Abstract
Sustained attention is essential in important behaviors in daily life. Many neuropsychiatric disorders are characterized by a compromised ability to sustain attention, making this cognitive domain an important therapeutic target. In this study, we tested a novel method of improving sustained attention. Monkeys were engaged in a continuous performance task (CPT) while the nucleus basalis of Meynert (NB), the main source of cholinergic innervation of the neocortex, was stimulated. Intermittent NB stimulation improved the animals’ performance by increasing the hit rate and decreasing the false alarm rate. Administration of the cholinesterase inhibitor donepezil or the muscarinic antagonist scopolamine alone impaired performance, whereas the nicotinic antagonist mecamylamine alone improved performance. Applying NB stimulation while mecamylamine or donepezil were administered impaired CPT performance. Methylphenidate, a monoaminergic psychostimulant, was applied in conjunction with intermittent stimulation as a negative control, as it does not directly modulate cholinergic output. Methylphenidate also improved performance, and it produced further improvement when combined with NB stimulation. The additive effect of the combination suggested NB stimulation altered behavior independently from methylphenidate effects. We conclude that basal forebrain projections contribute to sustained attention, and that intermittent NB stimulation is an effective way of improving performance.
Keywords: Acetylcholine, Deep brain stimulation, Nonhuman primate, Nucleus basalis of meynert, Sustained attention
1. Introduction
In daily life, a large amount of visual information meets our eyes at every moment, but only a fraction can be processed by our brains. Attention plays an important role in selecting visual information relevant to the task at hand. The allocation of attention is imperfect. Information may be ignored or processed slowly due to boredom, fatigue, or distraction (Grier et al., 2003; Pattyn et al., 2008). Failures in information processing are particularly common after prolonged continuous periods of attention. The effortful maintenance of attention is called sustained attention or vigilance (Roberton and Garavan, 2004; Clayton et al., 2015). Occasionally, failures in sustained attention can lead to calamities. For example, a driver’s lack of focus on a traffic sign can lead to a serious collision. In addition, failures in sustained attention measured in the lab predict real world failures in attention and motor function (Manly et al., 1999). Understanding the neural mechanisms of sustained attention can inform efforts to improve clinical populations with impaired attention, and those suffering from age-related declines in attention.
Many cerebral cortex regions are involved in sustained attention (Langner and Eickhoff, 2013). Neural activity in the nucleus basalis of Meynert releases acetylcholine throughout the cerebral cortex (Gielow and Zaborszky, 2017; Mesulam et al., 1992; Selden et al., 1998) and is critical for many executive functions (Bartus, 2000; Goard and Dan, 2009; Pehrson et al., 2016; Richardson and DeLong, 1991; Sarter and Bruno, 1997; Terry and Buccafusco, 2003). Acetylcholine has a broad role in attention that has been thoroughly studied (Freitas et al., 2015; Howe et al., 2017; McQuail and Burk, 2006; Pehrson et al., 2016; Rezvani et al., 2009). Lesions of cholinergic projections result in 30% drop in hit rate during a sustained attention task (Dalley et al., 2001; McGaughy et al., 2002, 1996). Although there are other projection neurons in the nucleus basalis, and activation of these neurons does impact cortical functioning (Lin et al., 2015), our focus was on cholinergic neurons because of their intimate ties to growth factors (Knipper et al., 1994) and neural plasticity (Bakin and Weinberger, 1996; Juliano et al., 1991; Kilgard and Merzenich, 1998; Webster et al., 1991) which suggest a role in regulating the function of the cerebral cortex.
Deep brain stimulation exists as a means to alter selectively the activity of neurons in a spatially limited brain region. Its use in motor pathologies is thought to functionally decrease the efficacy of those areas by creating adaptation through a high sustained output (Vitek, 2002). A common current use treats Parkinson’s Disease (Wichmann and DeLong, 2006). Today, deep brain stimulation is being tested to remediate essential tremor, dystonia, obsessive-compulsive disorder, and Alzheimer’s disease (Kuhn et al., 2015; Laxton et al., 2010; Nowak et al., 2011; Wichmann and DeLong, 2006). In a recent study, intermittent NB stimulation in adult monkeys improved working memory performance through a cholinergic-dependent mechanism (Blake et al., 2017; Liu et al., 2017). Intermittent stimulation, unlike continuous stimulation, increased the functional efficacy of the brain area in which stimulation occurred (Blake et al., 2017; Liu et al., 2017). Unlike cholinesterase inhibitors which also target cholinergic neurons outside the basal-cortical cholinergic pathways (Kaasinen et al., 2002; Okamura et al., 2008), NB stimulation should modulate neocortical acetylcholine in addition to non-cholinergic ascending projections. Accordingly, we were motivated to test whether NB stimulation would improve sustained attention.
In the current project, we tested two rhesus monkeys using an adapted version of the Continuous Performance Task, or CPT (Riccio et al., 2001; Shalev et al., 2011). We combined the CPT with intermittent NB stimulation to investigate the function of the basal forebrain in sustained attention. Modest doses of cholinergic antagonists, and a cholinesterase inhibitor, were used to probe the necessity of cholinergic receptor activation for stimulation to exert behavioral effects, and to assess the potential balance of the roles of cholinergic and non-cholinergic projection neurons in the stimulation region. As a negative control, methylphenidate, a drug that increases noradrenaline and dopamine levels, was used to boost sustained attention performance. Methylphenidate has known positive impacts on sustained attention that do not come from a direct modulation of acetylcholine levels, and we predicted its modulation of sustained attention would be independent of any effects of deep brain stimulation.
2. Materials and methods
2.1. Subjects
Two six year old male rhesus monkeys (Macaca mulatta) were subjects in the experiment. The two animals are labelled in this work by abbreviating their lab names to Chf and Dit. These monkeys had taken part in our previous working memory task (Liu et al., 2017). Chf and Dit were naive to the current task before the experiment. The animals weighed 8 −11 kg, and lived together in a room (~20 m2) with a 12h light and 12h dark cycle. The animals were food-regulated during experimental days, and typically worked to satiety. Water was provided ad libitum throughout. The monkey weights were monitored every day, and they were supplied food matching experimental average caloric content on non-experiment days i.e., weekends. On experimental days, each animal was placed in a primate chair, a Lexan-made cube, with its head extended out through a neck yoke. The chair floor was height adjusted for comfort. One arm was restrained at the elbow to prevent rotation in the chair. The other arm extended from the front of the chair to operate the touchscreen and engage in the task.
All procedures on the animals were conducted in accordance with the Guide for the Care and Use of Animals, 8th Edition, and were approved by the IACUC at Augusta University.
