Abstract
Background:
No comprehensive collection of routine therapeutic drug monitoring data for antipsychotic drugs has been published.
Methods:
In this compilation, data on 12 antipsychotics are presented. The drugs included are amisulpride (n = 506), aripiprazole (n = 1610), clozapine (n = 1189), flupentixol (n = 215), haloperidol (n = 390), olanzapine (n = 10,268), perphenazine (n = 1065), quetiapine (n = 5853), risperidone (n = 3255), sertindole (n = 111), ziprasidone (n = 1235), and zuclopenthixol (n = 691). Because only one sample per patient is included, the number of patients equals the number of samples. For each drug, median serum concentrations as well as that of the 10th and 90th percentiles are given for a range of daily doses. Comparisons are made between males and females, between patients younger than 65 years and 65 years and older, and between those treated with a low and a high dose of each drug. The concentration-to-dose (C/D) ratio is the primary variable used in these comparisons. Coefficients of variation (CVs) for the serum concentrations of each drug within and between subjects are presented.
Results:
In general, the C/D ratios were higher in females than in males, higher in those 65 years and older than in younger subjects, and lower in those treated with higher doses than in those treated with lower doses. CVs between individuals were larger than within subjects, and the CVs were highest for the drugs with short elimination half-lives.
Conclusions:
For each antipsychotic drug, the results presented can serve as a reference tool for pharmacokinetic interpretation of the individual patient's serum drug level. The compiled serum concentrations and the C/D ratios can support the physician's decision when individualizing dosing and determining treatment strategies for a specific patient.
Key Words: antipsychotic drugs, TDM, age, sex, dose
INTRODUCTION
Antipsychotic drugs are used to treat a wide range of disorders including schizophrenia and other psychoses, bipolar disorder, severe anxiety and depression, behavioral disorders, and dementia.1 A high proportion of patients treated with antipsychotics do, however, not achieve an adequate clinical response and/or experience adverse drug reactions.1,2 Moreover, differences in response and adverse effects have been reported between men and women.3–5 Desired and undesired effects of drugs are related to their concentration at the site of action (ie, for antipsychotics in the central nervous system). Plasma concentrations of antipsychotics have been shown to correlate well with the concentration in the brain.6 By contrast, because drug concentrations are highly variable when administering the same dose of a drug to a group of patients, the dose given to a patient is a poor predictor of clinical effect.6 Differences in serum concentrations of antipsychotics at a constant dose within and between individuals are caused by an array of factors, including patient adherence, genetic polymorphisms of drug transporters and metabolizing enzymes, age, sex, concurrent disease, hepatic and renal function, and use of concomitant medications.7
Therapeutic drug monitoring (TDM) uses the quantification of drug concentrations in plasma or serum to assist the physician in treatment decisions related to an individual patient. By adjusting the dose, a drug concentration associated with the highest probability of response and the lowest risk adverse drug reactions and toxic effects can be achieved. Serum concentrations of drug metabolites are of importance if the metabolites contribute to the overall clinical effect, but can also be used to calculate the ratio between the concentration of the parent drug and the metabolite, thereby providing a measure of the activity of the enzyme(s) involved in the metabolic step in question.8 By presenting a comprehensive compilation of a large number of serum concentrations for a drug, a reference tool can be created for appropriate pharmacokinetic interpretation of an individual patient's serum drug level. In addition, by compiling serum concentration data found in patients in treatment situations, reference levels for toxicological assessments can be established.9
The primary aim of the this study was to present serum concentrations obtained at different daily doses for commonly used antipsychotic drugs by means of TDM in a naturalistic setting. Secondary aims were to describe the serum concentration variability within and between individuals and to compare serum concentrations in women and men, in patients younger than 65 years and 65 years and older, and in patients using high and low doses of a drug.
METHOD
Samples
In the TDM service at the Department of Clinical Pharmacology, St. Olav University Hospital, Trondheim, Norway, serum samples from patients treated with antipsychotic drugs are analyzed on request from the responsible physician. Key patient information and the results of the TDM analyses for all samples received since 1999 are stored in a database.
