Abstract.
Anemia in HIV-infected patients improves with highly active antiretroviral therapy (HAART); however, it may still be associated with mortality among patients receiving treatment. We examined the associations of anemia severity and iron deficiency anemia (IDA) at HAART initiation and during monthly prospective follow-up with mortality among 40,657 adult HIV-infected patients receiving HAART in Dar es Salaam, Tanzania. Proportional hazards models were used to examine the associations of anemia severity and IDA at HAART initiation and during follow-up with mortality. A total of 6,261 deaths were reported. Anemia severity at HAART initiation and during follow-up was associated with an increasing risk of mortality (trend tests P < 0.001). There was significantly higher mortality risk associated with IDA at HAART initiation and during follow-up versus no anemia or iron deficiency (both P < 0.001). These associations differed significantly by gender, body mass index, and iron supplement use (all interaction test P < 0.001). The magnitude of association was stronger among men. Mortality risk with severe anemia was 13 times greater versus no anemia among obese patients, whereas it was only two times greater among underweight patients. Higher mortality risk was observed among iron supplement users, irrespective of anemia severity. Anemia and IDA were significantly associated with a higher mortality risk in patients receiving HAART. Iron supplementation indicated an increased mortality risk, and its role in HIV infections should be examined in future studies. Given the low cost of assessing anemia, it can be used frequently to identify high-risk patients in resource-limited settings.
INTRODUCTION
Anemia is the most common hematological complication in HIV-infected patients globally, with prevalence estimates ranging from 11% to 69%—severe anemia being more prevalent in the advanced disease stage.1–5 Causes of anemia are multifactorial, and iron deficiency (ID) is the most significant factor in approximately 50% of the cases.3,6 Another prevalent cause is the anemia of chronic disease (ACD), which is characterized by the release of pro-inflammatory cytokines. These cytokines disrupt the iron homeostasis of the body, resulting in anemia.7,8
Anemia in antiretroviral therapy (ART)-naive, HIV-infected patients has been associated with increased mortality, independent of CD4 cell count and other prognostic markers.1,9–12 Several studies in patients initiating highly active antiretroviral therapy (HAART) have also identified anemia as a prognostic marker.2,13 A collaboration of 10 HIV cohort studies in North America and Europe analyzing data from 12,100 subjects demonstrated that anemia at HAART initiation was independently associated with higher mortality, after adjusting for age, gender, CD4 cell count, plasma viral load, and disease stage (adjusted hazard ratio [95% CI] for mild anemia [1.42; 1.17–1.73], moderate anemia [2.56; 2.07–3.18], and severe anemia [5.26; 3.55–7.81]).13 Studies also suggest that anemia improves with HAART.1,5,13,14 Despite these findings, studies examining the association between anemia severity during follow-up and the risk of mortality are not available.
Oral iron is recommended for the treatment of non-severe forms of anemia.15 However, there are concerns regarding the use of iron in infectious disease, as it has been associated with increased morbidity.16,17 Experimental studies have identified that iron is critical for the transcription of HIV.17 Supportive findings from epidemiological studies conducted in developed countries have also shown an increased risk of HIV disease progression with increased iron stores.18 By contrast, others have not demonstrated significant associations, resulting in inconclusive evidence.17–19
Given the paucity of studies examining the relative contribution and potential interaction of anemia, iron deficiency anemia (IDA), and iron supplementation for HIV-infected individuals receiving HAART, we conducted an observational study of patients enrolled in a large urban HIV care and treatment program in Dar es Salaam, Tanzania. We examined the association of anemia severity and IDA at HAART initiation and during the course of treatment with mortality and explored their interaction with iron supplementation.
SUBJECTS AND METHODS
Subjects.
This prospective cohort study consisted of HIV-infected adults attending HIV care and treatment clinics supported by the Management and Development for Health (MDH) between November 2004 and September 2012 in Dar es Salaam, Tanzania. The Management and Development for Health is supported by the U.S. President’s Emergency Plan for AIDS Relief and provides HIV care and treatment, including HAART, follow-up for side effects, preventive and therapeutic treatment for disease complications, and laboratory and technical support, for HIV-infected patients in Dar es Salaam. Clinical care followed the guidelines of the Tanzanian National AIDS Control Program and the WHO.20,21 The criteria for HAART initiation were WHO stage IV, WHO stage III with CD4 cell count < 350 cells/µL, or CD4 cell count < 200 cells/µL. The initiation criteria were modified in 2012 to include adults in WHO stage IV or III, regardless of the CD4 cell count, or with CD4 cell count < 350 cells/µL, regardless of the clinical symptoms.22 The standard first-line ART regimen included two nucleoside reverse-transcriptase inhibitors, stavudine or zidovudine (AZT) and lamivudine, plus one nonnucleoside reverse-transcriptase inhibitor, efavirenz or nevirapine. To avoid drug interactions, efavirenz was substituted for nevirapine in patients receiving rifampin anti-tuberculosis (anti-TB) therapy. All drugs were supplied free of charge by the Tanzanian government if the patient was found eligible. Adult patients found to be anemic on hemoglobin test were given iron supplements daily, ferrous sulfate 200 mg and folic acid 5 mg, until follow-up test indicated no anemia or up to 6 months. Patients aged 15 years or older enrolled in the MDH who initiated HAART between November 2004 and September 2012, were ART naive and not pregnant, and had at least one hemoglobin measurement in the 3 months before or within 14 days of HAART initiation were included in this study.
Study procedures.
Detailed background information was obtained, and physical examination was conducted for all participants at the time of MDH enrollment. After HAART initiation, participants were seen monthly by a physician who performed a complete clinical examination. A counselor assessed adherence to the regimen and provided nutrition counseling, if necessary. Study physicians assessed the HIV disease stage according to the WHO staging criteria.23 At each visit, study nurses performed anthropometric measurements using calibrated instruments and standardized techniques and recorded any illness at the time of visit or in the previous 1 month. Laboratory tests, including CD4 cell counts (using FACS Calibur; Becton Dickinson [BD], San Jose, CA), were conducted and blood chemistry values (using Cobas Integra 400 Plus chemistry analyzer; Roche, Basil, Switzerland) measured at HAART initiation and repeated every 4 months. Viral load testing was not routinely performed in this cohort. All data on sociodemographic characteristics, clinical information, physical examination, laboratory measurements, and therapeutics were entered by physicians and nurses on standard structured forms with unique patient identifiers. Data forms were assessed by reviewers for completeness and accuracy of information, followed by entry into a secure computerized database.
