Abstract.
Vaginal self-sampling and human papillomavirus (HPV) DNA testing can be useful tools for women with limited access to health care living in sub-Saharan Africa. To assess the feasibility and acceptability of vaginal self-sampling and high-risk HPV prevalence in two villages of central Senegal, women were asked to self-sample vaginal swabs for HPV detection in May, 2016. Vaginal swabs were collected from 133 women and were tested for HPV genotyping. The acceptability rate of vaginal self-sampling was 98.5%, and 99.2% of the women (133/134) used the device correctly. The quality of self-sampling was satisfactory in 100% of the samples; 10.5% of the samples were positive for HPV, including 6% with high-risk HPV types and 4% with low-risk HPV types. This preliminary study indicates that vaginal self-sampling is a valuable strategy for high-risk HPV detection and cervical cancer screening in a population of women not attending gynecologic screening in rural areas of Senegal.
In sub-Saharan Africa, cervical cancer (CC) is the most common cancer in women.1 In Senegal, 1,197 Senegalese women are diagnosed with CC every year,1 with a standardized incidence rate of CC of 34.7 per 100,000 women-years and 795 deaths.1 In this country, 3.2 million females aged 15 and older are at risk of developing CC. A total of 70.9% of invasive CC are related to human papillomavirus (HPV) 16 and 18, whereas other high-risk (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59)—or probably high-risk HPV types (HPV 26, 53, 66, 67, 68, 70, 73, and 82) (International Agency for Research on Cancer 2011) are carcinogenic or probably carcinogenic. Few data are available regarding the prevalence and distribution of HPV types in Senegal.2 These epidemiological studies have potential implications for vaccine development.3 However, HPV vaccines are not yet available in Africa, and, therefore, an easy and feasible CC screening is mandatory. Among the screening tools, HPV DNA testing is useful for primary CC screening among women aged 30 years and older, and vaginal self-sampling is considered valuable for detecting oncogenic high-risk HPV infection,4 especially in women with limited access to health care living in low-resource countries.5 The two villages of Dielmo and Ndiop are located 280 km southeast of Dakar (capital of Senegal).6 Since 1990, these villages are part of the Dielmo project recruiting the whole population in a long-term survey of host–parasite associations.6 In 2010, a point-of-care (POC) laboratory was implemented in Dielmo for diagnosing pathogens causing misunderstood fevers.7 Point-of-care laboratory could be extended to HPV detection. With this setting, the feasibility of HPV vaginal self-sampling and high-risk HPV prevalence among Senegalese women in the two villages of central Senegal, Dielmo and Ndiop, were assessed in this preliminary study. A meeting was organized to explain the protocol, and all the women who are enrolled in the Dielmo–Ndiop health-care protocol who agreed to participate in this specific study were included.6
Vaginal swabs were collected from 133 nonvirgin women. The ethical agreement was obtained from the national Ethics Committee of Senegal (n°83/MSAS/DPRS/CNFRS). The women were explained the technique of vaginal self-sampling. Briefly, it consists of the women introducing the swab into their vagina without medical or paramedical presence.
The samples were collected with the Abbott Cervi-Collect Specimen Collection Kit and then stored at 4°C, transported in a cool box to Dakar laboratory where they were stored at −20°C, and then sent to Marseille University Hospital by DHL at −20°C for molecular analysis. Human papillomavirus genotyping was assessed by polymerase chain reaction (PCR) (MY09/MY11 primers), sequencing, phylogenetic analysis, and cloning if necessary as described.8 Human papillomavirus types were classified as high risk, probably high risk, and low risk according to the classification by de Villiers et al.9 Quantitation of HPV 16 and 18 viral load DNA was performed using a quantitative duplex real-time PCR method as reported previously.10 A plasmid that contained the three target sequences of interest was used: HPV 16 (on E6 gene), HPV 18 (on E7 gene), and human albumin (on exon 12). This method allowed the HPV 16, 18, and albumin gene copy number to be quantified in the same assay. Human papillomavirus viral load was expressed as HPV copies per million cells.
