Abstract
Recent research has demonstrated that survivors of childhood cancer are at risk for a myriad of late effects that affect physical and mental quality of life. We discuss the patterns and prevalence of neurocognitive problems commonly experienced by survivors of CNS tumors and acute lymphoblastic leukemia, the two most commonly researched cancer diagnoses. Research documenting the direct effects of tumor location and treatment type and intensity is presented, and patient characteristics that moderate outcomes (eg, age at diagnosis and sex) are discussed. Potential biologic mechanisms of neurotoxic treatment exposures, such as cranial irradiation and intrathecal and high-dose antimetabolite chemotherapy, are reviewed. Genetic, brain imaging, and neurochemical biomarkers of neurocognitive impairment are discussed. Long-term survivors of childhood cancer are also at risk for physical morbidity (eg, cardiac, pulmonary, endocrine) and problems with health behaviors (eg, sleep); research is reviewed that demonstrates these health problems contribute to neurocognitive impairment in survivors with or without exposure to neurotoxic therapies. We conclude this review with a discussion of literature supporting specific interventions that may be beneficial in the treatment of survivors who already experience neurocognitive impairment, as well as in the prevention of impairment manifestation.
INTRODUCTION
Long-term survivors of childhood cancer are at increased risk for neurocognitive problems, which seem related to direct effects of cancer and cancer therapy and are moderated by patient demographic and medical factors. Children who develop neurocognitive problems after diagnosis and treatment experience impact on long-term development, including attainment of major societal goals (eg, education, employment, functional independence). This manuscript presents a review of recent literature on the prevalence and pattern of neurocognitive deficits, cancer and treatment factors associated with risk of deficits, brain imaging and neurochemical biomarkers of deficits, medical complications and genetic predispositions that moderate deficits, and treatment options to facilitate recovery and/or prevent emergence of deficits.
EPIDEMIOLOGY
Prevalence and Patterns of Neurocognitive Deficits
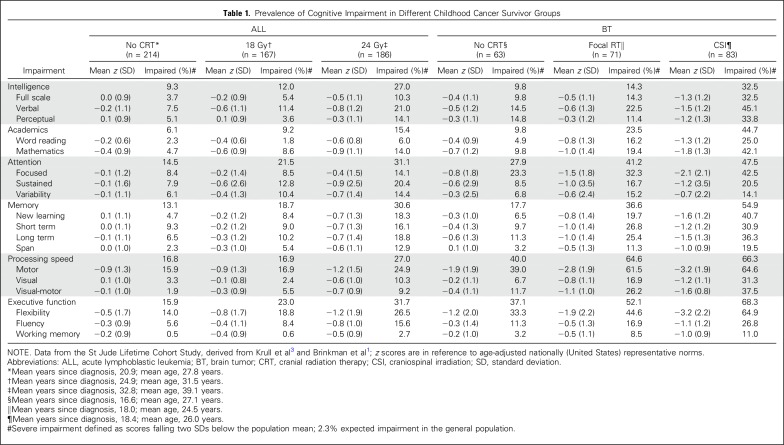
Neurocognitive impairment in long-term survivors is determined by type and intensity of treatment. Treatment of the CNS is performed to affect the tumor directly or prevent relapse. Survivors of CNS tumors are at greatest risk for neurocognitive impairment (Table 1). Impaired intelligence, processing speed, and executive function are most salient, followed by deficits in memory and attention.1 Younger age at diagnosis, higher cranial irradiation dose, larger brain volume irradiated, and longer time since treatment are risk factors for worse neurocognitive outcomes. Perioperative complications, hydrocephalus, and vasculopathy increase impairment risk.2
Table 1.
Prevalence of Cognitive Impairment in Different Childhood Cancer Survivor Groups
Acute lymphoblastic leukemia (ALL) was historically treated with CNS prophylaxis, resulting in neurocognitive impairment, dependent on dose of cranial radiation therapy (CRT; Table 1). Elevated rates of severe impairment are reported in intelligence, attention, memory, processing speed, and executive function after chemotherapy-only treatment.3,4 Dose-response patterns are demonstrated or intravenous and intrathecal methotrexate and for dexamethasone.3,4 Dose response is demonstrated for CRT, although impact can be exacerbated by younger age at diagnosis, female sex, and longer time since diagnosis.3,5-7
Progression of Impairment Over Time
Neurocognitive dysfunction progresses with time since CRT.3,6,8 Brain imaging demonstrates decline in white matter integrity with increasing age after CRT, a decline not present in same-age controls or chemotherapy-treated survivors.6 Global slowing of brain activity has been demonstrated in survivors, a pattern that characterizes old age and neurodegenerative disease.9 This similarity may suggest accelerated aging, which could increase risk of early-onset dementia.6,9 No clear indications of accelerated aging after chemotherapy have been reported.6
The effects of therapeutic radiation can be detected for at least 50 years after exposure,10 indicating the possibility for persistent impact on proliferating oligodendrocytes (myelin) and/or progenitor cells (precursors of other cell types).11 Telomere shortening occurs with normal aging but seems accelerated by radiation therapy.12-14 Proliferation of neural precursor cells is highest shortly after birth and declines with age.15 This may explain why CRT at younger ages is associated with worse outcomes. Inhibited neurogenesis may limit restorative capacity of the brain for life.11
BIOLOGY
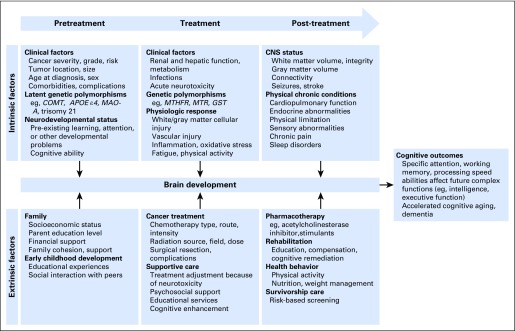
Increased focus on neurocognitive outcomes has resulted in identification of important disease and treatment risk factors. The survivor’s neurocognitive trajectory is determined by multiple direct and indirect disease- and treatment-related effects (Fig 1).