Assessment of anemia, ID, and mortality.
Blood samples for the assessment of anemia and ID were collected by trained phlebotomists at the time of HAART initiation and then repeated every 4 months. Patients receiving AZT also had hemoglobin assessed after 2 weeks of HAART initiation to assess for toxicity. Anemia and ID were defined using hemoglobin concentration and mean corpuscular volume (MCV) obtained from the complete blood count (using ACT5 DIFF hematology analyzer; Beckman Coulter, Brea, CA). Anemia was categorized as mild, moderate, and severe using the following definitions: mild (hemoglobin 10 to < 12 g/dL in women and 10 to < 13 g/dL in men), moderate (hemoglobin 7.0 to < 10 g/dL), and severe (hemoglobin < 7 g/dL). No anemia was defined as hemoglobin ≥ 12 g/dL in women and ≥ 13 g/dL in men.24 Iron deficiency was defined as MCV < 80 fL.25 Each individual’s IDA status was then identified by assessing anemia and ID, thereby creating four groups: no anemia or ID, ID without anemia, anemia without ID, and IDA.
Study participants were followed up to assess mortality due to any cause using a comprehensive tracking system. Early mortality was defined as death within the first 3 months of HAART initiation. If participants missed a scheduled clinic visit, they were contacted via phone or by a home visit to encourage a return visit to the clinic. Deaths were recorded after notification by family members or friends, through review of medical records, by a clinician treating a patient at the time of death, by patient tracking teams, or by community-based health-care workers. In case of loss to follow-up, a network of community-based health-care workers and volunteers actively traced patients and ascertained their vital status. Patients were followed up until death, loss to follow-up, or September 30, 2012. If the patient was known to have died but the date of death was unknown, date of the last patient visit was used as the date of death.
Covariates.
Detailed information on the following covariates was collected at the time of HAART initiation and subsequent clinic visits: age, body mass index (BMI), district in Dar es Salaam, facility level, WHO clinical disease stage, use of iron supplements, TB treatment, oral candidiasis, diarrhea, HAART regimen, and nonadherence to therapy. Gender, history of pulmonary TB, and calendar year of HAART initiation were recorded at the time of the initial visit. Nonadherence was measured using the drug pickup data and defined as days late for the scheduled drug pickup appointment as a proportion of the total days since last visit, expressed as a %. If this measure was ≥ 5%, the patient was labeled as nonadherent.26 Assessment for pulmonary TB was performed at enrollment in the program using chest X-ray and sputum smear examination for acid-fast bacilli. These were repeated in patients with symptoms suggestive of TB at follow-up visits.
Ethical considerations.
This study was ethically approved by the Institutional Review Boards of the Harvard School of Public Health and the Muhimbili University of Health and Allied Sciences.
Statistical analysis.
The distribution of baseline characteristics of the study participants was described using proportions and medians (interquartile ranges [IQRs]). Cox proportional hazards regression models were used to examine the associations of anemia severity and IDA at the time of HAART initiation with time to early mortality, and those of time-dependent anemia severity and IDA with time to overall mortality.27 Trend for the associations of anemia severity was calculated using the median hemoglobin value for each anemia category and by treating it as a continuous variable. For the association of IDA with mortality, P-values were calculated using the likelihood ratio tests. We also examined anemia severity and mortality associations in a subset of patients who did not have ID at HAART initiation as a proxy for anemia due to chronic inflammation.28 The Andersen–Gill data structure with a row representing observations for the exposure and covariates measured at a visit were used.29 Hemoglobin and other laboratory measures were repeated every 4 months and were carried forward until the next measurement became available. Follow-up for death was calculated as time from HAART initiation until the outcome, last visit during follow-up, or September 30, 2012, whichever occurred first. The proportional hazards assumption was tested by assessing the significance of time since HAART initiation by covariate interactions in the multivariate model using the partial likelihood ratio tests.
The following potential confounders and categorizations were included in the multivariate models: gender, age (< 30, 30 to < 40, 40 to < 50, and ≥ 50 years), district in Dar es Salaam (Ilala, Kinondoni, and Temeke), facility level (hospital, health center, and dispensary), BMI categories (< 18.5, 18.5 to < 25, ≥ 25 to < 30, and ≥ 30 kg/m2), CD4 cell count (< 50, 50 to < 100, 100 to < 200, and ≥ 200 cells/µL), WHO disease stage (I, II, III, and IV), alanine aminotransferase (< 40 and ≥ 40 IU/L), HAART regimen (stavudine, lamivudine, and nevirapine; stavudine, lamivudine, and efavirenz; zidovudine, lamivudine, and nevirapine; zidovudine, lamivudine, and efavirenz; tenofovir, lamivudine/emtricitabine, and nevirapine; tenofovir, lamivudine/emtricitabine, and efavirenz; and others/missing), nonadherence to therapy (< 5% or ≥ 5%), calendar year since HAART initiation, use of iron supplements (yes or no), TB history (yes or no), treatment for TB (yes or no), diarrhea (yes or no), oral candidiasis (yes or no), and season of visit (long rainy [April–May], long dry [June–September], short rainy [October–November], and short dry [December–March]). All continuous potential confounders were also evaluated for nonlinearity of the association semi-parametrically using restricted cubic splines.30,31 When found significant, spline terms were selected by stepwise selection for inclusion in the model. Missing indicators were used when model covariates were unavailable.32 We assessed potential modifications of the association by gender, use of iron supplements, BMI categories, CD4 cell count (< 350 versus ≥ 350 cells/µL), and use of zidovudine, as there is evidence from earlier studies or potential biological mechanisms suggesting plausible modifications of the associations.33 Interaction terms between exposure categories and potential effect modifiers were created and assessed for significance in the multivariate models using a partial likelihood ratio test. If found significant, we calculated estimates of the associations stratified by this variable. All significance tests were two-sided, and the differences were considered significant at P < 0.05. Statistical analyses were performed using SAS, version 9.2 software (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of study participants.