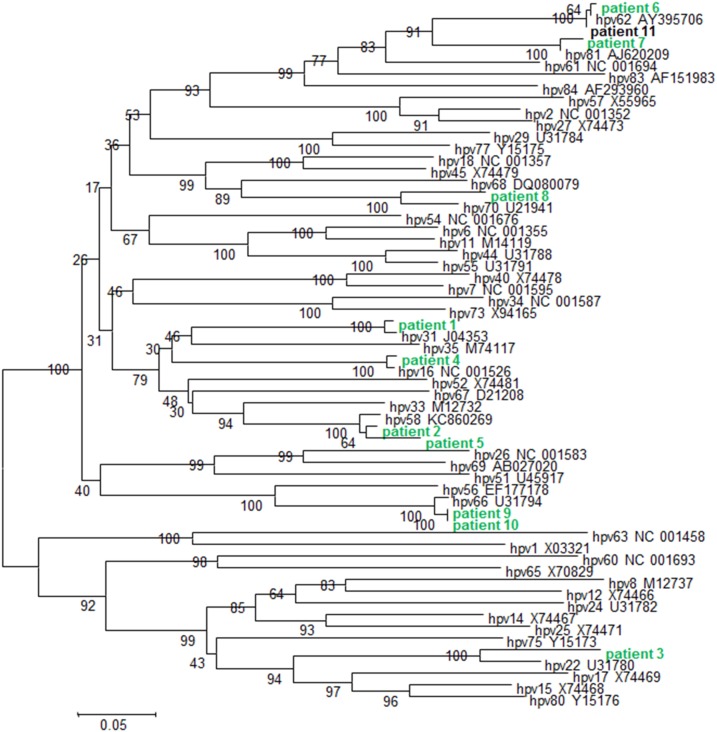
The location of the two villages of Dielmo and Ndiop is shown in Figure 1. One hundred thirty-six women responded to the invitation to go to the dispensary. One hundred thirty-four (98.5%) accepted to perform vaginal self-sampling. One hundred thirty-three of the 134 women performed it adequately. The quality of the collected vaginal samples was satisfactory in 100% of the samples (positive for human albumin). The frequency of HPV infection was 10.5% (14/133). Among them, 8/133 individuals had high-risk HPV types (6%), including two with HPV 18 and one with HPV 16 (Table 1). Among these high-risk HPV types, according to de Villiers classification,9 HPV 16, 18, 31, and 58 belong to group 1 of HPV types found most frequently in CC, whereas HPV type 66 belongs to group 2B classified as possibly carcinogenic according to the Bouvard classification.11 The frequency of low-risk HPV types was 4%, including HPV types 62 (1.5%), 22, 70, and 81 (0.7%). It is worth noting that according to the Bouvard classification,11 HPV type 70 should be included in the Group 2B and reclassified as possibly carcinogenic to humans. No multiple infections were observed (Table 1, Figure 2).
Figure 1.
Location of Dielmo and Ndiop villages in central Senegal. This figure appears in color at www.ajtmh.org.
Table 1.
Frequency of HPV types in 133 women tested in Dielmo and Ndiop areas
| n/Total | Frequency (%) | Detection methods | |
|---|---|---|---|
| HPV infection | 14/133 | 10.5 | qPCR and PCR sequencing |
| High-risk HPV types | 8/133 | 6 | qPCR and PCR sequencing |
| 16 | 1/133 | 0.7 | qPCR and PCR sequencing |
| 18 | 2/133 | 1.5 | qPCR |
| 31 | 1/133 | 0.7 | PCR sequencing |
| 58 | 2/133 | 1.5 | PCR sequencing |
| 66 | 2/133 | 1.5 | PCR sequencing |
| Low-risk HPV types | 6/133 | 4 | PCR sequencing |
| 22 | 1/133 | 0.7 | PCR sequencing |
| 62 | 2/133 | 1.5 | PCR sequencing |
| 70 | 1/133 | 0.7 | PCR sequencing |
| 81 | 1/133 | 0.7 | PCR sequencing |
HPV = human papillomavirus; pPCR = real-time polymerase chain reaction.
Figure 2.
Phylogenetic tree of 11 patients’ HPV sequences based on L1 gene fragment. Phylogenetic tree was constructed using nucleotides of the L1 gene. The tree was generated by nearest neighbor-joining analysis. Bootstrap values are shown at the branch nodes. The reference strains are reported with accession number. This figure appears in color at www.ajtmh.org.
This preliminary study is the first in Senegal, to our knowledge, to show the excellent feasibility and acceptability (98.5%) of self-sampling for HPV detection, as previously recorded in studies from developed countries among women not attending regular screening for cultural or religious reasons or from low socioeconomic level4,8 or among women from developing countries.5,12 Altogether, these studies demonstrate that vaginal self-sampling is an acceptable option in low-income communities5,12,13 and is a reliable tool for CC screening.4,8 Moreover, in the present study, the quality of self-sampling was excellent, indicating that the self-sampling was performed adequately. This technique could be a first step for the management of women and then needing clinical and financial resources for the treatment of these women positively screened (triage test, colposcopy, surgery, etc.).
