Abstract.
In this retrospective cohort study, we aimed to assess whether introducing benznidazole at escalating doses reduces the probability of adverse events or treatment discontinuation compared with a full-dose scheme. We collected data from patients who had chronic Trypanosoma cruzi infection and underwent treatment from July 2008 to January 2017 in a referral center in Madrid. Dose was adjusted to body weight (5 mg/kg/day), with treatment introduction with full dose or escalating dose according to local consensus and protocols. Among the 62 patients treated, benznidazole was introduced at full dose in 28 patients and on escalating dose in the remaining 34. We found no statistical differences in the number of adverse events, treatment discontinuations, days of treatment, or sociodemographic profiles. There is insufficient evidence to support escalating dose as a strategy for reducing the adverse effects of benznidazole. Further research is needed to evaluate this approach.
INTRODUCTION
Chagas disease is a zoonosis caused by the parasite Trypanosoma cruzi. The WHO estimates that there are 6–7 million people infected worldwide.1 Most cases are in the endemic regions of Latin America, but an increasing number of cases are being diagnosed in other countries (mainly the United States and southern Europe) because of migration.2–4 In endemic areas, the primary vector for transmission is the triatomine bug, whose presence in households has been related to substandard building construction. Other means of transmission in endemic and non-endemic countries are vertical (mother to child) and parenteral transmission through blood transfusion and organ transplantation. After a typically self-limited acute phase with unspecific symptoms (therefore difficult to diagnose), the disease enters a chronic phase in which organ complications (mostly cardiac and digestive) occur in approximately one-third of patients.5
The therapeutic options available for antiparasitic treatment of Chagas disease have remained unchanged for around 50 years. The only two available treatments are nifurtimox and benznidazole, which were introduced in 1965 and 1971, respectively.6 The efficacy of these treatments is highly variable because it is affected by the drug dose, disease stage, and the age and area of origin of the patient, among other factors. Cure rates between 60% and 100% are reported when treatment with benznidazole is provided in the acute phase and in younger patients.7–9 The antiparasitic effect of the treatment in the chronic phase is well documented.10–14 However, clinical effectiveness (defined as reduction in clinical events) is still the subject of intense scientific debate.15 Treatment with antitrypanosomal drugs is currently indicated in acute infection, congenital infection, reactivations, and chronic infection in children aged less than 18 years. Although in recent years there seems to be some consensus regarding the absence of benefit of antiparasitic treatment in patients with advanced forms of cardiac or digestive involvement, most national and regional guidelines recommend offering treatment in the indeterminate chronic phase16,17 and in patients with mild to moderate determinate disease.
The biggest challenge associated with the available antitrypanosomal drugs is their safety profile. From 48% to 86% of patients experience adverse effects of benznidazole, resulting in treatment discontinuation in 9–31% of cases.18–21 This further limits global treatment coverage, which is already low given that only 4–6% of migrants with Chagas disease are aware of their condition4 and that treatment reaches less than 1% of patients with Chagas disease.22–25 The most frequently observed adverse events are dermatological, gastrointestinal, and neurological, usually mild and with acceptable response to symptomatic treatment or to benznidazole discontinuation.18–21 Serious adverse events such as drug rash with eosinophilia and systemic symptoms syndrome are rare, and life-threatening conditions such as severe neutropenia are extremely uncommon.18–21,26
Alongside the search for new and better tolerated drugs,14,27–29 efforts are being made to increase the tolerability of existing anti-trypanocidal drugs.30 Studies aimed at identifying risk factors for adverse responses to benznidazole have found associations with female gender,21 graduation from elementary school, and white and mixed race.31 In addition, carrying the HLA-B*3505 allele could be associated with moderate to severe cutaneous reactions.32 Another study found that adverse events, female gender, drug dose, and eosinophilia were the main predictors of treatment interruption.33 A study attempting to find a measurable proxy for toxicity failed to correlate adverse events with serum concentrations of benznidazole.34 Some researchers have proposed the use of corticosteroid therapy along with benznidazole35 to prevent cutaneous reactions. However, this strategy failed to show a clear advantage and raised concerns because of the high rate of Strongyloides stercoralis coinfection in patients with Chagas disease36 and risk of hyperinfection syndrome. A clinical trial evaluating shorter regimes and lower dosing of benznidazole is expected to soon provide some insight on the feasibility of this strategy.37 Moreover, some researchers have proposed the use of escalating doses of benznidazole during the first days of treatment to increase its tolerability.38 However, this strategy has not been compared with standard treatment with full (adjusted to weight) doses from the beginning of treatment.
