Abstract.
After a dramatic decline in the annual malaria incidence in Thailand since 2000, the Thai government developed a National Malaria Elimination Strategy (NMES) to end local malaria transmission by 2024. This study examines the expected costs and benefits of funding the NMES (elimination scenario) versus not funding malaria elimination programming (resurgence scenario) from 2017 to 2036. Two case projection approaches were used to measure the number of malaria cases over the study period, combined with a set of Thailand-specific economic assumptions, to evaluate the cost of a malaria case and to quantify the cost–benefit ratio of elimination. Model A projects cases based on national historical case data using a log-normal regression and change-point analysis model. Model B projects cases based on periodic Yala Province-level outbreak cycles and incorporating NMES political and programmatic goals. In the base case, both models predict that elimination would prevent 1.86–3.11 million malaria cases from 2017 to 2036, with full NMES implementation proving to be cost-saving in all models, perspectives, and scenarios, except for the health system–only perspective in the Model A base case and all perspectives in the Model A worst case. From the societal perspective, every 1 US dollars (US$) spent on the NMES would—depending on case projections used—potentially result in a considerable return on investment, ranging from US$ 2 to US$ 15. Although the two case projection approaches resulted in different cost–benefit ratios, both models showed cost savings and suggest that ending local malaria transmission in Thailand would yield a positive return on investment.
INTRODUCTION
In 2015, the World Health Assembly adopted the WHO Global Technical Strategy for Malaria 2016–2030.1 This strategy describes the global malaria community’s long-term vision of a world free of malaria, targeting 1) a reduction of malaria incidence and mortality rates globally by at least 90% by 2030; 2) elimination of the disease in at least 35 new countries; and 3) prevention of its reestablishment in countries that were malaria-free in 2015. Progress against malaria in the last 10 years has been significant: malaria incidence and mortality have been reduced by 14.2% and 34.4%, respectively, and—in the last 5 years alone—five countries have been certified as malaria free, including the Maldives (2015), Sri Lanka (2016), Kyrgyzstan (2016), Paraguay (2018), and Uzbekistan (2018).
Over the past decade, Thailand has dramatically reduced its malaria burden and has embarked on the path to malaria elimination, making Thailand a strong candidate to be one of the countries eliminating malaria by 2030. Between 2000 and 2016, the annual parasite incidence decreased by 89% (down from 2.61 to 0.28); in 2016, 18,758 malaria cases were reported nationally. Mortality also declined considerably in recent years, with 33 deaths reported in 2015.2 Commitments from the Thai government and support from the international community, particularly the Global Fund to Fight AIDS, Tuberculosis, and Malaria (GFATM), drove this decline in malaria burden.3,4 To build on this success, the Thai government developed a National Malaria Elimination Strategy (NMES) (2017–2026) to end the local transmission of malaria in Thailand by 2024.5
In 2015, 8% of the Thai population lived in districts of high malaria transmission (i.e., defined as districts where the annual parasite incidence is higher than 1 per 1,000 population), primarily along the borders with Myanmar, Cambodia, and Malaysia; 42% lived in districts of low malaria transmission (i.e., defined as districts where the annual parasite incidence is ≤ 1 per 1,000 population); and 50% lived in malaria-free districts (i.e., defined as districts where no case has been reported in the past year[s]), primarily in the center of the country.3,6 In 2016, Plasmodium falciparum (Pf) and Plasmodium vivax (Pv) accounted for 52% and 42% of all malaria cases in Thailand, respectively.2,3 The introduction of artemisinin-based combination therapies (ACTs) in 1995 was associated with a decrease in Pf burden, which led to a shift in the Pf/Pv ratio among reported cases.6,7 However, the emergence and spread of malaria parasite strains resistant to artemisinin and partner drugs included in ACTs threaten the declining trend in Pf infection.8
Despite the Thai government’s ambition and commitment to eliminate malaria, a main challenge for the country’s program is its funding gap, particularly after Thailand’s transition away from GFATM support. Such a gap could potentially weaken national malaria elimination efforts.9 As reported in a systematic review on malaria resurgence, the weakening of a malaria control program due to resource constraints was highlighted as the major cause of resurgence (defined as “increasing trend[s] in malaria incidence or prevalence following suppression achieved through implementation of control efforts”)10 in more than half of the case reports reviewed; other causes were complacency and other issues with poor program execution, war or disaster, and purposeful cessation of control activities. Indeed, malaria resurgence has already occurred in a few Thai provinces. For example, a lapse in malaria control in Ubon Ratchathani Province, combined with greater malaria exposure due to an expansion in forest-related activities, such as timber logging, and potentially circulating drug-resistant Plasmodium strains, led to a malaria increase from 2,368 to 12,244 cases between 2013 and 2015.2,11,12 Similarly, when political unrest in Yala Province disrupted malaria services, reported malaria cases increased from 1,070 to 6,141 between 2015 and 2016.2
Given the reduced external funding for malaria prevention and control efforts, Thailand will have to mobilize extensive domestic resources to meet the NMES’s goals and targets, and successfully achieve malaria elimination by 2024.5 The aim of the study presented here was to measure the cost–benefit of a complete implementation of the NMES and thus assess the justification to invest in malaria elimination in Thailand.
METHODS
Because there is currently no standard guiding which type of epidemiological modeling approach should be used in economic models determining the cost-effectiveness of malaria elimination, we used two approaches—Model A and Model B—to forecast post-2016 malaria scenarios. Model A uses time–trend statistical modeling and draws on historical data by province from all geographic areas at risk of malaria. Model B assumes malaria resurgence based on trends observed during outbreaks in Yala Province from 1997 to 2016. Each model compared two scenarios: 1) elimination, corresponding to the complete support for the NMES; and 2) resurgence, corresponding to no support for the NMES. Finally, to conduct the cost–benefit analyses, we then applied an economic model to estimate the expected economic costs and benefits of implementing the NMES over a 20-year period (2017–2036). Table 1 provides a summary of the scenarios and cases used in the models; Supplemental Table 1 provides a summary of the inputs used in the models, all of which are described in more detail in the following paragraphs.
Table 1.
