swi6 with a C-terminal GFP tag (Figure S2, A–C). We further confirmed this in a double-tagged strain containing Chp1-GFP and Swi6-chRFP. Using live-cell imaging, we found Chp1-GFP foci colocalized with Swi6-chRFP foci in interphase, as expected (Figure 6A). During mitosis, both Swi6-chRFP and Chp1-GFP leave the SPBs, but Swi6-chRFP moves to the spindle pole earlier than Chp1-GFP (Figure 6A), confirming our previous observations using different fluorescent tags (Li et al. 2013a). We further examined the interdependent relationship between Swi6 and Chp1 localization. In the absence of Chp1, Swi6-GFP still forms foci but these foci do not colocalize with Sad1-DsRed throughout the cell cycle, consistent with evidence that Chp1 is required for the centromere localization of Swi6-GFP (Figure 6B; Sadaie et al. 2004). In the absence of Swi6, Chp1-GFP still localizes to the centromere/SPB in interphase (Figure 6C), indicating that Swi6 is not required for the recruitment of Chp1-GFP to the centromere.
Figure 6.
Swi6 and Chp2 are required for the noncentromeric location of Chp1 in interphase. (A) Localization of Chp1-GFP and Swi6-chRFP was observed during mitosis in live WT cells (FY7973). Arrows indicate the position of Swi6 localized close to spindle poles. (B) Live-cell imaging of Swi6-GFP in relation to Sad1-DsRed was captured during mitosis in chp1∆ (FY7978). (C) Localization of Chp1-GFP and Sad1-DsRed in swi6∆ (FY7979). (D) Localization of Chp1-GFP and Sad1-DsRed in chp2∆ (FY8795). (E) Localization of Chp1-GFP and Sad1-DsRed in chp2∆ swi6∆ (FY8726). Time 0’ corresponds to the timepoint that precedes the first observed separation of the SPB and defines the initiation of mitosis. (F) Chp1-GFP signals at centromere and noncentromere regions before mitosis were measured in (A and C–E), and the ratio of Chp1-GFP signals at centromere vs. noncentromere sites is presented. Bar, 5 µm. A two-tailed Student’s t-test was used to determine significance with * P < 0.05, *** P < 0.001. chRFP, mCherry red fluorescent protein; WT, wild-type.
We also examined Chp1-GFP localization to the SPBs in chp2∆ in interphase and found no defects (Figure 6D), which was not a surprise given the modest phenotypes associated with chp2 (Sadaie et al. 2004). However, in a swi6∆ chp2∆ double-mutant strain, noncentromeric localization of Chp1 was greatly reduced, while the Chp1 signal at the centromere/SPB remained robust (Figure 6E, quantified in Figure 6F). Thus, Swi6 and Chp2 are redundant for localization of Chp1 to noncentromere regions, while Chp1 is required for Swi6 localization to the centromere.
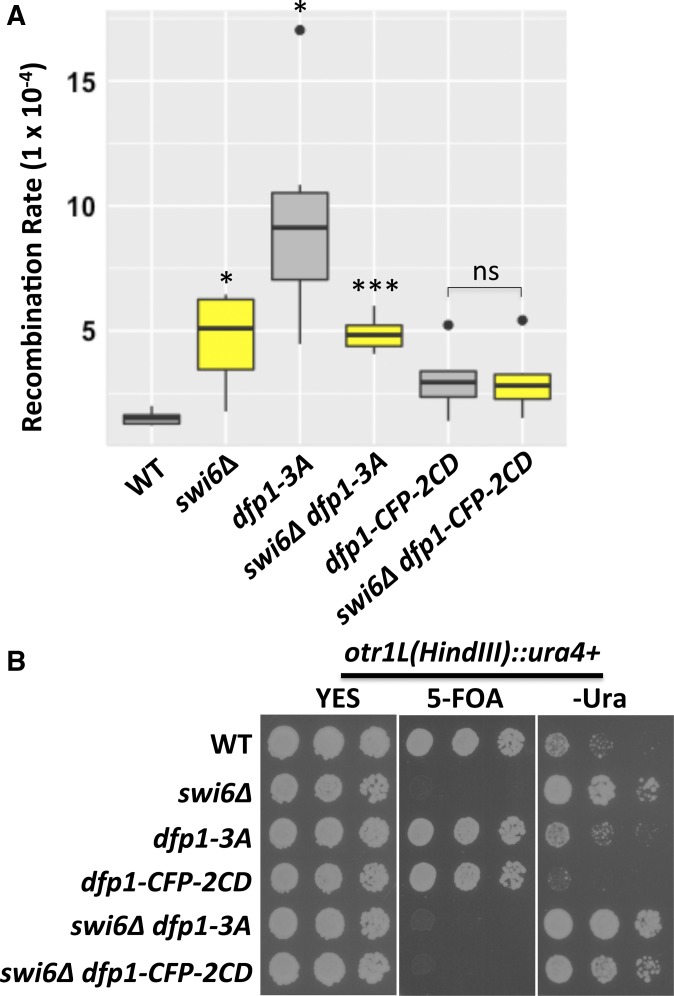
Replication timing and chromosome rearrangement
The two Dfp1 alleles have different effects on replication timing in the pericentromere (Hayashi et al. 2009). We used bromodeoxyuridine (BrdU) ChIP and quantitative PCR analysis to confirm that replication of the pericentromere occurred late in dfp1-3A and early in swi6∆ dfp1-CFP-2CD strains, as previously reported (Figure S3). We asked whether this replication timing correlated with genome instability in this domain. As in our earlier study (Li et al. 2013b), we used a genetically marked minichromosome to monitor chromosome rearrangement, which we showed reflects structural instability of repetitive sequences. Rearrangement occurs at a modestly higher