Abstract
Purpose
Although programmed death (PD)-1 pathway inhibitors are now used in nearly all patients with advanced non–small-cell lung cancer (NSCLC), the large number of patients with NSCLC and concurrent autoimmune disease (AID) have been universally excluded from immunotherapy clinical trials. Therefore, the safety of PD-1 and PD-ligand 1 (PD-L1) inhibitors in patients with NSCLC and underlying AID is currently unknown.
Methods
As part of a multi-institutional effort, we retrospectively collected clinicopathologic data from patients with NSCLC and a history of AID who received monotherapy with either a PD-1 or a PD-L1 (herein referred to as PD-[L]1) inhibitor. Qualifying AIDs included but were not limited to: rheumatologic, neurologic, endocrine, GI, and dermatologic conditions.
Results
We identified 56 patients with NSCLC and an AID who received a PD-(L)1 inhibitor. At the time of treatment initiation, 18% of patients had active AID symptoms and 20% were receiving immunomodulatory agents for their AID. A total of 55% of patients developed an AID flare and/or an immune-related adverse event (irAE). Exacerbation of the AID occurred in 13 patients (23% of the whole cohort), four of whom required systemic corticosteroids. Immune-related adverse events occurred in 21 patients (38%). Among irAEs, 74% were grade 1 or 2 and 26% were grade 3 or 4; eight patients required corticosteroids for irAE management. PD-(L)1 therapy was permanently discontinued in eight patients (14%) because of irAEs. The overall response rate to immunotherapy in this population was 22%.
Conclusion
In patients with NSCLC with AID treated with a PD-(L)1 inhibitor, exacerbation of AID occurred in a minority of patients. The incidence of irAEs was similar to reported rates in clinical trials where patients with AID were excluded. Adverse events were generally manageable and infrequently led to permanent discontinuation of immunotherapy.
INTRODUCTION
Two programmed death (PD)-1 inhibitors (nivolumab and pembrolizumab) and one PD-1 ligand (PD-L1) inhibitor (atezolizumab) are now approved by the US Food and Drug Administration for previously treated non–small-cell lung cancer (NSCLC), and pembrolizumab is approved in the first-line setting for NSCLCs with high PD-L1 expression (tumor proportion score ≥ 50%) and in combination with platinum and pemetrexed in nonsquamous NSCLCs regardless of PD-L1 expression.1-6 Moreover, the PD-L1 inhibitor durvalumab is approved for use after chemoradiation in unresectable stage III NSCLC.7 Thus, almost every patient with advanced NSCLC will likely receive a PD-1 or PD-L1 (herein referred to as PD-[L]1) inhibitor at some point over the course of their disease. However, because these drugs can be associated with serious and potentially fatal immune-related adverse events (irAEs),3,8,9 patients treated with immunotherapy must be monitored carefully for the development of toxicities.
In NSCLC clinical trials of immune checkpoint inhibitors, patients with a history of autoimmune disease (AID) have generally been excluded because of concerns that these individuals might be at greater risk for developing serious irAEs.1-6 This presents a tremendous knowledge gap, because an estimated 14% to 25% of patients with lung cancer also carry a diagnosis of AID.10 Because patients with AID have an increased risk of developing several malignancies, including lung cancer,11 there is a need to identify risks and benefits of immune checkpoint inhibitors in this population.
Two retrospective studies have evaluated the toxicities of immune checkpoint inhibitors in patients with advanced-stage melanoma and a history of autoimmune disease. Among 30 patients with melanoma and AID treated with ipilimumab, 27% experienced an exacerbation of their baseline AID, and 33% developed grade 3 to 5 irAEs, with one treatment-related death from colitis.12 By contrast, the use of PD-1 inhibitors among 52 patients with melanoma and AID seemed to be associated with milder toxicities, with 38% of patients experiencing an AID flare and 29% developing irAEs (10% grade 3 and no grade 4 or 5 irAEs).13
Although both of these studies suggest that the administration of immune checkpoint inhibitors in patients with melanoma and AID is generally safe,12,13 the findings may not necessarily apply to other cancers, because the adverse effects of immunotherapy may differ according to tumor type. For example, a recent meta-analysis of immunotherapy studies demonstrated that the incidence of PD-1 inhibitor-related pneumonitis was higher in NSCLC compared to melanoma.14 Because little is known about the use of PD-(L)1 inhibitors in patients with NSCLC and a history of AID, we conducted a multi-institutional retrospective analysis to examine the safety of immune checkpoint inhibitors in this population.