In this study, we aimed to ascertain whether introducing benznidazole at progressively higher dose until the target daily dose is reached reduces the probability of adverse events or treatment discontinuation.
METHODS
In this retrospective cohort study, we reviewed clinical records of patients referred to the Infectious Diseases department in Hospital 12 de Octubre (Madrid, Spain) from July 2008 to January 2017. The inclusion criteria were chronic infection with T. cruzi as defined by the WHO criteria (two positive serological tests),39 age 18 years or older, and previous treatment with benznidazole in our center. Those who were receiving it at the time of data collection were excluded. The usual evaluation of these patients includes a questionnaire about their country of origin and risk factors for Chagas disease. Patients’ medical history is obtained, and a physical examination aimed at detecting cardiac or digestive involvement is performed. Electrocardiogram and echocardiogram are routinely performed to rule out cardiac involvement. Tests to rule out digestive tract involvement are carried out according to symptoms.
In the evaluated time frame, some patients began treatment with benznidazole at full doses and others at progressive doses according to local protocols or consensus when the former were lacking. Changes in schemes of benznidazole treatment took place over time, both before and after the first protocol was launched in 2011. Thus, we divided the patients into two groups, according to the method of introduction of benznidazole treatment. In all cases, the standard dose of 5 mg per kilogram of body weight was calculated, and then the treatment was started according to the physicians’ criteria. The maximum daily dose was 300 mg in most patients, although some received 250 mg and others 400 mg. In the full-dose group, the previously calculated dose was divided into two daily doses for 60 days. In the progressive dose group, treatment was started with 50 mg per day (half a tablet) and then increased by 50 mg every day until the correct dosage according to weight was reached.
Statistical analysis.
Categorical variables were described by frequencies and percentages. Quantitative variables were described as means and SD or medians and interquartile ranges. A chi-square test or Fisher’s exact test was used to compare qualitative variables. Student’s t-test was used to compare normally distributed continuous variables, and the Mann–Whitney U test was used to compare non-normally distributed variables with qualitative variables. Statistical significance was set at P < 0.05. Data analysis was performed using Stata15 (Stata Corp., College Station, TX).
This work was submitted and approved under number 17/051by the Research Ethics Committee of Hospital 12 de Octubre, Madrid, Spain.
RESULTS
In this study, a total of 62 patients were treated with benznidazole adjusted to body weight: 28 on a full dose from the start and 34 with an escalating dose regime. As shown in Table 1, the two groups did not differ in baseline characteristics, comorbidities, or clinical stage of Chagas disease. A large majority of patients were from Bolivia (97%). One patient in the full-dose group was from Honduras, and one patient who received escalating dose treatment was from Brazil.
Table 1.
Baseline characteristics
| Characteristics | Full dose, N = 28 | Escalating dose, N = 34 | P-value |
|---|---|---|---|
| Age (years), mean (SD) | 40.7 (8.9) | 39.7 (8.2) | 0.68 |
| Male gender, no. (%) | 7 (25%) | 12 (35.3%) | 0.38 |
| Habits | |||
| Smoker, no. (%) | 3/18 (16.7%) | 6/22 (27.3%) | 0.42 |
| Alcohol consumption, no. (%) | 2/19 (10.5%) | 6/22 (27.3) | 0.18 |
| Comorbidities | |||
| Hypertension, no. (%) | 1 (3.6%) | 1 (2.9%) | 0.89 |
| Diabetes, no. (%) | 1 (3.6%) | 0 (0%) | 0.27 |
| Obesity, no. (%) | 3/26 (11.5%) | 2/33 (6.1%) | 0.45 |
| Cerebrovascular disease, no. (%) | 1 (3.6%) | 1 (2.9%) | 0.89 |
| Liver disease, no. (%) | 0 | 1 (2.9%) | 0.36 |
| Renal disease, coronary heart disease, HIV, and transplantation | 0 | 0 | – |
| Clinical stage | |||
| Indeterminate form | 22 (78.6%) | 25 (73.5%) | 0.64 |
| Cardiac form, no. (%) | 6 (21.4%) | 9 (26.4%) | 0.64 |
| Gastrointestinal form, no. (%) | 0 | 0 | – |
no. = number. Data are no./number tested (%) of patients, unless otherwise indicated.
