Abstract.
Lymph node tuberculosis (LNTB) is characterized by the enhanced baseline and antigen-specific production of type 1/17 cytokines and reduced baseline and antigen-specific production of interleukin (IL)-1β and IL-18 at the site of infection when compared with peripheral blood. However, the cytokine profile in the lymph nodes (LNs) of Mycobacterium tuberculosis culture–positive LNTB (LNTB+) and negative LNTB (LNTB−) has not been examined. To address this, we have examined the baseline and mycobacterial antigen–stimulated cytokine levels of type 1 (interferon gamma [IFNγ], tumor necrosis factor alpha [TNFα], IL-2), type 2 (IL-4, IL-5, and IL-13), type 17 (IL-17A, IL-17F, and IL-22), pro-inflammatory (IL-1α, IL-1β, IL-18, and granulocyte macrophage colony-stimulating factor [GM-CSF]), and regulatory cytokines (IL-10, transforming growth factor beta [TGF-β]) cytokines in the LN culture supernatants of LNTB+ and LNTB− individuals. We have observed significantly enhanced baseline levels of IL-13 and IL-10 and significantly reduced baseline levels of IL-4 and GM-CSF in LNTB+ individuals compared with LNTB− individuals. By contrast, we have observed significantly enhanced levels of type 1 (IFNγ, TNFα, and IL-2), type 17 (IL-17F and IL-22), and pro-inflammatory (IL-1α and GM-CSF) cytokines and significantly reduced levels of TGFβ in response to purified protein derivative, early secreted antigen-6, and culture filtrate protein-10 antigens in LNTB+ compared with LNTB− individuals. On phorbol 12-myristate 13-acetate/ionomycin stimulation, no significant difference was observed for any of the cytokines examined. Thus, our study revealed several interesting differences in the cytokine profiles of mycobacterial antigen–stimulated LN cultures in LNTB+ and LNTB− individuals. Therefore, we suggest the presence of mycobacteria plays a significant role in driving the cytokine response at the site of infection in LNTB.
INTRODUCTION
Lymph node tuberculosis (LNTB) is the most common form of extra-pulmonary tuberculosis. Extra-pulmonary tuberculosis comprises about 20% of all TB cases in India and LNTB constitutes nearly 35% of these cases.1–3 Extra-pulmonary tuberculosis has become more common since the advent of HIV infection. Lymph node tuberculosis is common in children; however, adults between the age of 20 and 40 years can also be commonly affected, with a predominant female bias. The clinical presentation depends primarily on the site of disease with LNTB in the cervical, axillary, and inguinal areas often presenting as non-tender swellings without any significant systemic symptoms.4 Lymph node tuberculosis is hard to diagnose because there are several other infectious and noninfectious diseases that can present with the same clinical profile. Excisional biopsy followed by bacterial culture has a high sensitivity for LNTB diagnosis, yet this procedure is invasive and not commonly performed. Fine-needle aspiration (FNA) biopsy is a much less invasive procedure, which provides a good clinical specimen for the initial pathologic assessment. The gold standard for diagnosis of LNTB still remains either culture (in solid or liquid media) or demonstration of the bacilli by nucleic acid amplification tests.5
However, very few studies have examined the utility of antigen-specific cytokine responses in the lymph nodes (LNs) as a diagnostic biomarker. The most well-studied antituberculosis immunity is the T cell–mediated response, which is known to be crucial against Mycobacterium tuberculosis (Mtb) infection. Type 1/pro-inflammatory cytokines such as interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) are able to activate either macrophages or other phagocytes to confer host protection by limiting bacterial growth. Tumor necrosis factor alpha is also essential for maintaining granuloma formation (early phase of infection), a well-organized assembly consisting of both innate and adaptive cells.6 Interleukin (IL)-2 is secreted by numerous immune cells and is also a key component of protective immunity.7 Similarly, type 17 (IL-17A, IL-l7F, and IL-22) cytokines, especially IL-17A, are essential in host protection against extracellular pathogens through the induction of other cytokines and chemokines.8 Furthermore, IL-22 has a defensive role in mucosal sites, by inducing antimicrobial proteins to mediate protection against extracellular pathogens.9 Collectively, published data suggest that both type 1 and type 17 cytokines are crucial in providing host protective immune responses against pulmonary tuberculosis (PTB).10
Apart from type 1/17 cytokines, several other effector molecules are able to contribute to host protection against TB disease. One such is the IL-1 family (including IL-1α, IL-1β, and IL-18) of cytokines, which contribute to diverse physiological functions such as host defense against bacterial and viral infection and activate the immune system (especially type 1 cytokines).11 Likewise, the pro-inflammatory cytokine, GM-CSF, has the potential ability to combat the bacterial growth in vitro.12 Natural killer (NK) T cells are the major source of GM-CSF, which is also produced by many other cell types.13 By contrast, the regulatory cytokines (IL-10 and TGFβ) have a deleterious effect on host immunity and help in establishing chronic infection.14 Thus, studying the T-cell effector cytokines produced on site-specific Mtb infection might offer new and potential immunotherapy targets for vaccine assessment. Therefore, we wanted to examine the function of T cells producing type 1, type 2, and type 17 cytokines and immune regulatory (IL-10 and TGFβ), IL-1 family (IL-1α and IL-1β), as well as pro-inflammatory (IL-18 and GM-CSF) cytokines in the Mtb antigen–stimulated LN cell culture supernatants of positive LNTB (LNTB+) and negative LNTB (LNTB−) individuals. Our findings have shown that enhanced type 1, type 17 (except IL-17A), IL-1α, GM-CSF, and diminished TGFβ cytokines were associated with LNTB+ individuals.