METHODS
Study Population
We retrospectively collected clinicopathologic data from patients with advanced stage IIIB (who were not candidates for definitive treatment with concurrent chemoradiation) or stage IV NSCLC with a preexisting diagnosis of AID and who received at least one dose of a commercially available PD-(L)1 inhibitor as monotherapy between May 1, 2015 and December 28, 2017 and had at least one follow-up visit at one of five participating academic cancer centers (Dana-Farber Cancer Institute, Massachusetts General Hospital, MD Anderson Cancer Center, Memorial Sloan Kettering Cancer Center, and University of California Davis Comprehensive Cancer Center). Qualifying AIDs included but were not limited to: rheumatologic (rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, polymyalgia rheumatica, seronegative arthritis, scleroderma, psoriatic arthritis, vasculitis), dermatologic (psoriasis, alopecia areata, discoid lupus), GI (Crohn disease, ulcerative colitis), endocrine (Graves disease, Hashimoto thyroiditis), neurologic (myasthenia gravis, multiple sclerosis), and other (autoimmune hemolytic anemia, rheumatic fever) conditions. We excluded patients with asthma and patients with hypothyroidism with no evidence of autoimmune thyroiditis. All patients consented to local institutional review board–approved protocols. Baseline AID symptoms, AID flares, and irAEs were retrospectively assessed by investigators at each participating institution through chart review of patient-reported symptoms and laboratory results; these were graded using the Common Terminology Criteria for Adverse Events version 4.03. Patient visits generally occurred every 2 to 3 weeks, and serial imaging was obtained every 6 to 9 weeks, depending on the immune checkpoint inhibitor used as well as variation in provider practices. Investigator-assessed response to immunotherapy was determined locally at each institution.
Statistical Analysis
Categorical and continuous variables were summarized descriptively using percentages and medians. Fisher’s exact test was used to test for associations between categorical variables. Time to immunotherapy treatment failure was determined from the start of PD-(L)1 therapy until treatment discontinuation for any reason, including disease progression, toxicity, or death. Patients who were alive and continuing treatment were censored on the date of their last adequate disease assessment. Kaplan-Meier methodology was used to estimate event-time distributions, and the Greenwood formula was used to estimate the SEs of the estimates. Median follow-up time was calculated using the reverse Kaplan-Meier estimator. Because patients may have discontinued anti–PD-(L)1 before the exacerbation of an AID, we estimated the cumulative incidence of AID exacerbation over time using the methodology of Fine and Gray; disease progression, death due to any cause in the absence of progression, and discontinuation of treatment were adjusted for as competing events. All P values are two-sided.
RESULTS
Study Population
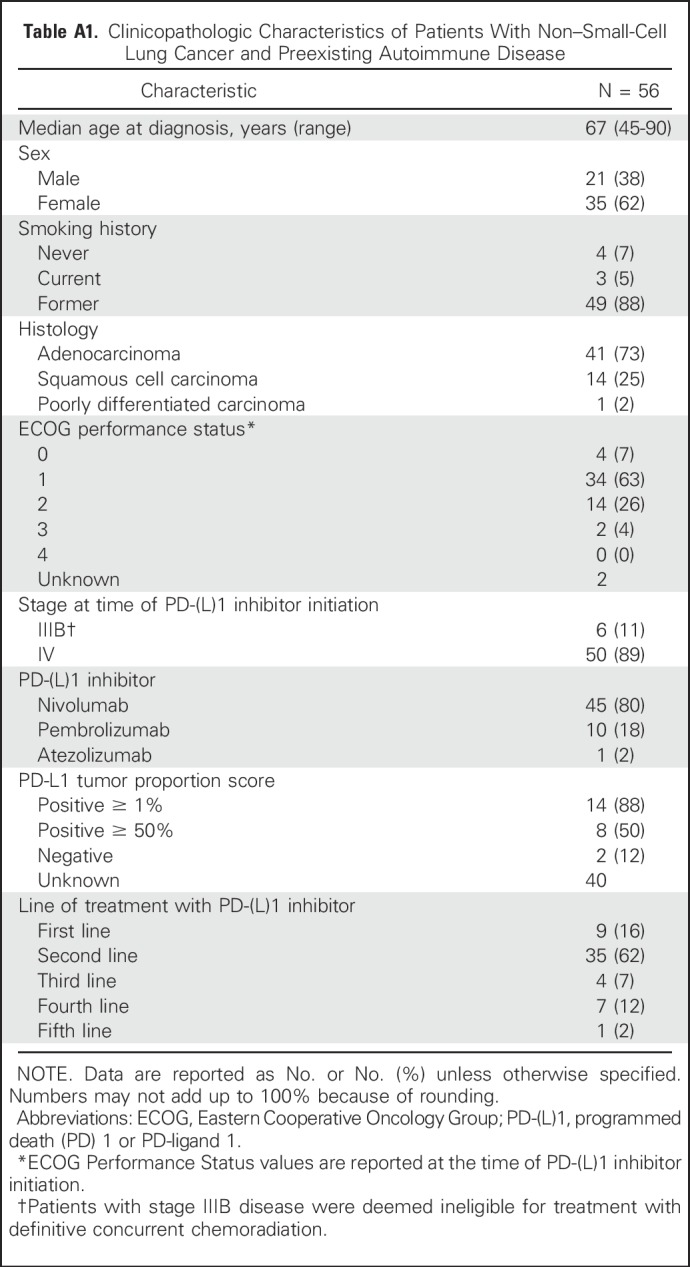
A total of 56 patients were identified with advanced NSCLC and a history of AID who were treated with a PD-(L)1 inhibitor as part of routine clinical care. The clinicopathological characteristics of these patients are summarized in Appendix Table A1. (online only). The median time receiving treatment was 3.1 months (95% CI, 1.8 to 5.1 months), and the median length of follow-up after PD-(L)1 initiation was 17.5 months.