The median maximum daily dose was approximately 300 mg, without differences between groups.
There were no significant differences between groups regarding the occurrence of adverse events (Table 2). At least one adverse event was observed in 88.7% of patients, with no differences between groups. The most frequent disturbances were cutaneous (61.3%). The second most frequent adverse reactions were neuromuscular (50%), which included headache, vertigo, insomnia, polyneuropathy, paresthesia, arthralgia, and myalgia. Other disturbances (hematologic, liver, digestive, and renal) occurred in smaller percentages of patients in both groups.
Table 2.
Adverse events
| Full dose, N = 28 | Escalating dose, N = 34 | P-value | |
|---|---|---|---|
| Adverse events | |||
| At least one adverse event, no. (%) | 25 (89.29) | 29 (85.29) | 0.64 |
| Skin disturbances, no. (%) | 17 (60.71) | 21 (61.76) | 0.93 |
| Neuromuscular side effects, no. (%) | 15 (53.57) | 16 (47.06) | 0.61 |
| Hematologic toxicity, no. (%) | 7 (25) | 5 (14.71) | 0.24 |
| Liver toxicity, no. (%) | 7 (25) | 5 (14.71) | 0.24 |
| Digestive disturbances, no. (%) | 7 (25) | 3 (8.82) | 0.08 |
| Kidney injury, no. (%) | 0 | 1 (2.94) | 1 |
| Management | |||
| Need of specific treatment, no. (%) | 12 (42.86) | 14 (41.18) | 0.89 |
| Referral to a specialist, no. (%) | 12 (42.86) | 15 (44.12) | 0.92 |
| Hospital admission, no. (%) | 1 (3.57) | 2 (5.88) | 0.67 |
| Treatment interruption, no. (%) | 7 (25) | 14 (41.18) | 0.18 |
| Treatment duration, days (SD) | 51.75 (15.4) | 49.4 (21.5) | 0.26 |
| Completed at least 80% of total dose, no. (%) | 21 (75) | 23 (67.65) | 0.53 |
| Completed at least 30 days, no. (%) | 25 (89.29) | 27 (79.41) | 0.29 |
| Maximum dose per day, median grams (SD) | 316.1 (38.7) | 304.5 (26.1) | 0.14 |
no. = number.
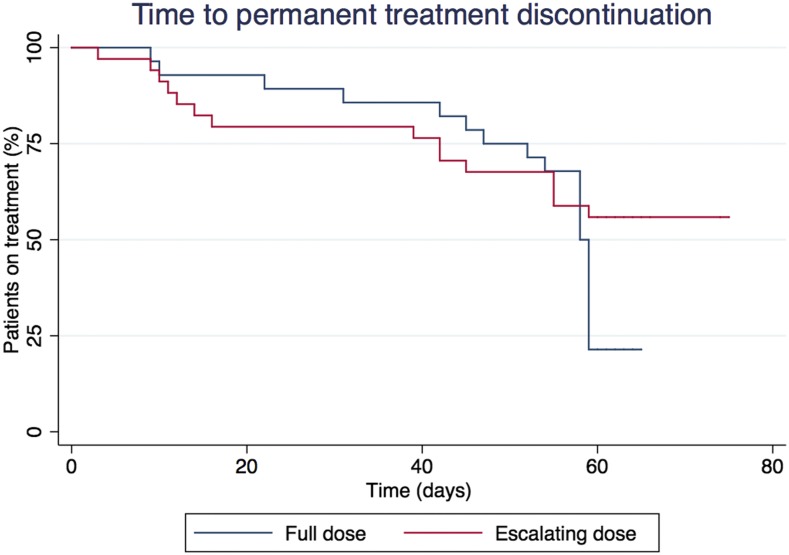
Treatment interruption due to adverse events occurred in 33.9% of all patients. The mean number of treatment days was 50.5 (SD: 18.9), with no differences between groups (Figure 1). Adverse events leading to early interruption (when established at 50% or 80% of the total dose) occurred in three to five of 25 patients in the full-dose group, and in seven to 11 of 29 patients in the escalating dose group. That is to say, 18.5% to 29.6% of adverse events (depending on the “early interruption” threshold used) led to early interruption of treatment that would need further antiparasitic treatment. Patients were referred to a specialist because of an adverse event in 43.5% of cases, with dermatology (85%), neurology (11%), and allergy (4%) being the most frequent. Specific treatment for an adverse event was prescribed in 42% of patients, with oral corticosteroids (50%) and antihistaminics (30.8%) as the most frequent prescriptions.
