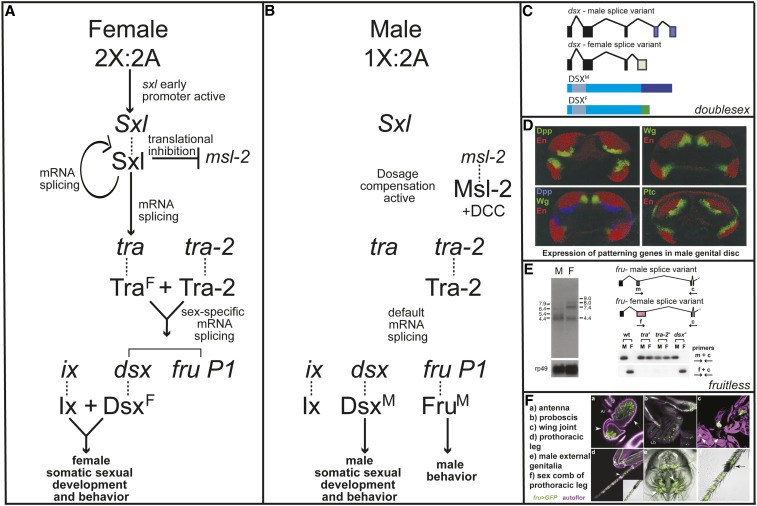
Figure 1.
Sex and the single fly: the somatic sex determination hierarchy. Bruce Baker and his laboratory, as well colleagues in the field, made many key discoveries about the genetic pathway that directs somatic sexual development. (A) Females with two X chromosomes produce Sxl, a pre-mRNA splicing factor. Sxl regulates the splicing of its own pre-mRNA and the tra pre-mRNA, resulting in the production of Sxl and TraF. TraF and Tra-2 regulate the pre-mRNA splicing of doublesex (dsx) and fruitless (fru; the product of the P1 promoter), resulting in the production of female-specific (DsxF) that binds Ix; the Dsx/Ix complex regulates transcription in females. Sxl also inhibits translation of msl-2, so dosage compensation is not active in females. (B) Males with one X chromosome do not produce Sxl. dsx and fru pre-mRNAs are spliced by the default pathway, resulting in the production of male-specific transcription factors DsxM and FruM. In males, DsxM does not require ix to direct male development. Dosage compensation is active in males, resulting in upregulation of genes that reside on the X chromosome in males, due to translation of msl-2. Msl-2 acts with a set of protein and noncoding RNAs (dosage compensation complex; DCC) to mediate upregulation of gene expression on the male X chromosome. In both males and females, Dsx directs somatic sex-specific development and aspects of behaviors. In males, FruM is required to specify the potential for male reproductive behaviors. (C) Alternative pre-mRNA splicing of dsx transcripts produces sex-specific transcription factors that share a common N-terminal region, containing the DNA-binding domain, but differ in the C-termini. This image is based on the original discovery (Burtis and Baker 1989). (D) The somatic sex determination hierarchy and conserved developmental patterning genes coordinate to bring about sex differences in genital morphology [reproduced from Chen and Baker (1997)]. Expression of patterning genes in the male genital disc is shown. Top is Engrailed (En) antibody staining (red), and decapentaplegic (dpp)-lacZ or wingless (wg)-LacZ reporter expression (green). Bottom left is En (red) and Wg (green) antibody staining, and dpp-LacZ reporter expression (blue). Bottom right is En antibody staining (red) and patched (ptc)-LacZ reporter expression (green). lacZ expression was detected with an anti-β-galactosidase antibody. (E) fruitless produces sex-specific transcripts due to sex hierarchy-regulated alternative pre-mRNA splicing. On the left, an RNA blot, with polyA(+) RNA from adult male and female heads; sex-specific transcripts are detected using a probe made from the ‘Broad-complex, Tramtrack, Bric-a-brac’ (BTB) domain coding region of a fru cDNA. The region of alternative pre-mRNA splicing of fru P1 transcripts is shown at the top right; splicing in females occurs downstream of where splicing occurs in males, due to sex hierarchy regulation. Primers used in RT-PCR are shown. Sex-specific splicing is downstream of tra and tra-2, but not dsx, as detected by Southern blot analysis of RT-PCR products [reproduced from Ryner et al. (1996)]. (F) fruitless is expressed in both the peripheral and central nervous system, in neurons important for reproductive behaviors in males and females [reproduced from Manoli et al. (2005)]. fruM-Gal4 drives expression of membrane-GFP (green) and autofluorescence (magenta). (a–f) Expression of fruM in olfactory neurons in the antenna (a), in gustatory neurons of the proboscis (b), in proprioceptive neurons in the wing joint (c), in gustatory and mechanosensory neurons of the prothoracic leg (d), in neurons of the male external genitalia (e), and in gustatory and mechanosensory neurons at base of male sex comb (f).