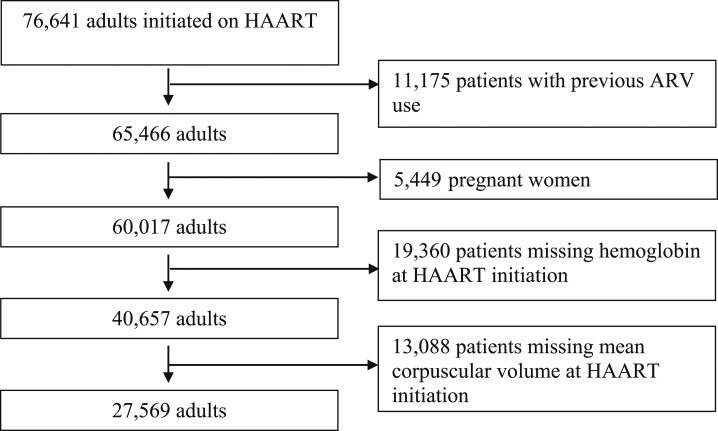
A total of 40,657 participants were eligible for the study (Figure 1). The median (IQR) age of study participants was 37 years (range 31–44 years), and 66% were females. Eighty-five percent of the participants were anemic at the time of HAART initiation; the proportion of participants with mild, moderate, and severe anemia was 41%, 36%, and 8%, respectively. More than 75% of patients were in WHO disease stage III or IV, and 73% had CD4 cell counts < 200 cells/µL. Approximately 10% of the patients received iron supplements. Characteristics of the study participants at HAART initiation are presented in Table 1. In all, 27,569 participants had MCV measured at HAART initiation. Of these, 44% had IDA, 41% had anemia without ID, and only 4% of the patients had ID without anemia. The primary reason identified for participants’ missing MCV measurements in this resource-limited setting was the nonavailability of laboratory reagents or nonfunctioning of the hematology analyzer.
Figure 1.
Flow diagram showing selection of study participants (November 2004 till September 2012).
Table 1.
Baseline characteristics of the study participants at HAART initiation (n = 40,657)
| Characteristics | N (%)* |
|---|---|
| Gender (F) | 26,954 (66) |
| Age, median (IQR) (years) | 37 (31–44) |
| Age category (years) | |
| < 30 | 7,102 (17) |
| 30 to < 40 | 17,888 (44) |
| 40 to < 50 | 10,780 (27) |
| ≥ 50 | 4,883 (12) |
| District | |
| Ilala | 16,637 (41) |
| Kinondoni | 12,245 (30) |
| Temeke | 11,648 (29) |
| Facility level | |
| Hospital | 32,349 (80) |
| Health center | 3,081 (8) |
| Dispensary | 5,100 (13) |
| Anemia† | |
| None | 6,369 (16) |
| Mild | 16,506 (41) |
| Moderate | 14,662 (36) |
| Severe | 3,120 (8) |
| IDA‡ | |
| None | 3,021 (11) |
| ID without anemia | 1,100 (4) |
| Anemia without ID | 11,235 (41) |
| IDA | 12,213 (44) |
| BMI, median (IQR) (kg/m2) | 20 (18–23) |
| BMI category | |
| Underweight | 12,403 (32) |
| Normal weight | 20,524 (53) |
| Overweight | 4,457 (11) |
| Obese | 1,589 (4) |
| CD4 T cell, median (IQR) (count/µL) | 125 (51–207) |
| CD4 T-cell category (count/µL) | |
| < 50 | 9,487 (24) |
| 50 to < 100 | 6,654 (17) |
| 100 to < 200 | 12,353 (32) |
| ≥ 200 | 10,350 (27) |
| Alanine aminotransferase, median (IQR) (IU/L) | 20 (14–30) |
| Alanine aminotransferase (IU/L) | |
| < 41 | 27,259 (86) |
| ≥ 41 | 4,280 (14) |
| WHO disease stage | |
| I | 2,781 (7) |
| II | 6,584 (16) |
| III | 22,933 (57) |
| IV | 7,892 (20) |
| HAART regimen | |
| Stavudine, lamivudine, and nevirapine | 9,818 (24) |
| Stavudine, lamivudine, and efavirenz | 2,683 (7) |
| Zidovudine, lamivudine, and nevirapine | 3,854 (9) |
| Zidovudine, lamivudine, and efavirenz | 13,224 (33) |
| Tenofovir, lamivudine/emtricitabine, and nevirapine/efavirenz | 1,637 (4) |
| Missing/others§ | 9,441 (23) |
| Season of visit | |
| Long rainy (April–May) | 6,821 (17) |
| Long dry (June–September) | 15,167 (37) |
| Short rainy (October–November) | 6,714 (17) |
| Short dry (December–March) | 11,955 (29) |
| Past TB history | |
| Yes | 13,163 (34) |
| No | 25,125 (66) |
| Receiving TB treatment | |
| Yes | 5,877 (17) |
| No | 29,610 (83) |
| Receiving iron supplement | |
| Yes | 1,870 (10) |
| No | 15,061 (89) |
| Oral candidiasis | |
| Yes | 1,315 (4) |
| No | 35,410 (96) |
| Diarrhea | |
| Yes | 1,444 (4) |
| No | 38,424 (96) |
| Calendar year of HAART initiation | |
| 2004 or 2005 | 1,628 (4) |
| 2006 | 4,529 (11) |
| 2007 | 6,210 (15) |
| 2008 | 7,413 (18) |
| 2009 | 7,779 (19) |
| 2010 | 6,278 (15) |
| 2011 | 4,213 (10) |
| 2012 | 2,607 (6) |
BMI = body mass index; HAART = highly active antiretroviral therapy; ID = iron deficiency; IDA = iron deficiency anemia; IQRs = interquartile ranges; TB = tuberculosis.
* Data are No. (%), unless otherwise indicated. Because of missing values, sums may be less than the total. Because of rounding, values may not add to 100%.
† Anemia defined as follows: mild (hemoglobin 10 to < 12 g/dL in women and 10 to < 13 g/dL in men), moderate (hemoglobin 7 to < 10 g/dL), and severe (hemoglobin < 7 g/dL). No anemia was defined as hemoglobin ≥ 12 g/dL in women or hemoglobin ≥ 13 g/dL in men.
‡ Iron deficiency anemia defined as follows: anemia as hemoglobin < 12 g/dL for women or hemoglobin < 13 g/dL for men, and ID as mean corpuscular volume < 80 fL. Anemia and ID exposures were cross-classified to define each individual’s exposure status.
§ Includes missing HAART regimen and combinations of abacavir, lopinavir, indinavir, saquinavir, atazanavir, didanosine, nelfinavir, ritonavir, zidovudine, lamivudine, stavudine, efavirenz, nevirapine, tenofovir, and emtricitabine.