Figure 1.
Time to permanent treatment discontinuation. This figure appears in color at www.ajtmh.org.
DISCUSSION
To our knowledge, this is the first study to compare how the strategy of progressively introducing benznidazole compares with initiating treatment at a full dose. We could not find any significant differences in the rate of adverse effects, treatment discontinuations, or number of treatment days completed, although the results showed numerically fewer hematologic, liver, and digestive disturbances in the escalating dose group. Although adverse events were not systematically rated for severity, it is possible to ascertain through indirect data such as need for specific treatment and need for treatment discontinuation that they were mostly mild. We found an unusually high referral rate to a specialist for adverse events (43.55%), which is not related to severity but to a local agreement of multidisciplinary evaluation of all benznidazole adverse events.
The global adverse event rate for benznidazole is in agreement with previous work, although we found a slightly higher rate of discontinuation than previous studies.18–21 With such a high rate of adverse events, an intervention such as the progressive introduction of the same drug is unlikely to provide major advantages. Hence, a larger sample size would be needed to detect whether these two treatment strategies yield different outcomes. There is currently no consensus regarding the ideal treatment schedule with benznidazole in terms of length, especially after it has been necessary to discontinue treatment. Some groups consider it sufficient to have received the treatment for 30 days and others 80% of the dose calculated for 60 days.10,14 In our study, the median duration of treatment in both groups exceeds even the most ambitious threshold of 48 days (80%). This means that although suspension is necessary in a high percentage of patients, treatment with a second drug would not be indicated in most of them due to having met the minimum dose requirement. As shown in Figure 1, most treatment discontinuations occurred after previously mentioned thresholds of 30 and 48 days. This is because clinicians would be more prone to discontinue treatment when a mild adverse event occurs after a sufficient duration of antiparasitic treatment has been reached, taking into account the risk/benefit balance.
The retrospective nature of this study and the fact that these two different strategies were put in place by different physicians may have introduced some measurement bias. Given that the patients were assigned to each doctor according to availability (without choice by the doctor or the patient), and treatments assigned according to local protocols (or consensus) at each point in time, we think that this would not constitute a source of selection bias. Nonetheless, data were collected using electronic medical records by different physicians than the ones who treated the patients to increase objectivity. Each time a benznidazole treatment is started, it constitutes both an opportunity and a challenge. Any doctor faced with this situation longs for alternatives that improve the safety of antiparasitic treatment, either with new drugs, dosing changes in existing drugs, or the use of adjuvant drugs. With currently available data, it cannot be asserted that a strategy of progressive doses is better than the use of full doses from the beginning. However, prospective randomized studies are needed to improve knowledge about this issue, given the possibility that different benznidazole treatment introduction strategies might improve tolerability and, therefore, might improve patient health outcomes.
The toxicity of antiparasitic treatment for Chagas disease continues to be a limiting factor. In this work, we explored the use of staggered doses versus complete doses in benznidazole treatment and its effect on the number and severity of adverse effects, and treatment discontinuations. To our knowledge, this is the first time these two strategies have been compared.