Fig 1.
Model of biobehavioral impact of cancer and cancer therapy on brain development and neurocognitive outcomes in long-term survivors of childhood cancer.
Direct Cancer and Treatment Effects
CNS tumor diagnosis alone increases risk for neurocognitive impairment.16 Before start of treatment, 20% to 50% of patients exhibit cognitive impairment.17 Treatment of brain tumors with surgery alone is associated with neurocognitive impairment,18-21 including severe impairment in intelligence (9.8%), academics (9.8%), attention (27.9%), memory (17.7%), processing speed (40.0%), and executive function (37.1%), with impairment influenced by tumor location and surgical complications (Table 1).20-24 Larger tumor size22 and infratentorial tumor location are associated with worse neurocognitive outcomes.17 The extent of risk attributable to tumor location versus treatment type or intensity is unclear. Risk increases with brain tumors that affect critical brain structures; for example, craniopharyngioma tumors are histologically benign but frequently involve critical structures (eg, hypothalamic-pituitary-adrenal axis, cranial nerves, circle of Willis) that complicate surgical resection and are unavoidable in radiation therapy planning.23 Surgical complications (eg, hemorrhage and vascular injury) can increase risk for neurocognitive impairment.24
Larger CRT fields are associated with greater neurocognitive impairment, with whole-brain CRT carrying greatest risk.25-28 Many survivors treated with whole-brain CRT exhibit severe impairment in memory (54.9%), processing speed (66.3%), and executive function (68.3%; Table 1). Better outcomes are observed in patients receiving reduced-dose (23.4 to 25.0 Gy) compared with high-dose CRT (35 to 36 Gy), although any whole-brain CRT seems to affect neurocognitive development.25 Reductions in boost dose volumes to the tumor bed have resulted in improved neurocognitive outcomes.26 Reducing dose to sensitive brain regions (including temporal lobes and hippocampi) have demonstrated better neurocognitive outcomes in medulloblastoma survivors.27 Younger age at CRT is a risk factor for neurocognitive impairment,25,28-31 even at lower CRT doses.27
Advanced CRT techniques (ie, intensity-modulated CRT, particle therapy) have improved precision of dose delivery, resulting in clinically significant reductions in dose to healthy tissue. Proton CRT minimizes dose to healthy tissue32 and is expected to provide similar disease control while yielding better neurocognitive outcomes; however, outcome studies are just emerging. A retrospective comparison found no significant intelligence quotient (IQ) decline or impairment in survivors treated with proton CRT, but significant IQ decline was seen in survivors treated with photon CRT.33 In pediatric medulloblastoma survivors, IQ decline was observed only in survivors younger than age 8 years after proton CRT.34 No evidence of clinically significant cognitive impairment in attention, processing speed, or executive functioning among survivors who received focal proton CRT has been reported, although whole-brain exposure was associated with impaired processing speed.35
The transition from CRT prophylaxis to treatment with chemotherapy only has reduced severity of neurocognitive impairments in ALL survivors.6,36-39 Nevertheless, ALL survivors treated with chemotherapy only demonstrate worse neurocognitive function compared with population norms36,40 and healthy controls.39,41-43 ALL survivors treated with chemotherapy only experience severe impairment in intelligence (9.3%), attention (14.5%), memory (13.1%), processing speed (16.8%), and executive function (15.9%; Table 1).