frequency in swi6∆ compared to that in wild-type cells (Figure 7A; Li et al. 2013b). Unexpectedly, in dfp1-3A cells rearrangement was increased relative to wild-type or swi6∆ cells, while the double mutant swi6∆ dfp1-3A resembled swi6∆ alone. In contrast, rearrangement is close to wild-type levels in dfp1-CFP-2CD mutant cells or in swi6∆ dfp1-CFP-2CD double mutants (Figure 7A). Thus, dfp1-3A causes instability even in the presence of wild-type Swi6, and recruiting dfp1-CFP-2CD to the pericentromere rescues both replication timing and genome stability defects associated with swi6∆.
Figure 7.
Late replication timing correlates with increased gross chromosome rearrangement. (A) The frequency of chromosome rearrangement was determined using a minichromosome with multiple genetic markers (see Figure 3C). Cells that survived on EMM +5-FOA +histidine +uracil +leucine + adenine +lysine plates have lost the right arm of the minichromosome and maintain the left arm (Leu+). Recombination rates were calculated according to the Lea–Coulson method in the indicated strains; P-values are reported as follows: * P < 0.05, *** P < 0.001. (B) Pericentromere heterochromatin silencing was measured by expression of an ura4+ gene inserted at otr1L in wt (FY4293), swi6∆ (FY4295), dfp1-3A (FY8144), dfp1-CFP-2CD (FY8221), swi6∆ dfp1-3A (FY8146), and swi6∆ dfp1-CFP-2CD (FY8191). Cells were spotted and grown on YES, YES+ 5-FOA, and EMM −Uracil plates at 32° for 3 days with 1:5 serial dilutions. Cells grow on EMM –Uracil plates, but not on YES + 5-FOA plates, when they lose heterochromatin silencing. CD, chromodomain; CFP, cyan fluorescent protein; WT, wild-type; YES, yeast extract with supplements.
Previous studies have suggested that the role of Swi6 in normal chromosome segregation is independent of its role in transcriptional silencing in the heterochromatin (Bernard and Allshire 2002; Nonaka et al. 2002; Yamagishi et al. 2008). The swi6-sm1 allele disrupts silencing without lagging chromosomes (Yamagishi et al. 2008) (Figure S4, A and B). We observed a similar frequency of lagging chromosomes in wild-type (1%) and swi6-sm1 mutants (1.03%). We also examined Rad21-GFP distribution in swi6-sm1 mutants and found that one Rad21-GFP focus colocalizes with Sad1-DsRed before mitosis in swi6-sm1, indicating that Rad21-GFP enrichment at the centromere is unaffected in swi6-sm1 (Figure S4C). This mutant also replicates the pericentromere with normal timing (Figure S3).
Finally, we examined pericentromeric silencing in the dfp1 mutant strains by monitoring the expression of an ura4+ reporter gene integrated in the otr1L of centromere 1 to test for silencing loss (Bernard et al. 2001; Freeman-Cook et al. 2005; Li et al. 2011). Cells that grow on 5-FOA have intact silencing, while cells that grow on −Ura plates are silencing-deficient. We observed that loss of heterochromatin silencing occurred in cells lacking swi6 regardless of the dfp1 allele (Figure 7B). Thus, the steady-state transcriptional silencing is independent of replication timing or Dfp1 recruitment. We found that swi6∆ cells show increased sensitivity to HU and camptothecin (CPT) (Figure S5), regardless of dfp1 alleles, while the dfp1 strains show no defects. This suggests that changes in centromere replication timing and DDK recruitment are not a key to drug sensitivity.