Model scenarios and cases
| Epidemiological model | Model A (based on statistical modeling with national data) | Model B (based on malaria elimination goals and trends from Yala Province) | |||
|---|---|---|---|---|---|
| Scenario | Elimination (NMES receives full support) | Resurgence (NMES receives no support) | Elimination (NMES receives full support) | Resurgence (NMES receives no support) | |
| Economic model | |||||
| Case | Base case (using point estimates for all inputs) | Model A elimination, base case | Model A resurgence, base case | Model B elimination, base case | Model B resurgence, base case |
| Best case (making elimination look as good as possible, defined as the highest cost–benefit ratio possible) | Model A elimination, best case | Model A resurgence, best case | n/a | n/a | |
| Worst case (making elimination look as bad as possible, defined as the lowest cost–benefit ratio possible) | Model A elimination, worst case | Model A resurgence, worst case | n/a | n/a | |
NMES = National Malaria Elimination Strategy; n/a = not applicable.
Malaria interventions in the two scenarios.
Under the elimination scenarios, all activities of the NMES receive full political and economic support. The goal of the NMES is a Thailand free from malaria by 2024.5 Objectives of the strategy are to—by 2021—reduce malaria morbidity to less than 0.20 cases per 1,000 population, reduce malaria mortality to less than 0.01 deaths per 1,000 population, eliminate malaria transmission in at least 95% of total districts, and prevent reintroduction of transmission in malaria-free areas. The NMES includes four key approaches to achieve this vision: eliminating local transmission through universal coverage with long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS) of households with insecticides, case management, case finding, and surveillance; developing new technologies and practices through monitoring, evaluation, and research; fostering a collaborative stakeholder network both within Thailand and internationally; and building the capacity of Thai communities to eliminate malaria in their regions.5,12 Our assumption is that by 2026, following successful implementation of the NMES, all malaria-related activities will continue and would be absorbed by the general health system.
Under the resurgence scenarios, the NMES receives no financial or political support. Without a vertical malaria program, the general public health system reacts to malaria cases and outbreaks through responsive treatment and limited prevention and control measures. The public health system does not conduct any active surveillance, all cases are passively detected at health facilities, and outbreaks are responded to as needed.
Epidemiological models.
The epidemiological modeling generates the number of malaria cases for each scenario described previously. To estimate the costs or benefits of malaria elimination, Model A and Model B used projections of the susceptible population between 2017 and 2036. The size of the population at risk was projected using annual growth rate estimates from 2015 onward.13 Our epidemiological models assume that malaria cases would occur in the 42 provinces of Thailand along the borders with Myanmar, Cambodia, Malaysia, and Lao People’s Democratic Republic; provinces without malaria were assumed to remain malaria-free. The 42 malaria-endemic provinces currently account for 35% of the Thai population.14 To forecast case counts under elimination and resurgence, we used different modeling approaches: 1) Model A, based on temporal statistical modeling applied to historical data from 1965 to 2016 for the entire country; and 2) Model B, based on NMES goals and on trends recorded in Yala Province during outbreaks between 1997 and 2016.
Model A.
For the elimination scenario in Model A, we assumed that malaria incidence in Thailand would follow its current downward trend, which began in the year 2000 after the major scale-up of malaria interventions. We assumed that intervention efforts will continue to reduce malaria incidence following the trend observed from 2000 to 2016. To describe this trend, we built a log-normal generalized linear regression model (LN-GLM),15,16 with 2000–2016 malaria incidence as the independent variable and years as the dependent variable. Given the aforementioned assumption, no other variables (e.g., with regard to intervention coverage) were included in the model. We used the model to project the incidence rates from 2017 through 2036 and then combined the projected malaria incidence with the projections of the population at risk to estimate the count of malaria cases from 2017 through 2036. A second LN-GLM was applied to model the ratio of reported cases due to Pf—versus Pv or other Plasmodium species—from 2000 to 2016. We then multiplied the projected fraction of Pf infections by the count of malaria cases to calculate the number of cases due to Pf from 2017 to 2036.
For the resurgence scenario, we assumed that the number of cases would increase following temporal trends of historical outbreaks. Thailand has experienced several periods of malaria resurgence since the 1960s. We applied change-point analysis to analyze the time series of malaria incidence from 1965 to 2017 and to identify resurgence periods.17 We identified three periods of significant malaria resurgence: 1969–1981, 1987–1989, and 1996–1999. The incidence change for each Plasmodium species within these resurgence periods was based on the year before the resurgence period. Fitting procedures were used to identify the type of curve which best explained the increase of malaria incidence during these three resurgence periods18; an exponential distribution was shown to be the best curve. The average trend of change incidence among all resurgence periods was quantified by an LN-GLM. In addition, a time series analysis using generalized additive models identified 3-year cyclical fluctuations in the historical malaria data, which were incorporated into the incidence projections as a sinusoidal function.19 We used these models to forecast the increase in malaria under resurgence from the reported incidence in 2016 through the projected incidence in 2036. We then multiplied these incidence rates by the projected population at risk to generate the number of cases expected each year between 2016 and 2036. An LN-GLM was also used to calculate the trend in the proportion of cases associated with Pf during the historical resurgence periods. The results from this model were applied to the case projections to calculate the proportion of cases associated with Pf versus those associated with other Plasmodium subspecies.
The mean estimate from the case projections described previously forms the Model A base case; the 95% confidence limits formed the bounds of the scenario analyses. In the best case, the number of cases under elimination is as low as possible and the number of cases under resurgence is as high as possible. In the worst case, the number of cases under elimination is as high as possible and the number of cases under the resurgence scenario is as low as possible. Therefore, in the best case, elimination prevents the maximum number of cases, and in the worst case, elimination prevents the minimum number of cases.
Model B.
For the elimination scenario, we assumed that the NMES succeeds in ending the local transmission of malaria in Thailand by 2024, requiring a yearly 35% incidence decrease of locally transmitted malaria between 2017 and 2024.5 We further assumed that the Southeast Asia region would eliminate the local transmission of malaria by 2030, in line with regional goals as stated in the Strategy for Malaria Elimination in the Greater Mekong Subregion 2015–2030.20 From 2030 to 2036, we assumed a constant number of 100 imported cases per year due to cases contracted outside of Southeast Asia. We also assumed that Pf will be eliminated sooner than Pv, and there will be no locally transmitted Pf from 2020 onward, whereas Pv transmission and dynamics would be related to the global efforts to eliminate malaria in neighboring countries.
