Abstract
Objective:
To estimate the prevalence of serious mental illness and dementia among Medicare beneficiaries in the Health and Retirement Study (HRS).
Methods:
This study utilizes HRS-linked Medicare claims data sets and inverse probability weighting to estimate overall and age-specific cumulative prevalence rates of dementia and serious mental illnesses among 18,740 Medicare beneficiaries. Two-way tabulations determine conditional probabilities of dementia diagnoses among beneficiaries diagnosed with specific mental illnesses, and binary logistic regressions determine conditional probabilities of dementia diagnoses among beneficiaries diagnosed with specific mental illnesses, controlling for covariates.
Results:
Weighted prevalence estimates for dementia, schizophrenia (SZP), bipolar disorder (BPD), and major depressive disorder (MDD) are similar to previous studies. Odds of dementia diagnosis are significantly greater for beneficiaries diagnosed with SZP, BPD, or MDD.
Conclusions:
Co-occurring mental disabilities require further investigation, as in the near future increasing numbers of mentally ill older adults will need appropriate and affordable community-based services and supports.
Keywords: mental health, dementia, Medicare, prevalence, aging
Although interest in geriatric mental health continues to grow in the field of gerontology, we have a limited understanding of the prevalence of severe and persistent mental illness among older Americans. The prevalence rates of these conditions in the adult population range from approximately 1% for schizophrenia (SZP; National Institute of Mental Health [NIMH], 2014c), from 1% to 9.8% for bipolar disorder (BPD), depending on the nature of the sample (Cerimele, Chwastiak, Dodson, & Katon, 2014; Das et al., 2005; Fagiolini et al., 2013; Thomas, 2004), and 6.7% for major depressive disorder (MDD; NIMH, 2014b). It is important to understand the risks faced by mentally ill older Americans, so that policy makers and long-term care providers can better prepare for the presence of these older adults in the long-term care system.
Psychiatric disorders such as MDD, BPD, and SZP may have differing degrees of impact on cognitive function in later (da Silva, Goncalves-Pereira, Xavier, & Mukaetova-Ladinska, 2013; Frisoni et al., 2011; Masouy et al., 2011; Shah, Qureshi, Jawaid, & Schulz, 2012; Silva et al., 2009; Singh-Manoux et al., 2010). Recent research using the nationally representative Health and Retirement Study (HRS) data shows that approximately 13% of HRS respondents over age 65 report having been told they have psychiatric, emotional, or nervous problems, and that the proportion is greater among respondents reporting a history of early life disadvantage (Brown, 2010). HRS respondents reporting a history of psychiatric, emotional, or nervous problems score significantly lower on assessments of cognitive function and show moderately but significantly steeper rates of cognitive decline as they age (Brown, 2010). These findings, however compelling in terms of revealing a potential vulnerability for cognitive impairment in this subset of older adults, are based on self-reports of a broad mental health category included in the HRS survey.
The use of a broad mental health category in the HRS data limited the ability of the previous analysis to identify specific serious mental illnesses or to identify which types of mental illness show stronger associations with poor cognitive function. By their nature, chronic mental illnesses may have a very different effect on cognitive function across the life course than would acute episodes of mental distress or later life depression. The inability to distinguish between different mental health conditions may have resulted in conservative estimates of the size of the relationship between psychiatric history and cognitive function and decline (Brown, 2010). Individuals with psychiatric disorders also face social stigma, which may result in the underreporting of psychiatric problems. Utilizing HRS-linked Medicare administrative records provides an opportunity to identify HRS respondents whose Medicare claims indicate a clinical diagnosis of a specific serious mental illness (Kilbourne et al., 2005).
This study contributes to the geriatric mental health literature by utilizing administrative data linked to a national probability sample of older adults to describe the prevalence rates of specific mental illness and dementia diagnoses. This study will address the following research questions:
Research Question 1: What is the prevalence of serious mental illness(i.e., SZP, BPD, and MDD) among Medicare beneficiaries in the HRS sample?
Research Question 2: What is the prevalence of dementia diagnoses (Alzheimer’s disease [AD] and related dementias) among Medicare beneficiaries in the HRS sample?
Research Question 3: What is the prevalence of the co-occurrence of serious mental illnesses and dementia among Medicare beneficiaries in the HRS sample?
Literature Review
Mental Illness in the General Population
Prevalence estimates of serious mental illness vary by population, by mental illness definitions, and by the measurements or methods used to calculate estimates. About 1% of the general population suffers from SZP, with disease onset usually occurring between the ages of 16 and 30 and only rarely after the age of 45 (NIMH, 2014c; Selvendra, Baetens, Trauer, Petrakis, & Castle, 2014). Prevalence estimates for BPD Types I and II range from 1% to 3.4% in community-based populations to as high as 9.8% among primary care patients, although evidence is growing that these prevalence rates are underestimated (Cerimele et al., 2014; Das et al., 2005; Fagiolini et al., 2013; Thomas, 2004). The NIMH (2015) estimates a 12-month prevalence rate for BPD of 2.6% among U.S. adults, with 83% of cases being classified as severe. BPD often develops in adolescence or early adulthood, with onset in 50% of cases occurring before age 25 (Beesdo et al., 2009; NIMH, 2014a; Thomas, 2004). Symptoms rarely begin in late life. Approximately 6.7% of adults in the United States experience MDD each year, with average age of onset at 32 (NIMH, 2014b). According to U.S. National Comorbidity Survey Replication data, the onset of most serious mental illnesses occurs prior to mid-life (Kessler et al., 2007). As a result, the majority of older adults with a serious mental illness have likely been living with that diagnosis for decades.