Discussion
Loss of heterochromatin in the fission yeast pericentromere causes defects in replication timing, and structural as well as numerical chromosome instability (Hayashi et al. 2009; Forsburg 2013; Li et al. 2013a,b). In this study, we investigated how association between the heterochromatin protein Swi6/HP1 and the conserved DDK kinase influences chromosome instability.
In fission yeast, Swi6 binds H3K9-methylated histones in the pericentromere to establish transcriptional silencing and promote proper chromosome segregation (Ekwall et al. 1995). Additional CD proteins, Chp1 and Chp2, are also implicated in promoting heterochromatin structure in fission yeast (Halverson et al. 2000; Thon and Verhein-Hansen 2000; Bannister et al. 2001; Partridge et al. 2002). Swi6 is implicated in the enrichment of cohesin at the pericentromere (Bernard et al. 2001; Nonaka et al. 2002). Enrichment of cohesin in this region is crucial to establish bioriented sister kinetochores and the proper microtubule attachment necessary to generate the mitotic pulling force for chromosome segregation (Kenney and Heald 2006). Consistent with this, loss of swi6 is associated with increased chromosome loss and lagging chromosomes resulting from merotelic attachments (Gregan et al. 2011).
DDK was first identified as an initiator of DNA replication, with an essential role in phosphorylating and activating the minichromosome maintenance (MCM) helicase [reviewed in Larasati and Duncker (2016)]. It is also implicated in the regulation of cohesion in mitosis and meiosis (Bailis et al. 2003; Takahashi et al. 2008; Marston 2009; Le et al. 2013; Hinshaw et al. 2017; Zheng 2018). DDK phosphorylates histone H3T45 (Baker et al. 2010) and topoisomerase TOP2A at the centromere (Wu et al. 2016). In fission yeast, early centromere replication and cohesion are promoted by the association of DDK with Swi6 (Bernard et al. 2001; Bailis et al. 2003; Hayashi et al. 2009). In contrast, in budding yeast, which lacks Swi6-mediated heterochromatin, there is a different mechanism to recruit DDK to the kinetochore, where it phosphorylates Ctf19 to promote both early replication and chromosome cohesion (Natsume et al. 2013; Hinshaw et al. 2017). We showed previously that DDK associates with Swi6 to promote normal chromosome segregation (Bailis et al. 2003). Temperature-sensitive alleles affecting the catalytic subunit hsk1+, or a truncation allele of the regulatory subunit dfp1+, are associated with increased frequency of lagging chromosomes and other phenotypes (Snaith et al. 2000; Bailis et al. 2003). These observations suggest that DDK-Swi6 association is important for normal chromosome segregation but do not exclude the possibility that these proteins have independent effects.
In this report, we have used two previously identified separation-of-function alleles of Dfp1 to manipulate the association of DDK at the pericentromere, to dissect its Swi6-dependent and -independent effects in chromosome stability. The dfp1-CFP-2CD allele targets Dfp1 to H3K9me independent of Swi6 (Hayashi et al. 2009). This is sufficient to restore early replication timing in a swi6∆ background (Hayashi et al. 2009; Figure S3). It has only minor phenotypes by itself. The dfp1-3A mutation disrupts the MIR motif that is implicated in binding the CSD domain of Swi6 (Bailis et al. 2003; Hayashi et al. 2009). This mutant suffers delayed DNA replication in the centromere, similar to swi6∆ (Hayashi et al. 2009) (Figure S3), and shows modest delays in cell cycle progression.
We examined chromosome segregation dynamics in the mutant strains. Kinetochore tension plays a key role in regulating the SAC activation that leads to a prolonged mitosis (Pinsky and Biggins 2005). We found a shortened spindle pole-to-pole distance in swi6∆ and to lesser extent dfp1-3A cells. The duration of the M-to-G1/S phase transition in the cell cycle is extended in swi6∆, dfp1-3A and swi6∆ dfp1-3A double mutants, and this depends upon the SAC protein Mad2 (Figure 2, B and C). The swi6∆ dfp1-CFP-2CD strain rescues the shortened spindle and loss of kinetochore tension associated with swi6∆ (Figure 2A), and also reduces the rate of minichromosome loss compared to swi6∆ alone (Figure 3C). This shows that the effect of Dfp1 recruitment on kinetochore function is independent of the presence of Swi6.