In this study, samples where the following 12 antipsychotic drugs were detected were assessed: amisulpride, aripiprazole, clozapine, flupenthixol, haloperidol, olanzapine, perphenazine, quetiapine, risperidone, sertindole, zuclopenthixol, and ziprasidone. Alimemazine, chlorpromazine, chlorprothixene, dixyrazine, levomepromazine, and thioridazine were not included in this compilation because these antipsychotic drugs are used mainly as needed with uncertain information related to the dose actually ingested. Samples analyzed from October 1999 to 2015 were included. The principles of collection were the same as used in a previously published compilation for antidepressants.10 In brief, after excluding intramuscular depot injections, one sample per patient was included in the final data set (Table 1). The sample chosen was the first sample from each patient where the daily dose was known. Information on whether the sample was a trough sample obtained under steady state-conditions or not could, in most cases, be derived from the running text found on the requisition forms. Information on concomitant medication was not possible to retrieve. Deliberate or unintentional overdoses were excluded. For the calculations of the coefficient of variation (CV) within and between individuals, data were retrieved from the original database.
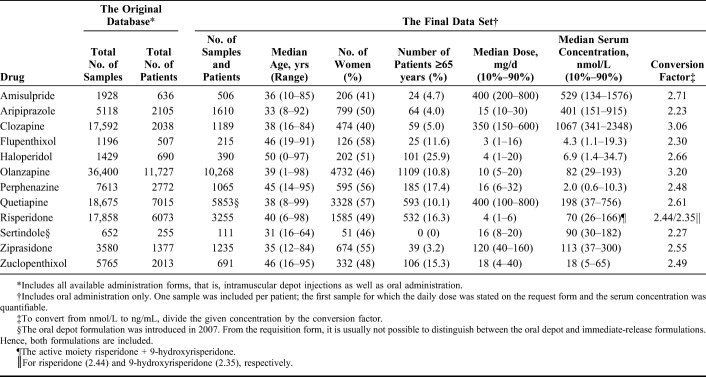
TABLE 1.
The Original Database and the Final Data Set Comprising the Number of Patients and Samples Included in the Analyses, Demographic Data of the Patients Evaluated, and Median Daily Doses and Serum Concentrations
The study was approved by the Regional Committee for Medical Research Ethics, Northern Norway, approval number 2016/994-3. According to Norwegian law, it is not necessary to obtain informed consent in scientific studies like the present. As far as the data are retrieved from laboratory routine sample database and anonymized before evaluation and compilation.
Analytical Methods
All antipsychotic drugs were analyzed with liquid chromatography-mass spectrometry methods described previously.11–14 In brief, after addition of the internal standards, the drugs were extracted from serum by organic solvents, the extracts were evaporated to dryness with air, and the residuals were reconstituted in methanol. Thereafter, the analytes were separated on C18 columns and quantified on an Agilent MSD 1100 system (Agilent, Palo Alto, CA). Internal standards, usually deuterated, were used. Together with the unknown patient samples, each analytical series contained 7 calibrators covering therapeutic, subtherapeutic, and toxic concentrations. In addition, 6 quality control samples with representative target levels were always included.
The limits of quantitation for the analytes were as follows: Amisulpride 25 nmol/L, aripiprazole and its main metabolite dehydroaripiprazole 25 nmol/L, clozapine and its main metabolite desmethylclozapine 25 nmol/L, flupenthixol 1 nmol/L, haloperidol 1 nmol/L, olanzapine 5 nmol/L, perphenazine 0.5 nmol/L, quetiapine 10 nmol/L, risperidone and its main metabolite 9-hydroxyrisperidone 2.5 nmol/L, sertindole 10 nmol/L, zuclopenthixol 2.5 nmol/L, and ziprasidone 10 nmol/L. Accuracy was controlled routinely with external control samples and precision was calculated from the quality control samples. In general, the interassay CVs were less than 10%. The methods were linear in the concentration ranges achieved by therapeutic use of the drug.
Statistical Analysis
All concentrations are given in nmol/L. To convert from nmol/L to ng/mL, conversion factors for each drug are presented in Table 1. For most calculations, serum concentrations were normalized for daily dose by calculating the concentration–dose (C/D) ratio, that is, the drug concentration (in nmol/L) per milligram drug administered daily.