Higher-intensity chemotherapy (eg, intravenous and/or intrathecal methotrexate) is associated with greater neurocognitive impairment.44 Comparisons of triple intrathecal chemotherapy (ie, methotrexate, cytarabine, and hydrocortisone) with single intrathecal methotrexate have shown comparable neurocognitive outcomes. Younger age at diagnosis (< 5 years) has been associated with 15% higher frequency of attention problems, and female sex has been associated with 10% higher frequency of executive dysfunction.42 Associations between dexamethasone and worse outcomes in memory, attention, executive functioning, and academic domains have been reported among adult survivors of pediatric ALL,3,45 although risk may be dependent on intensity of corticosteroid administered.46
Indirect Sources Neurocognitive Impairment
Survivors of CNS tumors are at risk for neurologic complications that influence neurocognitive outcomes. Hydrocephalus and shunt placement and revisions are associated with neurocognitive impairment, including lower intelligence, nonverbal reasoning, visual-motor integration, memory, and academic skills.30,47-50 CNS tumors and CRT are associated with increased risk for cerebrovascular complications, including stroke, caver-nomas, and cerebral microbleeds, which can further complicate neurocognitive development.51 Seizures are experienced by pediatric patients with brain tumors,52-55 particularly those with supratentorial tumors, and are associated with neurocognitive impairment.56 Uncontrolled seizures and use of antiseizure medications increase risk of neurocognitive impairment in the general population57 and may do so in cancer survivors as well.56
Childhood cancer survivors are at risk for morbidity in non-CNS systems. Long-term survivors of childhood Hodgkin lymphoma who are not exposed to neurotoxic therapies display increased frequency of neurocognitive impairment as a result of cardiopulmonary morbidity.58 In survivors of osteosarcoma and non-Hodgkin lymphoma who receive neurotoxic chemotherapies, neurocognitive impairment is associated with cardiac, pulmonary, and endocrine morbidity.59,60 Endocrine and pulmonary morbidity contribute to neurocognitive impairment, aside from CRT and neurotoxic chemotherapies.61
Compared with sibling controls, long-term survivors of childhood cancer are at increased risk for sleep disturbance and fatigue, particularly those diagnosed with Hodgkin lymphoma.62,63 After adjusting for neurotoxic therapies, risk of self-reported neurocognitive impairment is increased by 23% to 45% in survivors with sleep problems and 34% to 77% in survivors with clinically relevant fatigue.64 Sleep disturbance is also associated with lower cognitive flexibility and fluency in adolescent survivors of ALL.65,66
Although chronic health conditions in survivors are likely to emerge during adulthood, physiologic processes affecting brain function may begin much earlier. Low dehydroepiandrosterone sulfate, a marker of adrenal gland dysfunction, is associated with attention problems in long-term adolescent survivors of ALL.67 Elevated inflammatory serum biomarkers, which affect adrenal function,64 are associated with neurocognitive problems in these adolescents.66 Uric acid elevations are associated with increased inflammation.68,69 Elevations in uric acid in adolescent survivors are associated with cardiovascular morbidity as those survivors age, which in turn is associated with neurocognitive impairment.70
BIOMARKERS
Brain imaging, neurochemistry, and genetic polymorphisms have been examined as biomarkers of neurocognitive impairment in cancer survivors. These biomarkers have informed mechanisms and/or risk of impairment, although none are currently able to classify individuals at high or low risk.
Brain Imaging
Quantitative brain imaging includes measures of gray matter volume, white matter integrity, cerebral metabolism, neurochemistry, and functional activation. White matter pathways form structural scaffolding underlying functional networks and are essential for connectivity and integration of distributed information processing.71 Diffusion tensor imaging is a magnetic resonance imaging (MRI) sequence that assesses axonal organization from diffusion of water molecules along white matter tracts. Fractional anisotropy indicates diffusion preference, with lower values suggesting lower white matter integrity.72 Mean, axial, and radial diffusivities measure diffusion along different axes, with higher values indicating lower white matter integrity.72 Abnormalities of white matter can reflect changes in axon diameter, packing, myelin integrity, astrocytes, and vasculature, among others. Information processing occurs in gray matter regions, which can be measured from volumetric assessment of T1-weighted MRIs. The function of gray matter regions can be measured using functional MRI (fMRI), which measures the hemodynamic response to neural activity, or using positron emission tomography, which uses radiotracers that elucidate the cerebral metabolic rate of glucose.
Decades after treatment, childhood cancer survivors show smaller white matter volumes in distributed brain regions.73,74 Compared with noncancer controls, childhood cancer survivors show lower fractional anisotropy,6,75-82 although long-term adult survivors display higher fractional anisotropy, potentially because of glial scarring and/or white matter compaction.36 White matter damage is widespread, affecting frontal-striatal, frontal-occipital, periventricular, cerebellar, parietal, and temporal regions, and is detected decades after treatment. White matter integrity has been shown to be lowest in patients who received adjuvant therapy compared with surgery alone and those who received cranial irradiation6 or had higher methotrexate exposure.44
Gray matter abnormalities associated with childhood cancer include lower volumes of cortical surface area with thicker prefrontal cortex.74,44 Childhood cancer survivors demonstrate higher fMRI activation in prefrontal areas during memory and attention tasks compared with healthy controls.83 Higher frontal lobe fMRI activity and thicker prefrontal cortices are associated with higher methotrexate exposure,44 although higher dexamethasone exposure is associated with lower activation in retrosplenial regions.45 Atypically higher fMRI activation may reflect engagement of additional neural systems as a result of insufficient local processing capacity secondary to gray matter atrophy. Although higher activation suggests a compensatory adjustment, it also indicates increased burden on metabolic resources. Decreased white matter integrity may disrupt healthy constraint of functional network dynamics, resulting in higher than normal activation. Positron emission tomography studies have demonstrated lower glucose metabolism in cancer survivors.84 Some studies have shown a greater negative effect of CRT on cerebral metabolism compared with chemotherapy alone,80 and one group demonstrated higher metabolism in survivors treated with 24-Gy CRT compared with those treated with lower CRT dose.85
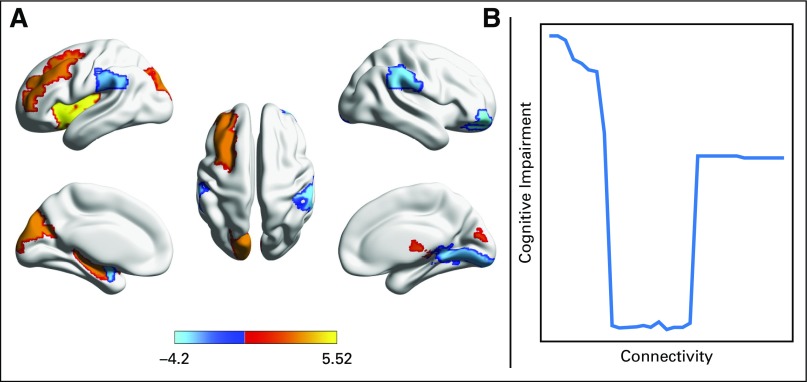
The interpretation of brain imaging metrics is complex and context dependent. Brain imaging focused on connectivity improves characterization of the complexity of the brain. These studies demonstrate both functional hypo- and hyperconnectivity among multiple regions in survivors of ALL.86 Reduced structural connectome organization and resilience have also been demonstrated in ALL survivors with regions of both hypo- and hyperconnectivity.87,88 Importantly, U-shaped relationships between local connectome organization and cognitive impairment suggest an optimal range of regional connectivity (Fig 2).87
Fig 2.