METHODS
Ethics.
The present study was approved by the National Institute of Research in Tuberculosis (NIRT) Institutional Ethics Committee (NIRTIEC2010007), and written informed consent was received from all the study participants.
Patient characterization.
A total of 18 LNTB+ and 10 LNTB− individuals were recruited in the study, and their demographics are listed in Table 1. All LNTB individuals were positive for interferon gamma release assay and diagnosed on the basis of excision biopsy showing positivity in liquid culture for Mtb or by histopathological diagnosis. All the study participants were HIV negative and not under any steroid treatment. Both LNTB+ and LNTB− individuals were given antituberculosis chemotherapy for 6 months and were successfully cured as determined by the disappearance of LN enlargement by X-ray and computed tomography (CT) scans. Therefore, LNTB− individuals are also LNTB patients, who happen to have negative bacterial cultures.
Table 1.
Demographics of study population
| Study demographics | LNTB+ | LNTB− | P value* |
|---|---|---|---|
| Number of subjects recruited (n) | 18 | 10 | – |
| Gender (M/F) | 7/11 | 3/7 | – |
| Median age in years (range) | 29 (18–51) | 35 (18–40) | NS (P = 0.5864) |
| Lymph node culture grade (0/1+/2+/3+) | 0/16/2/0 | 0/0/0/0 | – |
LNTB− = culture negative lymph node tuberculosis; LNTB+ = culture positive lymph node tuberculosis; NS = non significant.
* Calculated using the Mann–Whitney test.
Isolation of LN.
Excision biopsy was performed to collect the infected LNs, and biopsy material was meticulously transferred in the Roswell Park Memorial Institute (RPMI)-1640 medium, which was used for this study. The LNs were carefully transferred onto a petri dish and washed with the RPMI-1640 medium to remove the blood stains. The samples were dissected into minute pieces using sterilized surgical blade and forceps, and then treated with liberase (collagenase I and II, 0.1 mg/mL), deoxyribonuclease (0.1 mg/mL) enzyme (Roche Diagnostics, Indianapolis, IN), incubated for 20–30 minutes, and homogenized using a syringe plunger. The samples were then dissolved in the RPMI medium and filtered using an 80–100-μm filter (Becton Dickinson [BD] Biosciences, Franklin Lakes, NJ), inverted, and spun at 2,600 rpm for 10 minutes at 4°C. The supernatant was discarded, and the samples were once again washed with the RPMI medium. Finally, the cells were counted manually using trypan blue (BioWhittaker™, Walkersville, MD) and a hemocytometer and further used for in vitro culture stimulation.
Antigens.
Mycobacterial antigens used in the study were purified protein derivative (PPD) (Statens Serum Institute), recombinant early secreted antigen-6 peptide pools (ESAT-6) and culture filtrate protein-10 peptide pools (CFP-10) (both from Biodefense and Emerging Infections Research Resources Repository (BEI resources), National Institute of Allergy and Infectious Diseases [NIAID], NIH). In addition, HIV Gag peptide pools (HIVPP) were used as negative controls (AIDS Reagent Program, Division of AIDS, NIAID, NIH). The final concentrations of antigens are 10 µg/mL for PPD, 10 µg/mL for ESAT-6 pp, CFP-10 pp, and HIV Gag peptide pools. Phorbol 12-myristate 13-acetate (PMA)/ionomycin (I) (P/I) (Calbiochem, San Diego, CA) was used as a positive control stimulus with the final concentrations of 12.5 and 125 ng/mL.