Prevalence Among Older Adults
Mental illness. The NIMH estimates prevalence rates among U.S. adults in general but does not always make distinctions by age. The NIMH (2015) does estimate the lifetime prevalence of mood disorders (12%) and BPD (1%) among U.S. adults aged 60 and older. The Substance Abuse and Mental Health Services Administration (SAMHSA) distinguishes by age, and their definition of “older adults” includes everyone 50 and older. Rather than itemizing their prevalence rates by specific diagnoses like BPD or SZP, SAMHSA (2013) defines someone as being seriously mentally ill if they have at least one mental disorder, other than developmental or substance abuse disorders, resulting in serious impairment in the past 12 months. According to the 2012 National Survey on Drug Use and Health (SAMHSA, 2013), 2.8% of respondents aged 50 or older experienced a serious mental illness in the previous year and 15% experienced any mental illness in the previous year. Using 2000 census data, Hudson (2012) estimates that 1.2% of household-dwelling Americans aged 65 or older were diagnosed with at least one serious mental illness.
Most epidemiological data sets measure the prevalence of mental illness among persons living in individual households, omitting persons living in group quarters. This approach to measurement inherently excludes those who are so severely mentally ill as to require a group residential setting or institutionalization in either a psychiatric facility or a skilled nursing facility. According to the U.S. Census Bureau, at least 3.1% of Americans aged 65 or older would have been excluded from any household-based counts of mental illness prevalence because they were residing in a skilled nursing facility (Werner, 2011).
Cognitive impairment.
The Alzheimer’s Association (2014), using data from the U.S. census and the HRS Aging, Demographics, and Memory Study (ADAMS), estimates that 11% of Americans aged 65 and older have AD. Prevalence rates increase with age to as high as 32% of those 85 and older. Other prevalence estimates in adults aged 65 or older range from 4.7% with AD in the U.S. Census Bureau data, to 7.5% with any dementia in the Indianapolis-Ibadan Dementia Project data, to 13.67% with any dementia using the HRS ADAMS and U.S. Census Bureau data (Brookmeyer et al., 2011; Hebert, Weuve, Scherr, & Evans, 2013; Rocca et al., 2011). The majority (82%) of Americans with AD are 75 or older. Little information is available, however, about older adults with a history of mental illness who develop dementia (Perivoliotis, Granholm, & Patterson, 2004). Prevalence estimates of mental disabilities or disorders often combine psychiatric problems and age related cognitive disorders in the same category (Centers for Disease Control, 2006; National Library of Medicine, 2006; U.S. Census Bureau, 2006), while studies exploring psychosis accompanying mild cognitive impairment or dementia focus primarily on aging-related depression (Chan, Kasper, Black, & Rabins, 2003; Maddux, Delrahim, & Rapaport, 2003).
The literature suggests that demographic and socioeconomic factors may influence the prevalence and severity of psychiatric diagnoses (Fryers, Melzer, & Jenkins, 2003) and that adults with a history of mental illness may be at higher risk for developing cognitive decline or dementia (Gildengers, Butters, et al., 2004; Gildengers, Mulsant, et al., 2009; Wetherell, Gatz, Johansson, & Pederson, 1999; Zorrilla et al., 2000) However, studies exploring this possible connection are often based on small, nonrepresentative samples and produce mixed results. The majority of published studies involving patients with a combination of psychiatric problems and cognitive disorders tend to focus on older adults with aging-related depression (Bartels et al., 2003; Kunik et al., 1999; Maddux et al., 2003; Unützer & Bruce, 2002). This limits our understanding of how a broader array of mental illnesses may be related to later life cognitive functioning.
Using Administrative Data
Researchers often look to administrative data sets to estimate the prevalence of mental illness among older Americans. These administrative data sets can include data from the Centers for Medicare and Medicaid Services, such as Medicare beneficiary data or the national registry of nursing home resident admissions in the minimum data set (MDS). Grabowski, Aschbrenner, Feng, and Mor (2009) estimated the prevalence of mental illness (BPD, SZP, anxiety, or depression) and serious mental illness (BPD, SZP, or MDD) among new adult nursing home admissions in 2005 using data from the MDS. They identified 2.7% of 1.2 million new admission across the United States in the serious mental illness group and 27.4% in the more general mental illness group. Average age at first admission varied based on whether or not admissions were identified with mental illness (Grabowski, Aschbrenner, Feng, & Mor, 2009). The average age of all new admissions in 2005 was 77 compared to age 62 at first admission for people with serious mental illness.