2.2. Behavioral training
The animal in chair was placed in an acoustically insulated, electrically shielded metal chamber (Industrial Acoustics, North Aurora IL) and faced a touch screen attached to the wall. The distance from the eyes to the screen was 40 cm. A 36-cm diagonal visual touchscreen was adjusted to a comfortable height so that the subject could easily see it, and could see its hand when it touched the screen. The computer wiring was hidden from the animal’s view and access.
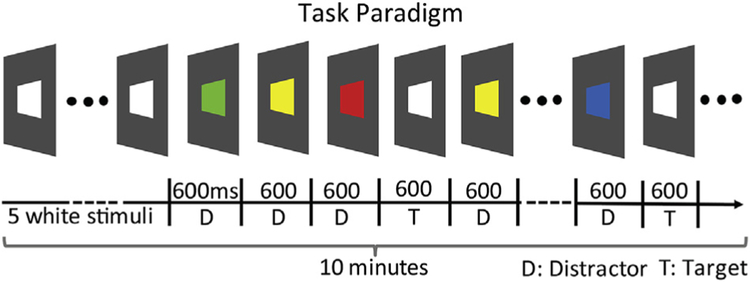
The behavioral task was a modified sustained and selective attention task adapted from a previously developed monkey CPT task (Aston-Jones et al., 1994; Golub et al., 2005). All the stimuli were created by custom-programmed software, which was adapted from the commercial software DTS-Gracewood (PBTLI Inc, http://www.pbtli.com, Augusta, GA). After the task started, a white square (of 15 degrees of visual angle) was presented centered on the screen. The task began with an initiation sequence. The animal was trained to touch a white stimulus and upon doing that, the stimulus disappeared. Following a brief delay (1 s), a second white square was presented. Touching a white square five times interleaved with 1 s waiting periods ended the initiation sequence and started a CPT test session (Fig. 1). After the initial five white stimuli, the animal was required to touch the screen whenever a white stimulus appeared and not to touch other color stimuli. Blue, red, yellow, green and white squares of the same size were pseudo-randomly presented. White squares were infrequent target stimuli; the other color stimuli were frequent non-targets. The targets were 20% of the stimuli. Each two target stimuli were separated by at least three non-target stimuli, and any adjacent stimuli were different in color. The stimuli were presented sequentially with no blank screen time. The monkey was rewarded with food slurry for correctly selecting the white squares in the stream. If the monkey selected a non-target stimulus, the sequence was interrupted with a 2 s blank screen timeout. Thereafter, a new initiation sequence started with a white square. If the animal missed a target stimulus, no reward was given, and the stimulus sequence continued without delay. If ten successive targets were missed, the stream stopped, and the initiation sequence started. The periods spanning ten such misses, and also the initiation sequences, were not included in analysis. Each testing session lasted roughly 10 min, and multiple sessions occurred each day in series.
Fig. 1.
The experimental paradigm. A white square was presented on the center of the screen at the beginning of the testing. Five successive taps on the white square started a CPT testing stimulus stream. Each stimulus duration was 600 ms. White squares in the stimulus stream were target stimuli. Any two adjacent target stimuli were separated by at least three non-target stimuli, and all colors were arranged pseudo-randomly. If the animal touched the target, food slurry reward was delivered to its mouth. Omission errors did not affect the stimulus stream, but inappropriate taps on the screen, errors of commission or false alarms, led to a 2–3 s timeout, after which a new stream was started. One testing session lasted 10 min. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The formal study began after a demonstration of adequate proficiency by the animals. Initially, each stimulus stayed on the screen for 1500 ms. When the animals achieved a hit rate above 85%, the stimulus duration was shortened. Eventually, the animals could perform the task with a 600 ms stimulus duration with high precision. In preliminary stimulation experiments in one animal, we tested NB stimulation with stimulus presentation durations of 600 ms, 750 ms, and 1000 ms. The data showed clear effects of the deep brain stimulation, on both hit and false alarm rates, with 600 ms stimulus presentation (Fig. 2). We used 600 ms in the main experiment, as longer durations presented a potential ceiling effect on hit rates.
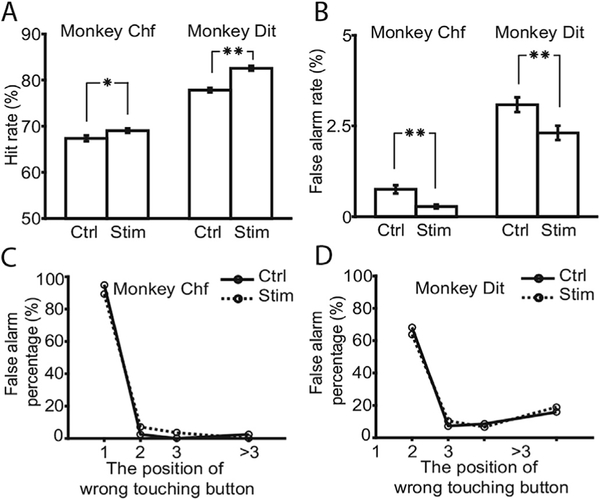
Fig.2.
Intermittent stimulation improved animals’ performance. A. Comparison of hit rates in control vs. stimulation. Left bars: animal Chf, right bars: animal Dit. B. Similar to A, but shows comparison of rates of commission errors (false alarms) in both animals. C. Distribution of false alarms on non-target to target stimulus. The horizontal axis indicates the position of the error of commission stimulus following a target stimulus. Solid line: data collected under the control condition, dotted line: data collected under stimulation. D. Data from animal Dit was shown as similar as section C. Error bars indicate the standard errors. The asterisks indicate the paired t-test results between any pair wised conditions. Single asterisk means 0.001 < p < 0.05, and double asterisk means p < 0.001.
2.3. Stimulation pulses and electrode
In the current study, we used only one set of stimulation parameters. They consisted of a biphasic pulse train, and each pulse had an initial negative phase of 200 mA, followed by a positive phase of 200 mA, and lasting for 100 ms per phase. The pulses were delivered at a rate of 60 Hz and applied for 20 s stimulation periods interleaved with 40 s stimulation-free periods. The stimulation cycle was set from the beginning of the test session to the end, and no attempt was made to synchronize the animal’s behavior to the onset or offset of the stimulation. We tested the impedances of each electrode each day to enable voltage controlled stimulation to deliver 200 mA and to verify electrode integrity. We found daily impedance checks indispensable to the research plan. The stimulation pulses were created by a multi-functional I/O device (USB-6211, National Instruments, Austin, TX), which was connected to a computer and controlled by a custom-programmed software (programmed by the authors in C#). The intermittent stimulation was the same as that used in a recent study (Liu et al., 2017), which documented that intermittent stimulation improved the animals’ working memory performance through cholinergic dependent mechanisms.