Under resurgence, we assumed that, without any investment in malaria elimination activities, the cycle of malaria resurgence will return to that seen before the scale-up of malaria prevention and control efforts in 1997. To generate the expected number of cases in resurgence years versus non-resurgence years, we examined malaria case reports from 1997 to 2016 by province.2 We assumed that malaria resurgence dynamics were based on the number of reported cases in Yala Province, where political instability had disrupted malaria prevention and control activities and resulted in suboptimal programmatic coverage of interventions. In this province, a cycle of resurgence once every 4 years was observed, with the first 2 years of the cycle showing a steady increase in incidence up to a peak, followed by 2 years of steady decrease in incidence down to initial incidence levels. The observed ratio of the number of cases in resurgence years versus the average number of cases in non-resurgence years was 2.47 to 1. We assumed that without the NMES, the cyclical pattern seen in Yala Province would apply in all 42 malaria-endemic provinces on an 8-year cycle basis. We estimated the non-resurgence incidence rate for each of the malaria-endemic provinces based on the mean number of cases reported and the corresponding total population size by province from 1997 to 1999; population size projections by province were based on the annual population growth from 2007 to 2011. We then multiplied the population counts by the appropriate incidence rates to create 8-year resurgence cycles, using the resurgence incidence rates in 2021 and 2029 and the low base-case incidence rates in 2025 and 2033. We interpolated the number of cases in the interim years in a straight line between the peaks and the valleys. Finally, we also took into account the increase in proportion of people residing in urban areas, as they are less exposed to malaria, and applied a statistical correction as described elsewhere.21
Calculation of case subtypes for elimination and resurgence scenarios.
For both Model A and Model B, after projecting the number of cases expected under each model and scenario, we applied proportions to the projected number of cases to estimate the number of cases occurring in pregnant women, the number of cases by treatment type, and the number of malaria deaths.
Under both scenarios, we assumed that 92.5% of Pf cases and 95.2% of Pv cases could be handled as outpatients.2,22 Under elimination, we assumed that 1.0% of cases would be severe and require inpatient treatment, which is the current rate.2 Because of the decline in population immunity coupled with the increase of malaria incidence under resurgence, we assumed that 1.8% of cases would require inpatient treatment under that scenario. All remaining cases require standard inpatient treatment. Under both scenarios, 10% of Pf treatments were assumed to fail because of antimalarial resistance,23 and 16.8% of Pv cases were assumed to relapse.2,24,25 We assumed a case fatality rate under the elimination scenario of 1.1 deaths per 1,000 cases based on 2012–2014 data.2 Under resurgence, we assume that the health system would struggle to handle the increased case load, so this rate would increase to 2.0 deaths per 1,000 cases, which was the average case fatality rate in 2007 (P. Sudathip, personal communication).
Case demographics, shown in Table 2, were drawn from the 2015 and 2016 Thailand Malaria Information System.2 We held these demographics constant throughout the analyses and did not vary them based on any other factors, such as region or pathogen type.
Table 2.
Age- and gender-specific incidence rates
| Age group (years) | Female | Male |
|---|---|---|
| < 15 | 12.2% | 16.3% |
| 15–60 | 17.0% | 46.7% |
| > 60 | 2.4% | 5.4% |
| Mean age of person with malaria | 35.9 | 34.3 |
Economic model.
The economic model examines the financial costs of the NMES versus the economic costs of malaria under both scenarios, expressed in US dollars (US$). In this cost–benefit model, the cost refers to the cost of the NMES under elimination. The benefits in the model refer to any costs averted by pursuing elimination. These costs averted are calculated by subtracting the expected value of the outcomes of elimination from the expected value of the outcomes of resurgence. Dividing these benefits by the cost of the NMES generates the benefit–cost ratio, the primary outcome of the model. When the benefit–cost ratio exceeds 1.0, elimination saves more money than it would cost to achieve it; when it is less than 1.0, elimination costs more money than it saves.
Expected costs and benefits are presented from three perspectives: 1) the health system alone (i.e., the provider perspective); 2) both health systems and directly affected households; and 3) the economy as a whole (i.e., the societal perspective). Health system costs include the costs of treatments, screening, and outbreak response through IRS and LLIN distribution. Household costs include the costs of seeking treatment, the value of caretakers’ time, the value of wages lost due to illness, and the costs of death. Costs to the overall economy include the loss of tourism revenues and the downstream costs of lost labor.
All costs are presented in 2017 US$ (1 US$ = 33.98 Thai baht).26 The economic model applied to Model A includes a standard 3% discount rate on all future costs, varying between 0% and 5% according to scenarios.27 Model B includes a 5% discount rate on all future costs.
Cost of the NMES.
The National Malaria Elimination Strategy costs include program costs and salary costs. The program costs were drawn from the National Malaria Operational Plan (2017–2021), in which the Bureau of Vector Borne Diseases (BVBD) projected the budget needs for the life of the NMES.12 After the end of the NMES in 2026, as it is expected that the BVBD will continue to conduct surveillance and provide technical support to the malaria community, program costs were extended up to 2036.
Salary costs are based on a survey conducted among program staff of the vertical malaria program at the BVBD, provincial vector-borne disease centers, district/subdistrict vector-borne disease units, and malaria clinics. The total number of staff members in each position, their salary, and the percent of their time spent on malaria work were collected. These staffing figures were projected throughout the life of the NMES with the assumption that no new position would be created for the NMES and that staffing levels would steadily decrease as the incidence of malaria declines. After the end of the NMES in 2026, the remaining staff will integrate into the general BVBD structure and continue some malaria activities. As with the program costs, the 2026 salary costs were applied up to 2036.
Drug costs and screening labor, which are both driven by the number of cases, appear in the outcome costs for all scenarios, but they also appear in the program costs for the NMES. To avoid double-counting, we subtracted these costs from the NMES operational costs. For this purpose, drug costs were calculated using the smaller of either the drug costs allocated in the BVBD budget or the expected drug costs for treatment. Screening labor costs were captured in discrete BVBD budget line items, which were subtracted in their entirety.