Associations of mild, moderate, and severe anemia with mortality.
There were a total of 6,261 deaths reported in the prospective follow-up of the study participants. Fifty-eight percent (n = 3,611) of the total deaths occurred within the first 3 months of follow-up (early mortality). A total of 31% of all deaths occurred in the mild anemia category (n = 1,933), 39% in the moderate anemia category (n = 2,429), 20% in the severe anemia category, and 11% of the total deaths among those with no anemia at HAART initiation (n = 662). The median (IQR) follow-up time for the study participants was 21 months (range 3–43 months).
The associations of anemia severity with mortality are shown in Table 2. Anemia at HAART initiation was significantly associated with an increased risk of mortality in the first 3 months of follow-up (trend test P-value < 0.001); the relative risk of mortality increased with increasing severity of anemia. A significant trend of increasing mortality with increasing anemia severity during follow-up was also observed (trend test P-value < 0.001). There was no indication of a change in the magnitude or direction of the association of anemia with mortality over time since HAART initiation.
Table 2.
Associations of anemia* with mortality in HIV-infected patients initiating HAART (n = 40,657)
| Exposure status | Deaths/person-months | Univariate RR (95% CI) | Trend test P-value | Multivariate RR† (95% CI) | Trend test P-value |
|---|---|---|---|---|---|
| Baseline anemia and early mortality‡ | 3,611/57,047 | ||||
| No anemia | 242/9,432 | 1.00 (ref) | < 0.001 | 1.00 (ref) | < 0.001 |
| Mild anemia | 956/24,106 | 1.53 (1.33, 1.76) | 1.26 (1.09, 1.45) | ||
| Moderate anemia | 1,592/20,189 | 2.90 (2.53, 3.32) | 1.90 (1.65, 2.18) | ||
| Severe anemia | 821/3,320 | 7.31 (6.33, 8.44) | 3.32 (2.86, 3.86) | ||
| Anemia during follow-up and overall mortality | 6,261/1,080,905 | ||||
| No anemia | 662/360,985 | 1.00 (ref) | < 0.001 | 1.00 (ref) | < 0.001 |
| Mild anemia | 1,933/495,826 | 1.44 (1.32, 1.58) | 1.28 (1.17, 1.40) | ||
| Moderate anemia | 2,429/200,062 | 2.70 (2.47, 2.95) | 2.00 (1.82, 2.19) | ||
| Severe anemia | 1,237/24,032 | 7.67 (6.96, 8.46) | 3.90 (3.51, 4.32) |
BMI = body mass index; HAART = highly active antiretroviral therapy; ref = reference; TB = tuberculosis.
* Baseline anemia defined as: mild (hemoglobin 10 to < 12 g/dL in women or 10 to < 13 g/dL in men), moderate (hemoglobin 7 to < 10 g/dL), and severe (hemoglobin < 7 g/dL). No anemia was defined as hemoglobin ≥ 12 g/dL in women or hemoglobin ≥ 13 g/dL in men.
† Baseline anemia model adjusted for gender, age, district, facility level, BMI and CD4 cell count splines, WHO disease stage, alanine aminotransferase, HAART regimen, iron supplement use, TB history, TB treatment, oral candidiasis, diarrhea, season of visit, and calendar year of HAART initiation. Follow-up anemia model adjusted for gender, district, calendar year of HAART initiation, and time-varying values of facility level, splines for age, BMI and CD4 cell count, WHO disease stage, alanine aminotransferase, HAART regimen, nonadherence to HAART, iron supplement use, TB treatment, oral candidiasis, and diarrhea.
‡ Early mortality defined as mortality in the first 3 months of follow-up.
We found significant independent modification of the associations of anemia severity at HAART initiation with early mortality by gender and BMI (Table 3). The magnitude of the associations was stronger in men than women (interaction test P-value = 0.01). Among men, severe anemia was associated with a 4-fold increased risk of mortality compared with no anemia (RR 4.33; 95% CI: 3.36, 5.57), whereas it was about three times greater among women (RR 2.81; 95% CI: 2.35, 3.37). We noted an increasing risk of mortality with increasing anemia severity, compared with no anemia in all strata of BMI. However, the relative risks were increasingly stronger with higher BMI (interaction test P-value < 0.01). Patients with severe anemia at baseline had 13 times the risk of mortality compared with those with no anemia if their baseline BMI was ≥ 30 kg/m2, but the risk of mortality was only two times greater when the baseline BMI was < 18.5 kg/m2. The association of baseline anemia with mortality was not modified by iron supplement use, CD4 cell count, and zidovudine use (interaction test P-values > 0.05).
Table 3.