The stimulation electrodes were custom-made in the laboratory based on published specifications (McCairn and Turner, 2009, 2015). A Teflon-insulated wire (50 μm Pt/Ir, A-M systems, Seattle, WA) was embedded within a hypodermic tube (30 ga. A-M systems, Seattle, WA). A polyimide sheath (28 ga. A-M systems, Seattle, WA) encased and insulated the hypodermic tube. The wire extended out of the hypodermic tube by 1e2 mm as the tip of the electrode. The insulation was stripped for a distance of 0.5e1 mm to achieve an impedance of 5–10 kOhm at 1 kHz. After adjusting impedance, all the components were fixed with insulated adhesive. The wire extended 5 cm from the rear end of the hypodermic, and was soldered to a connector fixed on a small chamber encasing the electrode.
2.4. Implantation surgery
After 12 h food deprivation, the animal was sedated with an I.M. injection of ketamine (15 mg/kg, Sigma/Aldrich). The animal was then moved to a sterile surgery room. The heart rate, respiratory rate, and body temperature were monitored, and anesthesia was maintained with oxygen and isoflurane mixture air (1–2%) through an endotracheal tube during the procedure. After surgery, the animals were treated with buprenorphine (0.01 mg/kg B.I.D.) for 48 hrs.
Pilot determination of optimal electrode position in stereotactic coordinates is described in our prior work (Liu et al., 2017). Electrodes were positioned at interaural AP 16 mm, ML 8 mm, and 29 mm vertically lower than the top of the dura. The target location of the stimulation electrode tip was the anterior portion of the NB, which densely contains cholinergic neurons and supplies acetylcholine to the cerebral cortex (Mesulam et al., 1983). The effective area of the stimulation is estimated to be a 4 mm diameter sphere centered at the electrode tip (Vitek, 2002). In addition to the NB, the stimulation sphere may have extended to portions of the anterior amygdala (including the central nucleus), the anterior commissure and the inferior internal globus pallidus. Although the NB extends more than 10 mm rostra-caudally in the rhesus monkey, our electrode was positioned in the anterior third of the nucleus near the site of the projection of the central nucleus of the amygdala to the NB.
2.5. Pharmacological administration
Donepezil, mecamylamine, scopolamine and methylphenidate (Sigma-Aldrich, St. Louis, MO) were used. The drugs were dissolved in sterile saline (vehicle). The doses of the drugs were 200 mg/kg for donepezil, 300 mg/kg for mecamylamine, 6.2 mg/kg for scopolamine, and 200 mg/kg for methylphenidate, and were selected based on previous publications (Bain et al., 2003; Liu et al., 2017). Because performance declines over time while performing the CPT task (Rosenberg et al., 2013), drug effects were tested on different days. Residual drug effects may be retained on the second day after drug testing, therefore non-drug behavior was tested on the first days of a week, and drugs were tested on following days. Accordingly, drug-free behavior was tested at least 72 h after the previous administration of a drug. Tests for each condition were applied for a minimum of two weeks. Injections were given 15 min preceding behavior (Bain et al., 2003). On control days, water was injected as placebo. For mecamylamine, scopolamine, and donepezil the impacts of the drug doses vs. control were tested for two weeks, and then in a different two weeks the impacts of the drug vs. the drug þ stimulation were assessed. The methylphenidate experiments were conducted together over a four week period.
2.6. Data acquisition and analysis
Data were collected by customized DTS-Gracewood software. The computer recorded the stimulus parameters: color, onset time, offset time, session start time and session end time, and the behavioral events (touches to the screen). Data were saved for offline analysis. Further data analyses and statistics were performed in MATLAB (MathWorks, Natick, MA).
2.7. Signal detection metric
We calculated d’ values based on signal detection theory (Green and Swets, 1966) to describe the signal discriminability as inferred from the difference between hit and false alarm rates. The following equation was used:
Where ‘norminv’ is a function provided by MATLAB to calculate the inverse of the normal distribution (Mathworks, Natick, MA). Accordingly, d’ is the z-score difference between the hit and false alarm rates.
3. Results
Two task-naive monkeys were trained in a sustained attention task (CPT). The task had an initiating and a testing sequence. The initiating sequence displayed a white square on the touchscreen, and the square extinguished on touch. Five squares were touched at 1 s intervals to complete the initiation sequence. Thereafter in the testing sequence, each 600 ms a new square appeared on the screen. Monkeys were rewarded for touching white squares, and touching any other color square interrupted the stream of stimuli and requiring completion of the initiation sequence after a brief timeout.
During the task, hits, misses (errors of omission) and false alarms (errors of commission) were recorded. After animals consistently achieved a 60% hit rate, they were entered in study. During data collection, the two animals’ average hit rates were 70% and 74%, average false alarm rates were 0.58% and 2.48% for subjects Chf and Dit, respectively.
3.1. Stimulation effect on animals’ performance
We have reported that 1200 NB pulses delivered in 20 s followed by 40 s free of stimulation was optimal among 1 min period stimulation patterns (Liu et al., 2017). Here, we used the same stimulation parameters to investigate sustained attention. The CPT period of 600 ms was chosen to minimize ceiling effects on performance.
Hit rates were increased by NB intermittent stimulation (Fig. 2A). Rates of the monkey Chf improved from 67.4% to 69.0%, and those of Dit improved from 77.8% to 82.6%. The changes in hit rates were significant (binomial statistic, p = 0.007, N=5758 trialsfor animal Chf, and p < 0.001, N =5893, for animal Dit). False alarm rates were also decreased by stimulation. As shown in Fig. 2B, the false alarm rate of Chf and Dit changed from 0.75% to 0.28% and 3.8%e2.3%, respectively. The binomial tests showed these decreases in the false alarm rates were significant (p < 0.001, N 5758 for animal Chf, and p < 0.001, N = 5893, for animal Dit). In this behavior, the signal discriminability can be described by d’, which is the z-score difference between the hit and false alarm rates (Green and Swets, 1966). The discriminability of the white squares in the stream was increased by intermittent stimulation. In Chf, d’ increased from 2.88 to 3.27, and in Dit, it increased from 2.64 to2.93. Thus, the d’ changes in the two animals were 0.39 and 0.29 for Chf and Dit respectively.
False alarm responses were analyzed for their position in the stimulus stream. The positions of non-target stimuli were numbered based on the number of stimuli preceding them but after the most recent target. For example, when a distractor stimulus was presented following a target, the number assigned that stimulus was ‘1’ (the first distractor after a target). A subsequent distractor would be assigned a ‘2’, and so on. The rates of errors at these positions were plotted in Fig. 2C (animal Chf) and Fig. 2D (animal Dit). Most false alarm errors occurred on the first non-target stimulus immediately following a target stimulus, which were possibly erroneously slow responses to the target.