Costs to the health system.
Costs to the health system include the costs of treatment, including the costs of drugs and care; the costs of screening; and the costs of outbreak response with IRS and ITN distribution. Treatment costs were based on the estimated number of cases by Plasmodium species, shown in Table 3.28 In addition to the standard treatment, pregnant women receive 3 days of supervised treatment and the corresponding transport fee at a government reimbursement rate of US$ 0.16 per kilometer (P. Sudathip, personal communication). Screening costs were based on the number of cases in each scenario by the screening-to-case ratio. Under elimination, a screening case ratio of 103.5:1 was used, which was the historical average from the Myanmar and Malaysian border regions. Because of the higher incidence rates under resurgence, we assumed that the screening case ratio would decrease 10-fold to 10.4:1. Under both scenarios, we assumed that 80% of screenings would use microscopy (US$ 0.37 per microscopic test) and 20% would use rapid diagnostic tests (RDTs) (US$ 1.29 per RDT), including a supply chain multiplier of 1.25.2,12,29 Each test also includes the labor cost of a laboratory technician who earns US$ 662.18 per 22-day month and spends 30 minutes on a microscopy slide and 20 minutes on a RDT (P. Sudathip, personal communication).
Table 3.
Costs of treatment to the health-care system, 2017 US$
| Plasmodium falciparum (Pf) and mixed infections | Plasmodium vivax (Pv) or other species | |||||
|---|---|---|---|---|---|---|
| Details | Cost of drugs | Cost of care | Details | Cost of drugs | Cost of care | |
| Outpatient | DHAPIP | 4.12 | 6.71 | Chloroquine/primaquine | 0.41 | 4.29 |
| Additional costs in pregnancy | 3 days of DOTS | 0 | 13.83 | 3 days of DOTS | 0 | 13.83 |
| Inpatient | IVATS | 66.75 | 238.88 | IVATS + DHAPIP | 70.87 | 31.28 |
| Relapse | – | – | – | DHAPIP | 4.12 | 6.71 |
| Severe | IVATS + ATQ | 119.72 | 1,219.46 | IVATS + DHAPIP | 70.87 | 1,268.32 |
| Resistant | IVATS + ATQ | 119.72 | 1,219.46 | – | – | – |
ATQ = atovaquone–proguanil; DHAPIP = dihydroartemisinin–piperaquine; DOTS = directly observed treatment; IVATS = intravenous artesunate.
Under the elimination scenario, the NMES conducts all IRS and LLIN distribution activities, so their costs are captured under the program costs described previously. Under resurgence, however, the general health system will continue to carry out IRS and LLIN distribution. As IRS occurs at the household level, we calculated the number of households in the susceptible population by assuming an average of 3.3 people per household.30 The expected costs of IRS were based on the number of households at risk and the current target coverage rate of 80% and the most recent procurement cost of US$ 7.65 per household structure sprayed.31 The expected costs of LLIN distribution were measured using a target of 1.8 nets per household, a target coverage rate of 80%, and a net cost based on the most recent procurement and distribution cost of US$ 19.92.31 In Model A, net distribution and IRS would occur each year, regardless of the number of cases in that year. Each LLIN has a 3-year life, which requires supplying 1/3 of the population with new nets each year, plus a 10% wastage rate due to loss or damage each year. Under Model B, the IRS and ITN responses would only occur during the peak outbreak years and the following year.
Costs to the household.
Costs to the household include the costs of treatment, the value of caretakers’ time, the value of labor lost due to malaria illness, and the costs of deaths. Costs of treatment to the household include the costs of transportation, food, and other costs associated with seeking treatment, but they do not include costs of malaria drugs or professional care because these are supported by the Thai government. For a Pf case, the household cost equals US$ 10.77 per outpatient and US$ 50.53 per inpatient; for other Plasmodium species, this cost was US$ 12.42 per outpatient and US$ 38.49 per inpatient.32
Caretakers’ time accounts for the value of time a person loses when caring for malaria patients. The 2009 International Labor Organization’s average monthly income per capita for the second lowest income quintile, US$ 82.49 per month,33 was used and adjusted to assume a 2-day malaria episode34 and 22 working days per month. Assuming all caretakers are women, a gender wage gap differential of 83% was included.33 Further, we assumed that half of the caretakers work in the informal sector and earn 50% of the wage value of a woman employed in the formal sector.35
Lost wages account for the wages or equivalent time that adult patients lose per malaria case because they cannot work. The mean wages are calculated as described as for caretakers’ time, including the adjustment for the wage gap; wages were multiplied by 4 days lost per outpatient case and 7 days lost per inpatient case.22 These values were then inflated each year by the average projected annual gross domestic product (GDP) growth rate from 2015 to 2019, 3.04% per year.13
Death costs include funeral and the costs of lost wages after death. We assume funeral costs of US$ 1,471 per death, an estimate reflecting lower incomes earned by the population susceptible to malaria.36 Assuming the workforce age ranges from 15 to 60 years in Thailand, and based on the mean age of patients (Table 2), a malaria death would be equivalent to an average loss of 24.1 years for women, 25.7 years of work for men, and 45 years for a child (under 15 years).
Costs to the economy.
Downstream costs of lost labor reflect the effects on the overall economy of missed work due to malaria beyond direct effects. Assuming that each US$ 1 of labor activity produces US$ 2.65 of downstream economic activity,37 the wages lost to the household each year were multiplied by 2.65 to calculate the downstream loss to society.
Tourism revenue loss was measured by the number of trips cancelled or not booked among foreign tourists due to malaria resurgence. Based on 2015 figures, we assumed that 52.4 million foreign tourists would visit Thailand per year (P. Chayajitchayawat, personal communication) and that an average of US$ 565 would be spent per visit. Under elimination, we assumed a conservative 1.5% annual increase in tourism revenue each year.38 Under resurgence, persistent increases in malaria burden would affect tourism. For Model A, which does not have clear delineations of assumed outbreak years, we assumed a constant 2% lower growth rate in tourism, which we varied between 1% and 5% in scenario analyses. For Model B, we assumed that negative effects would only be projected during the outbreak years, with tourist arrivals decreasing by 1% during outbreak years and the following year and rebounding back to expected levels for other years.