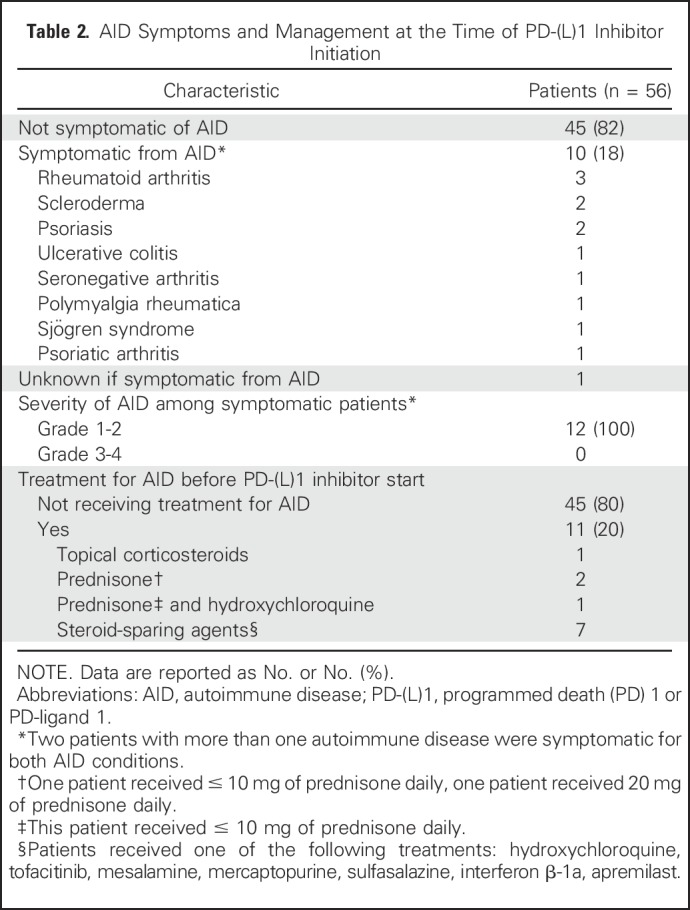
Among the baseline autoimmune conditions (Table 1), 45% of patients had a rheumatologic disorder, 29% had a dermatologic disorder, 16% had an endocrine disorder, 11% had inflammatory bowel disease, 5% had a neurologic condition, 3% had rheumatic fever, and one patient (2%) had autoimmune hemolytic anemia. Seven patients had more than one autoimmune disease. At the time of PD-(L)1 inhibitor initiation, 10 patients (18% of the cohort) had active symptoms from their AID, which were all grade 1 or 2. Eleven patients (20% of the cohort) were receiving immunosuppressant or immunomodulatory treatment for their AID at the time of PD-(L)1 initiation, and eight of them continued on their AID treatment regimen while receiving anti–PD-(L)1 therapy (Table 2). The Data Supplement contains additional details on baseline AID symptoms for each patient and their clinical course while receiving PD-(L)1 therapy.
Table 1.
Autoimmune Disease Types Among 56 Patients With Non–Small-Cell Lung Cancer
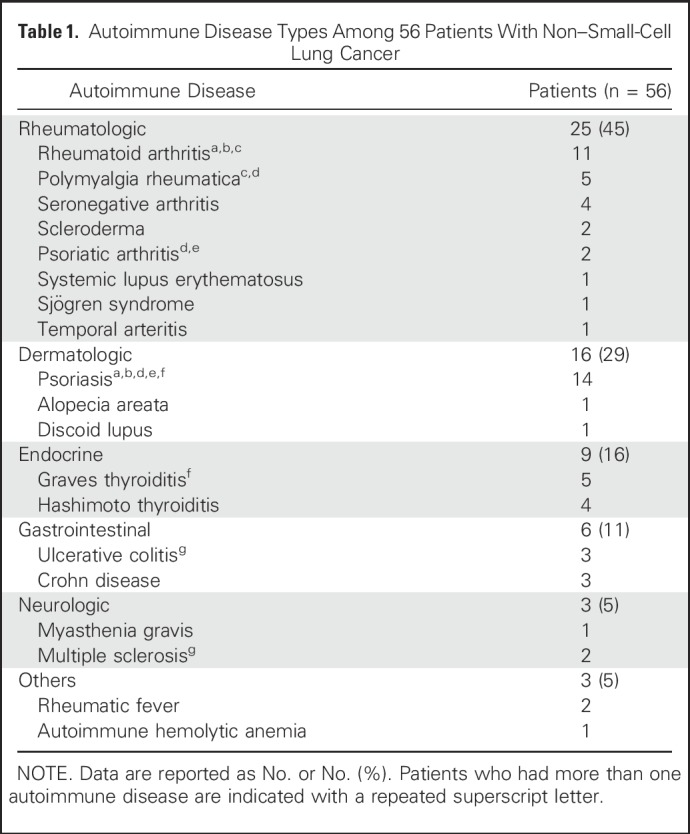
Table 2.