(A) Survivors of childhood acute lymphoblastic leukemia demonstrate a profile of both higher (warm colors) and lower (cool colors) white matter connectivity compared with healthy controls (color bar indicates T score). (B) Connectivity seems to have an optimal range with respect to cognitive function.87
Neurochemical Markers
Brain injury has also been demonstrated by MR spectroscopy, which measures metabolic markers of brain parenchymal integrity and function.89 These metabolites are considered markers of neuronal health, viability, and/or number (NAA), energy metabolism and homeostasis (Cr), and neuronal density and/or rate of membrane turnover (Cho).89 Reduced NAA/Cho and increased Cho/Cr from baseline to 20 weeks after diagnosis was demonstrated in survivors treated with CRT compared with healthy controls.90
Sphingomyelin and lysophosphatidylcholine are phospholipids found in cerebrospinal fluid (CSF) that are biomarkers of myelin and blood-brain barrier integrity.91 Sphingomyelin and lysophosphatidylcholine increase in newly diagnosed patients with ALL after induction and consolidation treatment. Increased sphingomyelin was related to slower motor speed, and increased lysophosphatidylcholine was associated with poorer verbal working memory. Declines in visual working memory were associated with elevations in sphingomyelin occurring later in therapy.91 Lipid peroxidation in CSF is considered an indicator of oxidative stress. Phosphatidylcholine and phosphatidylinositol, lipids abundant in neuronal cell membranes, increase in CSF across treatment phases, with the greatest increase occurring postinduction. Higher methotrexate dose was correlated with higher oxidized phosphatidylcholine, whereas older age at diagnosis was associated with higher oxidized phosphatidylinositol.91,92
Genetic Polymorphisms
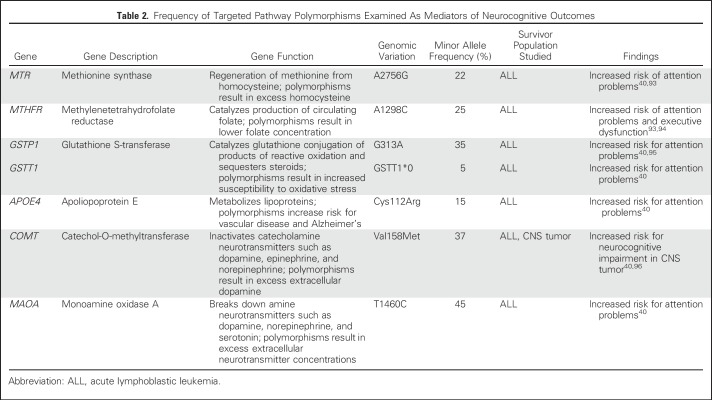
Emerging evidence suggests genetic predispositions moderate the effect of cancer therapy on neurocognitive outcomes in childhood cancer survivors (Table 2 summarizes polymorphisms examined). Polymorphisms in the folate pathway are associated with increased risk for problems in attention and executive function in survivors of ALL treated with chemotherapy only.40,93,94 Genes that regulate oxidative stress have also been associated with neurocognitive outcomes in survivors of ALL.95
Table 2.
Frequency of Targeted Pathway Polymorphisms Examined As Mediators of Neurocognitive Outcomes
Genetic predisposition for neurocognitive impairment may accelerate the onset of neurocognitive impairment in survivors. Polymorphisms in catechol-O-methyltransferase, an enzyme that helps regulate catecholamines (ie, dopamine, epinephrine, norepinephrine), have been associated with increased risk for neurocognitive problems in survivors of CNS tumors.96 In survivors of ALL treated with chemotherapy only, polymorphisms in monoamine oxidase A, an enzyme that catalyzes oxidative deamination of amines (ie, dopamine, norepinephrine, serotonin), are associated with increased risk for attention problems compared with survivors without such polymorphisms.40 Apolipoprotein E-epsilon 4 (APOE ε4) is a protein that affects lipids in the bloodstream and has been associated with dementia in the elderly. Polymorphisms in APOE ε4 are also associated with attention problems in survivors of childhood ALL.40 The APOE ε4 allele has been associated with accelerated telomere shortening, indicating accelerated cell aging. Additional collaborative research is needed to independently validate current association studies and to evaluate accuracy of risk prediction in prospective models.
