Abstract.
Human infection with Fasciola hepatica leads to obstruction of the common bile duct by adult worms and disease characterized by biliary colic, epigastric pain, and nausea. Recommended treatment is a single dose of triclabendazole (TCBZ) (10 mg/kg). Because in the 1990s the Bolivian Altiplano bordering Lake Titicaca was thought to have the highest prevalence of human fascioliasis worldwide, the Bolivian Ministry of Health instituted TCBZ mass drug administration (MDA). From 2008 to 2016 (excepting 2015), one dose of 250 mg was administered, usually in September/October, to each resident of highly endemic regions willing to participate. This is apparently the first reported use of MDA for Fasciola. The proportion of persons in key regions receiving TCBZ MDA was 87% in 2016. In 2017, we resurveyed key regions, and found that the MDA program had been dramatically successful. Whereas Fasciola prevalence was reported as 26.9% in Huacullani/Tiahuanaco and 12.6% in Batallas in 1999, there was 0.7% prevalence in Huacullani/Tiahuanaco and 1% in Batallas in 2017. However, lessons from schistosomiasis control efforts suggest that for sustained control of Fasciola infection, Fasciola MDA needs to be maintained and coupled with measures to control infection in the intermediary snail and in the animal hosts of F. hepatica.
INTRODUCTION
The flukes Fasciola hepatica and also Fasciola gigantica are the cause of fascioliasis. Fasciolid adult worms are parasites of the large biliary passages and the gallbladder of ruminants, mainly sheep and cattle, and incidentally humans. Eggs pass into the intestine and are excreted to become miracidia in fresh water. The intermediary hosts are specific freshwater gastropod snails of the family Lymnaeidae. The intragastropod development includes miracidium penetration, sporocyst formation, and production of cercariae. Cercariae swim until contacting a solid support, mostly leaves of water plants above or below the water line, to attach and encyst; metacercarial cysts become infective within 24 hours and are consumed by the mammalian host. After ingestion of contaminated water or water plants by ruminants or humans, the metacercariae excyst in the duodenum and migrate through the intestinal wall, the peritoneal cavity, and the liver parenchyma into the biliary ducts, where they develop into adult flukes which can live 9–13 years.1–4
Human fascioliasis is present in Andean countries (Bolivia and Peru), the Caribbean area (Cuba), northern Africa (Egypt), Western Europe (Portugal, France, and Spain), and the Caspian area (Iran and neighboring countries).2,4 There are two major clinical forms of fasioliasis.5 Acute disease, coincident with the 3–4 month migration of the immature parasites through the liver parenchyma, presents as fever, abdominal pain, tender hepatomegaly, and eosinophilia. Chronic disease, due to obstruction of the common bile duct by the adult worms, presents as biliary colic, epigastric pain, fatty food intolerance, nausea, intermittent jaundice, pruritus, and fever if bacterial superinfection causing cholangitis or cholecystitis occurs. The transient, nonspecific presentation of acute disease makes it hard to diagnose. Chronic disease is recognized by the presence of Fasciola eggs in the stool.
Triclabendazole (TCBZ), a benzimidazole compound used in veterinary practice since 1983 and in humans since 1989, is stated by the WHO to be the drug of choice against fascioliasis.5 Triclabendazole is active against adult parasites in the bile ducts and immature flukes migrating through the liver, and most efficacy studies are versus chronic disease.5 Although WHO recommends a single dose of TCBZ (10 mg/kg body weight) as the regimen of choice, the use of two doses of TCBZ (10 mg/kg followed by another equal dose 12–24 hours apart, for a total dose of 20 mg/kg) is recommended for treatment failures.5
To prepare for a clinical trial of TCBZ, we performed a stool survey at the southern coast of Lake Titicaca, Bolivia, reported to have the highest prevalence and intensity of Fasciola egg counts in the world in the 1990s6 and a region with precise geographic mapping of egg counts at that time.7 We were surprised to find virtually no human Fasciola in this region in 2017. In the intervening years, the Bolivian government had performed mass drug administration (MDA) of TCBZ. The success of this Bolivian program is the subject of the present report.
METHODS
Epidemiologic regions.