However, despite having defects in spindle elongation and cell cycle progression, the dfp1-3A mutation did not cause significant changes in the frequency of lagging chromosomes or chromosome loss (Figure 3, B and C), indicating that these phenotypes are not sufficient to significantly disrupt chromosome segregation. We conclude that recruitment of DDK to Swi6, and/or early replication in this domain, are not required for faithful segregation in otherwise normal cells.
Unexpectedly, however, the swi6∆ dfp1-3A double mutant has an increase of lagging chromosomes over that observed in swi6∆ single mutants. This suggests that Dfp1-3A disrupts some DDK activity that is required for proper chromosome segregation when Swi6 is missing. We asked whether Chp2, another HP1 homolog with a CSD, might provide an alternative recruitment platform. We observed that the chp2∆ mutant has the same frequency of lagging chromosomes as wild-type, while the phenotype of the chp2∆ swi6∆ double mutants resembles that of swi6∆ single mutants. This epistasis suggests that Swi6 plays the major role in promoting normal chromosome segregation while the contribution of Chp2 is negligible. However, chp2∆ dfp1-3A has an increased rate of lagging chromosomes relative to the single-mutant parents, which suggests that DDK and Chp2 make overlapping contributions. That these pathways are partly independent is further suggested because the triple mutant swi6∆ chp2∆ dfp1-3A is even more affected than single or double mutants. We conclude from this that Swi6 has a significant role in preventing lagging chromosomes, independent of Chp2 and DDK. In the absence of Swi6, both Chp2 and DDK become important to prevent lagging chromosomes in redundant pathways.
Cohesion is another key contributor to normal chromosome segregation and there is previous evidence that Swi6 mediates the phosphorylation of cohesin subunits by DDK (Bailis et al. 2003). Swi6 is also known to enrich cohesion in the centromere (Nonaka et al. 2002). We showed that Rad21-GFP enrichment at the dh locus depends upon Swi6, but is independent of Dfp1 recruitment. Importantly, the dfp1-CFP-2CD allele that restores early replication timing and independently recruits DDK to the pericentromere does not restore Rad21 enrichment (Figure 5). Nevertheless, even in the absence of this enrichment, the swi6∆ dfp1-CFP-2CD double mutant displays a significantly lower frequency of lagging chromosomes compared to a swi6∆ single mutant (Figure 3). Conversely, in the dfp1-3A strain where DDK cannot be recruited by Swi6, there are no defects in the enrichment of cohesion despite delayed replication. We conclude that: first, replication timing is not associated with cohesin enrichment, and second, that cohesion enrichment is not required for normal chromosome segregation (because swi6∆ dfp1-CFP-2CD rescues the chromosome segregation defect without restoring enrichment). Together, these observations suggest that cohesin enrichment and DDK recruitment play overlapping but nonidentical roles in promoting faithful chromosome segregation. Previous experiments showed that a C-terminal deletion mutant dfp1-(1-376) does reduce cohesion enrichment, but this also likely disrupts multiple interactions with other proteins and has numerous additional phenotypes (Fung et al. 2002; Bailis et al. 2003; Dolan et al. 2010). Importantly, this does not address cohesion activation.
Although dfp1-3A does not significantly affect chromosome segregation, we found that this mutant does cause a dramatic increase in the rate of minichromosome rearrangement, a phenotype previously shown to be associated with replication defects in heterochromatin mutants (Li et al. 2013b). It is possible that dfp1-3A does cause some replication defects that may account for the observed increased rearrangements, although the cells grow normally and are not HU-sensitive. Additionally, the rearrangement frequency in swi6∆ dfp1-3A resembles that of swi6∆, which suggests that the dfp1-3A allele is not causing additional replication stress [since replication stress has a synthetic phenotype in chromosome rearrangement with swi6∆ (Li et al. 2013b)]. Finally, cohesin enrichment does not prevent rearrangements (because dfp1-3A has dramatic rearrangements despite the presence of cohesion enrichment). Together, these observations separate the mechanisms associated with chromosome segregation defects (numerical instability) from that associated with gross chromosome rearrangements (structural instability)
Intriguingly, during the course of this study we also determined that visible recruitment of the CD protein Chp1 to noncentromere heterochromatin regions, including the telomeres, depends upon the presence of both Swi6 and Chp2 (Figure 6, C–F). This contrasts with the ability of Chp1 to bind the pericentromere in the absence of Swi6. H3K9me at the centromere is strongly reduced in chp1∆ mutants, while swi6∆ has a more modest effect (Sadaie et al. 2004). Loss of chp1∆ has little effect on H3K9me in the telomeres and there is detectable methylation even in a triple mutant (Sadaie et al. 2004). Consequently, recruitment of Swi6 and Chp2 to the telomeres appears to be interdependent (Sadaie et al. 2008). This underscores distinct differences in the mechanisms that establish and maintain heterochromatin at different genome domains.
