Abstract.
Pyrethroid resistance has been detected in Triatoma infestans, which in part has been attributed to increased oxidative metabolism of the insecticide by cytochrome P450s. Nicotinamide adenine dinucleotide phosphate (NADPH) cytochrome P450 reductase (CPR) catalyzes electron transfer from NADPH to all known cytochrome P450s. In this study, the expression of the CPR gene at transcriptional level was determined in different tissues and two life stages. The expression patterns showed differences in the tissues and stages studied, suggesting differential metabolic requirements. On the other hand, to investigate the presence of rhythms in the expression of genes related with insecticide resistance, we explored the daily expression profile of the CPR gene and a P450 gene (CYP4EM7) in fat body from adults of T. infestans under different dark/light regimes. The results suggest that CPR gene expression is under endogenous clock regulation and show a rhythmic profile in the expression of the CYP4EM7 gene.
INTRODUCTION
Triatoma infestans (Hemiptera, Reduviidae) is the main vector of Chagas disease in the Southern Cone of Latin America between latitudes 10°S and 46°S. Pyrethroid insecticides have been the major means to control the vector populations. However, during the past years, pyrethroid resistance in the vector insect has been reported as one of the main explanations of the unsatisfactory control observed.1 Cytochrome P450 monooxygenases (cytochrome P450s) are involved in the oxidative metabolism of various endogenous and exogenous substrates. They play a predominant role in the metabolism of insecticides, which often results in the development of insecticide resistance in insect populations. Increases of expression of cytochrome P450 genes at transcriptional level are often considered responsible for increasing the metabolism of insecticides and seem to be a common phenomenon in the evolution of resistance development in insects.2 All P450 monooxygenation reactions occurring in the endoplasmic reticulum require electrons supplied by nicotinamide adenine dinucleotide phosphate (NADPH) cytochrome P450 reductase (CPR), a diflavin enzyme that contains flavin mononucleotide and flavin adenine dinucleotide cofactors that shuttle electrons from the reduced form of NADPH through a series of redox-coupled reactions to P450.3 Expression analyses of cytochromes P450 and CPR genes in resistant and susceptible T. infestans populations to deltamethrin suggested that P450 genes would be involved in the development of resistance to pyrethroid insecticides and that the CPR gene would play an important role in P450-mediated resistance to insecticides.4,5
A large number of clock-controlled cycling genes that are involved in diverse physiological processes have been identified in Drosophila melanogaster.6–10 Among them, genes encoding detoxification enzymes of xenobiotics and endogenous compounds are well represented, suggesting that metabolism and detoxification of insecticides is likely to be under circadian clock control. In addition, a daily rhythm in the expression of genes with detoxification functions such as P450 genes has also been detected in other insect species.11–13 Because metabolic resistance of insecticides is believed to develop through upregulation of detoxification pathways, the time of day when expression of detoxification genes and subsequent enzyme activities is at their minimum may correspond to the time when insects are most susceptible to insecticides. To investigate the presence of rhythms in the expression of genes related to insecticide resistance in T. infestans, here we explored the daily expression profile of the CPR gene and a P450 gene (CYP4EM7) under different dark/light regimes.
MATERIALS AND METHODS
Triatoma infestans was reared at 28 ± 1°C at a relative humidity of 60–70% with a 6-hour light/18-hour dark cycle and fed once every 2 weeks on restrained chickens. For expression analysis of the CPR gene, fifth instar nymphs and adults were fed after 7–10 days of molting and 7 days later, the heads (nervous tissue), thoracic muscles, gonads, and fat bodies were extracted from female and male specimens pooled separately. Each sample was a pool of tissue from three adult specimens and five fifth instar nymphs. For the circadian expression analyses of the CPR and CYP4EM7 genes, T. infestans individuals were maintained under different dark/light regimes since the fifth instar nymph stage. Three experimental groups were subjected either to 1) light/dark cycle (LD), 2) constant light (LL), and 3) constant dark (DD). The LD cycle group consists of 12-hour light and 12-hour darkness. Time of day was reported in 24-hour zeitgeber time (ZT) with ZT12 (20:00 hours) defined as time of lights off and ZT0 (08:00 hours) defined as end of the dawn transition under the LD cycle. For LL and DD groups, subjective day was reported between ZT0 (08:00 hours) and ZT12 (20:00 hours). Fat body extraction was carried out between 40 and 45 days after molt from adult female and male specimens of the three experimental groups every 4 hours over 24 hours. In all cases, the tissues were dissected under aseptic conditions and stored in liquid nitrogen until used for RNA extraction.