AID Symptoms and Management at the Time of PD-(L)1 Inhibitor Initiation
Flares of Underlying Autoimmune Disease
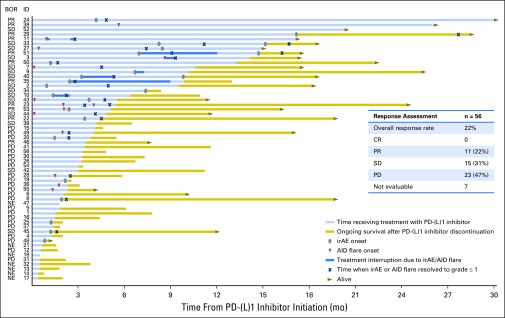
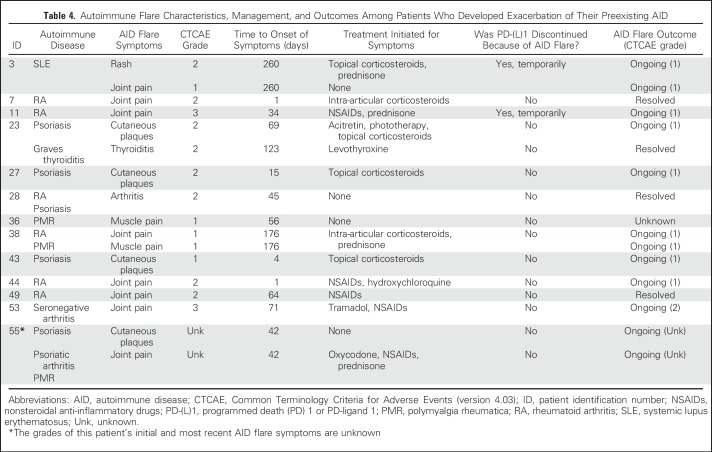
Exacerbations of underlying AID occurred in a minority of patients and were generally mild. Thirteen patients (23%) had an AID flare (Table 3), which in all cases affected the same anatomic sites where patients experienced prior AID symptoms. Of 17 unique AID flare symptoms, 87% were grade 1 or 2, 13% were grade 3, and 0% were grade 4 or 5. Only four patients required systemic corticosteroids for management of AID flares. One patient with rheumatoid arthritis experienced grade 3 arthralgias and required prednisone, nonsteroidal anti-inflammatory drugs, and temporary discontinuation of the PD-(L)1 inhibitor, with improvement of symptoms to grade 1. Another patient with seronegative arthritis also experienced grade 3 joint pains and was treated with tramadol and topical nonsteroidal anti-inflammatory drugs with no PD-(L)1 treatment interruption and ongoing grade 2 symptoms at the time of data censor. None of the patients required permanent PD-(L)1 inhibitor discontinuation because of an AID exacerbation. The onset of flare symptoms was highly variable, ranging from 1 to 260 days after PD-(L)1 inhibitor start (Fig 1). Details of AID flare characteristics and management are reported in Table 4.
Table 3.
Exacerbation of AID in Patients Treated With PD-(L)1 Inhibitors
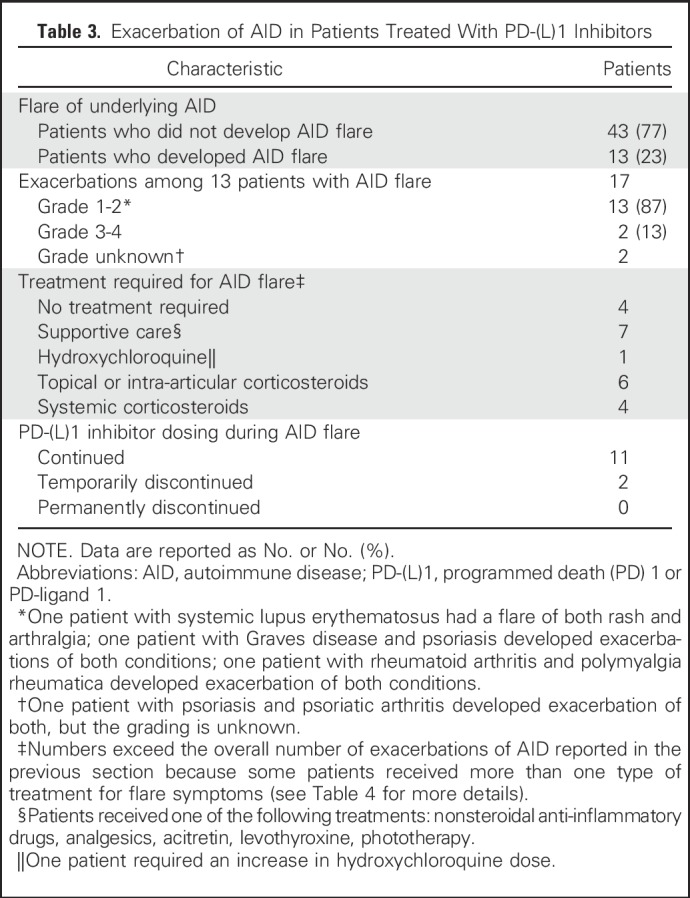
Fig 1.