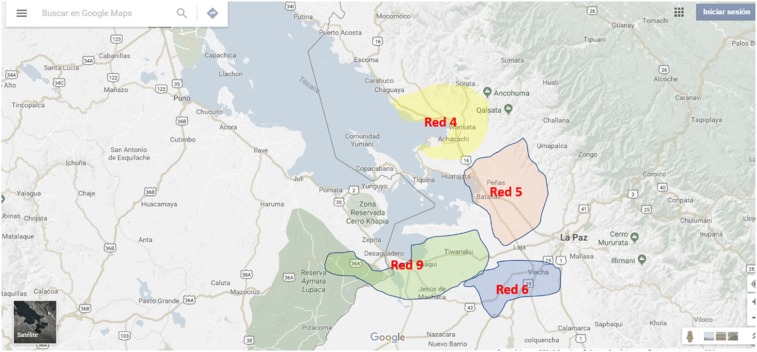
We collected stool samples from regions on the southern border of Lake Titicaca: region red 4 (region 4); region 5 including Batallas, Pucarani, and Puerto Perez; region 6 including Viacha; and region 9 including Huacullani and Tiahuanaco (Figure 1). These regions were included in the 1990s stool survey and in the subsequent Servicio Departamental de Salud, Bolivia (SEDES) TCBZ MDA program.
Figure 1.
Fasciola regions included in the Bolivian mass drug administration program. Note: Puerto Perez is located on Lake Titicaca close to Batallas. This figure appears in color at www.ajtmh.org.
Stool survey.
After obtaining ethical approval from SEDES and from the Bolivian Ministry of Health, we advertised our survey to schools in the region near Batallas (seven sites), Laja (16 sites), Puerto Perez (11 sites), Pucarani (33 sites), Tiahuanaco (nine sites including Huacullani), and Viacha (22 sites). Kato–Katz kits were purchased from Sterlitech Corporation (Kent, WA). A Kato–Katz collecting bottle (30 mL plastic, wide-mouthed, numbered container with a snap-on lid) was given to each person (child or parent) who was willing to provide a sample. Children who volunteered to provide a sample did so by bringing the sample to the school 1 day after receiving the Kato–Katz bottle. If a parent volunteered to provide a sample, the home was visited by a team member and the adult either provided a stool sample at that time or the adult brought the sample to a local health facility 1 or 2 days later. The bottles containing stool samples were kept on ice in plastic boxes until read either centrally (90% of samples) or locally (10% of samples) by the Kato–Katz technique.8 For specimens read at the central laboratory, the trip to the laboratory took between 1.5 and 2.5 hours; all samples were read within 24 hours (most samples were read in less than 12 hours) by one of three parasitology technicians, and ¼ of the slides were chosen at random to be reread by Dr. S. Mollinedo. The 10% of samples that were read locally constituted a training experience for local technicians, and all these samples were reread by Dr. S. Mollinedo.
Triclabendazole MDA.
In 2008, the Bolivian Ministry of Health initiated a program of massive drug administration with TCBZ in subjects aged 5 to 65 years. Every year (except 2015), one dose of 250 mg was administered, usually in September/October, to each subject. Children were treated at schools, and adults received treatment at home or during market days. The dose of 250 mg equates to 10 mg/kg for subjects weighing 25 kg and a lesser mg/kg dose for heavier persons. The proportion of persons in regions 4, 5, 6, and 9 covered by TCBZ MDA was 55–94% in the years 2009–2014 and was 87% in 2016. For 2016, the number of pills distributed (i.e., persons treated) was 116,000 in region “red 4,” 120,000 in “red 5,” 217,000 in “red 6,” and 63,000 in “red 9.”
RESULTS
Stool samples were collected from 5,243 individuals. Eighty-four percent were school-aged children (5–12 years), 11.5% were adolescents (13–18 years), and 4.5% were adults (> 18 years). Forty-four percent of the samples were provided by males.
We identified Fasciola eggs in 22 of the 5,243 samples (0.4%) (Tables 1 and 2). In the previously highly endemic region of Batallas, prevalence fell from 12.6% in 1999 to 1.05% in 2017 (P < 0.0001). Prevalence fell from 26.9% in 1997 in Huacullani to 0.7% in the conjoined town of Tiahuanaco in 2017 (P < 0.0001). In the previously lesser endemic regions of Pucarani and Viacha, prevalence fell from 1.3–2.7% to 0–0.6% (P = 0.001).
Table 1.
Prevalence of Fasciola eggs in stool in 1999 and in 2017
| Town | 1999 Report7 | 2017 Present study | |||
|---|---|---|---|---|---|
| No. of subjects | % Egg prevalence | No. of subjects | % Fasciola egg prevalence | % Other egg prevalence | |
| Batallas | 183 | 12.6 | 381 | 1.05 | 6.6 |
| Puerto Perez | nd | nd | 413 | 0.2 | 3.1 |
| Huacullani/Tiahuanaco | 690 | 26.9 | 819 | 0.7 | 4.3 |
| Laja | nd | nd | 909 | 0.7 | 0.9 |
| Pucarani | 73 | 2.7 | 1,837 | 0.6 | 1.7 |
| Viacha | 230 | 1.3 | 884 | 0 | 1.1 |
nd = not done.