Modification of the associations of anemia* with mortality in HIV-infected patients initiating HAART (n = 40,657)
| Exposure status | Baseline anemia and early mortality† | Anemia during follow-up and overall mortality | ||
|---|---|---|---|---|
| Multivariate RR‡ (95% CI) | P-value | Multivariate RR§ (95% CI) | P-value | |
| Gender | 0.01 | < 0.001 | ||
| Male | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 1.70 (1.34, 2.14) | 1.65 (1.43, 1.89) | ||
| Moderate anemia | 2.46 (1.95, 3.10) | 2.57 (2.22, 2.96) | ||
| Severe anemia | 4.33 (3.36, 5.57) | 4.69 (3.98, 5.53) | ||
| Female | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 1.03 (0.86, 1.23) | 1.06 (0.94, 1.19) | ||
| Moderate anemia | 1.61 (1.36, 1.91) | 1.67 (1.49, 1.87) | ||
| Severe anemia | 2.81 (2.35, 3.37) | 3.39 (2.99, 3.84) | ||
| BMI category | < 0.001 | < 0.001 | ||
| Obese | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 1.83 (0.65, 5.14) | 1.11 (0.72, 1.69) | ||
| Moderate anemia | 6.97 (2.67, 18.21) | 3.23 (2.13, 4.89) | ||
| Severe anemia | 13.09 (4.14, 41.36) | 7.73 (4.29, 13.92) | ||
| Overweight | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 1.57 (0.86, 2.88) | 1.32 (1.00, 1.74) | ||
| Moderate anemia | 3.96 (2.22, 7.04) | 2.77 (2.09, 3.67) | ||
| Severe anemia | 9.28 (4.84, 17.81) | 8.63 (6.04, 12.33) | ||
| Normal weight | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 1.46 (1.15, 1.86) | 1.27 (1.11, 1.46) | ||
| Moderate anemia | 2.38 (1.88, 2.99) | 2.09 (1.83, 2.39) | ||
| Severe anemia | 5.56 (4.33, 7.13) | 5.25 (4.49, 6.14) | ||
| Underweight | ||||
| No anemia | 1.00 (ref) | 1.00 (ref) | ||
| Mild anemia | 0.94 (0.77, 1.14) | 1.16 (1.00, 1.35) | ||
| Moderate anemia | 1.26 (1.04, 1.51) | 1.55 (1.34, 1.79) | ||
| Severe anemia | 2.04 (1.67, 2.49) | 2.82 (2.41, 3.29) | ||
| Iron supplement use | – | < 0.001 | ||
| Yes | ||||
| No anemia | – | 1.00 (ref) | ||
| Mild anemia | – | 1.08 (0.66, 1.76) | ||
| Moderate anemia | – | 1.07 (0.68, 1.69) | ||
| Severe anemia | – | 1.65 (1.04, 2.62) | ||
| No | ||||
| No anemia | – | 1.00 (ref) | ||
| Mild anemia | – | 1.34 (1.17, 1.53) | ||
| Moderate anemia | – | 2.02 (1.76, 2.31) | ||
| Severe anemia | – | 4.06 (3.46, 4.77) | ||
| Zidovudine use‖ | – | 0.22 | ||
| Yes | ||||
| No anemia | – | 1.00 (ref) | ||
| Mild anemia | – | 1.14 (0.99, 1.31) | ||
| Moderate anemia | – | 1.73 (1.50, 1.99) | ||
| Severe anemia | – | 3.62 (2.99, 4.38) | ||
| No | ||||
| No anemia | – | 1.00 (ref) | ||
| Mild anemia | – | 1.41 (1.24, 1.61) | ||
| Moderate anemia | – | 2.25 (1.98, 2.57) | ||
| Severe anemia | – | 4.32 (3.74, 4.97) | ||
BMI = body mass index; HAART = highly active antiretroviral therapy; ref = reference; TB = tuberculosis.
* Anemia defined as follows: mild (hemoglobin 10 to < 12 g/dL in women or 10 to < 13 g/dL in men), moderate (hemoglobin 7 to < 10 g/dL), and severe (hemoglobin < 7 g/dL). No anemia was defined as hemoglobin ≥ 12 g/dL in women or hemoglobin ≥ 13 g/dL in men.
† Early mortality defined as mortality in the first 3 months of follow-up.
‡ Baseline anemia model adjusted for gender, age, district, facility level, BMI and CD4 cell count splines, WHO disease stage, alanine aminotransferase, HAART regimen, iron supplement use, TB history, TB treatment, oral candidiasis, diarrhea, season of visit, and calendar year of HAART initiation.
§ Follow-up anemia model adjusted for gender, district, calendar year of HAART initiation, and time-varying values of facility level, splines for age, BMI and CD4 cell count, WHO disease stage, alanine aminotransferase, HAART regimen, nonadherence to HAART, iron supplement use, TB treatment, oral candidiasis, and diarrhea.
‖ Model also included interaction terms for missing categories of the effect modifier, but the results are not shown.
For the associations of anemia during follow-up with overall mortality, we identified similar modifications of the associations by gender and BMI as noted previously (interaction test P-values < 0.001); however, the magnitude of the associations for BMI ≥ 18.5 kg/m2 was stronger for anemia at HAART initiation than for the estimates for anemia during follow-up (Table 3). The anemia during follow-up and overall mortality association was modified by iron supplement use. Among iron supplement users, associations of anemia during follow-up with mortality were no longer statistically significant for non-severe anemia categories. The risk of mortality, however, was significantly higher for anemic patients not using iron supplements (interaction test P-value < 0.001). To explore the nonsignificant associations of mild and moderate anemia during follow-up with mortality among users of iron supplements, we estimated relative risks for anemia severity using patients with no anemia and no iron supplement use as a reference (Table 4). Higher risk of mortality was observed among users of iron supplements, irrespective of the anemia status. The magnitude of the associations with mortality was stronger among iron supplement users than in those who were not using iron supplements (Table 4).
Table 4.
Associations between anemia* with or without iron supplement use and mortality in HIV-infected patients initiating HAART (n = 40,657)
| Exposure status | Multivariate RR† (95% CI) | P-value |
|---|---|---|
| No anemia with no iron use | 1.00 (ref) | < 0.001 |
| Mild anemia with no iron use | 1.33 (1.17, 1.53) | |
| Moderate anemia with no iron use | 2.02 (1.76, 2.31) | |
| Severe anemia with no iron use | 4.06 (3.46, 4.77) | |
| No anemia with iron use | 3.84 (2.43, 6.06) | |
| Mild anemia with iron use | 4.14 (3.25, 5.28) | |
| Moderate anemia with iron use | 4.10 (3.43, 4.91) | |
| Severe anemia with iron use | 6.33 (5.23, 7.65) |
HAART = highly active antiretroviral therapy; ref = reference.
* Anemia defined as follows: mild (hemoglobin 10 to < 12 g/dL in women or 10 to < 13 g/dL in men), moderate (hemoglobin 7 to < 10 g/dL), and severe (hemoglobin < 7 g/dL). No anemia was defined as hemoglobin ≥ 12 g/dL in women or hemoglobin ≥ 13 g/dL in men.
† Adjusted for gender, district, calendar year of HAART initiation, and time-varying values of facility level, splines for age, body mass index and CD4 cell count, WHO disease stage, alanine aminotransferase, HAART regimen, nonadherence to HAART, iron supplement use, tuberculosis treatment, oral candidiasis, and diarrhea.
Associations of ID and anemia with mortality.
There were a total of 5,084 deaths in 27,569 patients who had both hemoglobin and MCV measured at the time of HAART initiation. Associations of IDA with mortality are presented in Table 5. We found significantly higher risk of mortality with IDA and anemia without ID than with no anemia or ID (P-value < 0.001); however, the association was not statistically significant for ID without anemia. The findings were less strong for the associations of IDA and anemia during follow-up than for no anemia or ID with overall mortality (P-value < 0.001).
Table 5.