Unlike the general admissions population, of whom only 14% were younger than 65, 31% of new nursing home admissions with serious mental illness were between the ages of 18 and 54, and 23% were between the ages of 55 and 64 (Grabowski et al., 2009). The age distribution of new admissions in the broader mental illness category was more similar to the distribution of the general admissions population, with over 50% between the ages of 65 and 84 and 20% below the age of 65. New admissions with mental illness and serious mental illness (32.6% and 45.6%, respectively) were also more likely to transition to long-stay status, or remain in the facility at least 90 days after admission, while only 24% of new admissions without a mental illness diagnoses were still residing in the facility 90 days after admission (Grabowski et al., 2009). Fullerton, McGuire, Feng, Mor, and Grabowski (2009), using MDS admissions data from 1999 through 2005, reported that the percentage of new admissions with a diagnosis of SZP or BPD were stable at .5% and .4%–.5%, respectively. New admissions diagnosed with anxiety disorders were more prevalent, ranging between 2.3% and 2.5%, but the most common mental illness in new nursing home admissions was depression, which grew from 11% in 1999 to 15.5% in 2005 (Fullerton, McGuire, Feng, Mor, & Grabowski, 2009).
These previous studies, which focus on administrative data from skilled nursing facilities, show that while a relatively small proportion of new admissions to skilled nursing facilities are diagnosed with serious mental illness, they are at greater risk of being admitted at younger ages and of long-term residence in facilities. Other research estimating the prevalence of mental illness in administrative claims data focus on depression or psychosis rather than other serious and persistent mental illness diagnoses like BPD or SZP (Bynum et al., 2004; Crystal, Sambamoorthi, Walkup, & Akincigil, 2003; Lin, Zhang, Leung, & Clark, 2011). What is not evident in these existing studies of serious mental illness among older adults is the prevalence of mental illness among older adults in the community or the prevalence of co-occurring mental illness and dementia. An improved understanding of these prevalence rates would provide a better understanding of the population of older adults at risk of premature admission to, and longer term residence in, skilled nursing facilities. Therefore, this study examines Medicare claims data from multiple care settings to estimate the prevalence of serious mental illness, dementia, and co-occurring mental illness and dementia among Medicare beneficiaries.
Research Design
Data
This project explores the prevalence of specific severe and persistent psychiatric conditions (MDD, BPD, and SZP) and cognitive disorders using diagnostic codes contained in Medicare claims and summary data files linked to respondents in the University of Michigan Asset and Health Dynamics Among the Oldest-Old (AHEAD) study and the HRS. The HRS is a longitudinal, nationally representative panel study, using a multistage area probability sample of households, with oversampling of Blacks, Hispanics, Floridians, and oldest old households (University of Michigan, 2009). The HRS began in 1992, and the AHEAD began in 1993; the two were merged into a single ongoing panel study in 1998. The Children of Depression and War Babies cohorts were also added in 1998, followed by the Early Baby Boomer cohort in 2004, and the Mid Baby Boomer cohort in 2010 (University of Michigan, 2009). The HRS collects a variety of information including labor force participation, assets, pension plans, disability, physical health and functioning, cognitive function, health insurance and health-care expenditure.
The current study uses data from the 1995, 1998, 2000, 2002, 2004, 2006, 2008, and 2010 AHEAD and HRS surveys. The Medicare beneficiary sample was constructed using the 1991–2010 data from the annual Medical Provider Analysis and Review (MedPAR), Outpatient, Home Health, and Hospice files (University of Michigan, 2014). We include all data records beginning in 1991 or the year of the beneficiary’s 65th birthday (whichever comes later) and ending in the beneficiary’s year of death or 2010 (whichever comes sooner). Thus, individuals contribute from 1 to 20 years of diagnostic indicators to our analysis. This research was approved by the institutional review board of a large Northeastern university.
Because the data come from administrative records, the problems of attrition and nonresponse, which are pervasive in panel survey designs, are largely eliminated. However, some HRS respondents refused permission to access their Medicare records, raising the possibility that the Medicare claims files available to researchers are biased in relation to the full HRS sample. Moreover, for those without linked Medicare records, all the diagnostic items used in our analysis are necessarily missing. It would be impractical to develop a missing data imputation model for every such diagnostic item. Therefore, we have used inverse probability weighting (Seaman & White, 2013) to correct for selection bias in our sample of linked HRS respondents. We linked our sample of Medicare beneficiaries to the HRS “tracker” file, which records each respondent’s history of survey participation and (if relevant) nonresponse and also provides several demographic and background variables. We then estimated a logistic regression model that predicts whether each person in the tracker file appears in the Medicare claims file, using as predictor variables age, gender, race, ethnicity, nativity, and level of education. The inverse of the predicted probability of appearing in the claims files is, finally, used as a weight in our prevalence analysis. This approach gives relatively more weight to individuals with a low estimated probability of appearing in the analysis and resulted in a final weighted sample of 18,740 beneficiaries.
Estimation Method
The HRS-linked Medicare files offer a variety of ways to identify beneficiaries who had at least one Medicare claim between 1991 and 2010, in which they were diagnosed with mental illness or cognitive disorders. This analysis defines these diagnoses using specific diagnostic categories (SZP, BPD, MDD, or dementia) from the International Classification of Disease Codes, Version 9, in the annual MedPAR, Outpatient, Hospice, and Home Health claims files. Each instance of any of these diagnoses can be associated with an age in the claims files. Once each data file was used to identify Medicare beneficiaries who have been coded at least once between 1991 and 2010 with conditions of interest to this study (SZP, BPD, MDD, or dementia), these codes were used to construct an “ever diagnosed with” variable for each condition (yes = 1, no =0).