RESULTS
Malaria cases.
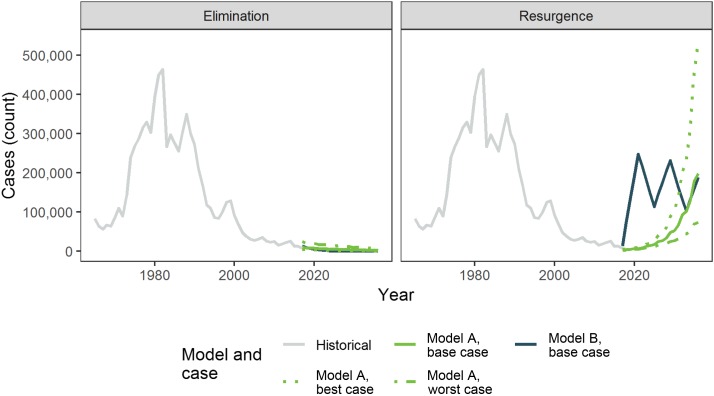
The expected numbers of malaria cases by model and scenario are shown in Figure 1, along with their best- and worst-case scenarios. For comparison, we also show the trends in historical cases from 1967 through 2016.
Figure 1.
Number of cases by scenario and case. This figure appears in color at www.ajtmh.org.
For Model A, under elimination, the expected number of cases declines from 10,407 in 2017 to 2,081 in 2036; under resurgence, the expected number of cases increases from 10,407 in 2017 to 198,671 in 2036. During the 20 years of the model, in the base case, elimination results in 101,357 cases of malaria and resurgence results in 1,029,279 cases—in Model A, elimination, therefore, prevents 0.93 million cases of malaria over 20 years. Model A also allows for best- and worst-case scenario analyses. In the best-case scenario analysis, the number of cases under elimination drops to only 598 in 2036, and the number of cases under resurgence rises beyond the historical peak of malaria incidence to 539,144 in 2036. In this case, elimination would prevent 4.59 million cases of malaria over 20 years. Under the worst-case analysis, pursuing elimination only reduces the number of cases to 7,238 in 2036 and resurgence would only lead to 73,209 cases in 2036. In the worst case, therefore, elimination only prevents 0.40 million cases of malaria over 20 years, more than 10 times fewer than the number of cases prevented in the best case.
For Model B, the number of cases under elimination declines from 13,425 in 2017 to 100 in 2036; under resurgence, the number of cases rises from 13,425 in 2017 to 189,107 in 2036. Over the 20 years of the model, elimination results in 46,598 cases of malaria, whereas resurgence leads to 3,155,632 cases—in Model B, elimination, therefore, prevents 3.11 million cases of malaria over 20 years.
Costs.
In the Model A base-case scenario, which assumes a 3% discount rate, NMES has an expected cost of US$ 134.93 million over 20 years. By varying the discount rate according to what makes elimination appear the best overall, the cost of the NMES over 20 years varies from US$ 124.92 to US$ 153.18 million. Because under the resurgence scenario the NMES receives no funding, by definition, its costs must be zero. In Model B, which assumes a 5% discount rate, the expected cost of the NMES is US$ 124.92 million.
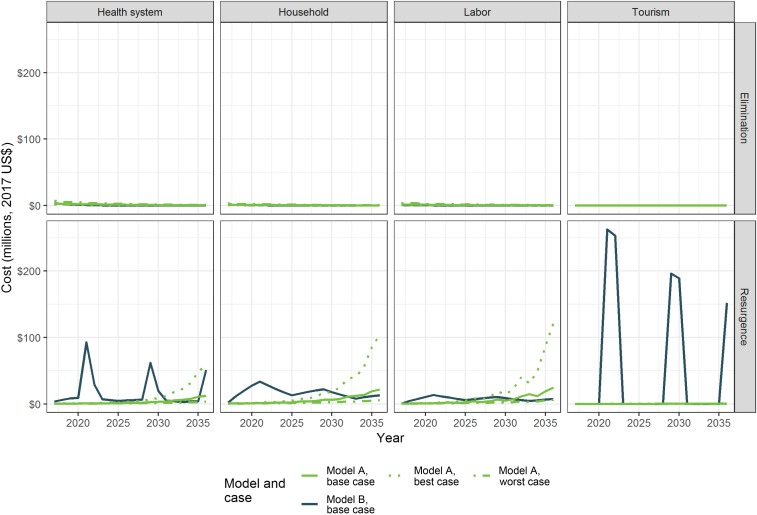
The expected costs for each year are shown by the sector that bears them in Figure 2. The costs generally follow the trends in expected cases for each model, scenario, and case, with the exception in Model B of lost tourism revenue and costs to the health system due to IRS and LLIN campaigns. For Model B, these costs spike dramatically every 8 years during and directly after assumed outbreaks.
Figure 2.
Expected costs by perspective and scenario. This figure appears in color at www.ajtmh.org.
The expected benefits, calculated by subtracting the costs of elimination from the costs of resurgence, are shown by perspective for each model in Table 4. In almost all cases, eliminating malaria leads to cost savings from every perspective examined. The only exception occurs in the worst-case scenario under Model A, in which the high costs of screening for malaria under elimination dwarf the cost savings from eliminating malaria.
Table 4.
Net benefits of elimination by model, scenario, and perspective (millions, 2017 US dollars)
| Health system | Household | Tourism revenue | Downstream productivity | ||
|---|---|---|---|---|---|
| Model A | Base case | $39.81 | $111.18 | $1.39 | $108.59 |
| Best case | $217.18 | $427.91 | $5.16 | $425.21 | |
| Worst case | −$31.30 | $18.96 | $0.54 | $9.89 | |
| Model B | Base case | $327.59 | $334.70 | $1,053.33 | $151.88 |
The benefit–cost ratios calculated by progressively broadening the perspective taken appear in Table 5. These ratios divide the cost spent on the NMES by the cost savings of reducing the incidence of malaria, given the following perspectives: first, the health system only; then the health system plus household costs; then the health system plus household costs and either lost tourism revenue or downstream productivity losses; and, finally, society as a whole. In the base case of Model A, elimination is expected to be cost-saving (defined by a benefit–cost ratio > 1) when compared with resurgence from every perspective except for the health system–only perspective. In the Model A best case, elimination is always cost-saving from every perspective. Under the worst-case scenario, however, elimination is expected to cost more than resurgence, which is driven by the negative “benefit” to the health system of elimination. As the benefits to larger sections of society are added to the model’s perspective, the overall ratio grows closer to 0, but it never approaches the break-even value of 1. Model B’s base-case outcomes find a much greater benefit of elimination than even the best-case scenario of Model A; from every perspective, Model B expects elimination to be highly cost-saving.