Does DNA replication timing account for these phenotypes? Both swi6∆ and dfp1-3A strains cause late replication in the pericentromere, which normally occurs early in S phase (Kim et al. 2003; Hayashi et al. 2009; Li et al. 2013a), and both mutants show substantial DNA rearrangements in this domain [Figure 7 and Li et al. (2013b)]. However, we previously showed that chp1∆ and clr4∆ mutants also undergo early replication timing in the pericentromere, but still suffer increased genomic rearrangements, lagging chromosomes, and segregation defects (Li et al. 2013a,b). Therefore, early replication timing per se does not prevent rearrangements. We note that Swi6 localization to the centromere (and by inference, cohesion enrichment) is abolished in chp1∆. Although we do not see obvious evidence for replication abnormalities or stress in the dfp1-3A mutant, we cannot exclude the possibility that this allele causes a low level of stress that triggers additional mitotic defects.
We propose that the recruitment of DDK to the centromere contributes to cohesin activation that is Swi6-dependent and independently contributes to kinetochore activity. Evidence in other systems identifies additional possible targets. In budding yeast, the kinetochore component Ctf19 recruits DDK (Natsume et al. 2013; Hinshaw et al. 2017). The fission yeast equivalent is Fta2, which is an essential kinetochore factor (Kerres et al. 2006). The FPC proteins Swi1 and Swi3 are also associated with centromere cohesion (Fernius et al. 2013) and bind DDK (Shimmoto et al. 2009). An additional candidate important for chromosome stability is Top2, which in humans is a DDK substrate. A recent paper suggests that centromere-associated DDK limits Top2 activity in this region (Wu et al. 2016). It is possible that this contributes to the rearrangements that we observe.
Recent studies suggest that there are mechanisms in mitosis that can rescue replication stress (Fragkos and Naim 2017). This is consistent with our observations suggesting that the proper coupling of S and M phase is necessary to reduce genome instability. Our work clearly separates pathways involved in reducing chromosome rearrangement in the fragile pericentromere from mechanisms that prevent lagging chromosomes, cohesin recruitment, and kinetochore activity. This study shows that the versatile DDK kinase influences multiple activities associated with chromosome dynamics across the cell cycle, beyond its interaction with the heterochromatin or the replication origin.
Acknowledgments
We thank Seong Min Kim, Vishnu Tripathi, and Wilber Escorcia for helpful comments on the manuscript, Hisao Masukata and Yoshinori Watanabe for strains, and Ji-Ping Yuan for technical assistance. This research was supported by the National Institutes of Health (grant R35 GM-118109 to S.L.F.).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8009408.
Communicating editor: D. Bishop
Literature Cited
- Aasland R., Stewart A. F., 1995. The chromo shadow domain, a second chromo domain in heterochromatin-binding protein 1, HP1. Nucleic Acids Res. 23: 3168–3173. 10.1093/nar/23.16.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire R. C., Nimmo E. R., Ekwall K., Javerzat J. P., Cranston G., 1995. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 9: 218–233. 10.1101/gad.9.2.218 [DOI] [PubMed] [Google Scholar]
- Alper B. J., Lowe B. R., Partridge J. F., 2012. Centromeric heterochromatin assembly in fission yeast–balancing transcription, RNA interference and chromatin modification. Chromosome Res. 20: 521–534. 10.1007/s10577-012-9288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis J. M., Bernard P., Antonelli R., Allshire R. C., Forsburg S. L., 2003. Hsk1-Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat. Cell Biol. 5: 1111–1116. 10.1038/ncb1069 [DOI] [PubMed] [Google Scholar]
- Baker S. P., Phillips J., Anderson S., Qiu Q., Shabanowitz J., et al. , 2010. Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae. Nat. Cell Biol. 12: 294–298. 10.1038/ncb2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister A. J., Zegerman P., Partridge J. F., Miska E. A., Thomas J. O., et al. , 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124. 10.1038/35065138 [DOI] [PubMed] [Google Scholar]
- Bernard P., Allshire R., 2002. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 12: 419–424. 10.1016/S0962-8924(02)02344-9 [DOI] [PubMed] [Google Scholar]
- Bernard P., Maure J. F., Partridge J. F., Genier S., Javerzat J. P., et al. , 2001. Requirement of heterochromatin for cohesion at centromeres. Science 294: 2539–2542. 10.1126/science.1064027 [DOI] [PubMed] [Google Scholar]