In vitro LN culture.
Lymph node culture supernatants were used to measure the different cytokine responses. The harvested LNs were diluted with the RPMI-1640 medium supplemented with penicillin/streptomycin (100 U/100 mg/mL), L-glutamine (2 mM), and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (10 mM) (Invitrogen, San Diego, CA) and equally dispersed (2 mL/well, i.e., 2 million cells/well) in 12-well tissue culture plates (Costar; Corning, Inc., Corning, NY). The samples were stimulated with the following antigens: unstimulated (UNS), PPD, ESAT-6, CFP10, HIVPP, and P/I and incubated at 37°C in 5% CO2 for 18 hours. After the incubation, the cells were transferred into sterile 50-mL falcon tubes and spun at 2,600 rpm for 10 minutes at 4°C. The culture supernatants were carefully transferred into 2-mL screw cap tubes and stored at −80°C.
Lymph node culture ELISA.
Fifty microliters of LN culture supernatants was used to measure the following cytokines using DuoSet ELISA kits purchased from R&D systems (Minneapolis, MN). Type 1 cytokines IFNγ, TNFα, and IL-2; type 17 cytokines IL-17A, IL-17F, and IL-22; type 2 cytokines IL-4, IL-5, and IL-13; regulatory cytokines IL-10, TGFβ, and IL-1 family; and pro-inflammatory cytokines IL-1α, IL-1β, IL-18, and GM-CSF. The UNS values were represented as baseline cytokines, whereas the net cytokines were represented for different antigenic (PPD, ESAT-6 PP, CFP-10 PP, HIV GAG PP, or PMA/I) stimulations (with the baseline cytokine values subtracted).
Statistics.
Statistical analysis was performed using GraphPad PRISM 6 (GraphPad Software, Inc., San Diego, CA). The geometric means were used to measure the central tendency. Nonparametric Mann–Whitney U test were performed to calculate the statistical significance between two different groups. receiver operating characteristic (ROC) analysis was performed to measure the significant correlation among different cytokines analyzed.
RESULTS
Hematological parameters.
The leukocyte and differential counts were performed for LNTB+ and LNTB− individuals using the Ac.T-5 Diff hematology analyzer (Beckman Coulter, Brea, CA). Positive lymph node tuberculosis individuals had significantly lower percentages of lymphocytes compared with LNTB− individuals. No other significant differences in hematological parameters were observed between the two groups (Table 2).
Table 2.
Hematological profile of study population
| Hematological profile | LNTB+ | LNTB− | P value* |
|---|---|---|---|
| Whole blood cells (103/L) | 7.417 (6.4–11.3) | 7.2 (3.8–10.3) | NS |
| Red blood cells (106/L) | 4.5161 (3.94–5.72) | 4.4 (4.61–5.41) | NS |
| Lymphocytes (%) | 28.01 (22.8–43.4) | 32.87 (23.9–44.8) | 0.0105 |
| Monocytes (%) | 7.8 (3.9–11.7) | 6.83 (4.5–10.5) | NS |
| Eosinophils (%) | 3.12 (1.1–11) | 3.34 (2–6.3) | NS |
| Basophils (%) | 1.05 (0.2–2.6) | 0.93 (0.3–1.5) | NS |
| Neutrophils (%) | 54.461 (42.9–71.3) | 46.03 (43.8–63.3 | NS |
| Platelets (103/L) | 321.4 (199–482) | 297.9 (163–654) | NS |
| Hemoglobin (g/dL) | 11.94 (8–16) | 11.02 (10.7–13.6) | NS |
LNTB− = negative lymph node tuberculosis; LNTB+ = positive lymph node tuberculosis. Bold value indicates P = < 0.05.
* Calculated using the Mann–Whitney test.
Positive lymph node tuberculosis individuals are associated with altered cytokine responses in UNS LN supernatants.