INTERVENTIONS
Compensatory interventions are offered for many survivors, including behavioral and cognitive strategies to help accommodate to deficits. These are delivered in the form of school-based accommodations (preferential seating, note taking, extended time for tests)97 and can include teaching organizational strategies, time management, and planning (eg, making lists, electronic organizers). Despite wide implementation, the efficacy of these interventions in long-term survivors is largely unknown. In the absence of efficacy data, there is a need for medical and psychosocial teams to provide advocacy for survivors during school reintegration and while establishing academic accommodations.98 Additionally, several types of interventions are being applied or investigated.
Pharmacologic Treatment
Pharmacologic agents targeting cholinergic (memory system) and dopaminergic (attention and executive function systems) neurotransmitters have been evaluated in survivors of childhood cancer. The acetylcholinesterase inhibitor donepezil has been associated with moderate improvements on performance-based tasks of executive functioning and visual memory in survivors of childhood brain tumors.99 The acute and long-term efficacy of the psychostimulant methylphenidate in pediatric cancer survivors have been supported in several trials.97 Methylphenidate is associated with improvement in attentional functioning, as evidenced by performance-based tasks and parent and teacher ratings. Although survivors have shown improvements on a variety of measures of attention with methylphenidate treatment, no improvements in academic functioning have been associated with methylphenidate therapy in this population.
Rehabilitation Programs
Researchers have investigated nonpharmacologic interventions to address neurocognitive deficits in childhood cancer survivors. These programs generally involve cognitive and/or behavioral skills acquisition approaches. Clinic-based cognitive remediation programs demonstrate improvements in academic achievement and parent ratings of attention, although participation rates and treatment adherence are suboptimal.100 There is evidence that computerized, home-based cognitive training is more feasible and acceptable to families and survivors.100,101 Targeted cognitive skills are amenable to improvement with successful completion of the training programs,102 and gains are associated with changes in brain function.102,103 Although the evidence for efficacy of cognitive interventions is still emerging, there is currently no evidence that cognitive training is harmful.
Health Behavior Programs
Interventions targeting health behavior and physical activity have been examined. Exercise training positively affects brain structure and function in pediatric brain tumor survivors.104 Specifically, after 12 weeks of group-based exercise, increased white matter and hippocampal volume were observed and reaction time improved.109
Prevention Efforts
There are limited studies examining prophylactic interventions during cancer treatment. In a randomized controlled trial of an intensive math intervention delivered to children during continuation/maintenance therapy for ALL to preserve survivors’ achievement over time, children who received math training evidenced gains in achievement over a 3-year period compared with children in a standard-of-care (ie, individualized recommendations for school-based interventions) control group.105 On the basis of success in trials of adults with metastatic brain cancer,106 clinical trials evaluating potential neuroprotective effects of memantine, an N-methyl-D-aspartate antagonist, are currently being planned or initiated for pediatric patients being treated with CRT.
DISCUSSION
Neurocognitive deficits are a relatively common long-term outcome of childhood cancer and cancer therapy. Many studies have characterized children at greatest risk and identified aspects of neurotoxic treatment exposures, although more work is needed to clarify sources of variability in outcomes. Guidelines for neuropsychological monitoring of children at risk have been detailed by a number of investigators and advocacy groups.104,107,108 For children with CNS-affecting cancers or treatment, there is broad consensus that neurocognition should be formally evaluated by the end of planned therapy at the latest, but recommendations differ on the best timing for a baseline assessment. Afterward, periodic testing for survivors with impairments is suggested, particularly at times of transition (eg, primary to secondary school). Recommendations include the medical team members performing routine clinical surveillance of neurocognitive outcomes in at-risk survivors using a combination of clinical interviewing, available data (eg, report cards, school testing), and rating scales.104 If indicated, survivors should then be referred for neuropsychological consultation. Strategies for improving or preventing neurocognitive late effects are relatively understudied. However, healthy dietary practices and especially physical exercise are appropriate for many survivors to prevent or mitigate cardiovascular and metabolic late effects that may ultimately contribute to neurocognitive health.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
Kristina K. Hardy
No relationship to disclose
Lisa S. Kahalley
No relationship to disclose
Ilse Schuitema
No relationship to disclose
Shelli R. Kesler
No relationship to disclose
REFERENCES
- 1.Brinkman TM, Krasin MJ, Liu W, et al. : Long-term neurocognitive functioning and social attainment in adult survivors of pediatric CNS tumors: Results from the St Jude Lifetime Cohort Study. J Clin Oncol 34:1358-1367, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffner PK: Risk factors for cognitive decline in children treated for brain tumors. Eur J Paediatr Neurol 14:106-115, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Krull KR, Brinkman TM, Li C, et al. : Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude Lifetime Cohort Study. J Clin Oncol 31:4407-4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung YT, Krull KR: Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev 53:108-120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelstein K, D’agostino N, Bernstein LJ, et al. : Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 33:450-458, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Schuitema I, Deprez S, Van Hecke W, et al. : Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol 31:3378-3388, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Schuitema I, de Sonneville L, Kaspers G, et al. : Executive dysfunction 25 years after treatment with