Associations of IDA* with mortality in HIV-infected patients initiating HAART (n = 27,569)
| Exposure status | Deaths/person-months | Univariate RR (95% CI) | P-value† | Multivariate RR‡ (95% CI) | P-value† |
|---|---|---|---|---|---|
| Baseline anemia and early mortality§ | 2,869/39,884 | ||||
| No anemia or ID | 122/4,716 | 1.00 (ref) | < 0.001 | 1.00 (ref) | < 0.001 |
| ID without anemia | 57/1,695 | 1.28 (0.94, 1.76) | 1.23 (0.90, 1.69) | ||
| Anemia without ID | 1,168/16,337 | 2.61 (2.16, 3.14) | 1.74 (1.44, 2.10) | ||
| IDA | 1,522/17,136 | 3.15 (2.62, 3.79) | 2.16 (1.79, 2.61) | ||
| Anemia during follow-up and overall mortality | 5,084/878,078 | ||||
| No anemia or ID | 549/305,408 | 1.00 (ref) | < 0.001 | 1.00 (ref) | < 0.001 |
| ID without anemia | 1,511/398,861 | 1.01 (0.84, 1.22) | 1.02 (0.85, 1.24) | ||
| Anemia without ID | 2,001/155,378 | 2.06 (1.85, 2.29) | 1.63 (1.46, 1.82) | ||
| IDA | 1,023/18,431 | 2.36 (2.12, 2.63) | 1.87 (1.68, 2.09) |
BMI = body mass index; HAART = highly active antiretroviral therapy; ID = iron deficiency; IDA = iron deficiency anemia; ref = reference; TB = tuberculosis.
* Iron deficiency anemia defined as follows: anemia as hemoglobin < 12 g/dL for women or hemoglobin < 13 g/dL for men, and ID as mean corpuscular volume < 80 fL. Anemia and ID exposures were cross-classified to define each individual’s exposure status.
† P-value calculated using the likelihood ratio test.
‡ Baseline exposure model adjusted for gender, age, facility level, BMI, CD4 cell count, WHO disease stage, alanine aminotransferase, HAART regimen, iron use, TB history, TB treatment, oral candidiasis, diarrhea, and calendar year of HAART initiation. The follow-up anemia model also adjusted for gender, facility level, calendar year of HAART initiation, and time-varying values of age, BMI and CD4 cell count splines, WHO disease stage, alanine aminotransferase, HAART regimen, iron use, TB treatment, oral candidiasis, diarrhea, and season of visit.
§ Early mortality defined as mortality in the first 3 months of follow-up.
We also found significant modification of the associations of IDA with mortality in the first 3 months by gender and BMI at HAART initiation (Supplemental Table 1; available online). The associations were significantly stronger among men (interaction test P-value = 0.03). Among overweight patients, IDA was associated with a 4-fold increased risk of mortality as compared with those with no anemia or ID (RR 4.61; 95% CI: 1.99, 10.67). By contrast, the risk of mortality was 1.41 times higher among IDA patients than in those with no anemia or ID when underweight (95% CI: 1.10, 1.81). Associations were not modified by CD4 cell count, iron supplement, and zidovudine use at the time of initiation of therapy (interaction test P-values > 0.05). We also found modification of the associations of IDA during follow-up with overall mortality, by gender and BMI as noted previously (Supplemental Table 1). Higher mortality risks were also noted among patients with zidovudine use than in those not using zidovudine, and in those with CD4 cell count < 350/µL than in those with ≥ 350/µL. No modification of the association was found by iron supplement use (interaction test P-value > 0.05).
We estimated the association of iron supplement use compared with no iron supplement use with mortality among patients with IDA and among those with anemia without ID. Iron supplement use was associated with significantly higher risk of mortality compared with no use among both groups (anemia with IDA RR 2.22; 95% CI: 1.94, 2.55; and anemia without ID RR 2.29; 95% CI: 1.95, 2.68).
Associations of anemia with mortality in patients without ID at HAART initiation.
We identified 14,223 patients without ID at HAART initiation. There were a total of 2,416 deaths, with 53% reported within the first 3 months of follow-up. Associations of anemia with mortality are presented in Supplemental Table 2. There was an increased risk of mortality with increasing anemia severity at HAART initiation (trend test P-value < 0.001). There was no indication of a change in the magnitude or direction of associations over time since HAART initiation. The association of baseline anemia with early mortality was modified by BMI with findings comparable with those presented earlier (interaction test P-value < 0.001). The associations of anemia during follow-up with mortality were modified by BMI as reported previously; however, the magnitude of associations for BMI ≥ 18.5 kg/m2 was stronger for anemia at HAART initiation than for that estimated for anemia during follow-up. The associations were also modified by gender and iron supplement use with findings similar to those reported for the overall analysis (Supplemental Table 3).
DISCUSSION
In this large cohort of HIV-infected patients in Tanzania, we demonstrated that anemia at the time of HAART initiation and anemia during follow-up were each independently associated with an increased risk of mortality; the strength of the association increased with the severity of anemia. Patients with IDA and anemia without ID at HAART initiation and during follow-up were also at a greater risk of mortality. However, ID alone did not increase the risk significantly as compared with no anemia or ID. Importantly, patients with iron supplement use during follow-up, irrespective of their anemia status, were at a higher risk than those who were not anemic and did not take iron supplements. In addition, the magnitude of the association of anemia and IDA with mortality was stronger among men and those with higher BMI.