Table 5.
Benefit–cost ratios by model, scenario, and perspective
| Health system costs only | Health system and household costs | Health system and household costs, plus tourism revenue | Health system and household costs, plus downstream productivity | Full model | ||
|---|---|---|---|---|---|---|
| Model A | Base case | 0.30 | 1.12 | 1.13 | 1.92 | 1.93 |
| Best case | 1.42 | 4.21 | 4.25 | 6.99 | 7.02 | |
| Worst case | −0.25 | −0.10 | −0.09 | −0.02 | −0.02 | |
| Model B | Base case | 2.61 | 5.29 | 13.72 | 6.50 | 14.93 |
DISCUSSION
In the base case, our study predicts that elimination would prevent 0.93–3.11 million malaria cases over the next 20-year period, with full implementation of the NMES proving to be cost-saving in all models, perspectives, and scenarios, except for the health system–only perspective in the Model A base case and all perspectives in the Model A worst case. From the societal perspective, every 1 US$ spent on the NMES would—depending on case projections used—potentially result in a considerable return on investment, ranging from US$ 2 to US$ 15.
Our study included two case projection approaches to measure the number of malaria cases over the 2016–2036 study period, which—combined with an economic model—allowed us to quantify the cost–benefit ratio of malaria elimination in Thailand. We used two different epidemiological models because the results of any cost–benefit analyses are highly variable depending on the assumptions that are made on how malaria transmission dynamics would evolve following the full implementation or non-implementation of the NMES. There currently is no standard guiding which type of epidemiological modeling approach should be used in economic models determining the cost-effectiveness of malaria elimination,39 and each approach—as evidenced here—will have its strengths and limitations. Although both models are based on the malaria incidence by province and populations at risk over the 1965–2016 period, model outputs result in different case projections. Model A projects cases based on national historical case data using a log-normal regression and change-point analysis model, and Model B projects cases based on periodic Yala Province-level resurgence cycles extrapolated to national scale and incorporating more of the NMES political and programmatic goals.
Notwithstanding, regardless of which epidemiological modeling approach was used, from most perspectives and scenarios examined, we find that the benefits of eliminating the domestic transmission of malaria in Thailand will exceed the costs of doing so. From the perspective of the health system, the Model A best case and Model B base case suggest that pursuing malaria elimination will be cost-saving; Model A base and worst cases estimate that elimination might cost more money than it saves. As the perspective broadens to include households and society as a whole, malaria elimination becomes cost-saving in every version of the models except for the worst-case scenario of Model A. Even in this worst-case scenario, however, malaria elimination prevents 400,000 cases of malaria over 20 years, which might carry a moral weight that justifies spending this money even if the efforts in doing so were to result in a financial loss.
The inclusion of post-elimination years in the model resulted in slightly lower than expected costs per capita of elimination over 20 years than in similar studies conducted in other countries. During the active period of the NMES, the costs per capita align with those of similar programs.39 The Model A base-case and worst-case scenarios produce cost–benefit ratios lower than all those found in a systematic review of cost–benefit analyses of malaria elimination; the Model A best case and Model B base case are comparable with malaria elimination cost–benefit ratios reported elsewhere, including for Paraguay (2.6–3.3), Iraq (6.3), and Greece (917.09).29,39,40
Under Model B, lost tourism revenue during outbreak periods accounts for much of the savings to the overall economy. Although the causal link between tourism rates and malaria outbreaks remains contested,41 evidence from South Africa suggests that international tourists will cancel their trips due to malaria outbreaks.42 Notably, during the 2003 severe acute respiratory syndrome outbreak, foreign tourist arrivals in Thailand dropped by 8.8% during the month after the outbreak, which suggests that foreign tourists to Thailand will change their plans based on disease outbreaks.43
The models and data used in our analyses have some limitations. First, the use of point estimates for all of our cost parameters prevents us from empirically assessing the uncertainty of our findings. Model A does, however, allow for a simple scenario analysis based on varying the expected number of cases over time, which affects the expected costs from the model’s perspectives. In addition, the structure of Model A allows us to vary a few key input parameters: the discount rate and the expected growth rate of tourism. Because of its structure, Model B does not allow for scenario or sensitivity analyses. Second, many of the models’ inputs rely on expert opinion and privately shared data rather than on published and/or peer-reviewed data. We chose these sources because of a dearth of publicly available data on the economics of malaria in Thailand. We wanted to build a model specific to the Thai context, and consulting members of the BVBD and other local Thai experts helped us to verify the appropriateness of our estimates. Third, in Model A, we created a country-wide model for all geographic regions at risk, which does not allow us to account for local variations in malaria transmission. Fourth, in Model B, we created our own case projections based on our assumptions for the expected number of cases under each scenario rather than building an epidemiological model or using one supported by the literature; incidence rates from resurgence cycles in Yala Province, much of which were due to political unrest and subsequent disruption of services, were extrapolated to the national level. We did, however, consult years of historical province-level malaria incidence data to inform our decisions. Fifth, the benefits to the economy as a whole may go beyond periods of missed work and tourism. There are broad economic literature studies—albeit with mixed findings—that have shown the impact of malaria on education outcomes, wages, and so on.44–46 Clearly, if those were included, the findings of the cost–benefit analysis presented here would be even more positive.
Finally, all of our findings hinge on the NMES receiving full political and financial support. Without adequate investment, the elimination scenario presented here would become much less likely.