cranial radiotherapy for pediatric lymphoid malignancies. J Int Neuropsychol Soc 21:657-669, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Edelstein K, Spiegler BJ, Fung S, et al. : Early aging in adult survivors of childhood medulloblastoma: Long-term neurocognitive, functional, and physical outcomes. Neuro-oncol 13:536-545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daams M, Schuitema I, van Dijk BW, et al. : Long-term effects of cranial irradiation and intrathecal chemotherapy in treatment of childhood leukemia: A MEG study of power spectrum and correlated cognitive dysfunction. BMC Neurol 12:84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veiga LH, Holmberg E, Anderson H, et al. : Thyroid cancer after childhood exposure to external radiation: An updated pooled analysis of 12 studies. Radiat Res 185:473-484, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monje ML, Mizumatsu S, Fike JR, et al. : Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955-962, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Stobbe CC, Park SJ, Chapman JD: The radiation hypersensitivity of cells at mitosis. Int J Radiat Biol 78:1149-1157, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Oeseburg H, de Boer RA, van Gilst WH, et al. : Telomere biology in healthy aging and disease. Pflugers Arch 459:259-268, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1016/j.mrrev.2014.01.001. Shim G, Ricoul M, Hempel WM, et al: Crosstalk between telomere maintenance and radiation effects: A key player in the process of radiation-induced carcinogenesis. Mutat Res Rev Mutat Res 760:1-17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn HG, Dickinson-Anson H, Gage FH: Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027-2033, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahalley LS, Conklin HM, Tyc VL, et al. : Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology 22:1979-1986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margelisch K, Studer M, Ritter BC, et al. : Cognitive dysfunction in children with brain tumors at diagnosis. Pediatr Blood Cancer 62:1805-1812, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Rocco C, Chieffo D, Pettorini BL, et al. : Preoperative and postoperative neurological, neuropsychological and behavioral impairment in children with posterior cranial fossa astrocytomas and medulloblastomas: The role of the tumor and the impact of the surgical treatment. Childs Nerv Syst 26:1173-1188, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Mabbott DJ, Penkman L, Witol A, et al. : Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology 22:159-168, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ris MD, Beebe DW, Armstrong FD, et al. : Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children’s Oncology Group. J Clin Oncol 26:4765-4770, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner CD, Chordas CA, Liptak CC, et al. : Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer 53:417-423, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Tonning Olsson I, Perrin S, Lundgren J, et al. : Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol 51:515-521, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Zada G, Kintz N, Pulido M, et al. : Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: A systematic literature review. PLoS One 8:e76562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullrich NJ, Embry L: Neurocognitive dysfunction in survivors of childhood brain tumors. Semin Pediatr Neurol 19:35-42, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Palmer SL, Armstrong C, Onar-Thomas A, et al. : Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. J Clin Oncol 31:3494-3500, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moxon-Emre I, Bouffet E, Taylor MD, et al. : Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol 32:1760-1768, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Merchant TE, Schreiber JE, Wu S, et al. : Critical combinations of radiation dose and volume predict intelligence quotient and academic achievement scores after craniospinal irradiation in children with medulloblastoma. Int J Radiat Oncol Biol Phys 90:554-561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ris MD, Walsh K, Wallace D, et al. : Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer 60:1350-1357, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight SJ, Conklin HM, Palmer SL, et al. : Working memory abilities among children treated for medulloblastoma: Parent report and child performance. J Pediatr Psychol 39:501-511, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Pinto M, Conklin HM, Li C, et al. : Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys 84:e363-e369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson KE, Fraley CE, Pearson MM, et al. : Neurocognitive late effects of pediatric brain tumors of the posterior fossa: a quantitative review. J Int Neuropsychol Soc 19:44-53, 2013 [DOI] [PubMed] [Google Scholar]
- 32. doi: 10.1038/ncponc0090. Yock TI, Tarbell NJ: Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol 1:97-103, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Kahalley LS, Ris MD, Grosshans DR, et al. : Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. J Clin Oncol 34:1043-1049, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yock TI, Yeap BY, Ebb DH, et al. : Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol 17:287-298, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Antonini TN, Ris MD, Grosshans DR, et al. : Attention, processing speed, and executive functioning in pediatric brain tumor survivors treated with proton beam radiation therapy. Radiother Oncol 124:89-97, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelmann MN, Krull KR, Liu W, et al. : Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain 137:2973-2983, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegler BJ, Kennedy K, Maze R, et al. : Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol 24:3858-3864, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Jacola LM, Edelstein K, Liu W, et al. : Cognitive, behaviour, and academic functioning in adolescent and young adult survivors of childhood acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study. Lancet Psychiatry 3:965-972, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harila MJ, Winqvist S, Lanning M, et al. : Progressive neurocognitive impairment in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 53:156-161, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Krull KR, Bhojwani D, Conklin HM, et al. : Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 31:2182-2188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genschaft