3.2. Effect of donepezil on behavioral performance
We hypothesized that NB stimulation increased cortical acetylcholine levels to improve sustained attention. To clarify the role of acetylcholine in sustained attention, we tested the animals with and without donepezil administration. Donepezil is a cholinesterase inhibitor which should prolong and amplify the effects of acetylcholine. We first used a donepezil dose of 200 mg/kg to maximize effects based on its impact on working memory performance (Liu et al., 2017). Unexpectedly, hit rates were decreased by donepezil application (Fig. 3A binomial statistic: p < 0.001, N = 5758 for animal Chf; p < 0.001, N 4498 for animal Dit). Similarly, false alarm rates were increased(Fig. 3B, binomial test: p <0.035, N = 1312 for animal Chf; p < 0.001, N=4498 for animal Dit). The value of d’ decreased by 0.65 and 0.29for Chf and Dit, respectively.
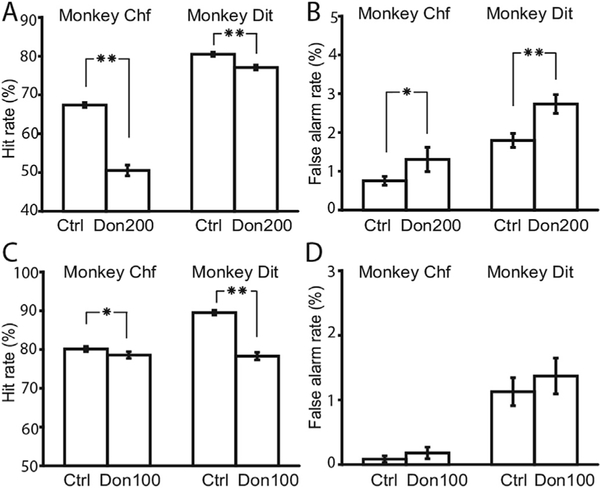
Fig. 3.
Donepezil decreased animals’ behavioral performance. A. The effect of a 200 mg/ kg dose donepezil on hit rate. B. The effect of 200 mg/kg dose donepezil on false alarm rate. C-D. The effects of a 100 mg/kg dose. Asterisks in the figure indicate the p values of paired t-test. Single asterisk means 0.001 < p < 0.05, and double asterisk means p < 0.001.
Subsequently, we tested the animals with the lower dose 100 mg/kg, and the data showed a similar but weaker result with a significant impairment in the hit rate (Fig. 3C, binomial statistic: p = 0.02, N =3500 for animal Chf; p < 0.001, N= 1768 for animal Dit). There was a non-significant increase of falsealarm rates with 100 mg/kg donepezil administration (Fig. 3D, binomial test: p = 0.23, N = 3500 for animal Chf; p = 0.366, N = 1768 for animal Dit). The value of d’ decreased by 0.30 and 0.55 for the two animals, respectively.
Performance under donepezil (100 μg/kg) was tested with and without stimulation (Fig. 4). Stimulation impaired animals’ hit rates significantly (binomial statistic, p < 0.001, N =2233 for animal Chf; p = 0.039, N = 1721 for animal Dit) and increased the animal’s falsealarm rates (Fig. 4B, p = 0.075, N = 2233 for animal Chf; p < 0.001, N = 1721 for animal Dit). The value of d’ decreased by 0.49 and 0.37for animals Chf and Dit, respectively.
Fig. 4.
Impact of intermittent stimulation on performance of animals with 100 mg/kg donepezil. A. Comparison of hit rates under donepezil vs. donepezil þ stimulation forboth animals. B. Comparison of rates of commission errors under donepezil vs. donepezil þ stimulation from both animals. Error bars indicate the standard errors. Asterisks in the figure indicate the p values of paired t-test. Single asterisk means0.001 < p < 0.05, and double asterisk means p < 0.001.
3.3. Effects of cholinergic antagonists
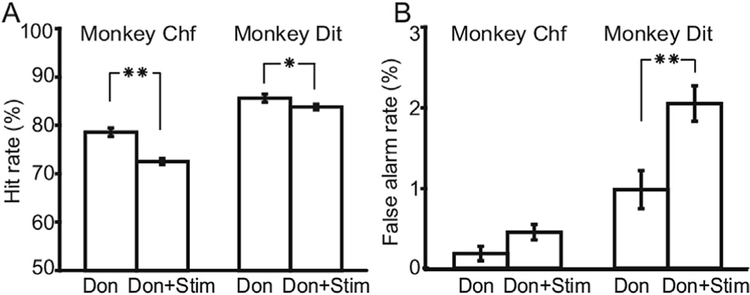
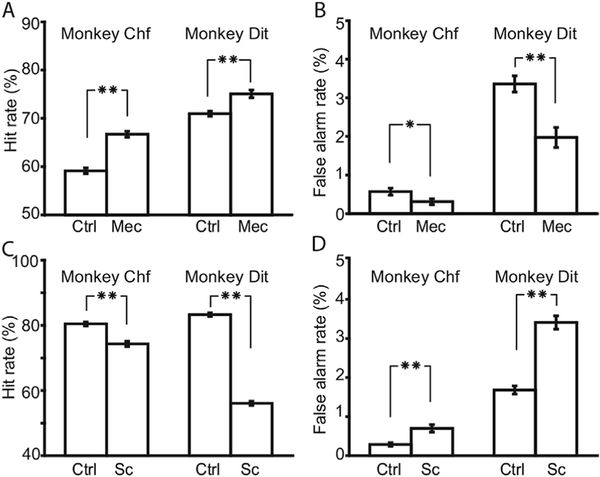
Acetylcholine affects neurons through ion channel nicotinic acetylcholine receptors (nAChR) and G-protein coupled muscarinic acetylcholine receptors (mAChR). To clarify the role of these receptors in sustained attention, we tested the animals’ performance with and without antagonists for these two receptor classes (Fig. 5A). Mecamylamine, a nAChR antagonist, improved hit rates (Fig. 5A, binomial test: p < 0.001, N = 5730 for Chf; p > 0.001, N = 2913, for Dit) and depressed false alarm rates in both animals (Fig. 5B, binomial test: p < 0.008, N = 5730 for Chf and p < 0.001, N = 2913 for Dit) Pharmacological treatment with mecamylamine increased d’ values by 0.41 and 0.35 for animal Chf and Dit, respectively (Fig. 5A). The mAChR antagonist scopolamine, in contrast, reduced hit rates (Fig. 5C, binomial test: p < 0.001, N =720, for Chf and p < 0.001, N = 5594, for Dit) and increased false alarm rates in both animals (Fig. 5D, binomial test: p < 0.001, N = 3720, for Chf and p < 0.001, N = 5594, for Dit). The values of d’decreased by 0.49 and 1.04 for Chf and Dit, respectively (Fig. 5C).