Total RNA was isolated from pools of insect tissues using MasterPure RNA Purification Kit (Epicentre, Madison, WI). Synthesis of cDNA was performed from total RNA using Oligo-dT20 and SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). The transcript levels of the CPR and CYP4EM7 genes were measured by quantitative polymerase chain reaction (qPCR) using Mx3005P qPCR System with Brilliant qPCR Core Reagent Kit (Stratagene, La Jolla, CA). The specific primers, Taqman probes, and the reactions conditions were described in previous works carried out in our laboratory.4,5 The relative copy number of CPR or CYP4EM7 mRNA was calculated according to 2−ΔΔCT.14 The threshold cycle value difference (ΔCT) between CPR or CYP4EM7 mRNA and β-actin mRNA of each reaction was used to normalize the level of total RNA. Two independent experiments were performed, and data for each point were registered by triplicate to account for intra-experimental variation. Graphs and statistical tests were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). One-way analysis of variance with Bonferroni post hoc test was used for comparisons. The results were presented as mean ± SD, and a P value < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
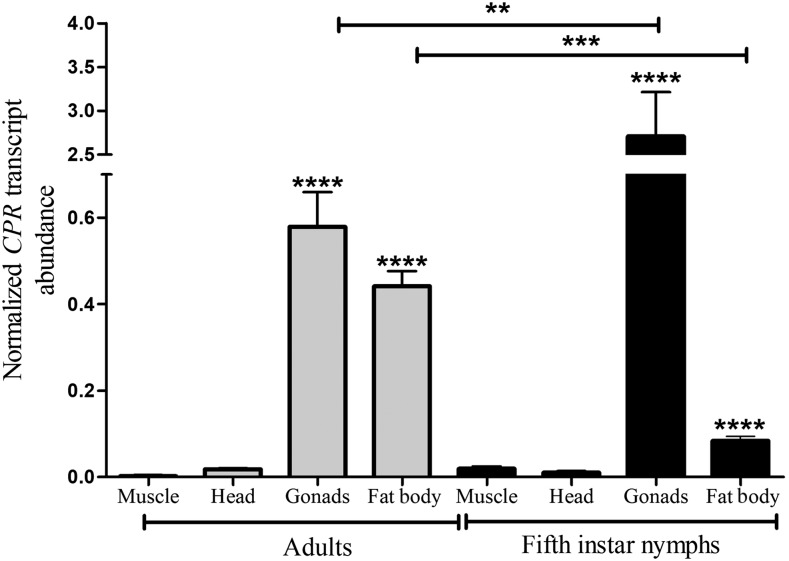
To investigate the tissue distribution and development variation of the CPR gene expression in T. infestans, the relative levels of the mRNA among various tissues and between two life stages and sexes were determined by qPCR. Because both sexes presented similar transcript levels, the gene expression of males and females are presented together in Figure 1. CPR mRNA was detected in all tested tissues of fifth instar nymphs and adults. The comparative analysis of the CPR gene expression in muscle, heads, gonads, and fat body of fifth instar nymphs and adults revealed that the gonads and fat body presented significant levels of expression in both stages. A higher level of expression in gonads of nymphs than in adults was detected, whereas the fat body presented a higher level of expression in adults. Tissue-specific and developmental expression of the CPR gene represents certain P450 activities in a life stage and in one part of the body. Cytochrome P450 reductase catalyzes electron transfer from NADPH to all known cytochrome P450s.3 However, CPR also serves as the electron donor protein for several oxygenase enzymes found in the endoplasmic reticulum of most eukaryotic cells. The expression patterns observed, which showed differences in the tissues and stages studied, would respond to differential metabolic requirements. For example, the higher CPR transcript levels detected in nymph gonads than in adult gonads would correspond to the P450 activity required for ecdysteroid biosynthesis involved in the insect ecdysis.15
Figure 1.