In this study, HPV prevalence was 10.5% in systematically screened women, which was close to worldwide data reporting that 11.4% of adult women are HPV infected.14 However, it was much lower than that found recently in other African countries such as Malawi (19.9%),15 Cameroon (18.5%),16 or Madagascar (31.6%).17 Of note, in the two villages of Dielmo and Ndiop, 100% of the women were Muslims, which could, according to the religious and traditional practices,17 explain the low HPV frequencies that were observed. Worldwide variation in HPV type distribution was reported18 with, for example, a higher frequency of HPV 45 in invasive CC in Africa, and a higher frequency of HPV 58 in eastern Asia. A wide variation in HPV prevalence through sub-Saharan African was reported ranging from 20% in Uganda, Kenya and Tanzania to 40–70% elsewhere.14 In Senegal, a study conducted by Mbaye el et al.2 shows that in the Dakar region, the prevalence of high-risk HPV infection was 17.4–23.2% according to the regions, and the most frequent types were HPV 52, followed by HPV 31 and HPV 16, 45, and 53. In another study in Dakar, the prevalence of HPV infection was 18% in women aged ≥ 35 years, whereas HPV 16 and 58 were the most frequent types detected in women with negative cytology (2.4% and 1.6%, respectively) and were also found strongly associated with risk of high-grade squamous intraepithelial lesions/CC.19 In the present study, the high-risk HPV types found were HPV 16, 18, 31, 58, and 66, which is in line with these previous studies in Dakar and also with screening studies conducted in developed countries.20 Of note, 4.5% of low-risk HPV types (HPV 22, 62, 70, and 81) were identified. Of interest, HPV 22 is a scarce cutaneous HPV type of the genus beta not classified as carcinogenic so far.9 In addition and contrasting with most of the previous studies published on HPV prevalence in comparable populations of women, no multiple HPV infection was found. This finding could be related first, to the genotyping technique used in this study, which may be problematic for detecting multiple HPV genotypes.21 Indeed, direct sequence analysis detects fewer multiple HPV types than methods such as reverse hybridizing method by linear array or DNA chips. Second, it could be related again to the cultural and religious customs of these women or rural areas of Senegal. Another limitation of our study was the small sampling (133 women) number, and the absence of cytological results, thus limiting our interpretation. This preliminary study was undertaken to demonstrate the good feasibility, acceptability, and accuracy of vaginal self-sampling to detect HPV types in the setting of a POC laboratory. In addition, HPV-based screening was recently proved to be more sensitive than cytology-based screening for CC screening.22
In conclusion, this preliminary study indicates that vaginal self-sampling is a valuable strategy for high-risk HPV detection and CC screening in a population of women not attending gynecologic screening in rural areas of Senegal. In the next future, recent tests such as Xpert HPV point-of-care test, which is more convenient, rapid, friendly to use, reliable, and appropriate for low-income countries, will be implemented.23,24 This warrants additional, larger, and more systematic studies in such areas and in addition to the implemented POC laboratories that help screen sexually transmitted diseases and vaginitis infectious agents.