In our cohort, similar to other studies in sub-Saharan Africa, about 75% of HIV-infected patients were mild and moderately anemic, and almost half of the patients had IDA.1,3–5 Our results also corroborate the findings from earlier studies which indicated an increased risk of mortality associated with anemia at the time of HAART initiation.13 We, however, also found that anemia and IDA during follow-up in patients receiving HAART increased the risk of mortality, independent of the associations of CD4 cell count, disease stage, HAART regimen, adherence to therapy, age, gender, BMI, and other important clinical factors. To our knowledge, this is the first study to evaluate the associations of anemia severity and IDA during follow-up in patients receiving HAART in sub-Saharan Africa. An earlier report on 18,271 patients enrolled in the MDH and also included in the current study showed that ≥ 10% decrease in hemoglobin in the first 3 months following HAART initiation was associated with increased mortality risk during the next 3- to 6-month interval following initiation of therapy. However, this initial decrease in hemoglobin was not associated with a significant increase in the risk subsequently during follow-up.4 A comparison of the findings of the current study with earlier studies emphasizes the prognostic importance of repeated anemia measurements during follow-up. In the current study cohort and several HIV care and treatment programs, complete blood counts are measured at 3- to 4-monthly regular intervals; patients with anemia were given iron supplements, ferrous sulfate 200 mg and folic acid 5 mg, once daily until follow-up test indicated no anemia or up to 6 months in the current cohort.34,35 With the onset of the test-and-treat program in Tanzania and other countries where all HIV-infected patients are immediately initiated on treatment, complete blood counts have been eliminated as a cost-saving strategy to allow for prioritization of treatment initiation for larger number of HAART-naive, HIV-infected individuals. Given the findings that we report here, we advocate for regular repeated assessments at least once per 6 months throughout the course of illness, as this may prove beneficial in monitoring these patients, a guideline also recommended by the U.S. Department of Health and Human Services and CDC.36
The anemia and IDA associations with mortality varied significantly by BMI; the magnitude of the association of anemia as compared with no anemia in underweight patients was smaller, whereas it was larger among overweight and obese patients. Obesity is a condition which is characterized by chronic, low-grade, systemic inflammation in which inflammatory markers are secreted by adipocytes.37 These inflammatory markers cause secretion of a hormone, hepcidin, from hepatocytes. Hepcidin causes internalization and degradation of a transmembrane protein, ferroportin, which is responsible for the export of iron. This serves as a potential protective mechanism of the body, blocking the availability of iron to the invading microorganisms by sequestration within the cells of the reticuloendothelial system. However, this may provide a favorable environment for the replication of microorganisms that target the reticuloendothelial cells.16 Recent experimental studies provide evidence of increased transcription of HIV within the macrophages due to the inhibitory effect of hepcidin on ferroportin expression38 In addition, studies have shown significantly higher hepcidin levels in obese than lean individuals.39 We hypothesize that hepcidin is implicated in anemia in overweight and obese patients, whereas anemia in underweight patients is more related to IDA.
The risk of mortality was also significantly greater in men. Furthermore, mild anemia was not significantly associated with mortality among women, whereas it significantly increased the risk of mortality in men. This can be explained by the physiological loss of blood during menstrual cycle which may cause mild anemia in women in the reproductive age. This mild anemia may not increase the risk of mortality in women. Recent studies have also found gender differences in the pharmacokinetics and metabolism of antiretroviral drugs.40
We found a higher risk of mortality among users of iron supplements, irrespective of their anemia status, than in those without anemia and no iron supplement use. Use of iron supplements is controversial in infectious and inflammatory disease conditions.41 Several studies have identified associations of increased iron stores with disease progression, viral load, and mortality in HIV-infected patients.18,42,43 By contrast, a study conducted in the same setting as ours did not find increased iron stores to be associated with HIV disease progression.3 A recent study in HIV-infected postpartum women in Zimbabwe demonstrated that the viral load increased 2.3-fold in nonanemic women for each unit increase in serum ferritin; however, no significant association was seen in anemic women.43 They also reported an increased risk of mortality in the subsequent year, but there was no significant difference by baseline anemia status. The association of increased iron stores with disease progression can also be explained by the pathway involving hepcidin through ferroportin. Hepcidin also affects the intestinal expression of ferroportin and iron absorption from the intestinal lumen.8 We postulate that chronic inflammation in HIV infection is significantly contributing to anemia in the current study through mechanisms described.
Our study has several limitations. First, this was an observational study. Although we adjusted extensively for potential confounders in the analysis, there may be residual confounding because of mismeasured or unmeasured confounders, such as education, income, and money spent on food per person per day. Second, viral load measurement, which is the standard test for monitoring HIV-infected patients in high-resource settings, was not routinely performed in this cohort. Third, as study participants were receiving HAART, the study findings will only be generalizable to those patient populations who are receiving HAART. Fourth, we used MCV for assessing the ID status of patients. Mean corpuscular volume is most widely used to assess ID, but low values are not specific to this condition and have been observed in other states, such as thalassemia and, less commonly, in ACD.44,45 Hence, the use of MCV could lead to exposure misclassification which, in the case of thalassemia, would be non-differential with bias toward the null. Because the carrier frequency of thalassemia ranges from 1% to 20% in affected countries,46 the magnitude of the bias is expected to be small. Nevertheless, there are several strengths to our study. One of the most important is the large sample size, which is one of the largest in resource-limited settings, allowing us to evaluate effect modification with adequate statistical power. Second, patients were followed up prospectively with repeated clinic visits, allowing for the examination of the exposures during follow-up with the outcomes along with the control of potential time-varying confounders.
Our study demonstrated that among patients receiving HAART, any degree of anemia and IDA were robustly associated with an increased risk of mortality. As a result, hemoglobin assessment, which is widely used in resource-limited settings, should be used by HIV programs to monitor patients receiving HAART, and the etiology of anemia investigated and appropriately managed. In many cases, anemia is associated with ID and provision of supplements is a rational intervention. Yet, in our study, use of iron supplements was associated with increased mortality, regardless of anemia and IDA status. The relationships of anemia, IDA, and iron supplementation among HIV-infected individuals appear to be complex. Future studies and potential clinical trials should further evaluate biological mechanisms involved and examine the safety and efficacy of iron, including lower doses of iron, among HIV-infected individuals receiving HAART. The impact of timing of iron supplementation with respect to HAART initiation and the role of regular monitoring of iron status and inflammatory biomarkers during iron supplementation should also be examined in this patient population.