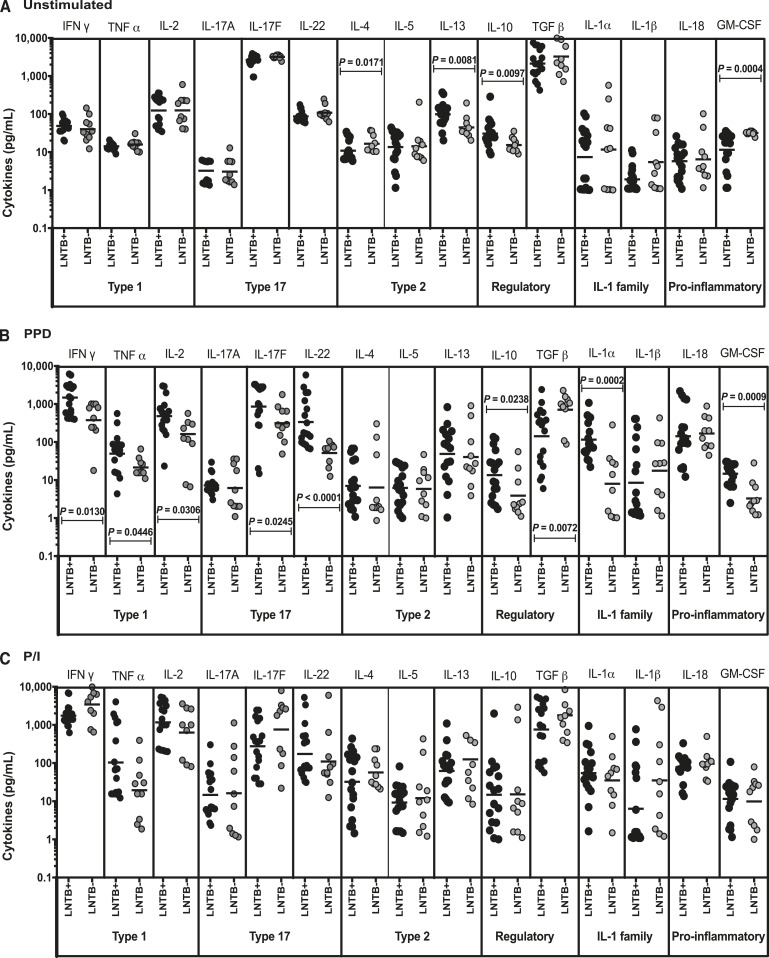
To determine the baseline cytokine profile, we measured the UNS LN supernatant levels of type 1 (IFNγ, TNFα, and IL-2), type 17 (IL-17A, IL-17F, and IL-22), type 2 (IL-4, IL-5, and IL-13), regulatory (IL-10 and TGFβ), IL-1 family, and pro-inflammatory cytokines (IL-1α, IL-β, IL-18, and GM-CSF) in LNTB+ and LNTB− individuals (Figure 1A). Among the different cytokines analyzed, we observed significantly decreased levels of IL-4 (P = 0.0171)and GM-CSF (P = 0.0004) and significantly enhanced levels of IL-13 (P = 0.0081) and IL-10 (P = 0.0097) in LNTB+ individuals when compared with LNTB− individuals. However, no significant differences in the levels of either type 1, type 17, or IL-1 family, and pro-inflammatory cytokines were found between the two study groups. Thus, LNTB+ individuals are associated with altered UNS cytokine levels.
Figure 1.
Baseline, purified protein derivative (PPD), and Phorbol 12-myristate 13-acetate/ionomycin (P/I) stimulated cytokine production in positive lymph node tuberculosis (LNTB+) and negative lymph node tuberculosis (LNTB−) individuals. The (A) unstimulated, (B) PPD and, (C) P/I stimulated culture supernatant levels of type 1 (interferon gamma [IFNγ], tumor necrosis factor alpha [TNFα], and interleukin [IL]-2), type 17 (IL-17, IL-17F, and IL-22), type 2 (IL-4, IL-5, and IL-13), regulatory (IL-10 and TGFβ), IL-1 family (IL-1α and IL-1β), and other pro-inflammatory (IL-18 and GM-CSF) cytokines were measured in lymph node cell suspensions of LNTB+ and LNTB− individuals. Each circle represents a single individual, and the bars represent the geometric mean values. Net cytokine levels were calculated by subtracting the baseline levels from the antigen-induced levels for each individual. P values were calculated using the Mann–Whitney U test.
Positive lymph node tuberculosis individuals are associated with enhanced type 1 and type 17 cytokines on TB antigen (PPD, ESAT-6 PP, and CFP-10 PP) stimulation in LN supernatants.