Beneficiaries may appear in the claims files from 1 to 20 times during the part of their life that they were at least 65 years old. In order to treat all individuals comparably, we produce for each beneficiary a single summary record, corresponding to the oldest age recorded in the claims file, and containing all the ever diagnosed with codes described above. Using this summary file, we develop age-specific cumulative prevalence estimates for each condition. For example, among individuals whose claims history ends at age 92, the mean of each diagnostic indicator reveals the cumulative prevalence of the respective indicators among those surviving to age 92.
We conducted several descriptive analyses of the cumulative prevalence measures. Age-specific cumulative prevalence for each condition is presented in graphical form. Two-way tabulations were performed to deter- mine co-occurrence rates for each specific mental illness and dementia. Finally, binary logistic regressions were run to determine the probability of dementia diagnoses among beneficiaries diagnosed with each specific mental illness. The logistic regressions introduce controls for age, race, sex, and education.
Results
On average, beneficiaries are 74 years old. The majority are female (58%), White non-Hispanics (73%), and with at least a high school diploma or equivalent level of education (67%). Using weighted counts from the Med- PAR, Outpatient, Hospice, and Home Health files to identify individuals with dementia or serious mental illness (SZP, BDP, or MDD) resulted in prevalence estimates in this sample (Table 1) that are comparable to those found in the literature. About 10.24% of the current sample had received at least one dementia diagnoses (Alzheimer’s or other type) between 1991 and 2010, while 1.11% have received an SZP diagnosis, 2.64% a BPD diagnosis, and 4.19% an MDD diagnosis during that 20-year period. Overall, 6% of the Medicare beneficiaries in this sample have received at least one diagnosis of a serious mental illness between 1991 and 2010.
Table 1.
Weighted Prevalence Estimates for Specific Psychiatric or Dementia Diagnoses, ICD-9a Definitions, Medicare 1991–2010.
| Percentage of Beneficiaries | |
|---|---|
| Diagnoses | (N= 18,740) |
| Any dementia | 10.24 |
| Any schizophrenia | 1.11 |
| Any bipolar disorder | 2.64 |
| Any MDD | 4.19 |
| Any episodic mood disorders—including MDD | 5.54 |
| Any SPMI—including MDD | 6.02 |
Note. MDD = major depressive disorder; SPMI = severe and persistent mental illness.
International Classifications of Disease, Version. 9 (ICD-9) diagnosis data from Medical Provider Analysis and Review, Outpatient, Home Health, and Hospice data files, 1991–2010.
Table 2 presents the conditional probabilities of having dementia given a specific form of mental illness in this sample. Weighted bivariate cross tabulations indicate significantly higher probabilities of a dementia diagnosis in beneficiaries who are diagnosed with SZP (21.08%), BPD (14.54%), or MDD (25.79%) than among the overall sample (10.24%). There is, however, a relatively low degree of association between these mental illness diagnoses and dementia, with Phi coefficients ranging from .02 for BPD to .11 for MDD. Conversely, the conditional probability of a dementia diagnosis was significantly lower (not presented) among beneficiaries without a diagnosis of SZP (10.12%), BPD (10.12%), or MDD (9.56%).
Table 2.
Weighted Conditional Probabilities of Dementia Diagnoses in Beneficiaries With Serious Mental Illness, ICD-9a Definitions, Medicare 1991–2010.
| Conditional Probabilityb | Conditional Probability With Controlsc | |||||
|---|---|---|---|---|---|---|
| Co-Occurring Diagnoses | Percentage | Phi Coefficients | χ2 Probability | OR | 95% Cl [Minimum, Maximum] | χ2 Probability |
| Schizophrenia and dementia | 21.08 | 0.04 | *** | 5.822 | [3.942, 8.598] | *** |
| Bipolar disorder and dementia | 14.54 | 0.02 | *** | 5.399 | [3.982, 7.321] | *** |
| Major depressive disorders and dementia | 25.79 | 0.11 | *** | 4.895 | [4.027, 5.950] | *** |
Note. N = 18,740. OR = odds ratio; CI confidence interval.
International Classifications of Disease, Version 9 (ICD-9) diagnosis data from Medical Provider Analysis and Review, Outpatient, Home Health, and Hospice data files, 1991–2010.
Bivariate cross tabulations.
Binary logistic regression models include controls for age, sex, race, and education.
~p < .10. *p < .05. **p < .01. ***p < .0001.
The logistic regression results shown in Table 2 confirm the associations between mental illness and dementia diagnoses and indicate that Medicare beneficiaries in this sample face significantly greater odds of being diagnosed with dementia if they are diagnosed with SZP (odds ratio [OR] 5.822, 95% confidence interval [CI] = [3.942, 8.598]), BPD (OR=5.399, 95% CI [3.982, 7.321], or MDD (OR = 4.895, 95% CI [4.027, 5.950], after controlling for covariates.