Parameters used for group comparisons (women vs. men; patients <65 versus ≥ 65 years; patients using a high versus a low dose) were the median parent compound concentration and the median metabolite/parent compound (M/P) concentration ratio at the most common daily doses used in the population. For comparisons between groups, the Mann–Whitney U test was used. The daily dose–serum concentration correlation and, when appropriate, daily dose–M/P concentration ratio correlation were analyzed by linear regression.
The CVs for serum concentration and M/P concentration ratio within and between subjects were estimated using a components-of-variance model15 performed on log10-transformed data here schematically and briefly outlined: The between-subjects mean sum of squares (M) and the within-subject error component (E) based on the number of repeated samples per patient (n) were calculated using analysis of variance. Thereafter, the respective variations were calculated:
Between-individual variation =
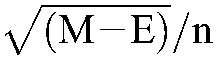
Within-individual variation =
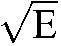
To achieve approximate CVs within and between individuals, the respective square root was multiplied by the natural logarithm of 10 (ln 10).
The computer software GraphPad PRISM, version 7.04 (GraphPad Software, La Jolla CA) and IBM SPSS Statistics 24 for Windows (IBM, Armonk, NY) were used for the statistical computations. P values < 0.05 were considered statistically significant.
RESULTS
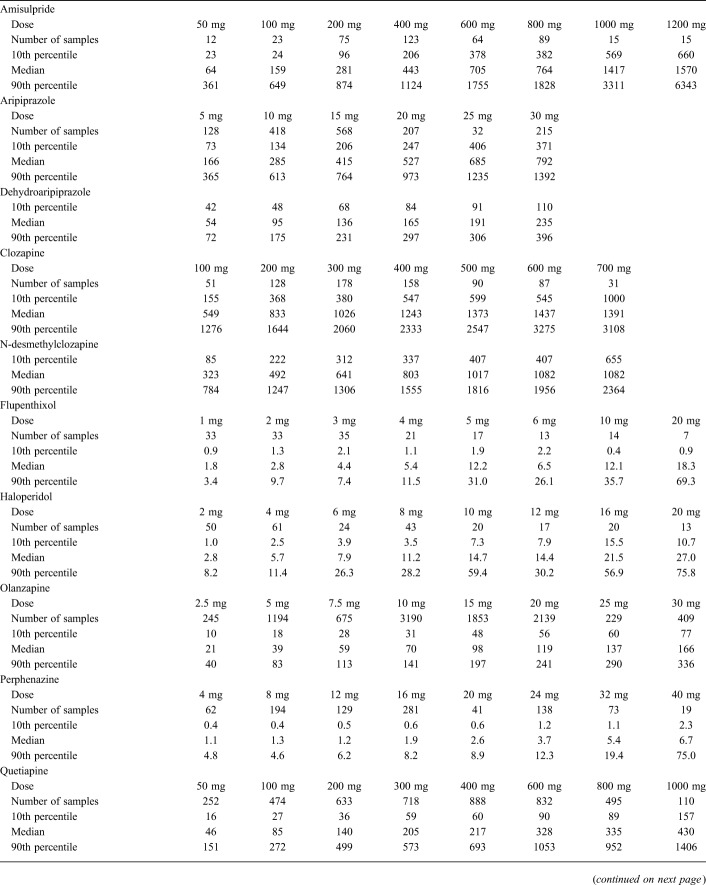
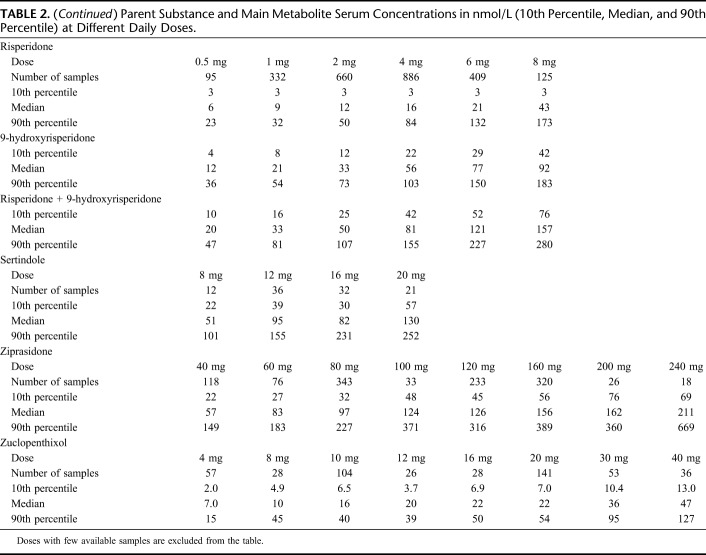
Numbers of samples retrieved from the original database and included in the final data set are presented in Table 1. The 3 drugs with the largest number of patients (and samples) were olanzapine (n = 10,268), quetiapine (n = 5853), and risperidone (n = 3255). Key demographic data for the patients included and overall median daily doses and serum concentrations can be found in Table 1. The concentrations measured (expressed as 10th percentiles, medians, and 90th percentiles) at various daily doses of the drugs are displayed in Table 2.