Table 2.
Fasciola egg counts in subjects whose stool was positive
| No. | Age (years) | Gender | Town | Density (egg/g) |
|---|---|---|---|---|
| 1 | 13 | F | Pucarani | 216 |
| 2 | 10 | M | Pucarani | 72 |
| 3 | 44 | F | Pucarani | 120 |
| 4 | 9 | F | Pucarani | 1,147 |
| 5 | 8 | F | Pucarani | 192 |
| 6 | 6 | F | Pucarani | 144 |
| 7 | 4 | M | Pucarani | 696 |
| 8 | 8 | F | Pucarani | 48 |
| 9 | 10 | F | Pucarani | 480 |
| 10 | 9 | M | Pucarani | 216 |
| 11 | 10 | M | Pucarani | 24 |
| 12 | 6 | F | Batallas | 652 |
| 13 | 8 | M | Batallas | 288 |
| 14 | – | F | Batallas | 576 |
| 15 | 10 | F | Batallas | 168 |
| 16 | F | Puerto Perez | 96 | |
| 17 | 7 | M | Tiahuanaco | 145 |
| 18 | 7 | M | Tiahuanaco | 222 |
| 19 | 11 | F | Tiahuanaco | 60 |
| 20 | 8 | M | Tiahuanaco | 458 |
| 21 | 12 | M | Tiahuanaco | 642 |
| 22 | 14 | M | Tiahuanaco | 120 |
F = female; M = male.
In the 22 subjects with Fasciola eggs in stool, the mean number was 308 eggs/g (SD = 285) (Table 2). Seven of the 22 individuals had egg intensities greater than 400/g. None of the Fasciola subjects volunteered gastrointestinal symptoms suggestive of Fasciola infection.
Other parasites were seen at a prevalence of 0.9–6.6%. The majority of other parasites (69%) were Himenolepis nana and Enterobius vermicularis, but Ascaris lumbricoides and Trichuris trichiura were also seen. In general, these parasites were seen at low densities (24 egg/g for H. nana and 48 egg/g for E. vermicularis).
DISCUSSION
In the Bolivian Altiplano previously stated to have “the highest prevalences and intensities of human fascioliasis known,”9 we found it impossible to identify sufficient subjects for a Fasciola treatment trial in 2017. Whereas Fasciola prevalence was 26.9% in Huacullani/Tiahuanaco and 12.6% in Batallas in 1999, we found 0.7% prevalence in Huacullani/Tiahuanaco and 1% in Batallas in 2017.
We attribute this dramatic decrease in human fascioliasis to the Bolivian Ministry of Health’s TCBZ mass drug administration program between 2008 and 2016. In this program, each contacted person received one 250-mg dose of TCBZ, and 87% of the resident population was covered in 2016, the year before our survey. Fascioliasis was predominately a pediatric disease in the Altiplano7 and remained so in 2017: 21 of the 22 subjects with Fasciola infection were aged between 4 and 14 years. Because 250 mg represents 10 mg/kg for a 25-kg child, most of the individuals for whom TCBZ MDA was intended are likely to have received a curative dose of this drug.
Other Bolivian Ministry of Health activities that might have contributed to countering fascioliasis in the Altiplano include annual deworming with albendazole and mebendazole for the last 40 years, and recent improvement of housing conditions, creation of new health centers, and a ministry of water program to improve water quality. Although albendazole does have anti-Fasciola activity,10,11 and there is a case report on cure of Fasciola infection with mebendazole,12 the Bolivian deworming efforts were not specifically directed against Fasciola and Fasciola “would not be expected to respond to” MDA albendazole regimens.13 There was no Bolivian program to specifically treat the sheep and cattle that are the natural hosts for Fasciola nor the freshwater snail intermediary host. Thus, we attribute the virtual eradication of human fascioliasis to the Ministry of Health’s new initiative of TCBZ MDA.