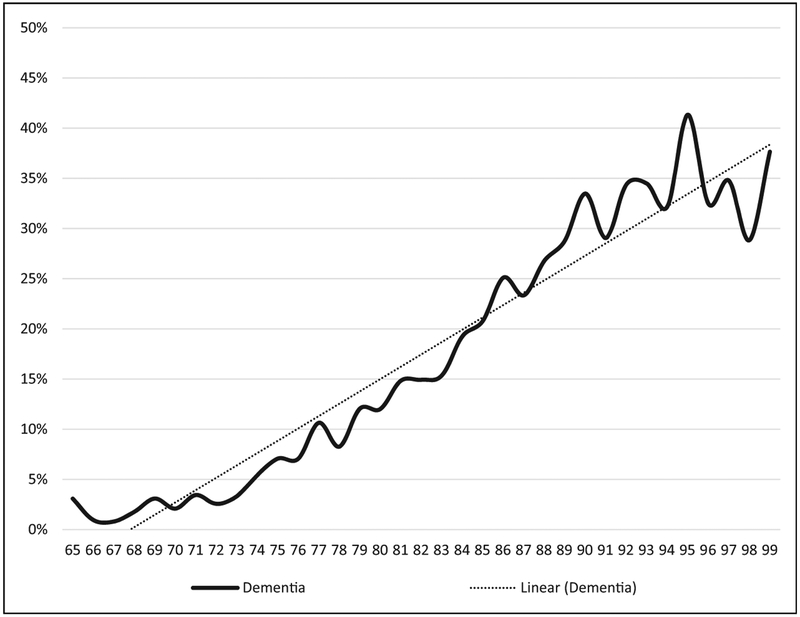
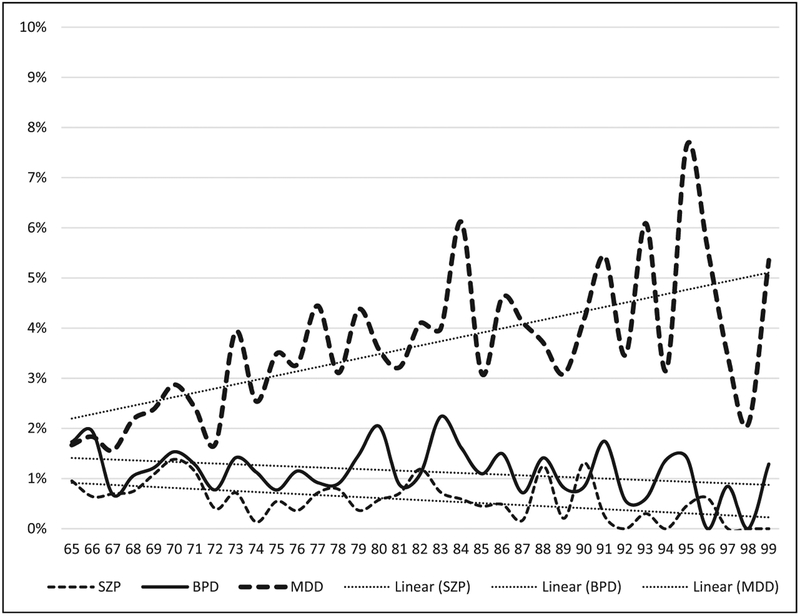
Figure 1 illustrates that the age-specific cumulative prevalence of dementia increases with age, from about 3% among beneficiaries aged 65–73 to about 35% among beneficiaries aged 92–99. A linear regression of cumulative prevalence on age is included in order to summarize the relationship; its slope indicates that the cumulative prevalence of dementia rises by 1.2 percentage points per year of age, on average. Figure 2 illustrates that the age-specific cumulative prevalence of MDD increases with age, while the age-specific cumulative prevalence of SZP and BPD declines with age. These age-specific estimates span the ages of 65–99; data for ages 100–113 are excluded from the reported results because the relatively small numbers of individuals in each age-group (ranging from 0 to 41) result in erratic prevalence estimates at these older ages.
Figure 1.
Age-specific cumulative prevalence of dementia by age, ICD-9 definitions, Medicare 1991–2010.
Source: International Classifications of Disease, Version 9 (ICD-9) diagnosis data from Medical Provider Analysis and Review, Outpatient, Home Health, and Hospice data files, 1991–2010.
Figure 2.
Age-specific cumulative prevalence of MDD, BPD, and SZP by age, ICD-9 definitions, Medicare 1991–2010. SZP= schizophrenia; BPD = bipolar disorder;MDD =major depressive disorder.
Source:International Classifications of Disease, Version 9 (ICD-9) diagnosis data from Medical Provider Analysis and Review, Outpatient, Home Health, and Hospice data files, 1991—2010.
Discussion
Our prevalence estimates for dementia and serious mental illness were similar to prevalence rates from previous studies (Alzheimer’s Association, 2014; Brookmeyer et al., 2011; Cerimele et al., 2014; Crystal et al., 2003; Das et al., 2005; Fagiolini et al., 2013; Hebert et al., 2013; NIMH, 2014a, 2014b; Rocca et al., 2011; Thomas, 2004). Our results indicate that the odds of being given a diagnosis of dementia, and the prevalence of dementia diagnoses, are higher among older adults with a diagnosis of serious mental illness (SZP, BPD, or MDD). These analyses confirm previous research finding associations between cognitive impairment and self-reports of more broadly defined psychiatric problems (Brown, 2010). The correlation between these two groups of conditions places individuals at greater risk of nursing home admission and long-term residency than the rest of the population of older adults.