Fig. 5.
Impacts of cholinergic antagonists on animals’ performance. A. Mecamylamine improved hit rates of animals. B. Mecamylamine decreased error of commission rates of animals. C. Scopolamine decreased hit rates of both animals. D. Scopolamine increased error of commission rates of both animals. Error bars indicate the standard errors. Asterisks in the figure indicate the p values of paired t-test. Single asterisk means 0.001 < p < 0.05, and double asterisk means p < 0.001.
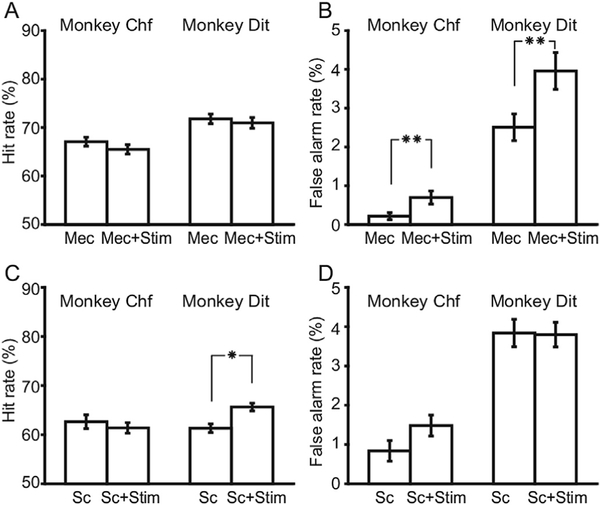
Performance was compared in the presence of these cholinergic antagonists with and without concurrent intermittent stimulation, as shown in Fig. 6A and B. The hit rates did not change significantly by adding NB stimulation to mecamylamine (binomial test: p < 0.104, N = 2413, for Chf; and p < 0.449, N=1690, for Dit). However, the animals’ false alarm rate was increasedby stimulation (0.58% for Chf and 1.8% for Dit, binomial test: p < 0.001, N 2413 for Chf; p < 0.001,N = 1690, for Dit). Stimulation decreased d’by 0.45 in Chf, and 0.23 in Dit.
Fig. 6.
Impacts of intermittent stimulation on animals’ performance with cholinergic antagonists. A. Hit rates of both animals with mecamylamine vs. mecamylamine þ stimulation. B. False alarm rates of both animals with mecamylaminevs. mecamylamine þ stimulation. C-D. Similar to A-B, comparisons under scopolaminevs. scopolamine þ stimulation. Error bars indicate the standard errors. Asterisks in thefigure indicate the p values of paired t-test. Single asterisk means 0.001 < p < 0.05, and double asterisk means p < 0.001.
For scopolamine, adding intermittent stimulation significantly improved the hit rate of animal Dit (by 4.3%, binomial statistic, p < 0.002, N = 3009) but not animal Chf (binomial statistic, p < 0.373, N = 1195). No significant change was found in the false alarm rates in either animal (binomial test: p < 0.071, N = 1195 for Chf;and p < 0.849, N 3009 for Dit). Adding stimulation toscopolamine led to a decreaseof d’ by 0.27 in Chf, and an increase of 0.11 in Dit.
3.4. Performance changes caused by intermittent stimulation and methylphenidate
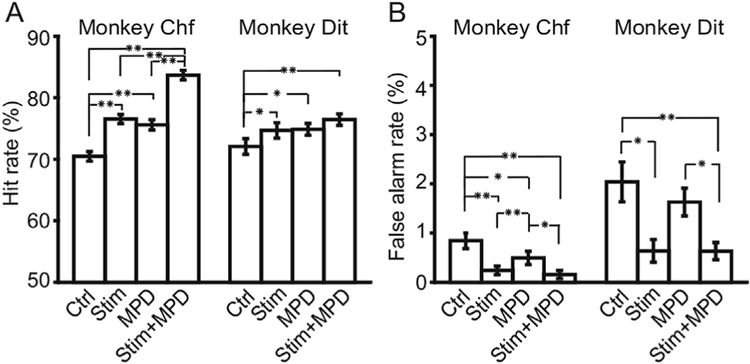
The effects of methylphenidate, with and without intermittent stimulation, were tested next. Methylphenidate is a non cholinergic agent that positively influences sustained attention (Losier et al., 1996). As shown in Fig. 7, the four experimental conditions were control, stimulation, methylphenidate, and stimulation þ methylphenidate, and these conditions were interleaved day by day. Significance was assessed by a 2-way ANOVA using the condition and animal as factors, and also by a binomial statistic with an exact P value reported in lieu of a posthoc test. In both animals, the hit rates were significantly elevated by NB stimulation or by methylphenidate, and the effects were greater when stimulation and methylphenidate were both applied to the animals (see Table 1). A 2-way ANOVA showed the significant effect of condition (F (3,3)=3.39, p=0.02 for main effect of conditionsF(1,3)=0.92, p = 0.34 for main effect of animals) (Fig. 7A). Falsealarm rates were reduced from control levels significantly in the other three conditions (Table 2). A 2-way ANOVA showed a significant effect in false alarm rates (F(3,3)=5.43, p= 0.002 for main effect of conditions, F(1,3)=17.09, p < 0.001for maineffect of animals) (Fig. 7B). The results demonstrate additive effects of methylphenidate and NB stimulation and suggest they operate through independent mechanisms.
Fig. 7.
Impacts of stimulation and methylphenidate on animals’ performance. A. comparison of hit rates of both animals under control, stimulation, methylphenidate and stimulation þ methylphenidate conditions. B. Comparison of false alarm rates ofboth animals under control, stimulation, methylphenidate and stimulation þ methylphenidate conditions. Error bars indicate the standard errors. Asterisks in the figure indicate the p values of paired t-test. Single asterisk means0.001 < p < 0.05, and double asterisk means p < 0.001. 2-way ANOVA shows significant difference between experimental conditions in both A and B.
Table 1.
Binomial statistic between conditions in Fig. 7A.
| Animal Chf | ||||
| Ctrl | Stim | MPD | ||
| Ctrl | ||||
| Stim | P < 0.001, N = 3253 | |||
| MPD | P < 0.001, N = 2676 | P = 0.245, N = 2676 | ||
| Stim+MPD | P < 0.001, N = 2354 | P < 0.001, N = 2354 | P < 0.001, N = 2354 | |
| Animal Dit | ||||
| Ctrl | Stim | MPD | ||
| Ctrl | ||||
| Stim | P = 0.0425, N = 1198 | |||
| MPD | P = 0.025, N = 1224 | P = 0.8940, N = 1198 | ||
| Stim+MPD | P < 0.001, N = 1224 | P = 0.1627, N = 1198 | P = 0.1047, N = 2028 | |
Table 2.