M, Huebner T, Plessow F, et al. : Impact of chemotherapy for childhood leukemia on brain morphology and function. PLoS One 8:e78599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacola LM, Krull KR, Pui CH, et al. : Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol 34:1239-1247, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kingma A, van Dommelen RI, Mooyaart EL, et al. : Slight cognitive impairment and magnetic resonance imaging abnormalities but normal school levels in children treated for acute lymphoblastic leukemia with chemotherapy only. J Pediatr 139:413-420, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Krull KR, Cheung YT, Liu W, et al. : Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 34:2644-2653, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edelmann MN, Ogg RJ, Scoggins MA, et al. : Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: A report from the SJLIFE cohort. Pediatr Blood Cancer 60:1778-1784, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadan-Lottick NS, Brouwers P, Breiger D, et al. : A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood 114:1746-1752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardy KK, Bonner MJ, Willard VW, et al. : Hydrocephalus as a possible additional contributor to cognitive outcome in survivors of pediatric medulloblastoma. Psychooncology 17:1157-1161, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Reimers TS, Ehrenfels S, Mortensen EL, et al. : Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Med Pediatr Oncol 40:26-34, 2003 [DOI] [PubMed] [Google Scholar]
- 49. doi: 10.3171/ped.2004.101.2.0159. Merchant TE, Lee H, Zhu J, et al: The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg 101:159-168, 2004 (suppl) [DOI] [PubMed] [Google Scholar]
- 50.Pietilä S, Korpela R, Lenko HL, et al. : Neurological outcome of childhood brain tumor survivors. J Neurooncol 108:153-161, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Morris B, Partap S, Yeom K, et al. : Cerebrovascular disease in childhood cancer survivors: A Children’s Oncology Group report. Neurology 73:1906-1913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Packer RJ, Gurney JG, Punyko JA, et al. : Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood Cancer Survivor Study. J Clin Oncol 21:3255-3261, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Khan RB, Hunt DL, Boop FA, et al. : Seizures in children with primary brain tumors: Incidence and long-term outcome. Epilepsy Res 64:85-91, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Ibrahim K, Appleton R: Seizures as the presenting symptom of brain tumours in children. Seizure 13:108-112, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Armstrong GT, Liu Q, Yasui Y, et al. : Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 101:946-958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landau E, Boop FA, Conklin HM, et al. : Supratentorial ependymoma: Disease control, complications, and functional outcomes after irradiation. Int J Radiat Oncol Biol Phys 85:e193-e199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vingerhoets G: Cognitive effects of seizures. Seizure 15:221-226, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Krull KR, Sabin ND, Reddick WE, et al. : Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol 30:3618-3624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edelmann MN, Daryani VM, Bishop MW, et al. : Neurocognitive and patient-reported outcomes in adult survivors of childhood osteosarcoma. JAMA Oncol 2:201-208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehrhardt MJ, Mulrooney DA, Li C, et al. : Neurocognitive, psychosocial, and quality-of-life outcomes in adult survivors of childhood non-Hodgkin lymphoma. Cancer 124:417-425, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung YT, Brinkman TM, Li C, et al. : Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 110:411-419, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulrooney DA, Ness KK, Neglia JP, et al. : Fatigue and sleep disturbance in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study (CCSS). Sleep 31:271-281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou ES, Recklitis CJ: Insomnia in adult survivors of childhood cancer: A report from project REACH. Support Care Cancer 22:3061-3069, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Tkachenko IV, Jääskeläinen T, Jääskeläinen J, et al. : Interleukins 1α and 1β as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids 76:1103-1115, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Clanton NR, Klosky JL, Li C, et al. : Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 117:2559-2568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung YT, Brinkman TM, Mulrooney DA, et al. : Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 123:3410-3419, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung YT, Chemaitilly W, Mulrooney DA, et al. : Association between dehydroepiandrosterone-sulfate and attention in long-term survivors of childhood acute lymphoblastic leukemia treated with only chemotherapy. Psychoneuroendocrinology 76:114-118, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruggiero C, Cherubini A, Ble A, et al. : Uric acid and inflammatory markers. Eur Heart J 27:1174-1181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feig DI, Kang DH, Johnson RJ: Uric acid and cardiovascular risk. N Engl J Med 359:1811-1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung YT, Edelmann MN, Mulrooney DA, et al. : Uric acid and neurocognitive function in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Cancer Epidemiol Biomarkers Prev 25:1259-1267, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filley CM, Fields RD: White matter and cognition: Making the connection. J Neurophysiol 116:2093-2104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soares JM, Marques P, Alves V, et al. : A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7:31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesler SR, Tanaka H, Koovakkattu D: Cognitive reserve and brain volumes in pediatric acute lymphoblastic leukemia. Brain Imaging Behav 4:256-269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeller B, Tamnes CK, Kanellopoulos A, et al. : Reduced neuroanatomic volumes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 31:2078-2085, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Khong PL, Kwong DL, Chan GC, et al. : Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: A pilot study. AJNR Am J Neuroradiol 24:734-740, 2003 [PMC free article] [PubMed] [Google Scholar]