To determine the mycobacterial antigen stimulated cytokine profile in LNTB+, we measured the levels of those cytokines following stimulation with TB antigens (PPD, ESAT-6 PP, and CFP-10 PP) in LNTB+ and LNTB− individuals (Figure 1B and Supplemental Figure 1A and B). Among the cytokines analyzed, significantly increased levels of IFNγ (P = 0.0130 in PPD, P = 0.0466 in ESAT-6 PP, and P = 0.0043 in CFP-10 PP), TNFα (P = 0.0446 in PPD, P = 0.0129 in ESAT-6 PP, and P = 0.0069 in CFP-10 PP), IL-2 (P = 0.0311 in PPD, P = 0.0037 in ESAT-6 PP, and P = 0.0140 in CFP-10 PP), IL-17F (P = 0.025 in PPD and P = 0.0111 in ESAT-6 PP), IL-22 (P < 0.0001 in PPD, P = 0.0135 in ESAT-6 PP, and P = 0.0009 in CFP-10 PP), IL-1α (P = 0.0002 in PPD, P < 0.0001 in ESAT-6 PP, and P = 0.0002 in CFP-10 PP), and GM-CSF (P = 0.0009 in PPD, P = 0.0024 in ESAT-6 PP, and P = 0.0002 in CFP-10 PP) were observed in LNTB+ individuals in comparison with LNTB− individuals.
Similarly, TGFβ (P = 0.0072 in PPD, P = 0.0025 in ESAT-6 PP, and P = 0.0452 in CFP-10 PP) levels were diminished and IL-10 (P = 0.0238 in PPD) levels were enhanced in LNTB+ individuals when compared with LNTB− individuals. However, no significant differences in the levels of type 2 cytokines (IL-4, IL-5, and IL-13) or IL-1β and IL-18 were found between LNTB+ and LNTB− individuals. Hence, tuberculous lymphadenitis (TBL) is associated with elevated levels of type 1, type 17, IL-1, or pro-inflammatory (IL-1α and GM-CSF) and regulatory cytokines (IL-10) in the LNTB+ individuals on stimulation with different TB (PPD, ESAT-6 pp, and CFP-10 PP) antigens.
No significant differences in cytokine production on HIV GAG PP and P/I stimulation.
To understand the positive (P/I) or non-TB specific or negative control (HIV GAG PP) antigen stimulated cytokine profile, we again measured the different cytokine levels in LNTB+ and LNTB− individuals. As shown in Figure 1C, we did not observe any significant difference in the LN supernatants for any of those cytokines analyzed on stimulation with P/I. Similarly, as shown in Supplemental Figure 1C, the non-TB specific or negative antigen control stimulated levels did not exhibit any significant differences (except IL-4 [decreased in LNTB+] and IL-13, increased in LNTB+) among the panel of cytokines analyzed between the LNTB+ and LNTB− individuals. Therefore, there are no significant differences observed in LN supernatants following HIV Gag PP or P/I stimulation between LNTB+ and LNTB− individuals.
Baseline and TB antigen stimulated type 1, type 17, regulatory, and IL-1 family or pro-inflammatory cytokines clearly distinguish LNTB+ from LNTB− individuals.
To understand the discriminatory power of different cytokines which distinguish LNTB+ from LNTB− individuals, we performed ROC analysis of type 1 (IFNγ, TNFα, and IL-2), type 17 (IL-17A, IL-17F, and IL-22), type 2 (IL-4, IL-5, and IL-13), regulatory (IL-10 and TGFβ), IL-1 family, and pro-inflammatory cytokines (IL-1α, IL-β, IL-18, and GM-CSF) in UNS, different TB (PPD, ESAT-6 PP, and CFP-10 PP) or non-TB (positive P/I or negative, HIV Gag PP) specific antigen stimulation (Supplemental Figure 2A–E). In UNS, IL-4, IL-13, GM-CSF, and IL-10 exhibited significant discriminatory power along with high area under the curve values, sensitivity, and specificity (Supplemental Figure 2A). Similarly, in different antigenic (PPD, ESAT-6 PP, and CFP-10 PP) stimulations, IFNγ, TNFα, IL-2, IL-17F (not for CFP-10 PP), IL-22 IL-1α, GM-CSF, and TGFβ were shown to exhibit clear discrimination and significance (Supplemental Figure 2B–D). However, in the HIV GAG PP stimulation, only IL-4, IL-13, and IL-1α were found to have potential discrimination (Supplemental Figure 2E). Hence, we demonstrate that type 1/type 17 along with IL-1 family or pro-inflammatory (IL-1α and GM-CSF) and regulatory (TGFβ) cytokines could potentially serve as discriminatory markers between the LNTB+ and LNTB− infection.