REFERENCES
- 1.Louie KS, de Sanjose S, Mayaud P, 2009. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Trop Med Int Health 14: 1287–1302. [DOI] [PubMed] [Google Scholar]
- 2.Mbaye el HS, Gheit T, Dem A, McKay-Chopin S, Toure-Kane NC, Mboup S, Tommasino M, Sylla BS, Boye CS, 2014. Human papillomavirus infection in women in four regions of Senegal. J Med Virol 86: 248–256. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM, 2007. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 121: 621–632. [DOI] [PubMed] [Google Scholar]
- 4.Sancho-Garnier H, Tamalet C, Halfon P, Leandri FX, Le Retraite L, Djoufelkit K, Heid P, Davies P, Piana L, 2013. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int J Cancer 133: 2681–2687. [DOI] [PubMed] [Google Scholar]
- 5.Kunckler M, Schumacher F, Kenfack B, Catarino R, Viviano M, Tincho E, Tebeu PM, Temogne L, Vassilakos P, Petignat P, 2017. Cervical cancer screening in a low-resource setting: a pilot study on an HPV-based screen-and-treat approach. Cancer Med 6: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trape JF, et al. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg 51: 123–137. [DOI] [PubMed] [Google Scholar]
- 7.Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape JF, Drancourt M, Raoult D, 2013. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis 7: e1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamalet C, Richet H, Carcopino X, Henry M, Leretraite L, Heid P, Leandri FX, Sancho-Garnier H, Piana L, 2010. Testing for human papillomavirus and measurement of viral load of HPV 16 and 18 in self-collected vaginal swabs of women who do not undergo cervical cytological screening in southern France. J Med Virol 82: 1431–1437. [DOI] [PubMed] [Google Scholar]
- 9.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H, 2004. Classification of papillomaviruses. Virology 324: 17–27. [DOI] [PubMed] [Google Scholar]
- 10.Carcopino X, Henry M, Benmoura D, Fallabregues AS, Richet H, Boubli L, Tamalet C, 2006. Determination of HPV type 16 and 18 viral load in cervical smears of women referred to colposcopy. J Med Virol 78: 1131–1140. [DOI] [PubMed] [Google Scholar]
- 11.Bouvard V, et al. WHO International Agency for Research on Cancer Monograph Working Group , 2009. A review of human carcinogens—Part B: biological agents. Lancet Oncol 10: 321–322. [DOI] [PubMed] [Google Scholar]
- 12.Lagier JC, Diagne N, Fenollar F, Tamalet C, Sokhna C, Raoult D, 2017. Vaginal self-sampling as a diagnosis tool in low-income countries and potential applications for exploring the infectious causes of miscarriage. Future Microbiol 12: 609–620. [DOI] [PubMed] [Google Scholar]
- 13.Jones HE, et al. 2007. Home-based versus clinic-based self-sampling and testing for sexually transmitted infections in Gugulethu, South Africa: randomised controlled trial. Sex Transm Infect 83: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruni L, et al. 2014. Human Papillomavirus and Related Diseases in the World, Summary Report. X. ICO Information Centre on HPV and Cancer (HPV Information Centre), 12–18. Available at: https://www.sexrightsafrica.net/wp-content/uploads/2016/04/ICO-World-HPV-report-2015.pdf. Accessed April 11, 2019. [Google Scholar]
- 15.Cubie HA, et al. 2017. HPV prevalence in women attending cervical screening in rural Malawi using the cartridge-based Xpert(R) HPV assay. J Clin Virol 87: 1–4. [DOI] [PubMed] [Google Scholar]
- 16.Catarino R, Vassilakos P, Jinoro J, Broquet C, Benski AC, Meyer-Hamme U, Petignat P, 2016. Human papillomavirus prevalence and type-specific distribution of high- and low-risk genotypes among Malagasy women living in urban and rural areas. Cancer Epidemiol 42: 159–166. [DOI] [PubMed] [Google Scholar]
- 17.Gultekin M, Akgul B, 2017. HPV screening in Islamic countries. Lancet Infect Dis 17: 368. [DOI] [PubMed] [Google Scholar]
- 18.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, Clifford GM, 2012. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 131: 2349–2359. [DOI] [PubMed] [Google Scholar]
- 19.Xi LF, Toure P, Critchlow CW, Hawes SE, Dembele B, Sow PS, Kiviat NB, 2003. Prevalence of specific types of human papillomavirus and cervical squamous intraepithelial lesions in consecutive, previously unscreened, West-African women over 35 years of age. Int J Cancer 103: 803–809. [DOI] [PubMed] [Google Scholar]
- 20.De Vuyst H, Clifford G, Li N, Franceschi S, 2009. HPV infection in Europe. Eur J Cancer 45: 2632–2639. [DOI] [PubMed] [Google Scholar]
- 21.Nilyanimit P, Chansaenroj J, Poomipak W, Praianantathavorn K, Payungporn S, Poovorawan Y, 2018. Comparison of four human papillomavirus genotyping methods: next-generation sequencing, INNO-LiPA, electrochemical DNA chip, and nested-PCR. Ann Lab Med 38: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayrand MH, et al. 2007. Human papillomavirus DNA versus Papanicolaou screening test for cervical cancer. N Engl J Med 357: 1579–1588. [DOI] [PubMed] [Google Scholar]
- 23.Toliman P, et al. 2016. Field evaluation of Xpert HPV point-of-care test for detection of human papillomavirus infection by use of self-collected vaginal and clinician-collected cervical specimens. J Clin Microbiol 54: 1734–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuzick J, et al. 2015. Performance of the Xpert HPV assay in women attending for cervical screening. Papillomavirus Res 1: 32–37. [Google Scholar]