TABLE 2.
Parent Substance and Main Metabolite Serum Concentrations in nmol/L (10th Percentile, Median, and 90th Percentile) at Different Daily Doses.
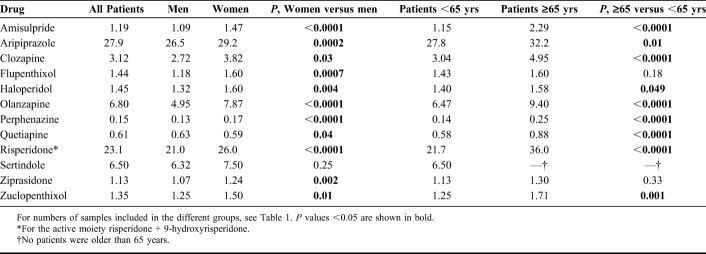
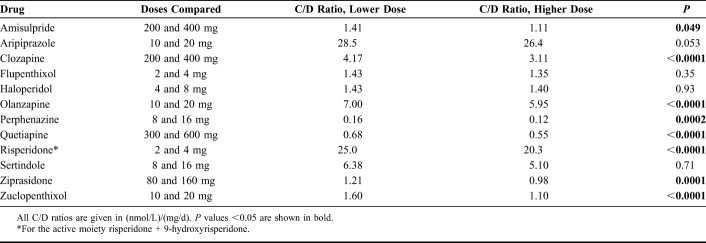
Comparisons of C/D ratios between men and women and between those younger and ≥65 years of age are shown in Table 3. With the exception of sertindole, women had significantly higher C/D ratios than men. Patients 65 years and older had significantly higher C/D ratios than younger ones for all drugs except flupenthixol and ziprasidone. When comparing C/D ratios for the 12 drugs at 2 common dose levels where the higher dose was twice the lower dose, the C/D ratios were in most cases significantly increased at the lower dose level as compared to the higher dose level (Table 4). The relationship between daily doses and M/P ratios for aripiprazole, clozapine, and risperidone is illustrated in Figure 1. The slopes of all regression lines were significantly different from zero (r2 = 0.075; P = 0.0008 for dehydroaripiprazole/aripiprazole; r2 = 0.171; P < 0.0001 for desmethylclozapine/clozapine; r2 = 0.070; P < 0.0001 for 9-hydroxyrisperidone/risperidone).
TABLE 3.
Median Concentration/Dose (C/D) Ratios in (nmol/L)/(mg/D) for all Subjects, Men and Women, and Subjects Younger and Older Than 65 Years.
TABLE 4.
Comparison of Concentration/Dose (C/D) Ratios for the Parent Substances at Two Common Dose Levels Where the Higher Daily Dose is Twice the Lower
FIGURE 1.