DISCUSSION
It is postulated that type 1, type 17, and pro-inflammatory cytokines are protective against TB infection and disease.15,16 However, the role of these cytokines in the context of immunity to the extra-pulmonary form of TB is less studied. We have previously reported that the expansion of mono- and multifunctional Th1 and Th17 cells in response to TB antigens is associated with LNTB. Similarly, CD8+ T cells expressing type 1/17 cytokines were significantly enhanced in LNTB when compared with PTB.17,18 Furthermore, we have previously shown that LNTB was associated with significantly diminished systemic or mycobacterial antigen stimulated levels of pro-inflammatory cytokines (IL-1β and IL-18) but not type 1/17 cytokines in comparison with LTB individuals. Similarly, we have also shown that LNTB individuals exhibit significantly elevated levels of type 1/type 17 cytokines and diminished baseline and antigen-specific levels of pro-inflammatory cytokines (IL-1β and IL-18) in the whole blood/LN.19,20 Despite all these studies, the cytokine profile in the LNs of culture confirmed LNTB+ individuals in comparison to culture negative LNTB− has not been investigated. Hence, we sought to explore those cytokine levels in the site-specific infected LN samples.
Interestingly, our analysis revealed that type 1/type 17 (except IL-17A) cytokines were significantly increased in the LN supernatants of LNTB+ individuals. This observation suggests that the altered levels of cytokine production were antigen or pathogen specific because a similar trend was not observed in the UNS supernatants of the same individuals. Type 1/17 cytokines (IFNγ and TNFα or IL-17) are arguably well-established surrogate markers involved in the host protection by delivering the counter mechanisms against tuberculosis pathogenesis.10,21 In addition, high levels of IFNγ have been correlated with disease severity, and they diminish after the completion of successful anti-TB treatment.22 Individuals with IFNγ deficiency have disseminated form of TB infections with high bacterial burden.23,24 On the whole, these observations are consistent with our findings where we have shown increased IFNγ expression in the LNTB+ individuals on stimulation with different Mtb antigens. Elevated IFNγ levels had been associated with increased rates of disease progression and may inversely correlate with protection. Thus, a concerted action of multiple cytokines might be necessary for protection.25 Tumor necrosis factor alpha, a key type 1 cytokine, is crucial in the granuloma biogenesis. Higher TNFα levels were observed in the peripheral blood mononuclear cells (PBMCs) or whole blood culture supernatants or systemic levels of PTB or PTB diabetes mellitus comorbidity.26,27 Similar responses were seen in the LN culture supernatants of LNTB individuals.20 It is well known that IL-2 can promote T cell–dependent immune responses, and its deficiency results in exaggerated bacterial load.7 Our study results demonstrate that LNTB+ individuals have enhanced IL-2 levels on Mtb antigen stimulation and, therefore, the capacity to activate antimycobacterial responses. In general, IL-2 activation results in the proliferation of NK, memory, and newly activated effector cells, which are required for the host immunity.28 Elevated levels of Type 1 cytokines could indicate disease severity or reflect the presence of antigen load in LNTB+ individuals.
Like type 1 cytokines, other immune markers may have the potential ability to provide the host protection against Mtb. In vaccine-induced models, IL-17– or IL-17RA–deficient mice have diminished Th1 cytokine responses.29 In HN878 Mtb infection, IL-17–deficient mice are more susceptible and have substantial defects in the localization of T cells and development of lung lymphoid follicles inside the TB granuloma.30,31 Our data reveal the presence of increased IL-17F in LNTB+ individuals, demonstrating that IL-17F might be correlated with the immune response against TB. Likewise, elevated IL-22 production has been reported at disease sites of active TB patients.32 This is delineated in our study as well, wherein we observe elevated IL-22 levels in LNTB+ compared with LNTB− individuals. Interleukin-1 family of cytokines is one of the most prominent factors in host defense against Mtb. A previous study suggested that IL-1–deficient mice have higher susceptibility rate when exposed to TB infection.33 Interleukin-1 is very important for the fibroblast formation and indeed could have a role in granuloma formation.34 Our data demonstrate the enhanced levels of IL-1α on stimulation with different TB antigens in the supernatants of LNTB+ individuals. Most importantly, priming by IL-1 cytokine increases the granzyme B expression, antigen-specific cytotoxic T-cell function, and IFNγ production capacity of antigen-specific CD8+ T cells.35 Therefore, increased IL-1α levels could reflect the increased host immunity in TBL. Our data also reveal enhanced GM-CSF levels in response to TB antigens in LNTB+ individuals. We speculate that the increased level of this cytokine could contribute to the activation of leukocytes in LNTB disease because GM-CSF–deficient mice are highly susceptible to the infection.36–38 Surprisingly, unlike our previous data, two important cytokines (IL-1β and IL-18) that had exhibited decreased levels systemically as well as following TB antigen stimulation in blood and LN did not exhibit any significant difference between LNTB+ and LNTB− individuals.