Binomial statistic between conditions in Fig. 7B.
| Animal Chf | ||||
| Ctrl | Stim | MPD | ||
| Ctrl | ||||
| Stim | P < 0.001, N = 3253 | |||
| MPD | P = 0.043, N = 676 | P > 0.001, N = 2676 | ||
| Stim+MPD | P < 0.001, N = 2354 | P = 0.672, N = 2354 | P = 0.018, N = 2354 | |
| Animal Dit | ||||
| Ctrl | Stim | MPD | ||
| Ctrl | ||||
| Stim | P = 0.052, N = 1198 | |||
| MPD | P = 0.257, N = 1224 | P = 0.252, N = 1198 | ||
| Stim+MPD | P < 0.001, N = 1224 | P = 0.567, N = 1198 | P = 0.011, N = 2028 | |
4. Discussion
In the present study, we tested the hypothesis that the basal forebrain projection to the cerebral cortex contributes to sustained attention performance, and the pharmacological basis of its effects. The experimental paradigm is a CPT in which a visual stimulus stream is presented over an extended time period. The animals were trained to select infrequent targets in a stream with frequent distractors. We found that NB intermittent electrical stimulation improved the animals’ sustained attention by increasing hit rates and decreasing false alarm rates. Donepezil, a cholinesterase inhibitor, decreased hit rates and increased false alarm rates. Scopolamine, an mAChR antagonist, impaired sustained attention performance while mecamylamine, a nAChR antagonist, improved it. The addition of stimulation during administration of mecamylamine or donepezil impaired performance, while results from combining scopolamine with stimulation were mixed. Methylphenidate was used as a negative control that modulates sustained attention. It boosted animals’ sustained attention performance, and the effect was independent of the effects of NB stimulation.
Our primary pharmacological hypothesis was that cholinergic pathways mediated the behavioral improvements. Acetylcholine facilitates attention and signal detection in the brain (Guillem et al., 2011; Parikh et al., 2007; Sarter et al., 2009). Either cholinesterase inhibitors or modest cholinergic blocks prevented clear positive impacts of stimulation, and in some cases led stimulation to impair behavior. Overall, positive effects of stimulation were not achieved with any pharmacological interference with the brain’s cholinergic systems, which supports the positive effects being cholinergically dependent.
Our work also bears on hypotheses that non-cholinergic neurons in the basal forebrain contribute to cognition. The primate nucleus basalis contains non-cholinergic projection neurons (Walker et al., 1989). In mice, activation of parvalbumin sensitive GABAergic projection neurons from the analogous nucleus results in increases in cortical gamma band activity (Kim et al., 2015). In our experiments with concurrent cholinergic pharmacological modulation (i.e. inhibition of nAChR or mAChR receptors or cholinesterase inhibition), the stimulation of these non-cholinergic projections may have an increased impact on behavior relative to stimulation during the unmodulated state. The impairment caused by adding intermittent stimulation to donepezil could have been caused by activation of these non-cholinergic cells. Such results must be interpreted with caution, as the normal role of the noncholinergic projection neurons is unlikely to occur independently from the cholinergic projections. Alternatively, cholinergic modulation in this task may follow a U-shaped dose response curve, in which optimal levels facilitated performance, but higher levels, as would be the case where NB stimulation is combined with done-pezil, increased ACh levels even further, and led to impaired performance.
Our method of stimulating the basal forebrain with electrical pulses applied on a microsecond scale with intermittent periods of stimulation might be expected to enhance the release of acetylcholine, and improve attention tonically (Descarries et al., 1997; Ren et al., 2011; Sarter et al., 2009; Zoli et al., 1999). Our stimulation was not synchronized with the behavior, which implies cognitive function was altered up to tens of seconds after stimulation. Electrophysiological evidence of such prolonged effects of cholinergic activation exist (Dannenberg et al., 2015; Pabst et al., 2016). The contrasting of rapid electrophysiological effects on the timescale of up to seconds with tonic effects lasting much longer plays into the existing debate on wiring vs. volume transmission of acetylcholine (Sarter et al., 2009). The volume transmission hypothesis suggests extrasynaptic acetylcholine receptors are activated by synaptic spillover, which has a time constant of at least seconds, while the wiring transmission mechanism operates using the synaptic cleft and has a faster time course. Of course, as second messenger activation can also have a reasonably slow time course, in vivo behavioral considerations of time course alone do not strongly argue the hypothesis.
In the present study, we only applied intermittent stimulation, which limits insight into comparative actions of continuous and intermittent stimulation from the current data. In our prior study of working memory (Liu et al., 2017), continuous stimulation impaired performance, and this impairment was reversed by donepezil. Those data suggested that continuous stimulation impaired acetylcholine release. As a result of that study, continuous stimulation parameters were not explored in the present study.
An important caveat in the interpretation of these results is that cholinergic drugs were administered systemically and exerted their effects throughout the intermittent NB stimulation procedure i.e. when the cholinergic neurons were on and off. Therefore cholinergic agents could be influencing cortical activity by altering baseline cholinergic transmission when the NB cholinergic neurons remained unstimulated. The effects of intermittent stimulating during cholinergic antagonism might have remained limited for this reason.
Methylphenidate was used as a negative control. This central neural system stimulant increases dopamine and norepinephrine concentrations within synapses by blocking the reuptake transporters (Wagner et al., 2009; Whyte et al., 2004) to achieve the goal of executive function disorder treatment (Losier et al., 1996). Because it does not directly modulate acetylcholine release, its known effects on sustained attention may not interact with the effects of deep brain stimulation. Methylphenidate improved sustained attention similarly to deep brain stimulation. Deep brain stimulation improved performance with or without methylpheni-date with comparable effect sizes consistent with independent mechanisms of effect.
Prior work has interpreted the positive modulation of working memory by donepezil as caused by cortical acetylcholine (Liu et al., 2017). In sustained attention work, nicotinic modulation often finds positive impacts (Rezvani and Levin, 2001) while muscarinic boosts tend to impair performance (Bushnell et al., 1997; Levin et al., 2011). Our work is complementary to this body of work, and we suggest the balance of effects, impairment, during donepezil administration was caused by muscarinic activation. The impairment caused by adding stimulation to donepezil may be caused by activating noncholinergic neurons, as discussed above. It was notable that in the mecamylamine administration experiments, intermittent stimulation decreased behavioral performance. These findings stand in contrast with the effects observed in a recent study on working memory (Liu et al., 2017), using the same animal subjects. Working memory performance improved by donepezil at the same high dose, and adding stimulation to mecamylamine did not impair performance. The disparate effects of these drugs suggest specific actions of acetylcholine in the two cognitive domains.