Relative expression (mRNA) of the CPR gene in the muscle, head, gonads, and fat body of Triatoma infestans fifth instar nymphs and adults. Relative abundance of CPR mRNA expression was referred to the ratio of CPR mRNA versus β-actin mRNA expression. The error bars represent the SD of the mean. Two, three, or four asterisks indicate significant difference between the means at P < 0.01, P < 0.001, and P < 0.0001, respectively.
Several studies suggested that insect CPRs play central roles in stimulating the catalytic activity of P450 to metabolize a wide variety of endogenous and exogenous chemicals and that knockdown of CPRs in insects result in enhanced susceptibility to insecticides.16,17 Consistent with these observations, the expression at transcriptional level of three cytochrome P450 genes (CYP4EM7, CYP3085B1, and CYP3092A6 genes) was induced by deltamethrin in resistant and susceptible strains of T. infestans and the levels of CPR mRNA were also upregulated in a susceptible strain after topical application of deltamethrin. Besides, as it was observed in the CYP4EM7 gene, it was detected overexpression of the CPR gene in the most resistant strain of T. infestans included in the study. These results suggested that the P450-mediated metabolic detoxification of xenobiotics might be an important mechanism for deltamethrin resistance and that CPR plays an essential role in P450-mediated resistance of T. infestans to insecticides.4,5
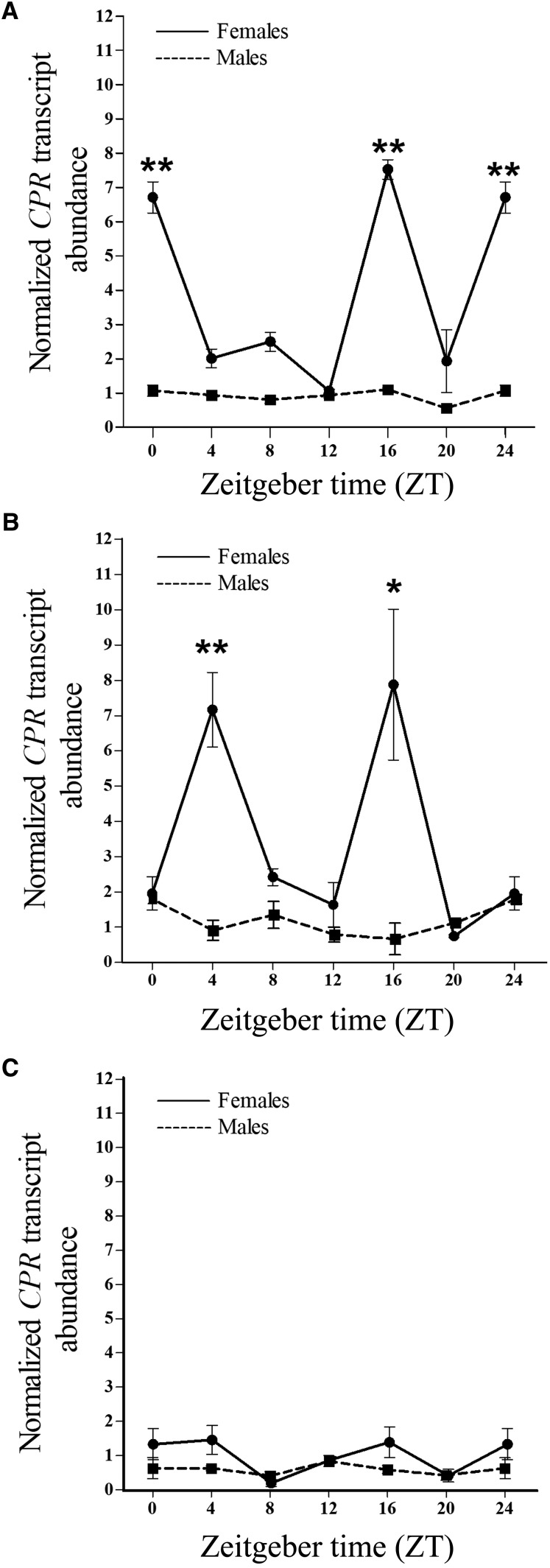
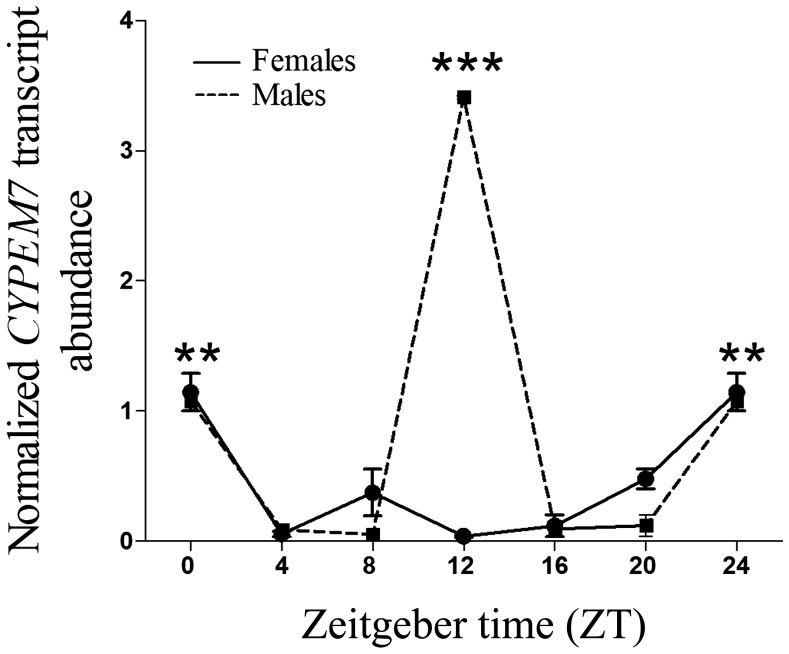
The importance of studying the daily expression patterns of genes involved in the resistance lies in its potential for exploitation in novel insect control strategies. Because in adults the CPR gene expression was highest in fat body, the daily variation in the expression of this gene was investigated in this tissue. In LD condition, females showed two