- Brasher S. V., Smith B. O., Fogh R. H., Nietlispach D., Thiru A., et al. , 2000. The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 19: 1587–1597. 10.1093/emboj/19.7.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. S., Zhang K., Nicolas E., Cam H. P., Zofall M., et al. , 2008. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451: 734–737. 10.1038/nature06561 [DOI] [PubMed] [Google Scholar]
- Choi S. H., McCollum D., 2012. A role for metaphase spindle elongation forces in correction of merotelic kinetochore attachments. Curr. Biol. 22: 225–230. 10.1016/j.cub.2011.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan W. P., Le A. H., Schmidt H., Yuan J. P., Green M., et al. , 2010. Fission yeast Hsk1 (Cdc7) kinase is required after replication initiation for induced mutagenesis and proper response to DNA alkylation damage. Genetics 185: 39–53. 10.1534/genetics.109.112284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont S., Mitchison T. J., 2009. Force and length in the mitotic spindle. Curr. Biol. 19: R749–R761. 10.1016/j.cub.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Elgin S. C., 2000. The HP1 protein family: getting a grip on chromatin. Curr. Opin. Genet. Dev. 10: 204–210. 10.1016/S0959-437X(00)00058-7 [DOI] [PubMed] [Google Scholar]
- Ekwall K., Javerzat J. P., Lorentz A., Schmidt H., Cranston G., et al. , 1995. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269: 1429–1431. 10.1126/science.7660126 [DOI] [PubMed] [Google Scholar]
- Fang G., Yu H., Kirschner M. W., 1998. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12: 1871–1883. 10.1101/gad.12.12.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernius J., Nerusheva O. O., Galander S., Alves Fde L., Rappsilber J., et al. , 2013. Cohesin-dependent association of scc2/4 with the centromere initiates pericentromeric cohesion establish Curr. Biol. 23: 599–606. 10.1016/j.cub.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., 2013. The CINs of the centromere. Biochem. Soc. Trans. 41: 1706–1711. 10.1042/BST20130146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Rhind N., 2006. Basic methods for fission yeast. Yeast 23: 173–183. 10.1002/yea.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Shen K. F., 2017. Centromere stability: the replication connection. Genes (Basel) 8: 37, . 10.3390/genes8010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkos M., Naim V., 2017. Rescue from replication stress during mitosis. Cell Cycle 16: 613–633. 10.1080/15384101.2017.1288322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Cook L. L., Gomez E. B., Spedale E. J., Marlett J., Forsburg S. L., et al. , 2005. Conserved locus-specific silencing functions of Schizosaccharomyces pombe sir2+. Genetics 169: 1243–1260. 10.1534/genetics.104.032714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung A. D., Ou J., Bueler S., Brown G. W., 2002. A conserved domain of Schizosaccharomyces pombe dfp1(+) is uniquely required for chromosome stability following alkylation damage during S phase. Mol. Cell. Biol. 22: 4477–4490. 10.1128/MCB.22.13.4477-4490.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E. B., Espinosa J. M., Forsburg S. L., 2005. Schizosaccharomyces pombe mst2+ encodes a MYST family histone acetyltransferase that negatively regulates telomere silencing. Mol. Cell. Biol. 25: 8887–8903. 10.1128/MCB.25.20.8887-8903.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. D., Sabatinos S. A., Forsburg S. L., 2015. Microscopy techniques to examine DNA replication in fission yeast. Methods Mol. Biol. 1300: 13–41. 10.1007/978-1-4939-2596-4_2 [DOI] [PubMed] [Google Scholar]
- Gregan J., Polakova S., Zhang L., Tolic-Norrelykke I. M., Cimini D., 2011. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 21: 374–381. 10.1016/j.tcb.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson D., Gutkin G., Clarke L., 2000. A novel member of the Swi6p family of fission yeast chromo domain-containing proteins associates with the centromere in vivo and affects chromosome segregation. Mol. Gen. Genet. 264: 492–505. 10.1007/s004380000338 [DOI] [PubMed] [Google Scholar]
- Hayashi M. T., Takahashi T. S., Nakagawa T., Nakayama J., Masukata H., 2009. The heterochromatin protein Swi6/HP1 activates replication origins at the pericentromeric region and silent mating-type locus. Nat. Cell Biol. 11: 357–362. 10.1038/ncb1845 [DOI] [PubMed] [Google Scholar]
- Hentges P., Van Driessche B., Tafforeau L., Vandenhaute J., Carr A. M., 2005. Three novel antibiotic marker cassettes for gene disruption and marker switching in Schizosaccharomyces pombe. Yeast 22: 1013–1019. 10.1002/yea.1291 [DOI] [PubMed] [Google Scholar]