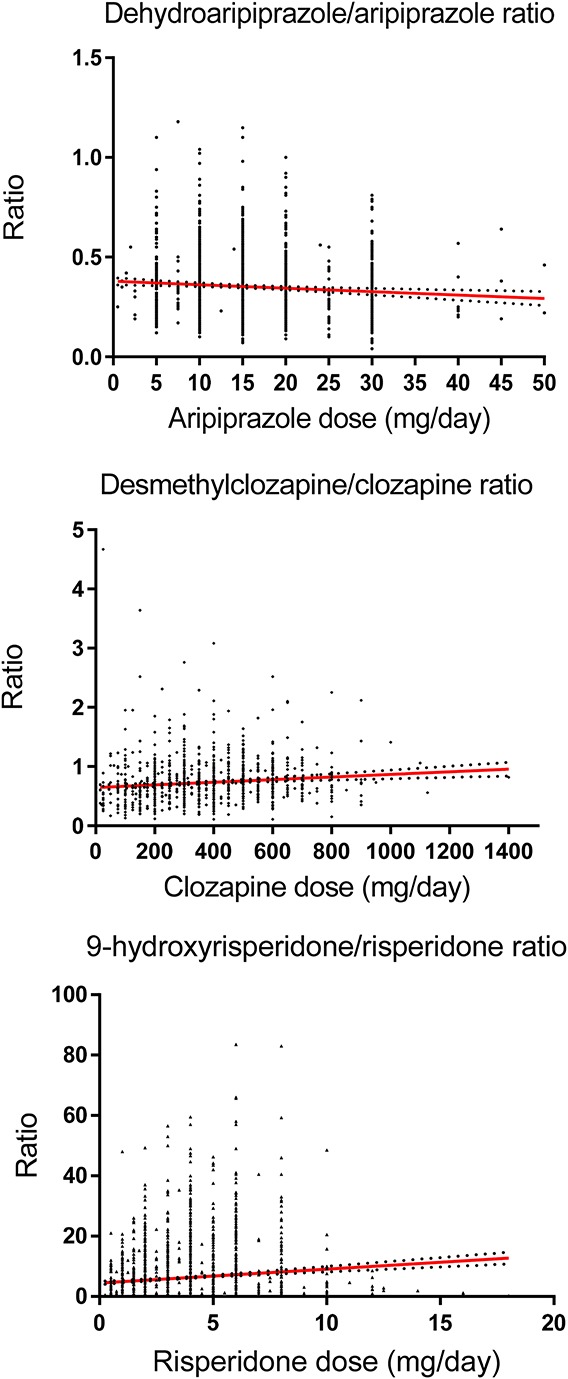
The daily dose–metabolite/parent substance ratios for aripiprazole, clozapine, and risperidone correlation were analyzed by linear regression (regression lines with 95% confidence intervals). Two extreme ratios (3.71 and 1.52, respectively) were excluded from the dehydroaripiprazole/aripiprazole figure, one ratio (36.5) was excluded from the desmethylclozapine/clozapine figure, and one ratio (300) was excluded from the 9-hydroxyrisperidone/risperidone figure. The slopes of the regression lines were significantly different from zero (r2 = 0.007; P = 0.0008 for dehydroaripiprazole/aripiprazole; r2 = 0.01 P < 0.0001 for desmethylclozapine/clozapine; r2 = 0.01; P < 0.0001 for 9-hydroxyrisperidone/risperidone).
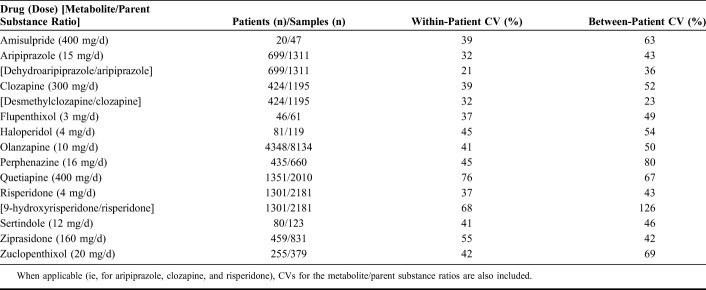
CVs within and between individuals for the concentrations of the parent antipsychotic drug at the most common daily dose and, when applicable, for the metabolite/parent antipsychotic drug ratio are shown in Table 5. For most drugs, the within-individual CVs were in the range of 30%–50%, with a notable exception for quetiapine, where it was 76%. The between-individual CVs were for most drugs even higher than the corresponding within-individual CVs (Table 5).
TABLE 5.
Coefficients of Variation Within and Between Individuals for the Concentrations Found at the Most Typical Dose Used for Each of the Antipsychotic Drugs Included
DISCUSSION
In this study, we present the most comprehensive compilation of TDM reference concentrations of antipsychotic drugs in a naturalistic setting. The AGNP (Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie) group has recently published updated consensus guidelines for TDM in psychopharmacology.6 In that review, therapeutic reference ranges of antipsychotics have been recommended when used for their primary indication. A rough comparison with the serum concentrations found in our TDM population shows that most of these concentrations were within the recommended ranges. Concentrations outside these intervals could be caused by numerous factors including noncompliance, genetically determined or disease-related excessively slow (or ultrarapid) drug metabolism, pharmacokinetic interactions, use of doses higher or lower than those recommended for the main indication of the drug, and concentrations not representing trough levels of the drugs.