CONCLUSION
Using two different malaria case projection approaches, combined with context-specific programmatic and economic data, we analyzed the expected costs and benefits of malaria elimination in Thailand. In the base case, both approaches show that eliminating malaria—as per the Thai NMES5 and National Malaria Elimination Operational Plan12—will result in cost savings from the perspective of society as a whole. Even in Model A’s worst-case scenario, where the health system would face increased costs that would make malaria elimination more expensive from a societal perspective, eliminating malaria would still lead to cost savings to households and prevent losses to the overall economy from lost tourism revenue and decreases in downstream productivity. Assuming full support for the Thai NMES and the success of its proposed strategies, the cost–benefit analyses presented here strongly suggest that pursuing elimination of malaria will both prevent hundreds of thousands of cases and save the Thai economy money in the mid to long term.
One of the goals of the WHO Malaria Global Technical Strategy is to eliminate the disease in at least 35 new countries by 2030.1 Given its progress in reducing malaria over the past decade, Thailand is certainly a strong candidate country to make that list of 35 countries. The results of this cost–benefit analysis provides the Thai government and stakeholders with compelling evidence that malaria elimination would yield considerable return on investment and that advocacy efforts to prioritize malaria elimination programming—including the full funding of the NMES—should continue.
Supplementary Files
Acknowledgments:
We thank BVBD staff, whose malaria programmatic data were used in the epidemiological and cost models, and Julien Zwang for reviewing the manuscript.
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.WHO , 2015. Global Technical Strategy for Malaria 2016–2030. Geneva, Switzerland: World Health Organization; Available at: https://www.who.int/malaria/areas/global_technical_strategy/en/. Accessed February 19, 2019. [Google Scholar]
- 2.BVBD , 2017. Thai Malaria Information System. Nonthaburi, Thailand: BVBD. [Google Scholar]
- 3.WHO , 2016. World Malaria Report 2016: Country Profiles. Available at: http://www.who.int/malaria/publications/world-malaria-report-2016/WMR-2016-profiles.pdf. Accessed July 13, 2018. [Google Scholar]
- 4.The Global Fund to Fight AIDS, Tuberculosis and Malaria 2017. Thailand. Available at: https://www.theglobalfund.org/en/portfolio/country/?k=0ce9c9f6-40cb-429a-a51f-93cd10773722&loc=THA. Accessed June 23, 2017.
- 5.BVBD , 2016. National Malaria Elimination Strategy. Nonthaburi, Thailand: BVBD. [Google Scholar]
- 6.Corbel V, Nosten F, Thanispong K, Luxemburger C, Kongmee M, Chareonviriyaphap T, 2013. Challenges and prospects for dengue and malaria control in Thailand, Southeast Asia. Trends Parasitol 29: 623–633. [DOI] [PubMed] [Google Scholar]
- 7.Lin JT, Juliano JJ, Wongsrichanalai C, 2010. Drug-resistant malaria: the era of ACT. Curr Infect Dis Rep 12: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imwong M, et al. 2017. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 17: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Regional Office for South-East Asia , 2016. Programmatic Review of the National Malaria Programme in Thailand: 31st August–11th September 2015. Available at: http://apps.who.int/iris/bitstream/10665/253958/1/9789290225133-eng.pdf. Accessed July 13, 2018. [Google Scholar]
- 10.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B, 2012. Malaria resurgence: a systematic review and assessment of its causes. Malar J 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imwong M, Jindakhad T, Kunasol C, Sutawong K, Vejakama P, Dondorp AM, 2015. An outbreak of artemisinin resistant falciparum malaria in Eastern Thailand. Sci Rep 5: 17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BVBD , 2017. National Malaria Elimination Operational Plan 2017–2021. Nonthaburi, Thailand: BVBD. [Google Scholar]
- 13.World Bank , 2017. Health, Nutrition, and Population Statistics: Population Estimates and Projections. Available at: http://databank.worldbank.org/data/reports.aspx?source=Health%20Nutrition%20and%20Population%20Statistics:%20Population%20estimates%20and%20projections. Accessed June 21, 2017. [Google Scholar]
- 14.Ministry of the Interior , 2015. The Number of People throughout the Kingdom According to the Proof of Residence Registration. Available at: http://stat.bora.dopa.go.th/stat/y_stat58.htm. Accessed July 13, 2018. [Google Scholar]
- 15.Brockwell PJ, Davis RA, Calder MV, 2002. Introduction to Time Series and Forecasting, Vol. 2 New York, NY: Springer. [Google Scholar]
- 16.Kutner MH, Nachtsheim C, Neter C, 2004. Applied Linear Regression Models, 4th edition New York, NY: McGraw-Hill/Irwin. [Google Scholar]
- 17.Killick R, Eckley IA, 2014. changepoint: an R package for changepoint analysis. J Stat Softw 58: 1–19. [Google Scholar]
- 18.Karian ZA, Dudewicz EJ, 2016. Handbook of Fitting Statistical Distributions with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 19.Fahrmeir L, Tutz G, 2013. Multivariate Statistical Modelling Based on Generalized Linear Models. New York, NY: Springer Science + Business Media. [Google Scholar]
- 20.WHO , 2015. Strategy for Malaria Elimination in the Greater Mekong Subregion (2015–2030). Manila, Philippines: World Health Organization Regional Office for the Western Pacific; Available at: http://iris.wpro.who.int/bitstream/handle/10665.1/10945/9789290617181_eng.pdf. Accessed February 19, 2019. [Google Scholar]
- 21.Knoema , 2016. Health Nutrition and Population Statistics: Population Estimates and Projections, 1960–2050. Available at: https://knoema.com/WBPEP2017/health-nutrition-and-population-statistics-population-estimates-and-projections-1960-2050. Accessed June 21, 2017. [Google Scholar]