Time receiving treatment with PD-(L)1 inhibitor, onset, and resolution of autoimmune disease (AID) exacerbation or immune-related adverse event (irAE) in 56 patients with non–small-cell lung cancer with preexisting autoimmune disease. Investigator-assessed best objective response (BOR) to immunotherapy for each patient (listed by identification [ID] number) is indicated in the left column. The table inset summarizes the response assessment among the 56 patients. Follow-up details on the AID flare for patient 36 and on the irAE for patients 19, 25, and 48 were not available at the time of data censoring. Patients who were still alive at the time of data censoring are indicated by a gray arrowhead pointing to the right. CR, complete response; NE, not evaluable, including six patients who were not radiologically evaluated because of clinical decline and one patient with no available tumor assessment imaging at the time of data analysis; PD, progressive disease; PR, partial response; SD, stable disease.
Table 4.
Autoimmune Flare Characteristics, Management, and Outcomes Among Patients Who Developed Exacerbation of Their Preexisting AID
Half of patients (50%) who were symptomatic from their AID at the time of PD-(L)1 initiation developed an AID flare, which was a significantly higher rate than the 18% of patients who were initially asymptomatic from their AID and experienced a flare (P = .04). The rate of AID flare was similar among patients who were receiving immunosuppressive or immunomodulatory treatment at the time of PD-(L)1 start compared with those who were not (36% v 20%, respectively; P = .43). A higher proportion of patients with rheumatologic AID experienced flares compared with patients with nonrheumatologic AID (40% v 10%, respectively; P = .01). Among the 25 patients with rheumatologic autoimmune disorders, eight (32%) had active symptoms from their AID at the time of PD-(L)1 inhibitor initiation, and four were receiving immunosuppressive or immunomodulatory treatment of their symptoms (Data Supplement). None of the six patients with inflammatory bowel disease or the three patients with neurologic AID developed a disease flare.
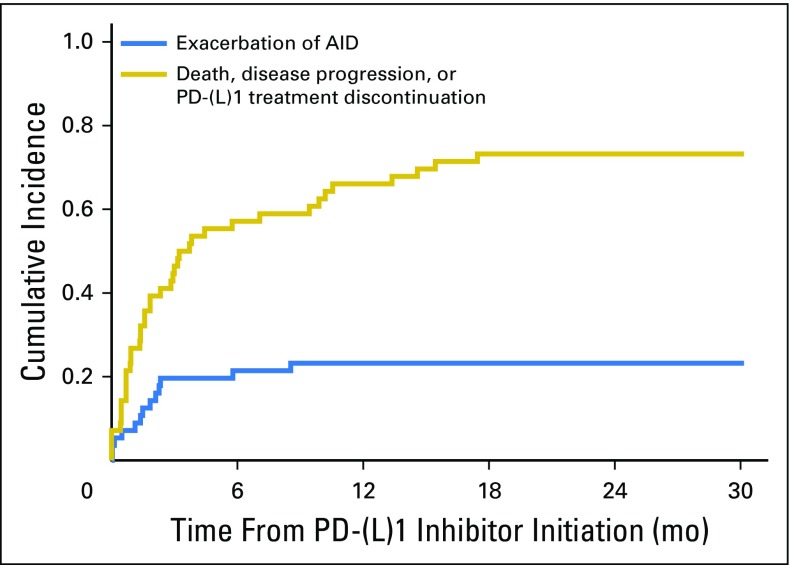
To identify the likelihood of developing an AID flare over the course of treatment, we estimated the cumulative incidence of exacerbation of an AID, adjusting for death, progression, or discontinuation of PD-(L)1 treatment as competing events (Appendix Fig A1, online only). The 6-month cumulative incidence rate of an AID exacerbation was 0.21 (95% CI, 0.10 to 0.32), and the 6-month rate of death, progression, or discontinuation of treatment was 0.57 (95% CI, 0.44 to 0.70). The 18-month cumulative incidence rate of an AID exacerbation was 0.23 (95% CI, 0.12 to 0.34), and the 18-month rate of death, progression, or discontinuation of treatment was 0.73 (95% CI, 0.61 to 0.85).