Dose-adjusted serum concentrations were significantly higher in women than in men for all drugs except sertindole and quetiapine. For sertindole, the difference in the median C/D ratio was about the same numerically as for the other drugs. Because there is no biological rationale that sertindole should be an exception with this respect, we consider this nonsignificant result to be caused by a power issue due to the low number of patients (n = 111) included. Several factors might explain the generally higher concentrations in women than in men, including differences in hepatic clearance of drugs, caused by a lower liver volume in women and/or by differential expression of cytochrome P-450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) enzymes.16,17 Interestingly, the differences were numerically larger for typical CYP1A2 substrates (eg, clozapine and olanzapine) than for typical CYP2D6 substrates (eg, perphenazine), consistent with the larger difference in expression between sexes for CYP1A2 than for CYP2D6. A factor adding to the CYP1A2 difference could be that males more often than females are smokers. Notably, quetiapine was the only drug for which the concentration was lower in women than in men. This is consistent with the fact that, in contrast to other CYP enzymes, the activity of CYP3A4 has been found to be higher in women than in men, and quetiapine is the most typical CYP3A4 substrate among the drugs included in this study. The same general pattern as in our study has been observed in previous studies of the most commonly used second-generation antipsychotics,3,5,18–21 although studies on ziprasidone, amisulpride, and aripiprazole have not been able to demonstrate any sex-related differences.5,22–24 Finally, it should be taken into account that possible variations in compliance for antipsychotics between males and females13 could add complexity to the understanding of the sex differences observed.
Dose-adjusted serum concentrations were significantly higher in patients 65 years and older for all antipsychotic drugs except flupenthixol and ziprasidone (for sertindole, no subjects older than 65 years were included in the study). Again, we consider these apparent exceptions caused by a type II error due to the low number of elderly included (n = 25 for flupenthixol and n = 39 for ziprasidone). The fact that elderlies have higher C/D ratios is generally consistent with the results from previous studies.18–20,25,26 The most comprehensive previous study of age effects on clozapine, olanzapine, risperidone, and quetiapine concentrations has been published by our group, using information from the same database as in this study.21 That study illustrates that “elderly” should not be viewed as a homogenous group and that the increases in concentrations are particularly prominent from about 80 years of age. The age effect was most pronounced for clozapine, where subjects aged 80 and 90 years, respectively, on average had dose-adjusted concentrations 2-fold and 3-fold higher than those aged 40 years. Thus, in patients of advanced age, dose reductions should be even larger than what could be anticipated based on the differences in C/D ratios between younger and older subjects presented in Table 3.
All drugs except aripiprazole, flupenthixol, haloperidol, and sertindole displayed lower C/D ratios at the higher dose levels compared with the lower dose levels. Again, we suspect that the nonsignificant differences for these 4 drugs are related to type II errors. For drugs exhibiting linear (first-order) kinetics, including those included in this study, the C/D ratio should principally be the same irrespective of dose. In general, for drugs with zero-order kinetics, the C/D ratio should increase and not decrease with increasing dose. As the C/D ratio expresses the inverse value of the oral clearance, what we have found is an increased clearance with higher dose (Css = (dose/Δt)/Cl; where Css is the concentration in steady state, Δt is the time interval between 2 doses, and Cl is clearance). We consider this effect to be a logical consequence of the naturalistic and nonrandomized design of our study, where those having an inherent higher clearance tend to be treated with a higher dose just to compensate for their increased clearance, thereby achieving the same therapeutic effect as in those having a lower clearance. It can also be speculated whether the use of TDM in fact could amplify this effect as it might be tempting to adjust the dose when the serum concentration of a drug is outside what could be considered as “normal”.
The correlation between daily dose and the M/P ratio for an antipsychotic drug is expected to be zero when linear pharmacokinetics prevails. In our study, however, the M/P ratio decreased with dose for aripiprazole, whereas it increased for clozapine and risperidone. As increased clearance of the parent antipsychotic drug causes a decrease in the M/P ratio, the effect seen for clozapine and risperidone could be caused by the same phenomenon as described above. It is harder to explain the effect on aripiprazole, but is should be noted that although statistically significant, the slopes were close to zero.