REFERENCES
- 1.WHO , 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90: 33–43. [PubMed] [Google Scholar]
- 2.Gascon J, Bern C, Pinazo M-J, 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115: 22–27. [DOI] [PubMed] [Google Scholar]
- 3.Requena-Méndez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DA, Gascon J, Muñoz J, 2015. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis 9: e0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conners EE, Vinetz JM, Weeks JR, Brouwer KC, 2016. A global systematic review of Chagas disease prevalence among migrants. Acta Trop 156: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Molina JA, Molina I, 2018. Chagas disease. Lancet 391: 82–94. [DOI] [PubMed] [Google Scholar]
- 6.Bern C, et al. 2007. Evaluation and treatment of Chagas disease in the United States. JAMA 298: 2171–2181. [DOI] [PubMed] [Google Scholar]
- 7.Russomando G, de Tomassone MM, de Guillen I, Acosta N, Vera N, Almiron M, Candia N, Calcena MF, Figueredo A, 1998. Treatment of congenital Chagas’ disease diagnosed and followed up by the polymerase chain reaction. Am J Trop Med Hyg 59: 487–491. [DOI] [PubMed] [Google Scholar]
- 8.Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H, 2003. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother 52: 441–449. [DOI] [PubMed] [Google Scholar]
- 9.Cançado JR, 1999. Criteria of Chagas disease cure. Mem Inst Oswaldo Cruz 94 (Suppl 1): 331–335. [DOI] [PubMed] [Google Scholar]
- 10.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A, 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 144: 724–734. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Molina JA, Pérez-Ayala A, Moreno S, Fernández-González MC, Zamora J, López-Velez R, 2009. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J Antimicrob Chemother 64: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 12.Morillo CA, et al. BENEFIT Investigators , 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373: 1295–1306. [DOI] [PubMed] [Google Scholar]
- 13.Viotti R, et al. Latin American Network for Chagas Disease, NHEPACHA , 2014. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother 58: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrico F, Gascon J, Ortiz L, Alonso-Vega C, Pinazo M-J, Schijman A, Almeida IC, Alves F, Strub-Wourgaft N, Ribeiro I; E1224 Study Group , 2018. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: a proof-of-concept, randomised, placebo-controlled trial. Lancet Infect Dis 18: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villar JC, Perez JG, Cortes OL, Riarte A, Pepper M, Marin-Neto JA, Guyatt GH, 2014. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev 5: CD003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrade JP, et al. 2011. I Latin American guidelines for the diagnosis and treatment of Chagas’ heart disease: executive summary. Arq Bras Cardiol 96: 434–442. [DOI] [PubMed] [Google Scholar]
- 17.Pérez de Ayala A, Pérez-Molina JA, Navarro M, López-Vélez R, 2009. Enfermedad de Chagas en Personas Procedentes de Latinoamérica Residentes en España. Madrid, Spain: Ministerio de Sanidad y Política Social, Centro de Publicaciones. [Google Scholar]
- 18.Aldasoro E, Posada E, Requena-Méndez A, Calvo-Cano A, Serret N, Casellas A, Sanz S, Soy D, Pinazo MJ, Gascon J, 2018. What to expect and when: benznidazole toxicity in chronic Chagas’ disease treatment. J Antimicrob Chemother 73: 1060–1067. [DOI] [PubMed] [Google Scholar]
- 19.Hasslocher-Moreno AM, do Brasil PE, de Sousa AS, Xavier SS, Chambela MC, Sperandio da Silva GM, 2012. Safety of benznidazole use in the treatment of chronic Chagas’ disease. J Antimicrob Chemother 67: 1261–1266. [DOI] [PubMed] [Google Scholar]