Decreases in the age-specific cumulative prevalence of SZP and BPD can be explained by the fact that people with serious mental illness have higher mortality rates than their nonmentally ill counterparts (Colton & Manderscheid, 2006). However, as all older Americans live longer, including individuals with a history of mental illness, the proportion of mentally ill residents requiring long-term care can be expected to increase (Fullerton et al., 2009). Without a corresponding increase in appropriate community-based services for older adults with serious mental illness, this population will continue to be at risk for placement and long-stay residency in skilled nursing facilities (Grabowski et al., 2009).
Limitations
This analysis demonstrates the predictive power and limitations of using administrative claims data for identifying individuals with serious mental illness or dementia in the Medicare population. The limitations inherent in using claims data include incomplete, inaccurate, or missing data, particularly for beneficiaries in Medicare Health Maintenance Organizations (HMOs), or billing codes that lack the specificity needed to identify certain conditions (Stein, Lum, Lee, Rich, & Coleman, 2014; Taylor, Ostbye, Langa, Weir, & Plassman, 2009; Weir, Faul, & Langa, 2011). A beneficiary in a health claims database may have many claims over a period of several years, but if health-care providers do not determine that their psychiatric illness is relevant to a particular episode of medical care, that diagnosis will not appear on claims related to that episode of care. These omissions, which may make sense in the context of care provision, can result in an underestimation of psychiatric illnesses in that administrative claims data and may compromise researcher’s ability to examine issues related to quality, costs, and utilization. Our use of cumulative rates rather than age-specific prevalence or incidence rates represents an attempt to minimize any biases that might be attributable to such lapses in the use of diagnostic codes. The advantages to using health claims data to study the prevalence of physical or mental health diagnoses include access to large and diverse samples, access to follow-up data for longitudinal studies, the absence of selection bias, and the potential for conducting complex, multivariate analyses (Stein et al., 2014).
Conclusion
This study provides a basis for understanding the cognitive challenges facing older adults with a history of serious mental illness, which can inform policy supports and home- and community-based long-term care services for older adults with psychiatric problems and different types of cognitive impairment. Prevalence estimates for mental illness, cognitive disorders, and dual diagnoses of these two conditions vary based on the method used to collect data and to calculate estimates. Future research should compare these prevalence estimates to the prevalence of self-reported psychiatric, emotional, or nervous problems among the same beneficiaries in the HRS sample. Additionally, future studies can examine associations between serious mental illness and the Telephone Interview for Cognitive Status scores of these HRS beneficiaries, and model differences in nursing home utilization based on singular and dual diagnoses of serious mental illness and dementia, controlling for life-course factors, socioeconomic status, health conditions, and health behaviors.
Dual diagnoses of mental disabilities require further investigation, as these interrelated conditions could potentially impact the availability of natural social supports at critical points in later life, leaving increasing numbers of mentally ill adults dependent on long-term care services (Maddux et al., 2003). Data from the Alzheimer’s Association (“How Many Americans Have AD?,” 2014) indicate that without significant medical breakthroughs in preventing, slowing, or curing AD, the number of older adults with Alzheimer’s and other dementias is projected to triple by the year 2050. This dramatic rise in the prevalence of dementia, and the potential for higher prevalence rates among older adults with serious mental illness, will further strain an already overburdened long-term care system as well as contribute to Medicaid’s financing problems.
From a public health perspective, a better understanding of the relationship between psychiatric conditions and dementia can inform programs designed to promote early diagnosis of dementia in affected older adults, improve mental health in adults with dementia, and enable older adults with dementia to remain independent in the community. Geriatric social workers at every level of care need appropriate training to better care for this population and should advocate for those supports necessary to allow mentally ill older adults to age in community rather than in institutions. Strengthening supports for this population will improve the quality of life for older adults with mental illness who develop dementia and potentially prevent unnecessary and premature institutionalization in skilled nursing facilities.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging under Grant 5P30AG034464–04 (principal investigator: Douglas A. Wolf).
Biographies
Maria Teresa Brown is an assistant research Professor at Syracuse University’s Aging Studies Institute. She is a social gerontologist who uses the life-course perspective to research the later life experiences of socioeconomically disadvantaged individuals, women, and racial, ethnic, sexual, and gender minorities.