The within-individual variations in the serum concentrations were generally lower than the between-individual variations. Moreover, for aripiprazole and clozapine, the variability of the M/P ratio was lower than that of respective parent drug. A larger-than-normal within-individual variation has previously been suggested as a tool to identify noncompliant patients.8 The within-individual perspective is also useful when a possible interacting medication is introduced or stopped in a patient, or if somatic comorbidity occurs. Not surprisingly, the variability was largest for drugs with short elimination half-lives such as quetiapine and ziprasidone (about 7 hours for both). The even shorter elimination half-life of risperidone (about 3 hours) is not mirrored in the variability of this drug, as the CV presented in Table 5 is based on the sum of risperidone and its active metabolite 9-hydroxyrisperidone, which have a considerably longer elimination half-life. However, these differential elimination half-lives also explained the high variability in the M/P ratio of risperidone because risperidone itself is found in the denominator of this ratio.
This study has some strengths and weaknesses that should be addressed. One of the main limitations is that no structured or detailed information were available on time intervals from last dose to sampling, concomitant medication used, or whether steady state was achieved. Other shortcomings are the lack of information on body weight and smoking habits of the patients. Information on ethnic background and CYP enzyme genotype could also have added interesting data. It is also unknown whether the subjects included are representative for the whole population of patients using antipsychotic drugs, and it is not known to what degree the patients were adherent to the treatment. However, the naturalistic design of this study could also be considered an advantage, and the study included more than 26,000 patients in the final data set, which may counterbalance the possible impact the inaccuracies mentioned above would have on the principal results found. We also consider it a strength that only one sample per patient has been included because this would reduce the influence of outliers, which could otherwise be expected to be represented with a higher number of samples than the average patient.
Although we had a large total sample, it would have been advantageous to have more samples for some drugs, such as sertindole and flupenthixol. However, the few samples for these drugs reflect that they are infrequently used, thereby reducing the impact of the uncertainties related to the results for these drugs in clinical practice. Nevertheless, for most drugs, there were too few subjects of advanced age to be able to subdivide elderly patients according to exact age. No data were available for the antipsychotics approved most recently, such as paliperidone and lurasidone. However, data for these drugs are emerging, as exemplified by the recently published preliminary report from our group on 310 TDM samples obtained after administration of the long-acting injectable formulation of paliperidone.27
CONCLUSIONS
The data shown in this study represent a naturalistic population of patients using antipsychotics comprising both sexes, all ages, various comorbidities, and all possible concomitant medications. For each of the 12 antipsychotic drugs evaluated, the results presented can serve as a reference tool for pharmacokinetic interpretation of an individual patient's drug level. The compiled serum concentrations and the C/D ratios can thus support the physician when individualizing dosing and determining treatment strategies for a specific patient.
ACKNOWLEDGMENTS
The authors thank Ludvig Johannessen for retrieving the original TDM data files.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remington G, Chong SA. Conventional versus novel antipsychotics: changing concepts and clinical implications. J Psychiatry Neurosci. 1999;24:431–441. [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman MV. Secondary effects of antipsychotics: women at greater risk than men. Schizophr Bull. 2009;35:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford MB, DeLisi LE. Issues related to sex differences in antipsychotic treatment. Curr Opin Psychiatry. 2016;29:211–217. [DOI] [PubMed] [Google Scholar]