- 20.Pinazo M-J, Munoz J, Posada E, Lopez-Chejade P, Gallego M, Ayala E, del Cacho E, Soy D, Gascon J, 2010. Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob Agents Chemother 54: 4896–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina I, Salvador F, Sánchez-Montalvá A, Treviño B, Serre N, Sao Avilés A, Almirante B, 2015. Toxic profile of benznidazole in patients with chronic Chagas disease: risk factors and comparison of the product from two different manufacturers. Antimicrob Agents Chemother 59: 6125–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirtz VJ, Manne-Goehler J, Reich MR, 2015. Access to care for Chagas disease in the United States: a health systems analysis. Am J Trop Med Hyg 93: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartor P, Colaianni I, Cardinal MV, Bua J, Freilij H, Gürtler RE, 2017. Improving access to Chagas disease diagnosis and etiologic treatment in remote rural communities of the Argentine Chaco through strengthened primary health care and broad social participation. PLoS Negl Trop Dis 11: e0005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manne JM, Snively CS, Ramsey JM, Salgado MO, Bärnighausen T, Reich MR, 2013. Barriers to treatment access for Chagas disease in Mexico. PLoS Negl Trop Dis 7: e2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro I, Sevcsik A-M, Alves F, Diap G, Don R, Harhay MO, Chang S, Pecoul B, 2009. New, improved treatments for Chagas disease: from the R&D pipeline to the patients. PLoS Negl Trop Dis 3: e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A, 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7: 157–163. [DOI] [PubMed] [Google Scholar]
- 27.Chatelain E, 2017. Chagas disease research and development: is there light at the end of the tunnel? Comput Struct Biotechnol J 15: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drugs for Neglected Diseases initiative (DNDi) Fexinidazole (Chagas)—DNDi. Available at: https://www.dndi.org/diseases-projects/portfolio/fexinidazole-chagas/. Accessed May 20, 2018. [Google Scholar]
- 29.Pinazo MJ, Espinosa G, Gallego M, Lopez-Chejade PL, Urbina JA, Gascon J, 2010. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am J Trop Med Hyg 82: 583–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Álvarez MG, et al. 2016. New scheme of intermittent benznidazole administration in patients chronically infected with Trypanosoma cruzi: a pilot short-term follow-up study with adult patients. Antimicrob Agents Chemother 60: 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperandio da Silva GM, Mediano MFF, Alvarenga Americano do Brasil PE, da Costa Chambela M, da Silva JA, de Sousa AS, Xavier SS, Rodrigues da Costa A, Magalhães Saraiva R, Hasslocher-Moreno AM, 2014. A clinical adverse drug reaction prediction model for patients with chagas disease treated with benznidazole. Antimicrob Agents Chemother 58: 6371–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvador F, et al. 2015. Evaluation of cytokine profile and HLA association in benznidazole related cutaneous reactions in patients with Chagas disease. Clin Infect Dis 61: 1688–1694. [DOI] [PubMed] [Google Scholar]
- 33.Olivera MJ, Cucunubá ZM, Valencia-Hernández CA, Herazo R, Agreda-Rudenko D, Flórez C, Duque S, Nicholls RS, 2017. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS One 12: e0185033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinazo M-J, Guerrero L, Posada E, Rodríguez E, Soy D, Gascon J, 2013. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic Chagas disease. Antimicrob Agents Chemother 57: 390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Górgolas M, Robles I, Cabello A, Pérez-Tanoira R, Peremarch CP-J, Fernández-Roblas R, Williams F, Rincón JM, 2013. The use of steroids to prevent cutaneous reactions to benznidazole in patients with Chagas disease. Pathog Glob Health 107: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puerta-Alcalde P, Gomez-Junyent J, Requena-Mendez A, Pinazo MJ, Álvarez-Martínez MJ, Rodríguez N, Gascon J, Muñoz J, 2018. High prevalence of S. Stercoralis infection among patients with Chagas disease: a retrospective case-control study. PLoS Negl Trop Dis 12: e0006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov NCT03378661. BENDITA BEnznidazole New Doses Improved Treatment and Associations. Available at: https://clinicaltrials.gov/ct2/show/NCT03378661. Accessed May 16, 2018.
- 38.Navarrete MN, Gutiérrez-Gutiérrez B, de Arellano-Ramos R, del Castillo S-F, Domínguez-Castellano Á, 2016. Low incidence of adverse effects using a progressive regimen of benznidazole in Chagas disease. Clin Infect Dis 62: 1052. [DOI] [PubMed] [Google Scholar]
- 39.WHO Expert Committee , 2002. Control of Chagas Disease, Vol. 2. World Health Organization Technical Report Series No. 905. Geneva, Switzerland: World Health Organization, 1–109. [PubMed] [Google Scholar]