As mentioned above, our data showed that the nAChR antagonist mecamylamine had a positive effect on sustained attention. This result is at odds with the traditional view that mecamylamine impairs cognitive functions (Gitelman and Prohovnik, 1992; Little et al., 1998; Newhouse et al., 1992; Pickworth et al., 1997; Stolerman et al., 1973). Our result is not the only outlier; some recent papers have also reported similar positive effects of nAChRs antagonists, including mecamylamine, methyllycaconitine and dihydro-b-erythroidine, to executive functions (Terry et al., 1999; Levin and Caldwell, 2006; Hahn et al., 2011; Levin et al., 2013; Liu et al., 2017). Higher doses support the traditional view that mecamylamine impairs behavior, while lower doses support the converse view. A study aimed to address this issue reported that lower doses of mecamylamine did not affect human subjects’ performance significantly (Yuille et al., 2016). The dose of the drug used is one of many possible causes to the contrasting results. Another view is that application of such a low dose of an antagonist may alter the system similarly to nAChR desensitization (Yuille et al., 2016).
In summary, we supported our hypothesis that stimulation in nucleus basalis of Meynert improved sustained attentional performance most likely by altering neocortical cholinergic function.
Acknowledgments
We wish to thank N. Rodriguez and P. Otovic for veterinary support. Advice on methods for deep brain stimulation in primates came from the RS Turner laboratory. Helpful discussions on the manuscript came from NE Lambert.
Funding
This work was supported by the National Institutes of Health (R01MH097695 to CC and DTB).
References
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T, 1994. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci 14, 4467e4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JN, Prendergast MA, Terry AV, Arneric SP, Smith MA, Buccafusco JJ, 2003. Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology (Berl) 169, 150e160 10.1007/s00213-003-1483-1 [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM, 1996. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Nat. Acad. Sci. USA 93, 11219e11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, 2000. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol 163, 495e529 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Blake DT, Terry AV, Plagenhoef M, Constantinidis C, Liu R, 2017. Potential for intermittent stimulation of nucleus basalis of meynert to impact treatment of Alzheimer’s disease. Commun. Integr. Biol 889, 00e00 10.1080/19420889.2017.1389359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK, 1997. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 134, 230e241 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, Cohen Kadosh R, 2015. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci 19 (4), 188e195 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW, 2001. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J. Neurosci 21, 4908e4914 https://doi.org/21/13/4908[pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H, 2015. Synergy of direct and indirect cholinergic septohippocampal pathways coordinates firing in hippocampal networks. J. Neurosci 35, 8394e8410 10.1523/JNEUROSCI.4460-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M, 1997. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol 00050e00056 10.1016/S0301-0082(97. [DOI] [PubMed] [Google Scholar]
- Freitas KC, Hillhouse TM, Leitl MD, Negus SS, 2015. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev. Res 76, 194e203 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielow MR, Zaborszky L, 2017. The input-output relationship of the cholinergic basal forebrain. Cell Rep. 18, 1817e1830 10.1016/j.celrep.2017.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Prohovnik I, 1992. Muscarinic and nicotinic contributions to cognitive function and cortical blood flow. Neurobiol. Aging 13, 313e318 10.1016/0197-4580(92)90044-X. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y, 2009. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci 12, 1444e1449 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B, 2005. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol. Teratol 27, 615e627 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA, 1966. Signal Detection Theory and Psychophysics. Wiley, New York. [Google Scholar]
- Grier RA, Warm JS, Dember WN, Matthews G, Galinsky TL, Szalma JL, Parasuraman R, 2003. The vigilance decrement reflects limitations in effortful attention, not mindlessness. Hum. Factors j. Hum. Factors Ergon. Soc 45, 349e359 10.1518/hfes.45.3.349.27253. [DOI] [PubMed] [Google Scholar]
- Guillem K, Bloem B, Poorthuis, Loos M, Smit AB, Maskos U, Spijker S, Mansvelder HD, 2011. Nicotinic acetylcholine receptor. Science (80-) 333, 888e892 10.3109/10799899909036672. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP, 2011. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psycho-pharmacology (Berl) 217, 75e82 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Howe WM, Gritton HJ, Lusk NA, Roberts EA, Hetrick VL, Berke JD, Sarter M, 2017. Acetylcholine release in prefrontal cortex promotes gamma oscillations and thetaegamma coupling during cue detection. J. Neurosci 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Eslin D, 1991. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc. Nat. Acad. Sci. USA 88, 780e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Järvenpää T, Roivainen A, Yu M, Oikonen V, Kurki T, Rinne JO, 2002. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J. Clin. Psychopharmacol 22, 615e620 10.1097/00004714-200212000-00012. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM, 1998. Cortical map reorganization enabled by nucleus basalis activity. Science (80-.) 279, 1714e1718. [DOI] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW, 2015. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc.Natl. Acad. Sci 112, 3535e3540 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D, Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D, 1994. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur. J. Neurosci 6, 668e671 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, Mai JK, Zilles K, Bauer A, Matusch A, Schulz R-J, Noreik M, Bührle CP, Maintz D, Woopen C, H äussermann P, Hellmich M, Klosterkotter J, Wiltfang J, Maarouf M, Freund H-J, Sturm V, 2015. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry 20, 353e360 10.1038/mp.2014.32. [DOI] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB, 2013. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull 139, 870e900 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM, 2010. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol 68, 521e534 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- Levin ED, Caldwell DP, 2006. Low-dose mecamylamine improves learning of rats in the radial-arm maze repeated acquisition procedure. Neurobiol. Learn. Mem 86, 117e122 10.1016/j.nlm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bushnell PJ, Rezvani AH, 2011. Attention-modulating effects of cognitive enhancers. Pharmacol. Biochem. Behav 99, 146e154 10.1016/j.pbb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Rezvani AH, 2013. Improvement of attentional function with antagonism of nicotinic receptors in female rats. Eur. J. Pharmacol 702, 269e274 10.1016/j.ejphar.2013.01.056.Improvement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-C, Brown RE, Hussain Shuler MG, Petersen CCH, Kepecs A, 2015. Optogenetic dissection of the basal forebrain neuromodulatory control of cortical activation, plasticity, and cognition. J. Neurosci 35, 13896e13903 10.1523/JNEUROSCI.2590-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JT, Johnson DN, Minichiello M, Weingartner H, Sunderland T, 1998. Combined nicotinic and muscarinic blockade in elderly normal volunteers: cognitive, behavioral, and physiologic responses. Neuropsychopharmacology 19, 60e69 10.1016/S0893-133X(98)00002-5. [DOI] [PubMed] [Google Scholar]
- Liu R, Crawford J, Callahan PM, Terry AV, Constantinidis C, Blake DT, 2017. Intermittent stimulation of the nucleus basalis of meynert improves working memory in adult monkeys. Curr. Biol 27, 2640e2646 10.1016/j.cub.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM, 1996. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. J. Child Psychol. Psychiatry 37, 971e987 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Galloway M, Hawkins K, 1999. The absent mind: further investigations of sustained attention to response. Neuropsychologia 37, 661e670 10.1016/S0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- McCairn KW, Turner RS, 2009. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J. Neurophysiol 101, 1941e1960 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS, ID-ORCID: http://orcid.org/0000-0002-8546-4095.