- Hinshaw S. M., Makrantoni V., Harrison S. C., Marston A. L., 2017. The kinetochore receptor for the cohesin loading complex. Cell 171: 72–84.e13. 10.1016/j.cell.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R. S., Sanulli S., Tibble R., Hornsby M., Ravalin M., et al. , 2017. Biochemical basis for distinct roles of the heterochromatin proteins Swi6 and Chp2. J. Mol. Biol. 429: 3666–3677. 10.1016/j.jmb.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A., van der Burg M., Szuhai K., Kops G. J., Medema R. H., 2011. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333: 1895–1898. 10.1126/science.1210214 [DOI] [PubMed] [Google Scholar]
- Kenney R. D., Heald R., 2006. Essential roles for cohesin in kinetochore and spindle function in Xenopus egg extracts. J. Cell Sci. 119: 5057–5066. 10.1242/jcs.03277 [DOI] [PubMed] [Google Scholar]
- Kerres A., Jakopec V., Beuter C., Karig I., Pohlmann J., et al. , 2006. Fta2, an essential fission yeast kinetochore component, interacts closely with the conserved Mal2 protein. Mol. Biol. Cell 17: 4167–4178. 10.1091/mbc.e06-04-0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang L., Heichinger C., Watt S., Bahler J., Nurse P., 2009. Cyclin-dependent kinase inhibits reinitiation of a normal S-phase program during G2 in fission yeast. Mol. Cell. Biol. 29: 4025–4032. 10.1128/MCB.00185-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. M., Dubey D. D., Huberman J. A., 2003. Early-replicating heterochromatin. Genes Dev. 17: 330–335. 10.1101/gad.1046203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc A., Zaratiegui M., Nora E., Martienssen R., 2008. RNA interference guides histone modification during the S phase of chromosomal replication. Curr. Biol. 18: 490–495. 10.1016/j.cub.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Gonzalez P., Westhorpe F. G., Taylor S. S., 2012. The spindle assembly checkpoint. Curr. Biol. 22: R966–R980. 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Larasati, Duncker B. P., 2016. Mechanisms governing DDK regulation of the initiation of DNA replication. Genes (Basel) 8: E3 10.3390/genes8010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A. H., Mastro T. L., Forsburg S. L., 2013. The C-terminus of S. pombe DDK subunit Dfp1 is required for meiosis-specific transcription and cohesin cleavage. Biol. Open 2: 728–738. 10.1242/bio.20135173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune E., Bayne E. H., Allshire R. C., 2010. On the connection between RNAi and heterochromatin at centromeres. Cold Spring Harb. Symp. Quant. Biol. 75: 275–283. 10.1101/sqb.2010.75.024 [DOI] [PubMed] [Google Scholar]
- Li P. C., Chretien L., Cote J., Kelly T. J., Forsburg S. L., 2011. S. pombe replication protein Cdc18 (Cdc6) interacts with Swi6 (HP1) heterochromatin protein: region specific effects and replication timing in the centromere. Cell Cycle 10: 323–336. 10.4161/cc.10.2.14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. C., Green M. D., Forsburg S. L., 2013a Mutations disrupting histone methylation have different effects on replication timing in S. pombe centromere. PLoS One 8: e61464 (erratum: PLoS One 8). 10.1371/journal.pone.0061464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. C., Petreaca R. C., Jensen A., Yuan J. P., Green M. D., et al. , 2013b Replication fork stability is essential for the maintenance of centromere integrity in the absence of heterochromatin. Cell Rep. 3: 638–645. 10.1016/j.celrep.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. L., 2009. Meiosis: DDK is not just for replication. Curr. Biol. 19: R74–R76. 10.1016/j.cub.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi M. R., Hong E. J., Li X., Gerber S., Denison C., et al. , 2008. HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol. Cell 32: 778–790. 10.1016/j.molcel.2008.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D., 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8: 379–393. 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Natsume T., Muller C. A., Katou Y., Retkute R., Gierlinski M., et al. , 2013. Kinetochores coordinate pericentromeric cohesion and early DNA replication by Cdc7-Dbf4 kinase recruitment. Mol. Cell 50: 661–674. 10.1016/j.molcel.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka N., Kitajima T., Yokobayashi S., Xiao G., Yamamoto M., et al. , 2002. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 4: 89–93. 10.1038/ncb739 [DOI] [PubMed] [Google Scholar]
- Paro R., 1990. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 6: 416–421. 10.1016/0168-9525(90)90303-N [DOI] [PubMed] [Google Scholar]