significant peaks in the daily CPR transcript expression, occurring at midnight (ZT16) and at dawn transition (ZT0/ZT24) (Figure 2A). The two peaks profile in the CPR gene expression observed in LD persisted in DD condition with peaks at subjective midnight (ZT16) and at subjective morning (ZT4), suggesting that gene expression is under endogenous clock regulation (Figure 2B), whereas in LL condition, the CPR gene expression damped out (Figure 2C). However, in males, a rhythmic profile in the expression of the CPR gene under the LD, DD, and LL conditions was not observed (Figure 2). On the other hand, the expression analysis of a P450 gene involved in insecticide resistance in T. infestans (CYP4EM7 gene) showed daily significant variations of the transcript level under LD condition (Figure 3). The expression in females presented a peak at dawn transition (ZT0/ZT24) and in males showed two significant peaks, one at dawn transition (ZT0/ZT24) and other at sunset (ZT12). Rhythmic RNA expression levels are likely to correspond to rhythmic protein abundance. Consequently, these temporal changes in gene expression would confer time-of-day–specific changes in metabolic detoxification and responses to insecticide challenge. Further studies will shed light to better understand the relationship between the endogenous clock and the expression of genes involved in the insecticide metabolisms.
Figure 2.
Circadian expression of CPR gene in fat body of Triatoma infestans adults under light/dark cycle (A), constant dark (B), and constant light (C) conditions. Relative abundance of CPR mRNA expression was referred to the ratio of CPR mRNA versus β-actin mRNA expression at each time point. The error bars represent the SD of the mean. One or two asterisks on the standard error bar indicate significant differences between the means at P < 0.05 and P < 0.01, respectively.
Figure 3.