- 22.Attanayake N, Fox-Rushby J, Mills A, 2000. Household costs of ‘malaria’ morbidity: a study in Matale district, Sri Lanka. Trop Med Int Health 5: 595–606. [DOI] [PubMed] [Google Scholar]
- 23.BVBD , 2017. Therapeutic Efficacy Study. Nonthaburi, Thailand: BVBD; (unpublished). [Google Scholar]
- 24.Saifi MA, Wajihullah, Siddiqui MI, Al-Khalifa MS, 2010. Malaria: patterns of relapse and resistance. J King Saud Univ Sci 22: 31–36. [Google Scholar]
- 25.Manandhar S, Bhusal CL, Ghimire U, Singh SP, Karmacharya DB, Dixit SM, 2013. A study on relapse/re-infection rate of Plasmodium vivax malaria and identification of the predominant genotypes of P. vivax in two endemic districts of Nepal. Malar J 12: 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oanda.com , 2018. Historical Exchange Rates. Available at: https://www.oanda.com/fx-for-business/historical-rates. Accessed June 15, 2018. [Google Scholar]
- 27.Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL, eds, 2003. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Available at: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. Accessed July 13, 2018. [Google Scholar]
- 28.Vannaphan S, Saengnetswang T, Suwanakut P, Kllangbuakong A, Klinnak W, Rungmatcha P, Looareesuwan S, 2005. The epidemiology of patients with severe malaria who died at the Hospital for Tropical Diseases, 1991–2004. Southeast Asian J Trop Med Public Health 36: 385–389. [PubMed] [Google Scholar]
- 29.Shretta R, Baral R, Avanceña ALV, Fox K, Dannoruwa AP, Jayanetti R, Premaratne R, 2017. An investment case to prevent the reintroduction of malaria in Sri Lanka. Am J Trop Med Hyg 96: 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Japan International Cooperation Agency , 2013. Data Collection Survey on Housing Sector in Thailand: Final Report. Available at: http://dhds.nha.co.th/gisfs/internet/content/620_166_0.pdf. Accessed June 21, 2017. [Google Scholar]
- 31.BVBD , 2016. National Malaria Budget. Nonthaburi, Thailand: BVBD. [Google Scholar]
- 32.Baingern S, Kongsin S, Silachamroon U, Chamroonsawasdi K, 2011. Activity-based costing of malaria patient admissions (fiscal year 2009), Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University, Thailand. J Trop Med Parasitol 34: 70–78. [Google Scholar]
- 33.International Labour Organization , 2013. Thailand: A Labor Market Profile. Available at: http://www.ilo.org/wcmsp5/groups/public/---asia/---ro-bangkok/documents/publication/wcms_205099.pdf. Accessed June 21, 2017. [Google Scholar]
- 34.Dasgupta S, Bhula-or R, Fakthong T, 2015. Earnings Differentials between Formal and Informal Employment in Thailand (ILO Asia-Pacific Working Paper Series). Bangkok, Thailand: International Labor Organization Regional Office for Asia and the Pacific; Available at: http://www.ilo.org/wcmsp5/groups/public/---asia/---ro-bangkok/documents/publication/wcms_436903.pdf\. Accessed June 22, 2017. [Google Scholar]
- 35.Thailand National Statistical Office , 2017. The Informal Employment Survey. Available at: http://www.nso.go.th/sites/2014/DocLib13/%E0%B8%94%E0%B9%89%E0%B8%B2%E0%B8%99%E0%B8%AA%E0%B8%B1%E0%B8%87%E0%B8%84%E0%B8%A1/%E0%B8%AA%E0%B8%B2%E0%B8%82%E0%B8%B2%E0%B9%81%E0%B8%A3%E0%B8%87%E0%B8%87%E0%B8%B2%E0%B8%99/%E0%B9%81%E0%B8%A3%E0%B8%87%E0%B8%87%E0%B8%B2%E0%B8%99%E0%B8%99%E0%B8%AD%E0%B8%81%E0%B8%A3%E0%B8%B0%E0%B8%9A%E0%B8%9A/%E0%B9%81%E0%B8%A3%E0%B8%87%E0%B8%87%E0%B8%B2%E0%B8%99%E0%B8%99%E0%B8%AD%E0%B8%81%E0%B8%A3%E0%B8%B0%E0%B8%9A%E0%B8%9A_2560/Full_report2560.pdf. Accessed July 13, 2018. [Google Scholar]
- 36.Thepaksorn P, Pongpanich S, 2014. Occupational injuries and illnesses and associated costs in Thailand. Saf Health Work 5: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pholphirul P, Rukumnuaykit P, Kamlai J, 2013. Do Immigrants Improve Thailand’s Competitiveness? Available at: http://www.unescap.org/sites/default/files/Migration-Productivity.pdf. Accessed July 13, 2018. [Google Scholar]
- 38.World Travel & Tourism Council , 2018. Travel & Tourism Economic Impact 2018: Thailand. Available at: https://www.wttc.org/-/media/files/reports/economic-impact-research/countries-2018/thailand2018.pdf. Accessed July 13, 2018. [Google Scholar]
- 39.Shretta R, Avanceña ALV, Hatefi A, 2016. The economics of malaria control and elimination: a systematic review. Malar J 15: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shretta R, et al. 2017. An Investment Case for Eliminating Malaria in the Greater Mekong Subregion. San Francisco, CA: The Global Health Group, University of California, San Francisco. [Google Scholar]
- 41.Modrek S, Liu J, Gosling R, Feachem RG, 2012. The economic benefits of malaria elimination: do they include increases in tourism? Malar J 11: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maartens F, Sharp B, Curtis B, Mthembu J, Hatting I, 2007. The impact of malaria control on perceptions of tourists and tourism operators concerning malaria prevalence in KwaZulu-Natal, 1999/2000 versus 2002/2003. J Travel Med 14: 96–104. [DOI] [PubMed] [Google Scholar]
- 43.Rittichainuwat BN, Chakraborty G, 2009. Perceived travel risks regarding terrorism and disease: the case of Thailand. Tourism Manag 30: 410–418. [Google Scholar]
- 44.Bleakely H, 2010. Malaria eradication in the Americas: a retrospective analysis of childhood exposure. Am Econ J Appl Econ 2: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas AM, 2010. Malaria eradication and educational attainment: evidence from Paraguay and Sri Lanka. Am Econ J Appl Econ 2: 46–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutler D, Fung W, Kremer M, Singhal M, Vogl T, 2010. Early-life malaria exposure and adult outcomes: evidence from malaria eradication in India. Am Econ J Appl Econ 2: 72–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.