- Paro R., Hogness D. S., 1991. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc. Natl. Acad. Sci. USA 88: 263–267. 10.1073/pnas.88.1.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge J. F., Scott K. S., Bannister A. J., Kouzarides T., Allshire R. C., 2002. cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr. Biol. 12: 1652–1660. 10.1016/S0960-9822(02)01177-6 [DOI] [PubMed] [Google Scholar]
- Petersen J., Hagan I. M., 2003. S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 13: 590–597. 10.1016/S0960-9822(03)00205-7 [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Biggins S., 2005. The spindle checkpoint: tension vs. attachment. Trends Cell Biol. 15: 486–493. 10.1016/j.tcb.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Rougemaille M., Shankar S., Braun S., Rowley M., Madhani H. D., 2008. Ers1, a rapidly diverging protein essential for RNA interference-dependent heterochromatic silencing in Schizosaccharomyces pombe. J. Biol. Chem. 283: 25770–25773. 10.1074/jbc.C800140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinos S. A., Forsburg S. L., 2010. Molecular genetics of Schizosaccharomyces pombe. Methods Enzymol. 470: 759–795. 10.1016/S0076-6879(10)70032-X [DOI] [PubMed] [Google Scholar]
- Sadaie M., Iida T., Urano T., Nakayama J., 2004. A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J. 23: 3825–3835. 10.1038/sj.emboj.7600401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie M., Kawaguchi R., Ohtani Y., Arisaka F., Tanaka K., et al. , 2008. Balance between distinct HP1 family proteins controls heterochromatin assembly in fission yeast. Mol. Cell. Biol. 28: 6973–6988. 10.1128/MCB.00791-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmoto M., Matsumoto S., Odagiri Y., Noguchi E., Russell P., et al. , 2009. Interactions between Swi1-Swi3, Mrc1 and S phase kinase, Hsk1 may regulate cellular responses to stalled replication forks in fission yeast. Genes Cells 14: 669–682. 10.1111/j.1365-2443.2009.01300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers J. F., Henikoff S., 2000. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 10: 27–30. 10.1016/S0960-9822(99)00260-2 [DOI] [PubMed] [Google Scholar]
- Snaith H. A., Brown G. W., Forsburg S. L., 2000. Schizosaccharomyces pombe Hsk1p is a potential cds1p target required for genome integrity. Mol. Cell. Biol. 20: 7922–7932. 10.1128/MCB.20.21.7922-7932.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. S., Basu A., Bermudez V., Hurwitz J., Walter J. C., 2008. Cdc7-Drf1 kinase links chromosome cohesion to the initiation of DNA replication in Xenopus egg extracts. Genes Dev. 22: 1894–1905. 10.1101/gad.1683308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. U., 2002. Bi-orienting chromosomes on the mitotic spindle. Curr. Opin. Cell Biol. 14: 365–371. 10.1016/S0955-0674(02)00328-9 [DOI] [PubMed] [Google Scholar]
- Tanaka T. U., Clayton L., Natsume T., 2013. Three wise centromere functions: see no error, hear no break, speak no delay. EMBO Rep. 14: 1073–1083. 10.1038/embor.2013.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G., Verhein-Hansen J., 2000. Four chromo-domain proteins of Schizosaccharomyces pombe differentially repress transcription at various chromosomal locations. Genetics 155: 551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga T., Nagao K., Kawasaki Y., Furuya K., Murakami A., et al. , 2000. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 14: 2757–2770. 10.1101/gad.832000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P. T., Paoletti A., Chang F., 2004. Imaging green fluorescent protein fusions in living fission yeast cells. Methods 33: 220–225. 10.1016/j.ymeth.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Wu K. Z., Wang G. N., Fitzgerald J., Quachthithu H., Rainey M. D., et al. , 2016. DDK dependent regulation of TOP2A at centromeres revealed by a chemical genetics approach. Nucleic Acids Res. 44: 8786–8798. 10.1093/nar/gkw626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y., Sakuno T., Shimura M., Watanabe Y., 2008. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455: 251–255 [corrigenda: Nature 563: E21 (2018)]. 10.1038/nature07217 [DOI] [PubMed] [Google Scholar]
- Yang L., Ukil L., Osmani A., Nahm F., Davies J., et al. , 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 3: 1359–1362. 10.1128/EC.3.5.1359-1362.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Kanchwala M., Xing C., Yu H., 2018. MCM2-7-dependent cohesin loading during S phase promotes sister-chromatid cohesion. Elife 7: e33920 10.7554/eLife.33920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains are available upon request. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8009408.