- McGaughy J, Kaiser T, Sarter M, 1996. Behavioral vigilance following infusions of192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav. Neurosci 110, 247e265. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW, 2002. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J. Neurosci 22, 1905e1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Burk JA, 2006. Evaluation of muscarinic and nicotinic receptor antagonists on attention and working memory. Pharmacol. Biochem. Behav 85, 796e803 10.1016/j.pbb.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH, 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol 214, 170e197 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, Geula C, 1992. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J. Comp. Neurol 318, 316e328 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R, 1992. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology (Berl) 108, 480e484. [DOI] [PubMed] [Google Scholar]
- Nowak K, Mix E, Gimsa J, Strauss U, Sriperumbudur KK, Benecke R, Gimsa U, 2011. Optimizing a rodent model of Parkinson’s disease for exploring the effects and mechanisms of deep brain stimulation. Parkinsons. Dis 2011, 414682 10.4061/2011/414682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Funaki Y, Tashiro M, Kato M, Ishikawa Y, Maruyama M, Ishikawa H, Meguro K, Iwata R, Yanai K, 2008. In vivo visualization of donepezil binding in the brain of patients with Alzheimer’s disease. Br. J. Clin. Pharmacol 65, 472e479 10.1111/j.1365-2125.2007.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, Schoch S, Beck H, 2016. Astrocyte intermediaries of septal cholinergic modulation in the Hippocampus. Neuron 90, 853e865 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M, 2007. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56, 141e154 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn N, Neyt X, Henderickx D, Soetens E, 2008. Psychophysiological investigation of vigilance decrement: boredom or cognitive fatigue? Physiol. Behav 93, 369e378 10.1016/j.physbeh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Hillhouse TM, Haddjeri N, Rovera R, Porter JH, Mørk A, Smagin G, Song D, Budac D, Cajina M, Sanchez C, 2016. Task- and treatment lengthedependent effects of vortioxetine on scopolamine-induced cognitive dysfunction and hippocampal extracellular acetylcholine in rats. J. Pharmacol. Exp. Ther 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Butschky MF, Henningfield JE, 1997. Effects of mecamylamine on spontaneous EEG and performance in smokers and non-smokers. Pharmacol. Biochem. Behav 56, 181e187. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M, 2011. Habenula “ cholinergic” neurons corelease glutamate and acetylcholine and activate post-synaptic neurons via distinct transmission modes. Neuron 69, 445e452 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED, 2001. Cognitive effects of nicotine. Biol. Psychiatry 49, 258–267. 10.1016/S0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Cauley MC, Dawson E, Levin ED, 2009. Attenuation of pharmacologically-induced attentional impairment by methylpheni-date in rats. Pharmacol. Biochem. Behav 92, 141e146 10.1016/j.pbb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P, 2001. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J. Neuropsychiatry Clin. Neurosci 13, 326e335 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR, 1991. Electrophysiological studies of the functions of the nucleus basalis in primates. Adv. Exp. Med. Biol 295, 233e252. [DOI] [PubMed] [Google Scholar]
- Roberton I, Garavan H, 2004. Vigilance attention In: Gazzaniga MS (Ed.), The Cognitive Neuroscience. MIT Press, pp. 631e640. [Google Scholar]
- Rosenberg M, Noonan S, DeGutis J, Esterman M, 2013. Sustaining visual attention in the face of distraction: a novel gradual-onset continuous performance task. attention, perception. Psychophys 75, 426e439 10.3758/s13414-012-0413-x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, 1997. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res. Rev 23, 28e46 10.1016/S0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM, 2009. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci 10, 383e390 10.1038/nrn2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam M-MM, 1998. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 121 (Pt 1), 2249e2257 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- Shalev L, Ben-Simon A, Mevorach C, Cohen Y, Tsal Y, 2011. ConjunctiveContinuous Performance Task (CCPT)ea pure measure of sustained attention. Neuropsychologia 49, 2584e2591 10.1016/j.neuropsychologia.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Goldfarb T, Fink R, Jarvik ME, 1973. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia 28, 247e259. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr., Buccafusco JJ, 2003. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol Exp. Ther 306, 821e827. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Prendergast MA, 1999. Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Dev. Res 47, 127e136 . [DOI] [Google Scholar]
- Vitek JL, 2002. Mechanisms of deep brain stimulation: excitation or inhibitionMov. Disord. 17, S69eS72 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Drewencki LL, Chen X, Santos FR, Khan AS, Harun R, Torres GE, Michael AC, Dixon CE, 2009. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury J. Neurochem. 108, 986e997 10.1111/j.1471-4159.2008.05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Price DL, Young WS 3, 1989. GABAergic neurons in the primate basal forebrain magnocellular complex. Brain Res. 499, 188e192 10.1016/0006-8993(89)91152-9. [DOI] [PubMed] [Google Scholar]
- Webster HH, Hanisch U-KK, Dykes RW, Biesoldt D, Biesold D, 1991. Basal forebrain lesions with or without reserpine injection inhibit cortical reorganization in rat hindpaw primary somatosensory cortex following sciatic nerve section. Somat. Mot. Res 8, 327e346 10.3109/08990229109144756. [DOI] [PubMed] [Google Scholar]
- Whyte J, Hart T, Vaccaro M, Grieb-Neff P, Risser A, Polansky M, Coslett HB, 2004. Effects of methylphenidate on attention deficits after traumatic brain injury: a multidimensional, randomized, controlled trial. Am. J. Phys. Med. Rehabil 83, 401e420. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR, 2006. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Yuille MB, Olmstead CK, Wells AK, Hahn B, 2016. A test of the cognitive-enhancing potential of low-dose mecamylamine in healthy non-smokers. Psychopharmacology (Berl) 234, 109e116 10.1007/s00213-016-4443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Sykova E, Agnati LF, Fuxe K, 1999. Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci 01343e01347. 10.1016/S0165-6147(99. [DOI] [PubMed] [Google Scholar]