This reduction in fascioliasis in the Bolivian Altiplano is more striking when considering the most recent prevalence reports of fascioliasis in the neighboring Altiplano region in Peru. Whereas a singular multinational epidemiologic study of fascioliasis in the Altiplano region is lacking, studies conducted in the 2010 and 2012 in communities located near Cusco, Peru, reported fascioliasis to be present in 9.7–10.3% of school-aged children.14,15
A search of the literature (PubMed searches in June 2018 for “TCBZ AND MDA” and for “Fasciola AND MDA”) failed to reveal any reports of additional TCBZ or fascioliasis MDA efforts. Selective chemotherapy (diagnose-and-treat) programs for fasciolisis have been historically used in other countries with some success. In Egypt, a screening and treatment program targeting school-aged children in six districts in the Nile Delta successfully reduced the incidence of fascioliasis from 5.6% to 1.2%.16,17
Mass diagnosis followed by treatment of the few individuals with infection is however much more expensive than simple MDA. Thus, we believe the scope and breadth of Bolivian anti-Fasciola MDA program to be unique in its efficiency in controlling human infection.
Other antihelminthic MDA programs include WHO-promulgated programs to eliminate filariasis, soil-transmitted helminthiasis, and schistosomiasis.18 Of these, the most relevant program is the schistosomiasis program because schistosomes and Fasciola share the obligatory use of freshwater snails as an intermediate host. Antischistosomal MDA involves administration of one dose of praziquantal (PZQ), 40 mg/kg, to each human subject. A recent report from five schools near Lake Victoria, Kenya, shows the dramatic effect that PZQ MDA can have on human schistosome infection. In approximately 1,000 school children, four annual rounds of MDA with PZQ were associated with reduced Schistosoma mansoni prevalence (44.7–14.0%), mean intensity of infection (91–8 eggs/g), and prevalence of high-intensity infection (> 400 eggs/g: 6.8–0.3%).19 Nevertheless, the literature also reports issues with PZQ MDA. 1) Multiple studies and programs now find that even within well-implemented, multiyear, annual MDA programs, there often remain locations that do not decline in prevalence and/or intensity to the expected levels.20,21 2) Compliance with MDA may be low,22,23 and the 40 mg/kg recommended dose may be far too low, resulting in only a 52% cure rate.22 3) Reinfection rates after successful human treatment can be high,24 and to eliminate human infection, attention to infected cattle/snail control/improved water quality is also needed.22,25 The efficacy of one dose of TCBZ for Fasciola in Bolivia was 78%26 and in neighboring Peru was 95%,27 which is higher than the 52% rate of PZQ for schistosomiasis. Nevertheless, the cautionary notes with respect to schistosomiasis control suggest that for sustained elimination of Fasciola infection, past efforts of the Bolivian Ministry of Health need to be maintained in the future and coupled with attention to control of the intermediary (snail) and animal hosts of F. hepatica. Triclabendazole was originally a veterinary drug and is widely used to treat the animal reservoir. Triclabendazole-resistant Fasciola in animals is now recognized28 and could lead to TCBZ-resistant human infection. The cases of resistant human infection,29,30 although presently few, are concerning and further emphasize the importance of addressing animal and intermediary hosts if human infection is to be controlled.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) , 2018. Parasites—Fascioliasis (Fasciola Infection): Biology. Available at: http://www.cdc.gov/parasites/fasciola/biology.html. Accessed December 7, 2018. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) , 2018. Parasites—Fascioliasis (Fasciola Infection): Epidemiology and Risk Factors. Available at: http://www.cdc.gov/parasites/fasciola/epi.html. Accessed December 7, 2018. [Google Scholar]
- 3.Ashrafi K, Bargues MD, O’Neill S, Mas-Coma S, 2014. Fascioliasis: a worldwide parasitic disease of importance in travel medicine. Trav Med Infect Dis 12: 636–649. [DOI] [PubMed] [Google Scholar]
- 4.Mas-Coma S, 2005. Epidemiology of fascioliasis in human endemic areas. J Helminthol 79: 207–216. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) , 2006. Report of the WHO Informal Meeting on Use of Triclabendazole in Fascioliasis Control. Available at: http://www.who.int/neglected_diseases/preventive_chemotherapy/WHO_CDS_NTD_PCT_2007.1.pdf. Accessed December 7, 2018. [Google Scholar]
- 6.Esteban JG, Flores A, Angles R, Strauss W, Aguirre C, Mas-Coma S, 1997. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop Med Int Health 2: 695–699. [DOI] [PubMed] [Google Scholar]
- 7.Mas-Coma S, Anglés R, Esteban JG, Bargues MD, Buchon P, Franken M, Strauss W, 1999. The northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop Med Int Health 4: 454–467. [DOI] [PubMed] [Google Scholar]
- 8.1991. Available at: http://microbeonline.com/kato-katz-technique-principle-procedure-results. Accessed December 7, 2018.