Circadian expression of CYP4EM7 gene in fat body of Triatoma infestans adults under light/dark cycle condition. Relative abundance of CYP4EM7 mRNA expression was referred to the ratio of CYP4EM7 mRNA versus β-actin mRNA expression at each time point. The error bars represent the SD of the mean. Two or three asterisks on the standard error bar indicate significant differences between the means at P < 0.01 and P < 0.001, respectively.
Acknowledgments:
We thank the Centro de Referencia de Vectores, Servicio Nacional de Chagas de Córdoba (Córdoba, Argentina), for providing insects used in our studies.
REFERENCES
- 1.Mougabure-Cueto G, Picollo MI, 2015. Insecticide resistance in vector Chagas disease: evolution, mechanisms and management. Acta Trop 149: 70–85. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Schuler MA, Berenbaum MR, 2007. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52: 231–253. [DOI] [PubMed] [Google Scholar]
- 3.Paine MJI, Scrutton NS, Munro AW, Roberts GCK, Wolf CR, 2004. Electron transfer partners of cytochrome P450. Ortiz de Montellano PR, ed. Cytochromes P450: Structure, Mechanism and Biochemistry. New York, NY: Kluwer Academic/Plenum Publishers, 115–148. [Google Scholar]
- 4.Grosso CG, Blariza MJ, Mougabure-Cueto G, Picollo MI, García BA, 2016. Identification of three cytochrome P450 genes in the Chagas’ disease vector Triatoma infestans: expression analysis in deltamethrin susceptible and resistant populations. Infect Genet Evol 44: 459–470. [DOI] [PubMed] [Google Scholar]
- 5.Grosso CG, Stroppa MM, Varela GM, García BA, 2018. cDNA isolation and expression of nicotinamide adenine dinucleotide phosphate-dependent cytochrome P450 reductase gene in the Chagas disease vector Triatoma infestans. Am J Trop Med Hyg 98: 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald MJ, Rosbash M, 2001. Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107: 567–578. [DOI] [PubMed] [Google Scholar]
- 7.Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S, 2002. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J Biol Chem 277: 14048–14052. [DOI] [PubMed] [Google Scholar]
- 8.Wijnen H, Naef F, Boothroyd C, Claridge-Chang A, Young MW, 2006. Control of daily transcript oscillations in Drosophila by light and the circadian clock. PLoS Genet 2: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan KP, Pradhan S, Wang JP, Allada R, 2007. Meta-analysis of Drosophila circadian microarray studies identifies a novel set of rhythmically expressed genes. PLoS Comput Biol 3: e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez J, Tang CH, Khodor YL, Vodala S, Menet JS, Rosbash M, 2013. Nascent-Seq analysis of Drosophila cycling gene expression. Proc Natl Acad Sci USA 110: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang YY, Liu Y, Teng HJ, Sauman I, Sehnal F, Lee HJ, 2010. Circadian control of permethrin-resistance in the mosquito Aedes aegypti. J Insect Physiol 56: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 12.Hamby KA, Kwok RS, Zalom FG, Chiu JC, 2013. Integrating circadian activity and gene expression profiles to predict chronotoxicity of Drosophila suzukii response to insecticides. PLoS One 8: e68472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balmert NJ, Rund SSC, Ghazi JP, Zhou P, Duffield GE, 2014. Time-of-day specific changes in metabolic detoxification and insecticide resistance in the malaria mosquito Anopheles gambiae. J Insect Physiol 64: 30–39. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 15.Brown MR, Sieglaff DH, Rees HH, 2009. Gonadal ecdysteroidogenesis in arthropoda: occurrence and regulation. Annu Rev Entomol 54: 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lycett GJ, McLaughlin LA, Ranson H, Hemingway J, Kafatos FC, Loukeris TG, Paine MJ, 2006. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol Biol 15: 321–327. [DOI] [PubMed] [Google Scholar]
- 17.Zhu F, Sams S, Moural T, Haynes KF, Potter MF, Palli SR, 2012. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug, Cimex lectularius. PLoS One 7: e31037. [DOI] [PMC free article] [PubMed] [Google Scholar]