- 5.Aichhorn W, Whitworth AB, Weiss EM, et al. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug Saf. 2006;29:587–598. [DOI] [PubMed] [Google Scholar]
- 6.Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51:9–62. [DOI] [PubMed] [Google Scholar]
- 7.Patteet L, Morrens M, Maudens KE, et al. Therapeutic drug monitoring of common antipsychotics. Ther Drug Monit. 2012;34:629–651. [DOI] [PubMed] [Google Scholar]
- 8.Reis M, Åberg-Wistedt A, Ågren H, et al. Compliance with SSRI medication during 6 months of treatment for major depression: an evaluation by determination of repeated serum drug concentrations. J Affect Disord. 2004;82:443–446. [DOI] [PubMed] [Google Scholar]
- 9.Reis M, Aamo T, Ahlner J, et al. Reference concentrations of antidepressants. A compilation of postmortem and therapeutic levels. J Anal Toxicol. 2007;31:254–264. [DOI] [PubMed] [Google Scholar]
- 10.Reis M, Aamo T, Spigset O, et al. Serum concentrations of antidepressant drugs in a naturalistic setting: compilation based on a large therapeutic drug monitoring database. Ther Drug Monit. 2009;31:42–56. [DOI] [PubMed] [Google Scholar]
- 11.Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry. 2007;68:1540–1545. [DOI] [PubMed] [Google Scholar]
- 12.Castberg I, Spigset O. Effects of comedication on the serum levels of aripiprazole: evidence from a routine therapeutic drug monitoring service. Pharmacopsychiatry. 2007;40:107–110. [DOI] [PubMed] [Google Scholar]
- 13.Castberg I, Westin AA, Spigset O. Does level of care, sex, age, or choice of drug influence adherence to treatment with antipsychotics? J Clin Psychopharmacol. 2009;29:415–420. [DOI] [PubMed] [Google Scholar]
- 14.Söderberg C, Wernvik E, Tillmar A, et al. Antipsychotics—postmortem fatal and non-fatal reference concentrations. Forensic Sci Int. 2016;266:91–101. [DOI] [PubMed] [Google Scholar]
- 15.Snedecor GW, Cochran WG. Analysis of variance: the random effects model. In: Statistical Methods. 7th ed Ames, IA: The IA State University Press; 1980:238. [Google Scholar]
- 16.Bartkowiak-Wieczorek J, Wolski H, Bogacz A, et al. Gender-specific implications for pharmacology in childbearing age and in postmenopausal women. Ginekologia polska. 2015;86:143–149. [DOI] [PubMed] [Google Scholar]
- 17.Meibohm B, Beierle I, Derendorf H. How important are gender differences in pharmacokinetics? Clin Pharmacokinet. 2002;41:329–342. [DOI] [PubMed] [Google Scholar]
- 18.Patel MX, Bowskill S, Couchman L, et al. Plasma olanzapine in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1999-2009. J Clin Psychopharmacol. 2011;31:411–417. [DOI] [PubMed] [Google Scholar]
- 19.Lane HY, Chang YC, Chang WH, et al. Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics. J Clin Psychiatry. 1999;60:36–40. [DOI] [PubMed] [Google Scholar]
- 20.Aichhorn W, Weiss U, Marksteiner J, et al. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19:395–401. [DOI] [PubMed] [Google Scholar]
- 21.Castberg I, Westin AA, Skogvoll E, et al. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr Scand. 2017;136:455–464. [DOI] [PubMed] [Google Scholar]
- 22.Bowskill SV, Patel MX, Handley SA, et al. Plasma amisulpride in relation to prescribed dose, clozapine augmentation, and other factors: data from a therapeutic drug monitoring service, 2002-2010. Hum Psychopharmacol. 2012;27:507–513. [DOI] [PubMed] [Google Scholar]
- 23.Molden E, Lunde H, Lunder N, et al. Pharmacokinetic variability of aripiprazole and the active metabolite dehydroaripiprazole in psychiatric patients. Ther Drug Monit. 2006;28:744–749. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann CJ, Rieger-Gies A, Heinzel-Gutenbrunner M, et al. Large variability of aripiprazole and dehydroaripiprazole serum concentrations in adolescent patients with schizophrenia. Ther Drug Monit. 2008;30:462–466. [DOI] [PubMed] [Google Scholar]
- 25.Bakken GV, Rudberg I, Molden E, et al. Pharmacokinetic variability of quetiapine and the active metabolite N-desalkylquetiapine in psychiatric patients. Ther Drug Monit. 2011;33:222–226. [DOI] [PubMed] [Google Scholar]
- 26.Molden E, Waade RB, Hoff M, et al. Impact of ageing on serum concentrations of risperidone and its active metabolite in patients with known CYP2D6 genotype. Basic Clin Pharmacol Toxicol. 2016;119:470–475. [DOI] [PubMed] [Google Scholar]
- 27.Helland A, Spigset O. Serum concentrations of paliperidone after administration of the long-acting injectable formulation. Ther Drug Monit. 2017;39:659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]