Douglas A. Wolf is a professor of Public Administration at Syracuse University’s Maxwell School and a faculty associate in the Aging Studies Institute. A primary theme of Wolf’s research is the role of family and kinship patterns in shaping living and care arrangement choices facing older people and their immediate kin.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alzheimer’s Association. (2014). 2014 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 10, e47–e92. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Dums AR, Oxman TE, Schneider LS, Arean PA, Alexopoulos GS, & Jest DV (2003). Evidence-based practices in geriatric mental health care: an overview of systematic reviews and meta-analyses. Psychiatric Clinics of North America, 26(2003), 971–990. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Höfler M, Leibenluft E, Lieb R, Bauer M, & Pfennig A (2009). Mood episodes and mood disorders: Patterns of incidence and conversion in the first three decades of life. Bipolar Disorders, 11, 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, & Kukull WA (2011). National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s & Dementia, 7, 61–73. doi: 10.1016/j.jalz.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT (2010). Early-life characteristics, psychiatric history, and cognition trajectories in later life. The Gerontologist, 50, 646–656. doi: 10.1093/geront/gnq049 [DOI] [PubMed] [Google Scholar]
- Bynum JPW, Rabins PV, Weller W, Niefeld M, Anderson GF, & Wu AW (2004). The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. Journal of the American Geriatrics Society, 52, 187–194. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (2006). Health Information for Older Adults [Published by the Centers for Disease Control]. Retrieved July 25, 2006, from: http://www.cdc.gov/aging/info.htm#3.
- Cerimele JM, Chwastiak LA, Dodson S, & Katon WJ (2014). The prevalence of bipolar disorder in general primary care samples: A systematic review. General Hospital Psychiatry, 36, 19–25. doi: 10.1016/j.genhosppsych.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D-C, Kasper JD, Black BS, & Rabins PV (2003). Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: The memory and medical care study. International Journal of Geriatric Psychiatry, 2003, 8. doi: 10.1002/gps.781 [DOI] [PubMed] [Google Scholar]
- Crystal S, Sambamoorthi U, Walkup JT, & Akincigil A (2003). Diagnosis and treatment of depression in the elderly Medicare population: Predictors, disparities, and trends. Journal of the American Geriatric Society, 51, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CW and Manderscheid RW (2006). Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing Chronic Disease, 3(2), 1–14. Retrieved from http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htm. [PMC free article] [PubMed] [Google Scholar]
- Das AK, Olfson M, Gameroff MJ, Pilowsky DJ, Blanco C, Feder A,…Weissman MM (2005). Screening for bipolar disorder in a primary care practice. Journal of the American Medical Association, 293, 956–963. doi: 10.1001/jama.293.8.956 [DOI] [PubMed] [Google Scholar]
- da Silva J, Goncalves-Pereira M, Xavier M, & Mukaetova-Ladinska EB (2013).Affective disorders and risk of developing dementia: Systematic review. British Journal of Psychiatry, 202, 177–186. doi: 10.1192/bjp.bp.111.101931 [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell’Osso MC,…Rossi A (2013). Prevalence, chronicity, burden and borders of bipolar disorder. Journal of Affective Disorders, 148, 161–169. doi: 10.1016/j.jad.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Geroldi C, Adorni A, Ghidoni R, Amicucci G,…Giannakopoulos P (2011). Alzheimer’s CSF markers in older schizophrenia patients. International Journal of Geriatric Psychiatry, 26, 640–648. doi: 10.1002/gps.2575 [DOI] [PubMed] [Google Scholar]
- Fryers T, Melzer D, & Jenkins R (2003). Social inequalities and the common mental disorders: A systematic review of the evidence. Social Psychiatry and Psychiatric Epidemiology, 38, 229–237. doi: 10.1007/s00127-003-0627-2 [DOI] [PubMed] [Google Scholar]
- Fullerton CA, McGuire TG, Feng Z, Mor V, & Grabowski DC (2009).Trends in mental health admissions to nursing homes, 1999–2005. Psychiatric Services, 60, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Seligman K, McShea M, Miller MD, Mulsant BH,…Reynolds CF (2004). Cognitive functioning in late-life bipolar disorder. American Journal of Psychiatry, 161, 736–738. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Mulsant BH, Begley A, Mazumdar S, Hyams AV,Reynolds CF,…Butters MA (2009). The longitudinal course of cognition in older adults with bipolar disorder. Bipolar Disorders, 11, 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski DC, Aschbrenner KA, Feng Z, & Mor V (2009). Mental illness in nursing homes: Variation across states. Health Affairs, 23, 689–700. doi: 10.1377/hlthaff.28.3.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weube J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CG (2012). Declines in mental illness over the adult years: An enduring finding or methodological artifact? Aging & Mental Health, 16, 735–752. doi: 10.1080/13607863.2012.657157 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, & Ustun TB (2007). Age of onset of mental disorders: A review of recent literature. Current Opinions in Psychiatry, 20, 359–364. doi: 10.1097/YCO.0b013e32816ebc8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne AM, Cornelius JR, Han X, Haas GL, Salloum I, Conigliaro J, & Pincus HA (2005). General-medical conditions in older patients with serious mental illness. American Journal of Geriatric Psychiatry, 2005, 250–254. doi: 10.1176/appi.ajgp.13.3.250 [DOI] [PubMed] [Google Scholar]