- 76.Leung LHT, Ooi G-C, Kwong DLW, et al. : White-matter diffusion anisotropy after chemo-irradiation: A statistical parametric mapping study and histogram analysis. Neuroimage 21:261-268, 2004 [DOI] [PubMed] [Google Scholar]
- 77. doi: 10.1200/JCO.2005.02.4505. Khong PL, Leung LH, Fung AS, et al: White matter anisotropy in post-treatment childhood cancer survivors: Preliminary evidence of association with neurocognitive function. J Clin Oncol 24:884-890, 2006 [Erratum: J Clin Oncol 28:4868, 2010] [DOI] [PubMed] [Google Scholar]
- 78.Dellani PR, Eder S, Gawehn J, et al. : Late structural alterations of cerebral white matter in long-term survivors of childhood leukemia. J Magn Reson Imaging 27:1250-1255, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Aukema EJ, Caan MW, Oudhuis N, et al. : White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys 74:837-843, 2009 [DOI] [PubMed] [Google Scholar]
- 80.Rueckriegel SM, Driever PH, Blankenburg F, et al. : Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys 76:859-866, 2010 [DOI] [PubMed] [Google Scholar]
- 81.ElAlfy M, Ragab I, Azab I, et al. : Neurocognitive outcome and white matter anisotropy in childhood acute lymphoblastic leukemia survivors treated with different protocols. Pediatr Hematol Oncol 31:194-204, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Baron Nelson M, Compton P, Macey PM, et al. : Diffusion tensor imaging and neurobehavioral outcome in children with brain tumors treated with chemotherapy. J Pediatr Oncol Nurs 33:119-128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson KE, Livesay KL, Campbell LK, et al. : Working memory in survivors of childhood acute lymphocytic leukemia: Functional neuroimaging analyses. Pediatr Blood Cancer 54:585-590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morioka S, Morimoto M, Yamada K, et al. : Effects of chemotherapy on the brain in childhood: Diffusion tensor imaging of subtle white matter damage. Neuroradiology 55:1251-1257, 2013 [DOI] [PubMed] [Google Scholar]
- 85.Krull KR, Minoshima S, Edelmann M, et al. : Regional brain glucose metabolism and neurocognitive function in adult survivors of childhood cancer treated with cranial radiation. J Nucl Med 55:1805-1810, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kesler SR, Gugel M, Pritchard-Berman M, et al. : Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer 61:1295-1299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kesler SR, Gugel M, Huston-Warren E, et al. : Atypical structural connectome organization and cognitive impairment in young survivors of acute lymphoblastic leukemia. Brain Connect 6:273-282, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hosseini SM, Hoeft F, Kesler SR: GAT: A graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One 7:e40709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soares DP, Law M: Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clin Radiol 64:12-21, 2009 [DOI] [PubMed] [Google Scholar]
- 90. doi: 10.1007/978-3-211-98811-4_36. Ficek K, Blamek S, Syguła D, et al: Evaluation of the late effects of CNS prophylactic treatment in childhood acute lymphoblastic leukemia (ALL) using magnetic resonance spectroscopy. Acta Neurochir Suppl (Wien) 106:195-197, 2010 (suppl) [DOI] [PubMed] [Google Scholar]
- 91.Krull KR, Hockenberry MJ, Miketova P, et al. : Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leuk Lymphoma 54:535-540, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ki Moore IM, Gundy P, Pasvogel A, et al. : Increase in oxidative stress as measured by cerebrospinal fluid lipid peroxidation during treatment for childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 37:e86-e93, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamdar KY, Krull KR, El-Zein RA, et al. : Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer 57:454-460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krull KR, Brouwers P, Jain N, et al. : Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr 152:101-105, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Cole PD, Finkelstein Y, Stevenson KE, et al. : Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 33:2205-2211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howarth RA, Adamson AM, Ashford JM, et al. : Investigating the relationship between COMT polymorphisms and working memory performance among childhood brain tumor survivors. Pediatr Blood Cancer 61:40-45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conklin HM, Reddick WE, Ashford J, et al. : Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol 28:4465-4472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Armstrong FD, Briery BG: Childhood cancer and the school, in Brown RT (ed): Handbook of Pediatric Psychology in School Settings. New York, NY, Lawrence Erlbaum, 2004, pp 263-282. [Google Scholar]
- 99.Castellino SM, Tooze JA, Flowers L, et al. : Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: A pilot study. Pediatr Blood Cancer 59:540-547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler RW, Copeland DR, Fairclough DL, et al. : A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol 76:367-378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardy KK, Willard VW, Allen TM, et al. : Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology 22:1856-1865, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conklin HM, Ashford JM, Clark KN, et al. : Long-term efficacy of computerized cognitive training among survivors of childhood cancer: A single-blind randomized controlled trial. J Pediatr Psychol 42:220-231, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kesler SR, Lacayo NJ, Jo B: A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj 25:101-112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baum KT, Powell SK, Jacobson LA, et al. : Implementing guidelines: Proposed definitions of neuropsychology services in pediatric oncology. Pediatr Blood Cancer 64:e26446, 2017 [DOI] [PubMed] [Google Scholar]
- 105.Moore IM, Hockenberry MJ, Anhalt C, et al. : Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer 59:278-284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown PD, Pugh S, Laack NN, et al. : Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-oncol 15:1429-1437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http://www.survivorshipguidelines.org/
- 108.Walsh KS, Noll RB, Annett RD, et al. : Standard of care for neuropsychological monitoring in pediatric neuro-oncology: Lessons from the Children’s Oncology Group (COG). Pediatr Blood Cancer 63:191-195, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riggs L, Piscione PJ, Laughlin S, et al. : Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncology 19:440-450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]