Elevated Th2 responses play a vital function in TB susceptibility, as type 2 cytokines (IL-4, IL-5, and IL-13) can downregulate Th1 cell–mediated immunity and inhibit macrophage activation and autophagy.39,40 The present study did not show any differential effect of TB antigen stimulation on type 2 cytokines between LNTB+ and LNTB− individuals, making it unlikely that type 2 cytokine contribute to disease pathogenesis in LNTB. Both IL-10 and TGFβ are the most important immunoregulatory cytokines capable of suppressive function. Increased IL-10 levels were reported in lungs and spleen of PTB and IL-10 acts by inhibiting Th1/Th17 immune responses and reducing the antimicrobial efficacy.41 TGFβ, the other mediator of immune suppression, is expressed at high levels in TB patients in response to Mtb antigens and is associated with diminished T-cell proliferation and production.42 Our data reveal increased levels of IL-10 and decreased levels of TGFβ in the culture supernatants of LNTB+ individuals. Thus, our data support a scenario wherein reduction in TGFβ levels could support enhanced pro-inflammatory cytokine responses in the affected LNs and perhaps enhanced pathology.
Finally, ROC analysis of the data revealed the importance of a variety of cytokines in discriminating between the LNTB+ and LNTB− individuals. Thus, TNFα, IFNγ, IL-2, IL-1α, IL-22, GM-CSF, and TGFβ are the potential cytokines that could discriminate between the respective study groups either at baseline or following antigen stimulation. This could be linked to the higher frequency of Mtb antigen–specific T cells that are highly detectable in the infection site in TB-positive but not in TB-negative individuals.43,44
Overall, our data suggest that the expression of type 1/17, IL-1α, and GM-CSF cytokines in the site of infection probably indicates an association of these cytokines with the immune response in LNTB. However, our study has some limitations, including small sample size, lack of data availability on bacterial burdens, lack of non-TB LN samples, and no way to determine causality. Nevertheless, our study provides important insights into the cytokine profile at the site of infection in this understudied form of TB.
Supplementary Files
Acknowledgments:
We thank V. Rajesh Kumar of NIH-NIRT-ICER and the staff members of the Department of Clinical Research, NIRT; Government Stanley Hospital; Government General Hospital; and Government Kilpauk Medical Hospital, Chennai, for providing valuable assistance in recruiting the patients for this study.
Note: Supplemental figures appear at www.ajtmh.org.
REFERENCES
- 1.Sharma SK, Mohan A, 2004. Extrapulmonary tuberculosis. Ind J Med Res 120: 316–353. [PubMed] [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention , 2014. Annual Report on the Notified Tuberculosis in Korea. Cheongwon, Korea: Korea Centers for Disease Control and Prevention, 2013. [Google Scholar]
- 3.Gothi D, Jaswal A, Spalgais S, 2016. Lymph node tuberculosis. EC Pulmonol Respir Med 2.5: 194–211. [Google Scholar]
- 4.Handa U, Mundi I, Mohan S, 2012. Nodal tuberculosis revisited: a review. J Infect Dev Ctries 6: 6–12. [DOI] [PubMed] [Google Scholar]
- 5.Knox J, Lane G, Wong JSJ, Trevan PG, Karunajeewa H, 2012. Diagnosis of tuberculous lymphadenitis using fine needle aspiration biopsy. Intern Med J 42: 1029–1036. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AM, 2009. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27: 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arenas-Ramirez N, Woytschak J, Boyman O, 2015. Interleukin-2: biology, design and application. Trends Immunol 36: 763–777. [DOI] [PubMed] [Google Scholar]
- 8.Kolls JK, Khader SA, 2010. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev 21: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudakov JA, Hanash AM, van den Brink MR, 2015. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33: 747–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP, 2013. The immune response in tuberculosis. Annu Rev Immunol 31: 475–527. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Barber KD, Sher A, 2015. Cytokine and lipid mediator networks in tuberculosis. Immunol Rev 264: 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothchild AC, Jayaraman P, Nunes-Alves C, Behar SM, 2014. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog 10: e1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin JD, Cannistra SA, Sullivan R, Demetri GD, Ernst TJ, Kanakura Y, 1990. The biology of GM-CSF: regulation of production and interaction with its receptor. Int J Cell Cloning 8 (Suppl 1): 35–44; discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 14.Ellner JJ, 2010. Immunoregulation in TB: observations and implications. Clin Transl Sci 3: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt K, Verma S, Ellner JJ, Salgame P, 2015. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol 22: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar NP, Gopinath V, Sridhar R, Hanna LE, Banurekha VV, Jawahar MS, Nutman TB, Babu S, 2013. IL-10 dependent suppression of type 1, type 2 and type 17 cytokines in active pulmonary tuberculosis. PLoS One 8: e59572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar NP, Sridhar R, Banurekha VV, Nair D, Jawahar MS, Nutman TB, Babu S, 2013. Expansion of pathogen-specific mono- and multifunctional Th1 and Th17 cells in multi- focal tuberculous lymphadenitis. PLoS One 8: e57123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar NP, Sridhar R, Hanna LE, Banurekha VV, Jawahar MS, Nutman TB, Babu S, 2014. Altered CD8(+) T cell frequency and function in tuberculous lymphadenitis. Tuberculosis (Edinb) 94: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kathamuthu GR, Moideen K, Bhaskaran D, Sekar G, Sridhar R, Vidyajayanthi B, Gajendraraj G, Babu S, 2017. Reduced systemic and mycobacterial antigen-stimulated concentrations of IL-1β and IL-18 in tuberculous lymphadenitis. Cytokine 90: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathamuthu GR, et al. 2017. Tuberculous lymphadenitis is associated with enhanced baseline and antigen-specific induction of type 1 and type 17 cytokines and reduced interleukin-1β (IL-1β) and IL-18 at the site of infection. Clin Vaccine Immunol 24: e00045-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper AM, Mayer-Barber KD, Sher A, 2011. A role of innate cytokines in mycobacterial infection. Mucosal Immunol 4: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahiratmadja E, et al. 2007. Plasma granulysin levels and cellular interferon-gamma production correlate with curative host responses in tuberculosis, while plasma interferon-gamma levels correlate with tuberculosis disease activity in adults. Tuberculosis (Edinb) 87: 312–321. [DOI] [PubMed] [Google Scholar]
- 23.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM, 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178: 2243–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR, 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178: 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandi B, Behar SM, 2011. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med 208: 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Attiyah R, El-Shazly A, Mustafa AS, 2012. Comparative analysis of spontaneous and mycobacterial antigen-induced secretion of Th1, Th2 and pro-inflammatory cytokines by peripheral blood mononuclear cells of tuberculosis patients. Scand J Immunol 75: 623–632. [DOI] [PubMed] [Google Scholar]
- 27.Kumar Nathella P, Babu S, 2017. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 152: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millington KA, Gooding S, Hinks TS, Reynolds DJ, Lalvani A, 2010. Mycobacterium tuberculosis-specific cellular immune profiles suggest bacillary persistence decades after spontaneous cure in untreated tuberculosis. J Infect Dis 202: 1685–1689. [DOI] [PubMed] [Google Scholar]
- 29.Khader SA, et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 30.Jurado JO, et al. 2012. IL-17 and IFN-γ expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol 91: 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang XO, et al. 2008. Regulation of inflammatory responses by IL-17F. J Exp Med 205: 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scriba TJ, et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 180: 1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer-Barber KD, et al. 2014. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I, 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Invest 80: 759–767. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Sasson SZ, et al. 2013. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med 210: 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ushach I, Zlotnik A, 2016. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol 100: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi Y, et al. 2006. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res 16: 126–133. [DOI] [PubMed] [Google Scholar]
- 38.Rothchild AC, et al. 2017. Role of granulocyte-macrophage colony-stimulating factor production by T cells during Mycobacterium tuberculosis infection. MBio 8: e01514-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, Kaufmann SH, 2006. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol 36: 631–647. [DOI] [PubMed] [Google Scholar]
- 40.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V, 2007. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27: 505–517. [DOI] [PubMed] [Google Scholar]
- 41.Redford PS, Murray PJ, O’Garra A, 2011. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol 4: 261–270. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z, 1997. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA 94: 3926–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, Marienfeld K, Lalvani A, Lange C, 2006. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med 174: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, Maskell N, Davies R, Pasvol G, Lalvani A, 2005. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis 40: 184–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.