- 9.Mas-Coma S, Funatsu IR, Bargues MD, 2001. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 123 (Suppl): S115–S127. [DOI] [PubMed] [Google Scholar]
- 10.Venturina VM, Alejandro MA, Baltazar CP, Abes NS, Mingala CN, 2015. Evidence of Fasciola spp. resistance to albendazole, triclabendazole and bromofenofos in water buffaloes (Bubalus bubalis). Ann Parasitol 61: 283–289. [DOI] [PubMed] [Google Scholar]
- 11.Novobilský A, Amaya Solis N, Skarin M, Höglund J, 2016. Assessment of flukicide efficacy against Fasciola hepatica in sheep in Sweden in the absence of a standardised test. Int J Parasitol Drugs Drug Resist 6: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugernier T, Geubel A, Bigaignon G, Cesbron JY, Coche E, 1986. Human fascioliasis: cure by mebendazole? A case report. Gastroenterol Clin Biol 10: 513–516. [PubMed] [Google Scholar]
- 13.Cabada MM, Lopez M, Arque E, Clinton White A, 2014. Prevalence of soil-transmitted helminths after mass albendazole administration in an indigenous community of the Manu jungle in Peru. Pathog Glob Health 108: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez M, White AC, Cabada MM, 2012. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg 86: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabada MM, Goodrich MR, Graham B, Villanueva-Meyer PG, Lopez M, Arque E, White AC, 2014. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg 91: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtale F, Abd-el Wahab Hassanein Y, El Wakeel A, Barduagni P, Savioli L, 2003. The school health programme in Behera: an integrated helminth control programme at governorate level in Egypt. Acta Trop 86: 295–307. [DOI] [PubMed] [Google Scholar]
- 17.Curtale F, Hassanein YA, Savioli L, 2005. Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Trans R Soc Trop Med Hyg 99: 599–609. [DOI] [PubMed] [Google Scholar]
- 18.WHO , 2012. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases. Available at: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf. Accessed December 7, 2018. [Google Scholar]
- 19.Abudho BO, Ndombi EM, Guya B, Carter JM, Riner DK, Kittur N, Karanja DMS, Secor WE, Colley DG, 2018. Impact of four years of annual mass drug administration on prevalence and intensity of schistosomiasis among primary and high school children in western Kenya: a repeated cross-sectional study. Am J Trop Med Hyg 98: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kittur N, Binder S, Campbell CH, King CH, Kinung’hi S, Olsen A, Magnussen P, Colley DG, 2017. Defining persistent hotspots: areas that fail to decrease meaningfully in prevalence after multiple years of mass drug administration with praziquantel for control of schistosomiasis. Am J Trop Med Hyg 97: 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rujeni N, Morona D, Ruberanziza E, Mazigo HD, 2017. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: an update on their epidemiology and control. Infect Dis Poverty 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AG, et al. 2015. Can mass drug administration lead to the sustainable control of schistosomiasis? J Infect Dis 211: 283–289. [DOI] [PubMed] [Google Scholar]
- 23.Inobaya MT, et al. 2018. Mass drug administration and the sustainable control of schistosomiasis: an evaluation of treatment compliance in the rural Philippines. Parasit Vectors 11: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lelo AE, et al. 2014. No apparent reduction in schistosome burden or genetic diversity following four years of school-based mass drug administration in Mwea, central Kenya, a heavy transmission area. PLoS Negl Trop Dis 8: e3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchuem Tchuenté LA, Rollinson D, Stothard JR, Molyneux D, 2017. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villegas F, et al. 2012. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl Trop Dis 6: e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maco V, Marcos L, Delgado J, Herrera J, Nestares J, Terashima A, Samalvides F, Gotuzzo E, 2015. Efficacy and tolerability of two single-day regimens of triclabendazole for fascioliasis in Peruvian children. Rev Soc Bras Med Trop 48: 445–453. [DOI] [PubMed] [Google Scholar]
- 28.Kelley JM, Elliott TP, Beddoe T, Anderson G, Skuce P, Spithill TW, 2016. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol 32: 458–469. [DOI] [PubMed] [Google Scholar]
- 29.Cabada MM, Lopez M, Cruz M, Delgado JR, Hill V, White AC, 2016. Treatment failure after multiple courses of triclabendazole among patients with fascioliasis in Cusco, Peru: a case series. PLoS Negl Trop Dis 10: e0004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb CM, Cabada MM, 2018. Recent developments in the epidemiology, diagnosis, and treatment of Fasciola infection. Curr Opin Infect Dis 31: 409–414. [DOI] [PubMed] [Google Scholar]