- Kunik ME, Snow-Turek AL, Iqbal N, Molinari VA, Orengo CA, Workman RH, & Yudofsky SC (1999). Contribution of psychosis and depression to behavioral disturbances in geropsychiatric inpatients with dementia. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 54A, M157–M161. doi: 10.1093/gerona/54.3.M157 [DOI] [PubMed] [Google Scholar]
- Lin W-C, Zhang J, Leung GY, & Clark RE (2011). Twelve-month diagnosed prevalence of behavioral health disorders among elderly Medicare and Medicaid members. The American Journal of Geriatric Psychiatry, 2011, 970–979. [DOI] [PubMed] [Google Scholar]
- Maddux RE, Delrahim KK, & Rapaport MH (2003). Quality of life in geriatric patients with mood and anxiety disorders. CNS Spectrums, 8, 35–47. [DOI] [PubMed] [Google Scholar]
- Masouy A, Chopard G, Vandel P, Magnin E, Rumbach L, Sechter D, & Haffen E (2011). Bipolar disorder and dementia: Where is the link? Psychogeriatrics, 11, 60–67. doi: 10.1111/j.1479-8301.2010.00348.x [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2014a). Bipolar disorder. Retrieved from http://www.nimh.nih.gov/health/topics/bipolar-disorder/index.shtml#part1
- National Institute of Mental Health. (2014b). Depression. Retrieved from http://www.nimh.nih.gov/health/topics/depression/index.shtml-part1
- National Institute of Mental Health. (2014c). Schizophrenia. Retrieved from http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
- National Institute of Mental Health. (2015). Bipolar disorder among adults. Retrieved from http://www.nimh.nih.gov/health/statistics/prevalence
- National Library of Medicine. (2006). Medical Subject Headings browser for theNational Library of Medicine’s controlled vocabulary thesaurus. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMDsearch&DB=mesh
- Perivoliotis D, Granholm E, & Patterson TL (2004). Psychosocial functioning on the independent living skills survey in older outpatients with schizophrenia. Schizophrenia Research, 69, 307–316. doi: 10.1016/j.schres.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Peterson RC, Knopman DS, Hebert LE, Evans DA, Hall KS, …White LR (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia, 7, 80–93. doi: 10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman SR, & White IR (2013). Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research, 22, 278–295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- Selvendra A, Baetens D, Trauer T, Petrakis M, & Castle D (2014). First episode psychosis in an adult area mental health service—A closer look at early and late onset first episode psychosis. Australasian Psychiatry, 22, 235–241. doi: 10.1177/1039856214532558 [DOI] [PubMed] [Google Scholar]
- Shah JN, Qureshi SU, Jawaid A, & Schulz PE (2012). Is there evidence for late cognitive decline in chronic schizophrenia? Psychiatric Quarterly, 83, 127–144. doi: 10.1007/s11126-011-9189-8 [DOI] [PubMed] [Google Scholar]
- Silva D, Santana I, Do Couto FS, Maroco J, Guerreiro M, & De Mendonca A(2009). Cognitive deficits in middle-aged and older adults with bipolar disorder and cognitive complaints: Comparison with mild cognitive impairment. International Journal of Geriatric Psychiatry, 24, 624–631. doi: 10.1002/gps.2166 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Akbaraly TN, Marmot M, Melchior M, Ankri J, Sabia S,& Ferrie JE (2010). Persistent depressive symptoms and cognitive function in late midlife: The Whitehall II study. Journal of Clinical Psychiatry, 71, 1379–1385. doi: 10.4088/JCP.09m05349gry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JD, Lum F, Lee RP, Rich WL, & Coleman AL (2014). Use of health care claims data to study patients with ophthalmologic conditions. Ophthalmology, 121, 1134–1141. doi: 10.1016/j.ophtha.2013.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2013). The NSDUH report: Revised estimates of mental illness from the National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality. [PubMed] [Google Scholar]
- Taylor DH, Ostbye T, Langa KM, Weir D, & Plassman BL (2009). The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. Journal of Alzheimer’s Disease, 17, 807–815. doi: 10.3233/JAD-2009-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P (2004). The many forms of bipolar disorder: A modern look at an old illness. Journal of Affective Disorders, 79, S3–S8. doi: 10.1016/j.jad.2004.01.001 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. (2006). Disability status: 2000—Census 2000 brief. Retrieved from http://factfinder.census.gov/servlet/SAFFPeople?_submenuIdpeople_4&_sse=on
- University of Michigan.(2009). Sample evolution: 1992–1998. Retrieved from http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf
- University of Michigan. (2014). HRS standard release Medicare data dictionary.Retrieved from http://hrsonline.isr.umich.edu/sitedocs/rda/cmsdocs/HRS_Standard_Release_Medicare.html#Filetypes
- Unützer J, & Bruce ML (2002). The elderly. Mental Health Services Research, 4,245–247. [DOI] [PubMed] [Google Scholar]
- Weir D, Faul J, & Langa KM (2011). Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: A comparison of HRS and ELSA. Longitudinal and Life Course Studies, 2, 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CA (2011). The older population: 2010. Retrieved from http://www.census.gov/2010census
- Wetherell JL, Gatz M, Johansson B, & Pederson NL (1999). History of depression and other psychiatric illness as risk factors for Alzheimer disease in a twin sample. Alzheimer Disease and Associated Disorders, 13, 47–52. doi: 10.1097/00002093-199903000-00007 [DOI] [PubMed] [Google Scholar]
- Zorrilla LTE, Heaton RK, McAdams LA, Zisook S, Harris MJ, & Jeste DV (2000). Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: No differences in age-related cognitive decline. The American Journal of Psychiatry, 157, 1324–1326. [DOI] [PubMed] [Google Scholar]