Supplementary Files
Acknowledgments:
We thank the Management and Development for Health (MDH), Dar es Salaam City Council, Muhimbili University of Health and Allied Sciences, Harvard School of Public Health (HSPH), and Ministry of Health and Social Welfare for the guidance and collaboration in implementing this national HIV care and treatment program in Dar es Salaam, Tanzania, and also all the patients and staff of the MDH-supported clinical sites who contributed to these findings.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Belperio PS, Rhew DC, 2004. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med 116 (Suppl 7A): 27S–43S. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann CJ, Fielding KL, Johnston V, Charalambous S, Innes C, Moore RD, Chaisson RE, Grant AD, Churchyard GJ, 2011. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr 58: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupka R, Msamanga GI, Mugusi F, Petraro P, Hunter DJ, Fawzi WW, 2007. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr 137: 2317–2323. [DOI] [PubMed] [Google Scholar]
- 4.Liu E, Spiegelman D, Semu H, Hawkins C, Chalamilla G, Aveika A, Nyamsangia S, Mehta S, Mtasiwa D, Fawzi W, 2011. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis 204: 282–290. [DOI] [PubMed] [Google Scholar]
- 5.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Gardner LI, Vlahov D; HER (Human Immunodeficiency Virus Epidemiology Research) Study Group , 2001. Highly active antiretroviral therapy associated with improved anemia among HIV-infected women. AIDS Patient Care STDS 15: 473–480. [DOI] [PubMed] [Google Scholar]
- 6.Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, Hunter DJ, Fawzi WW, 2000. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr 130: 1950–1957. [DOI] [PubMed] [Google Scholar]
- 7.Bain BJ, 1999. Pathogenesis and pathophysiology of anemia in HIV infection. Curr Opin Hematol 6: 89–93. [DOI] [PubMed] [Google Scholar]
- 8.Weiss G, Goodnough LT, 2005. Anemia of chronic disease. N Engl J Med 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 9.Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, Spiegelman D, Duggan C, Fawzi WW, 2012. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr 142: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RD, Keruly JC, Chaisson RE, 1998. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 19: 29–33. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW, 2005. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr 40: 219–225. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW, 1998. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood 91: 301–308. [PubMed] [Google Scholar]
- 13.Harris RJ, et al. Antiretroviral Therapy Cohort Collaboration , 2008. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther 13: 959–967. [PMC free article] [PubMed] [Google Scholar]
- 14.Takuva S, Maskew M, Brennan AT, Sanne I, Macphail AP, Fox MP, 2013. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med 2013: 162950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez K, Kulnigg-Dabsch S, Gasche C, 2015. Management of iron deficiency anemia. Gastroenterol Hepatol (N Y) 11: 241–250. [PMC free article] [PubMed] [Google Scholar]
- 16.Drakesmith H, Prentice AM, 2012. Hepcidin and the iron-infection axis. Science 338: 768–772. [DOI] [PubMed] [Google Scholar]
- 17.Nekhai S, Kumari N, Dhawan S, 2013. Role of cellular iron and oxygen in the regulation of HIV-1 infection. Future Virol 8: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordeuk VR, Delanghe JR, Langlois MR, Boelaert JR, 2001. Iron status and the outcome of HIV infection: an overview. J Clin Virol 20: 111–115. [DOI] [PubMed] [Google Scholar]
- 19.Drakesmith H, Prentice A, 2008. Viral infection and iron metabolism. Nat Rev Microbiol 6: 541–552. [DOI] [PubMed] [Google Scholar]
- 20.United Republic of Tanzania NACPN , 2009. National Guidelines for the Management of HIV and AIDS, 3rd edition Dar es Salaam, Tanzania: Government of Tanzania. [Google Scholar]
- 21.World Health Organization , 2010. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Dar es Salaam, Tanzania: WHO HIV/AIDS Programme. [PubMed] [Google Scholar]
- 22.United Republic of Tanzania NACPN , 2012. National Guidelines for the Management of HIV and AIDS, 3rd edition Dar es Salaam, Tanzania: Government of Tanzania. [Google Scholar]
- 23.World Health Organization , 2007. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva, Switzerland: WHO. [Google Scholar]
- 24.World Health Organization , 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: WHO. (WHO/NMH/NHD/MNM/11.1). [Google Scholar]
- 25.Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, Means RT, eds, 2009. Wintrobe’s Clinical Hematology, 12th edition Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 26.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N, 2000. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 133: 21–30. [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB, 1990. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 9: 1501–1515. [DOI] [PubMed] [Google Scholar]
- 28.Claster S, 2002. Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. J Infect Dis 185 (Suppl 2): S105–S109. [DOI] [PubMed] [Google Scholar]
- 29.Andersen P, Gill R, 1982. Cox’s regression model counting process: a large sample study. Ann Stat 10: 1100–1120. [Google Scholar]
- 30.Durrleman S, Simon R, 1989. Flexible regression models with cubic splines. Stat Med 8: 551–561. [DOI] [PubMed] [Google Scholar]
- 31.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA, 2007. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 26: 3735–3752. [DOI] [PubMed] [Google Scholar]
- 32.Miettinen OS, 1985. Theoretical Epidemiology. New York, NY: John Wiley & Sons. [Google Scholar]
- 33.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, Ewald T, Mugusi F, Mtasiwa D, Fawzi W, 2011. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS 25: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 34.Bussmann H, et al. 2008. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS 22: 2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization , 2006. Patient Monitoring Guidelines for HIV Care and Antiretroviral Therapy (ART). Geneva, Switzerland: WHO HIV/AIDS Programme. [Google Scholar]
- 36.Panel on Antiretroviral Guidelines for Adults and Adolescents , 2018. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Rockville, MD: Department of Health and Human Services; Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 1, 2018. [Google Scholar]
- 37.Trayhurn P, Wood IS, 2005. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans 33: 1078–1081. [DOI] [PubMed] [Google Scholar]
- 38.Xu M, Kashanchi F, Foster A, Rotimi J, Turner W, Gordeuk VR, Nekhai S, 2010. Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology 7: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, Braunschweig C, 2012. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet 112: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floridia M, Giuliano M, Palmisano L, Vella S, 2008. Gender differences in the treatment of HIV infection. Pharmacol Res 58: 173–182. [DOI] [PubMed] [Google Scholar]
- 41.Clark TD, Semba RD, 2001. Iron supplementation during human immunodeficiency virus infection: a double-edged sword? Med Hypotheses 57: 476–479. [DOI] [PubMed] [Google Scholar]
- 42.McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A, 2007. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr 46: 498–507. [DOI] [PubMed] [Google Scholar]
- 43.Rawat R, Humphrey JH, Ntozini R, Mutasa K, Iliff PJ, Stoltzfus RJ, 2009. Elevated iron stores are associated with HIV disease severity and mortality among postpartum women in Zimbabwe. Public Health Nutr 12: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg MH, Dreiling BJ, 1983. Microcytosis. Its significance and evaluation. JAMA 249: 85–87. [DOI] [PubMed] [Google Scholar]
- 45.Van Vranken M, 2010. Evaluation of microcytosis. Am Fam Physician 82: 1117–1122. [PubMed] [Google Scholar]
- 46.Weatherall DJ, Clegg JB, 2001. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ 79: 704–712. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.