Immune-Related Adverse Events
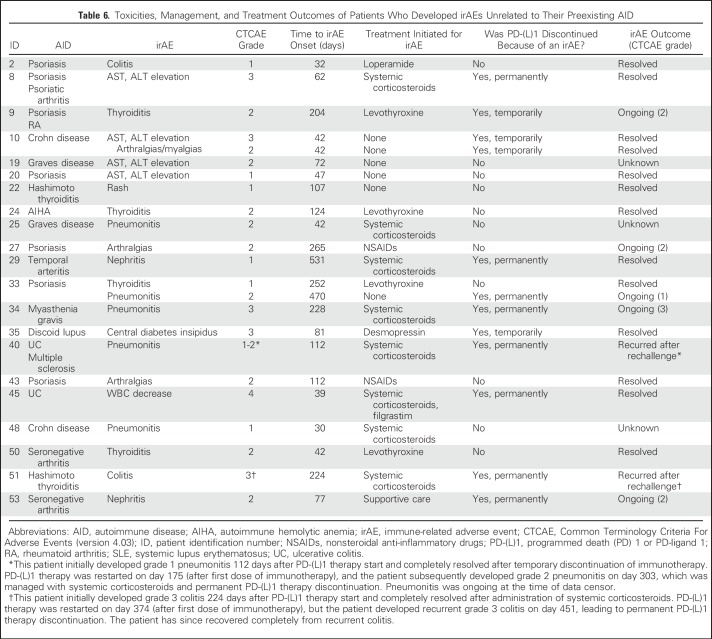
From the overall cohort, 21 patients (38%) developed a total of 23 unique irAEs while receiving PD-(L)1 therapy (Table 5). Fifteen patients developed grade 1 or 2 irAEs (representing 71% of those who developed an irAE and 27% of the overall cohort); 11 of these patients required only supportive care or no treatment to manage these irAEs, and four patients were treated with systemic corticosteroids for pneumonitis (three patients) and nephritis (one patient). Six of 21 patients developed grade 3 or 4 irAEs (representing 29% of those who developed an irAE and 11% of the overall cohort); four of these patients were treated with systemic corticosteroids. Grade 3 irAEs included two patients with elevated transaminases, one with pneumonitis, one with central diabetes insipidus, and one with colitis. One patient with ulcerative colitis who had been taking oral sulfasalazine and rectal mesalamine for several years developed grade 4 WBC decrease, which resolved after treatment with intravenous corticosteroids and filgrastim. No clear immunotherapy-related grade 5 irAEs occurred; one patient died 1 month after developing grade 3 pneumonitis, but this death was believed to be due to disease progression. Overall, eight patients (14% of the entire cohort of 56 patients) required permanent PD-(L)1 treatment discontinuation because of the development of an irAE. To determine whether there was an imbalance in the length of follow-up time among patients who did or did not develop an irAE, we calculated the median follow-up time in each group and found no significant difference between them (18.4 months v 17.3 months, respectively; P = .33). Detailed information on irAE features, treatment, and outcome are summarized in Table 6.
Table 5.
Immune-Related Adverse Events
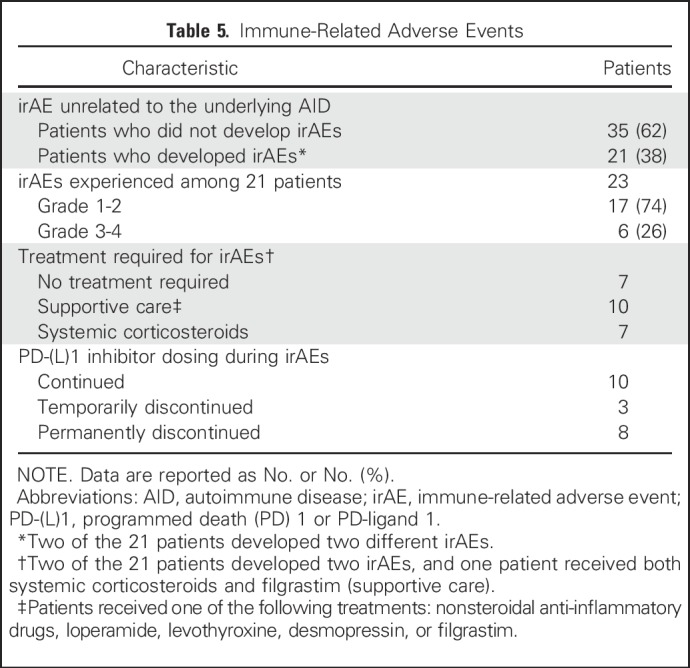
Table 6.
Toxicities, Management, and Treatment Outcomes of Patients Who Developed irAEs Unrelated to Their Preexisting AID
In total, 55% of patients developed either an AID flare or an irAE, and only three patients (5% of the cohort) developed both an AID flare and a separate irAE. Two patients with psoriasis both experienced worsening symptoms of psoriasis, managed with topical steroids, and also developed grade 2 arthralgias; in one patient, immunotherapy was temporarily discontinued because of arthralgias. One patient with seronegative arthritis developed grade 3 arthralgias but also developed grade 2 nephritis that led to permanent discontinuation of nivolumab.
At the time of data censoring, we did not observe any new AID flares or new irAEs after immunotherapy treatment discontinuation. The line of immunotherapy (first-line v second-line or greater) did not affect risk of AID flare (P = .66) or irAEs incidence (P = 1).
Response Assessment
Among the 56 patients included in this study, the best overall response was progressive disease in 23 patients (47%), stable disease in 15 patients (31%), partial response in 11 patients (22%), and complete response in no patients (0%). The overall response rate was 22%, and the disease control rate was 53% (Fig 1). Seven patients were not evaluable for response: six patients were not radiologically evaluated because of clinical decline, and for one patient there were no available data on tumor assessment. We found no association between the use of immunomodulatory treatment (corticosteroids and/or steroid sparing agent) at the time of PD-(L)1 inhibitor start and response to immune checkpoint inhibitor treatment (P = .66). We also found no association between the development of AID flare and response to immunotherapy (P = .45).
DISCUSSION
Because of the widespread use of immune checkpoint inhibitors in NSCLC, and the relatively high incidence of autoimmune conditions in this population,10 there is a need to understand whether PD-(L)1 inhibitors can safely be administered to patients with NSCLC and a history of AID. To the best of our knowledge, this is the largest study to evaluate the toxicity profile of anti–PD-(L)1 therapies in patients with NSCLC and autoimmune conditions. Although 55% of patients in this study developed an exacerbation of their AID and/or an irAE, most toxicities were mild and manageable, and immunotherapy was permanently discontinued in only 14% of patients.
Almost one quarter of patients in our study developed at least one flare of their AID, but flares were generally low grade and well controlled, without the need to add or escalate immunosuppressive medications. Only two patients required a PD-(L)1 treatment delay because of AID symptoms, and no patients required permanent PD-(L)1 discontinuation because of an AID flare. We found that patients with active AID symptoms at the time of PD-(L)1 initiation were at higher risk for experiencing an AID flare, and although these AID exacerbations were still manageable, caution should be taken while using immunotherapy in patients with active symptoms from their autoimmune condition.
Although 38% of patients in this study developed an irAE after exposure to an anti–PD-(L)1, the vast majority of irAEs were mild (grade 1 or 2), and most of these patients did not require treatment with systemic immunosuppression. Of the six patients who developed grade 3 or 4 irAEs, five were manageable with supportive treatment and systemic corticosteroids. None of the patients in this study required immunosuppressive agents other than systemic corticosteroids (eg, anti–tumor necrosis factor agents) for management of immunologic toxicity. The rate of grade 3 or 4 irAEs in clinical trials that excluded patients with preexisting autoimmune conditions ranges from 7% to 15%,1-4,6 which is comparable to the rate of grade 3 or 4 irAEs observed in this study (11% of 56 patients). The rate of PD-(L)1 therapy discontinuation in our study because of toxicity (14%) was slightly higher than drug discontinuation rates reported in clinical trials (between 3% and 8%).1-4,6
Certain limitations in this retrospective analysis should be considered when interpreting these data. This study relied on retrospective chart review of patients treated with commercially available PD-(L)1 inhibitors, so some characteristics of the baseline AID and subsequent toxicities may have not been documented or graded as thoroughly by the treating oncologist compared with patients who are being followed in clinical trials. In addition, at the time of PD-(L)1 initiation, 82% of the patients in this study had no active AID symptoms, and only 20% of the overall population was receiving treatment of their baseline AID. Therefore, the conclusions from this study may not be broadly applicable to patients with more severe and symptomatic AID who are about to start immunotherapy. Furthermore, some autoimmune conditions included in this study were only represented by small numbers of patients, so larger studies may be needed to identify risks of immune checkpoint inhibitors for specific AID subtypes.
The length of time receiving immunotherapy or in follow-up may also be potential confounders in this analysis, because immunologic toxicities might be more likely to be identified in patients with longer exposures to PD-(L)1 therapies. Because 84% of patients in this study received PD-(L)1 therapy in the second-line or greater setting, the response rate to PD-(L)1 in our cohort was 22%, similar to the overall response rate reported in phase III trials of PD-(L)1 inhibitors in unselected patients with NSCLC.1,6 This relatively low response rate and short duration of exposure to immunotherapy may underestimate the risk of immunologic toxicity compared with a more highly enriched patient population who might be more likely to respond to immunotherapy for longer periods of time (eg, NSCLCs with a high PD-L1 tumor proportion score or high mutational load). Taking into account the competing risk of PD-(L)1 discontinuation due to death, disease progression, or toxicity, we observed that the cumulative incidence rate for AID flare was 21% in the first 6 months and 23% at 18 months, suggesting that most of the AID flare events would be detected earlier in the course of immunotherapy treatment. With a median follow-up time in our study of 17.5 months, we aimed to capture any potentially late-occurring immunologic toxicities in this population.
Overall, our findings are similar to those reported in two recent studies in patients with melanoma with AID conditions, where treatment with CTLA-4 or PD-1 inhibitors was shown to be generally safe, with manageable toxicities.12,13 A recent systematic review of immune checkpoint inhibitor use among patients with cancer and AID, including 16 patients with lung cancer, also showed that these therapies can generally be administered safely without treatment discontinuation.15 Although larger retrospective and prospective analyses will be helpful in further delineating the risks of immunotherapy in patients with specific autoimmune diseases, our study adds to the growing body of evidence supporting the use of immunotherapy in patients with cancer with preexisting AID, albeit with close monitoring for adverse events. Additional studies are needed to determine the risk factors for developing immune-mediated toxicities in patients with cancer treated with immune checkpoint inhibitors.
Appendix
Fig A1.
Cumulative incidence of exacerbation of an autoimmune disease (AID) compared to PD-1 or PD-L1 therapy discontinuation due to death, disease progression, or treatment toxicity.
Table A1.
Clinicopathologic Characteristics of Patients With Non–Small-Cell Lung Cancer and Preexisting Autoimmune Disease
Footnotes
Supported by the Kaplan Research Fund and Jeni Fund; Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30 CA008748; Stand Up to Cancer (SU2C)–American Cancer Society Lung Cancer Dream Team Translational Research Grant No. SU2C-AACR-DT17-15 (SU2C is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C); generous philanthropic contributions to The University of Texas MD Anderson Lung Moon Shot Program; and MD Anderson Cancer Center Support Grant No. P30 CA01667.
Presented at the 2017 ASCO Annual Meeting, Chicago, IL, June 2-6, 2017.
Processed as a Rapid Communication manuscript.
None of the funding organizations were involved in data collection, analysis, or manuscript preparation. The corresponding author had full access to all data in the study, and had final responsibility for the decision to submit for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Giulia C. Leonardi, Mark M. Awad
Collection and assembly of data: Giulia C. Leonardi, Justin F. Gainor, Mehmet Altan, Roxana Azimi, Hira Rizvi, Jonathan W. Riess, Matthew D. Hellmann, Mark M. Awad
Data analysis and interpretation: Giulia C. Leonardi, Justin F. Gainor, Sasha Kravets, Suzanne E. Dahlberg, Lydia Gedmintas, Matthew D. Hellmann, Mark M. Awad
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety of Programmed Death–1 Pathway Inhibitors Among Patients With Non–Small-Cell Lung Cancer and Preexisting Autoimmune Disorders
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Giulia C. Leonardi
No relationship to disclose
Justin F. Gainor
Honoraria: Merck, Incyte, ARIAD Pharmaceuticals, Pfizer, Novartis
Consulting or Advisory Role: Boehringer Ingelheim, Clovis Oncology, Genentech, Bristol-Myers Squibb, Theravance Biopharma, Loxo Oncology, Genentech, Takeda, Array BioPharma, Amgen
Research Funding: Merck (Inst), Novartis (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Adaptimmune Therapeutics (Inst), AstraZeneca (Inst), ARIAD Pharmaceuticals
Travel, Accommodations, Expenses: Affymetrix
Mehmet Altan
No relationship to disclose
Sasha Kravets
No relationship to disclose
Suzanne E. Dahlberg
Consulting or Advisory Role: AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for a statistical model assessing tumor growth (Inst)
Lydia Gedmintas
No relationship to disclose
Roxana Azimi
No relationship to disclose
Hira Rizvi
No relationship to disclose
Jonathan W. Riess
Consulting or Advisory Role: Celgene, Takeda/ARIAD Pharmaceuticals, Medtronic, Abbvie
Research Funding: Millennium (Inst), Novartis (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Genentech
Matthew D. Hellmann
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech, AstraZeneca/MedImmune, Novartis, Janssen, Mirati Therapeutics, Shattuck Labs
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent filed by Memorial Sloan Kettering related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208)
Mark M. Awad
Consulting or Advisory Role: Genentech, Merck, Pfizer, AstraZeneca/MedImmune, Nektar, Bristol-Myers Squibb, Foundation Medicine
Research Funding: Bristol-Myers Squibb
REFERENCES
- 1.Brahmer J, Reckamp KL, Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer CJ, Gadgeel SM, Borghaei H, et al. : Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497-1508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonia SJ, Villegas A, Daniel D, et al. : Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Gettinger SN, Horn L, Gandhi L, et al. : Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33:2004-2012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metro G, Ricciuti B, Brambilla M, et al. : The safety of nivolumab for the treatment of advanced non-small cell lung cancer. Expert Opin Drug Saf 16:101-109, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Khan SA, Pruitt SL, Xuan L, et al. : Prevalence of autoimmune disease among patients with lung cancer: Implications for immunotherapy treatment options. JAMA Oncol 2:1507-1508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franks AL, Slansky JE: Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 32:1119-1136, 2012 [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, Sullivan RJ, Ott PA, et al. : Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2:234-240, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Menzies AM, Johnson DB, Ramanujam S, et al. : Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28:368-376, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Nishino M, Giobbie-Hurder A, Hatabu H, et al. : Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol 2:1607-1616, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. : Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med 168:121-130, 2018 [DOI] [PubMed] [Google Scholar]